Abstract
Objective
To evaluate the association of prevalent unilateral THA with worsening of degenerative knee abnormalities and clinical outcomes in the ipsilateral and contralateral knee.
Methods
Both knees of 30 individuals in the Osteoarthritis Initiative (OAI) with unilateral THA (n=14 left, n=16 right) at baseline were assessed at baseline and at 4-year follow-up for Whole-organ MR Imaging Scores (WORMS), cartilage T2 relaxation time (only available for right knees) Western Ontario and McMasters Universities Arthritis Index (WOMAC) scores and upper leg isometric strength. Right knees of 30 individuals without THA were analyzed as controls. Contralateral knees were compared to ipsilateral knees with paired t-tests and to control knees with multivariate regression analysis adjusting for covariates.
Results
In paired analyses, compared to ipsilateral knees, contralateral knees had higher WORMS total (P=0.008) and cartilage scores (P=0.007) at baseline. Over 4 years contralateral knees worsened more on WORMS total score (P=0.008). Cartilage T2 values were higher knees contralateral to the THA (baseline, P=0.02; follow-up, P<0.001). Contralateral knees had greater declines in knee extension strength (P=0.04) and had a trend for greater worsening in WOMAC pain, stiffness, function and total scores (P=0.04 to 0.09). Similar results were found comparing contralateral knees with control knees in multivariate regression models.
Conclusions
Prevalent unilateral THA is associated with an greater progression of degenerative findings for the knee contralateral to THA.
Keywords: Hip arthroplasty, Osteoarthritis, knee, cartilage, T2 measurements, WORMS
INTRODUCTION
Osteoarthritis (OA) of the knee or hip joint is a major cause for disability in our aging society [1]. Joint degeneration of one large joint is associated with degenerative changes in other large joints [2]. Interestingly, patients with unilateral total hip arthroplasty (THA) have a higher rate of total knee replacements on the contralateral side rather than the ipsilateral side [3][4]. However, the association of THA with degenerative changes in the ispi-versus contralateral knee is not well understood. No longitudinal follow-up studies have evaluated the influence of unilateral THA on knee pain and function or on degenerative changes at the knee joint assessed with MRI [5]. MRI provides detailed information on morphological abnormalities in OA [6][7]. Recently, T2 mapping techniques have been developed to visualize and quantitatively evaluate early cartilage matrix damage, mainly collagen disruption and elevation of cartilage water content [8, 9].
The Osteoarthritis Initiative (OAI) is an ongoing longitudinal, NIH initiated multi-center, prospective observational study of knee OA (https://oai.epi-ucsf.org/datarelease/StudyOverview.asp). The overall aim is to develop a public domain research resource to facilitate the scientific evaluation of biomarkers for OA as potential surrogate endpoints for disease onset and progression. Four clinical centers and a data coordinating center conduct the OAI, a public-private partnership, that bring together new resources and commitment to help find biochemical, genetic and imaging biomarkers for development and progression of OA. The OAI establishes and maintains a natural history database for OA that includes clinical evaluation data, radiological images, and a biospecimen repository from 4796 men and women [10].
The purpose of this study was to determine the association of prevalent unilateral THA with degenerative changes at the ipsilateral and contralateral knee at baseline and in a four year longitudinal follow-up. We hypothesized that unilateral THA is associated with more advanced degenerative changes (3T MRI assessment), knee symptoms and muscle weakness in the contralateral compared to the ipsilateral knee, and in particular, that these findings show greater progression in contralateral knees over 4 years.
METHODS
Subjects
Individuals from the OAI with a unilateral THA at baseline were included in the study if they had no metallic implants in either knee at either time-point, no additional THA at the contralateral hip at the 4 year follow-up and had complete MRIs in both knees at baseline and 4 year follow-up. There were 30 subjects with a unilateral THA (14 left, 16 right) who met these criteria (23/30 from the OAI incidence cohort, 7/30 from the OAI progression cohort). As a reference group, 30 subjects without THA were age, gender, BMI and OAI cohort matched (24/30 from the OAI incidence cohort, 6/30 from the OAI progression cohort). The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. The study protocol, amendments, and informed consent documentation were approved by the local institutional review boards. Specific OAI datasets used in this study were baseline datasets 0.2.2 and 0.E.1 and the 4 year follow-up datasets 6.2.1 and 6.E.1 (http://www.oai.ucsf.edu/).
Imaging: pelvic and knee radiography
Bilateral standing anterior-posterior pelvic radiographs and posterior-anterior knee radiographs were acquired (http://oai.epi-ucsf.org/datarelease/OperationsManuals.asp). Pelvic radiographs were screened for presence of unilateral THA at baseline and year 4. Baseline Kellgren-Lawrence (K-L) scores for both knees of each individual were obtained from the OAI database (central K-L readings).
Imaging: Knee MRI
MRI sequences for semiquantitative WORMS readings and for quantitative T2 mapping were acquired in right knees at 4 clinical sites using 3T MRI scanners (Siemens Magnetom Trio; Siemens, Erlangen, Germany) and quadrature transmit-receive knee coils (USA Instruments, Aurora, OH). Details of the acquisition protocol are given in table 1 [11].
Table 1.
MR pulse sequences.
| Parameter | 2D IM-w FSE | 3D DESS WE | 3D T1-w FLASH WE | 2D IM-w FS FSE | 2D T2-w MSME SE |
|---|---|---|---|---|---|
| Plane | Coronal | Sagittal | Coronal | Sagittal | Sagittal |
| Field of view (FOV; mm) | 140 | 140 | 160 | 160 | 120 |
| Matrix | 128×256 | 307×384 | 512×512 | 313×448 | 269×384 |
| Number of slices | 35 | 160 | 80 | 37 | 21 |
| Slice thickness (mm) | 3 | 0.7 | 1.5 | 3 | 3 |
| Repetition time (RT; msec) | 3700 | 16.3 | 20 | 2700 | 3200 |
| Echo time (ET; msec) | 29 | 4.7 | 7.6 | 10,20,30,40, 50,60,70 | 30 |
| Refocusing flip angle (°) | 180 | 25 | 12 | 180 | n/a |
| Bandwidth (Hz/ Pixel) | 352 | 185 | 130 | 248 | 250 |
| Echo train length | 7 | 1 | 1 | 5 | 1 |
| Acquisition time (min) | 3.4 | 10.6 | 8.6 | 4.7 | 10.6 |
IM-w, intermediate-weighted; FSE, fast spin echo; DESS, dual-echo steady-state; WE, water-excitation; FLASH, fast low-angle shot; 3D, three-dimensional; 2D, two-dimensional; FS, fat saturated; MSME, multislice-multiecho; SE, spin echo.
Semi-quantitative morphological Knee MRI analyses
Morphological MR images (Table 1) of both knees of the unilateral THA subjects and of right knees of those without a THA were assessed for OA-related abnormalities using a modified whole-organ magnetic resonance imaging score (WORMS) [12, 13]. MR images were reviewed on picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ). MRIs were evaluated by two musculoskeletal radiologists separately (P.M.J., L.N.; 6 and 8 years of experience, respectively), blinded for the THA side and for the time-point; if scores were not identical consensus readings by both radiologists and a third independent radiologist (T.M.L., 23 years of experience) were performed. A total WORMS summation score, with a potential maximum of 110 was calculated as the sum of grades for all of the knee features: (i) meniscus abnormalities (score 0–4 in 6 regions), (ii) cartilage lesions (score 0–6 in 6 regions), (iii) bone marrow lesions (score 0–3 in 6 regions) (iv) ligament abnormalities (score 0–4 in 6 locations), and (v) other abnormalities (effusion (0–3), intraarticular body (0–2), baker cyst (0–3). Additionally, meniscus scores were calculated for the medial and for the lateral meniscus (score 0, all meniscus subregions “0“; score 1, no >1; score 2, “2“ in one subregion; score 3, “2“ in >1 subregion; score 4, “3“ in ≥1 subregion; score 5, “4“ in one subregion; score 6, “4“ in ≥1 subregion). For the parameters cartilage, bone marrow lesions, ligament abnormalities and other abnormalities maximum scores were determined, indicating the maximum score of all subregions for each parameter. The total maxWORMS score with a potential maximum of 28 was defined as the sum of the meniscus scores and the maximum scores.
T2 relaxation time measurements
T2 mapping MSME spin echo sequences, available for all right knees were analyzed on a remote workstation (SPARC; Sun Microsystems, Mountain View, CA) using an in-house software implemented in MATLAB (The Mathworks Inc., Natick, MA) [14–16]. Semi-automated spline-based segmentation was performed by two radiologists (P.M.J. and H.L.; 6 and 2 years of experience) under supervision of an experienced radiologist (T.M.L.; 23 years of experience) in five compartments: (1) patella, (2) medial and (3) lateral femoral condyle and (4) medial and (5) lateral tibia. The trochlea was excluded because of interfering flow artifacts from the popliteal artery. Segmentation was performed on all slices of the first echo images. T2 maps were created using a monoexponential decay model as fitting function to calculate the signal intensity at the different echo times. T2 calculations were measured from the second (20 ms) to the last (70 ms) echo images, dropping the first echo time [17–19]. T2 values of baseline and four-year follow-up time points were calculated. “Global” T2 value was defined as mean of all compartments combined. Four-year progression was calculated as the difference (T2 follow-up-T2 baseline). Since T2 mapping sequences were only available for right knees, comparisons of T2 values between the groups were non-paired. The “contralateral THA“ group was represented by the 14 individuals with a left THA and the “ipsilateral THA“ group was represented by the 16 individuals with a right THA.
Reproducibility of MRI measurements
Reproducibility was calculated in a randomly selected sample of 10 OAI subjects. For WORMS measurements, images were graded twice by two radiologists (P.M.J., L.N.) on two separate occasions (2 weeks in-between) and linear weighted Cohen’s Kappa values were calculated. For cartilage defects, inter-observer kappa was 0.89, intra-observer kappa was 0.91 and 0.95, respectively. For meniscus defects, inter-observer kappa was 0.80, intra-observer kappa was 0.89 and 0.95. For ligament abnormalities, inter-observer kappa was 0.65, intra-observer kappa was 0.76 and 0.77. For bone marrow abnormalities, inter-observer kappa was 0.80, intra-observer kappa was 0.81 and 0.87. For other scorings, inter-observer kappa was 0.70, intra-observer kappa was 0.79 and 0.79. Inter-observer agreement for T2 measurements (coefficient of variation) was described previously with an inter-reader reproducibility error for mean T2 of 1.57%, respectively 0.53ms [13]. Mean intra-reader reproducibility for T2 measurements was 1.66%, respectively 0.55ms.
WOMAC
Knee symptoms assessed by WOMAC (University of Western Ontario and the McMaster University in Canada) in both knees [20, 21]. The WOMAC score is an established multidimensional health status instrument. It quantifies the degree of three different domains of disease status (pain, stiffness and functional impairment) through a 5-point scale (none, slight, moderate, severe and extreme), with higher scores indicating increasing disease severity
Physical Activity Scale for the Elderly
Physical activity levels were measured using the Physical Activity Scale for the Elderly (PASE) [22, 23]. The PASE score aims to assess general activity and health status in older adults. This established score includes questions on household chores and occupational activities, as well as individual activities such as knee bending, squatting and stair climbing. It has a scale range from 0 to 400 and was described as valid and reliable for epidemiologic studies.
Isometric strength
Isometric strength measurements were performed using a Good Strength apparatus (Metitur, Jyväskylä, Finland; www.oai.ucsf.edu/datarelease/OperationsManuals.asp) for knee flexion and extension. Two submaximal practice trials were completed before force was measured three times for 3 seconds, each separated by 30 seconds; the highest value for a limb is used for maximal strength reported (N).
Other baseline measurements
Heberden’s nodes were considered present if bony enlargements were found in ≥3 DIP joints in either hand during an examination of the hand at baseline (http://oai.epi-ucsf.org/datarelease/forms.asp). History of knee injury or surgery, familial predisposition of OA, defined as a total knee replacement for OA in a biological parent or sibling, were assessed by self-report (https://oai.epi-ucsf.org/datarelease/operationsManuals.asp). Body mass index (BMI) was calclulated as weight in kg/height m2.
Statistical analysis
Statistical analysis was performed with JMP software Version 9 (SAS Institute, Cary, NC, USA). The analyses compared knees contralateral to THA with knees ipsilateral to THA and with knees of controls without THA for change in measures of knee OA over four years, for baseline scores and for scores at year 4 of follow-up. Paired t-tests were used to compare outcomes between paired ipsilateral and contralateral knees in the same subject. Multivariable linear regression was used for comparisons between contralateral and control knees in different subjects and for comparisons of T2. From the regression models adjusted means and standard errors (SEM), adjusted mean differences and 95% confidence intervals were obtained. Covariates were OA risk factors including: self-reported history of knee injury or surgery, familial predisposition of OA, defined as a total knee replacement for OA in a biological parent or sibling, Heberden’s nodes, age, gender BMI and PASE score. Sensitivity analyses were also performed by including preexisting knee OA (K-L grade) at baseline as a covariate in mixed model fits (Stata Ver 13, College Station, TX, USA) and in the linear regression models. The level of significance was set at P<0.05.
RESULTS
Baseline characteristics
There were no statistically significant differences in gender, age, BMI or physical activity between subjects with unilateral THA and controls with no THA (P>0.05; table 2A). Subjects with a THA in the left hip (i.e. the right knee is ‘contralateral’ and also used in comparisons to right control knees) had lower PASE physical activity scores than controls (113±47 versus 158±73, P=0.04). K-L grades were significantly different between the groups (Table 2B).
Table 2.
Baseline Characteristics.
| 2A. Subject characteristics by baseline THA status. | ||||
|---|---|---|---|---|
| Subjects with | ||||
| BL characteristic | 1. Unilateral THA | 2. Unilateral THA in L hip | 3. Unilateral THA in R hip | 4. Controls, No THA |
| Subjects (n) | 30 | 14 | 16 | 30 |
| Gender (n; Male/ Female) | 43% (13/17) | 43% (6/8) | 44% (7/9) | 47% (14/16) |
| Age ±SD (years) | 67±10 | 67±10 | 67±11 | 66±10 |
| Body mass index ±SD (kg/m2) | 28.5±4.3 | 28.8±3.4 | 28.2±4.9 | 27.9±4.7 |
| PASE ±SD | 127±53 | 113±47 * | 140±56 | 158±73 |
| 2B. Knee K-L grade by baseline THA status. | |||||
|---|---|---|---|---|---|
| BL KL grade | Subjects with Unilateral THA | No THA (controls) | |||
| 1. All contralateral knees | 2. All ipsilateral knees | 3. Right contalateral knees * | 4. Right ipsilateral knees * | 5.Rt knees | |
| 0–1 | 50/100% (15/30) | 70/100%(21/30) | 43/100% (6/14) | 75/100% (12/16) | 73/100% (22/30) |
| 2 | 23/100% (7/30) | 20/100% (6/30) | 21/100% (3/14) | 19/100% (3/16) | 10/100% (3/30) |
| 3 | 17/100% (5/30) | 7/100% (2/30) | 29/100% (4/14) | 6/100% (1/16) | 17/100% (5/30) |
| 4 | 10/100% (3/30) | 3/100% (1/30) | 7/100% (1/14) | 0/100% (0/16) | 0/100% (0/30) |
| Knees (n) | 30 | 30 | 14 | 16 | 30 |
2 vs. 4, P=0.038. All other comparisons are NS, P > 0.05.
BL = Baseline; SD = Standard deviation; PASE = Physical Activity Scale for the Elderly; THA = Total hip arthroplasty; R = right; L = left
Only right knees are included in analyses of differences in T2 relaxation times.
1 vs 2, P=0.04 1 vs 5, P = 0.04; 3 vs 4, P=0.02; 3 vs 5, P=0.04; 4 vs. 5, P=0.56. THA = Total hip arthroplasty; K-L = Kellgren-Lawrence.
WOMAC
WOMAC scores of knees contralateral to prevalent THA worsened on average over 4 years (Total score, 2.0±2.6) while improving in ipsilateral knees (Total score, −3.4±2.6, P=0.06; Table 3). WOMAC scores in control knees also improved during follow-up (Total score, −2.3±2.6; contralateral versus control, P=0.13). Baseline WOMAC scores in contralateral knees were not significantly different compared to ipsilateral knees (Total scores, 8.4±2.3 versus 5.9±2.2, P=0.29) or compared to control knees (Total score, 8.8±2.3, P=0.38; Table 3). Adjusting for baseline K-L grade had little impact on these comparisons, except that worsening in WOMAC total (P=0.03) and function (P=0.03) scores were now significantly greater in contralateral versus ipsilateral knees.
Table 3. Baseline Knee pain and MRI findings, and change, in knees contralateral and ipsilateral to THA.
Adjusted mean baseline values and adjusted mean changes over 4 years ± standard error of the mean (SEM) for WOMAC and WORMS scores by THA status. Contralateral vs ipsilateral knee comparisons are paired within subject differences. Contralateral compared to right knees of subjects with No THA are adjusted means from multivariate linear regression models, adjusted for age, gender, PASE, BMI and risk factors.
| BASELINE | Unilateral THA | No THA) (controls) right knee | Contralateral vs ipsilateral | Contralateral vs control | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Contralatera knee | Ipsilateral knee | P-value (paired t-test) # | Paired Difference (95% CI) # | P-value ## (95% CI) | Adj. mean difference (95% CI) ## | |||
| WOMAC | Total score | 8.4±2.3 | 5.9±2.2 | 8.8±2.3 | 0.29 | 2.4 (−2.1; 6.9) | 0.38 | −2.3 (−7.6; 2.9) |
| Pain | 1.9±0.5 | 1.3±0.5 | 2.0±0.6 | 0.20 | 0.6 (−0.3; 1.5) | 0.42 | −0.5 (−1.8; 0.8) | |
| Stiffness | 1.1±0.4 | 1.0±0.4 | 1.5±0.4 | 0.61 | 0.2 (−0.5; 0.8) | 0.16 | −0.6 (−1.4; 0.2) | |
| Function | 5.7±1.7 | 4.1±1.7 | 5.3±1.7 | 0.39 | 1.5 (−2.0; 5.0) | 0.56 | −1.2 (−5.1; 2.8) | |
| WORMS | Total score | 28.1±2.4 | 21.3±2.4 | 19.7±2.4 | 0.008 | 6.8 (1.9; 11.7) | 0.07 | 5.4 (−0.4; 11.1) |
| Med. meniscus | 2.9±0.4 | 2.0±0.4 | 1.7±0.4 | 0.01 | 0.8 (0.0; 1.6) | 0.06 | 0.8 (−0.0; 1.6) | |
| Lat. meniscus | 2.0±0.4 | 1.6±0.4 | 0.9±0.4 | 0.28 | 0.4 (−0.3; 1.1) | 0.05 | 0.9 (0.0; 1.9) | |
| Ligaments | 3.8±0.7 | 3.0±0.7 | 3.8±0.7 | 0.25 | 3.0 (−0.6; 2.1) | 0.59 | −0.4 (−2.0; 1.1) | |
| Cartilage | 13.4±1.2 | 10.1±1.2 | 8.9±1.3 | 0.007 | 3.4 (1.0; 5.8) | 0.04 | 3.1 (0.2; 6.1) | |
| BME* | 3.4±0.6 | 2.6±0.7 | 2.9±0.7 | 0.21 | 0.7 (−0.4; 1.9) | 0.71 | −0.3 (−1.9; 1.3) | |
|
| ||||||||
| CHANGE BASELINE TO 4 YEARS | ||||||||
|
| ||||||||
| WOMAC | Total score | 2.0±2.6 | −3.4±2.6 | −2.3±2.6 | 0.06 | 5.1 (−0.2;10.4) | 0.13 | 4.8 (−1.4; 11.1) |
| Pain | 0.5±0.7 | −0.5±0.6 | −0.7±0.7 | 0.09 | 1.0 (−0.2; 2.2) | 0.31 | 0.8 (0.8; 2.4) | |
| Stiffness | 0.0±0.3 | −0.7±0.3 | −0.2±0.3 | 0.04 | 0.7 (0.1; 1.3) | 0.34 | 0.4 (−0.4; 1.2) | |
| Function | 1.8±1.9 | −2.3±1.9 | −1.5±1.9 | 0.06 | 3.9 (−0.1; 7.8) | 0.10 | 2.9 (−0.7; 8.5) | |
| WORMS | Total score | 7.9±1.1 | 4.6±1.1 | 5.7±1.2 | 0.008 | 3.3 (0.9; 5.7) | 0.03 | 2.8 (0.2; 5.4) |
| Med. meniscus | 0.8±0.2 | 0.4±0.2 | 0.2±0.2 | 0.06 | 0.4 (−0.0; 0.8) | 0.009 | 0.6 (0.2; 1.1) | |
| Lat. meniscus | 0.6±0.2 | 0.4±0.2 | 0.6±0.2 | 0.26 | 0.4 (−0.2; 0.6) | 0.70 | −0.1 (−0.6; 0.4) | |
| Ligaments | 2.4±0.5 | 0.8±0.5 | 0.6±0.5 | 0.01 | 0.8 (0.3; 2.8) | 0.009 | 1.7 (−0.4; 2.9) | |
| Cartilage | 3.8±0.6 | 3.0±0.6 | 3.2±0.6 | 0.25 | 3.0 (−0.6; 2.3) | 0.19 | 1.0 (−0.5; 2.5) | |
| BME* | −0.1±0.5 | −0.9±0.5 | 0.6±0.6 | 0.26 | 0.9 (−0.6; 2.3) | 0.69 | −0.3 (−1.6; 1.0) | |
paired difference = contralateral – ipsilateral; P-value and 95% from paired t-test
P-value and adjusted mean difference and 95% CI from the multivariate regression model, contralateral vs control knees. Covariates were OA risk factors including: self-reported history of knee injury or surgery, familial predisposition of OA, defined as a total knee replacement for OA in a biological parent or sibling, Heberden’s nodes, age, gender BMI and PASE score.
BME: bone marrow edema-like lesions; P-values are given for the sum of the compartmentspecific BME scores. P-values for maximum BME scores: Pbaseline=0.19; Pincrease=0.45 (ipsi-versus contralateral).
Morphological analysis of knee abnormalities (WORMS)
While WORMS scores tended to worsen during follow-up in all knees, the increase in contralateral knees was greater than ipsilateral knees for total score (7.9±1.1 versus 4.6±1.1, P=0.008; replacing the sum score with the maximum score of bone marrow lesions in the total WORMS score, P=0.03; total maxWORMS score, P=0.05) and ligaments (2.4±0.5 versus 0.8±0.5, P=0.01; ligament maximum score, P=0.10) and compared to control knees for total score (P=0.03; total maxWORMS score, P=0.04), medial meniscus (P=0.009; medial meniscus score, P=0.008) and ligaments (p=0.009; ligament maximum score, P=0.13) (Table 3). Baseline WORMS scores in contralateral knees were significantly worse compared to ipsilateral knees for cartilage of the whole knee (13.4±1.2 versus 10.1±1.2, P=0.007; cartilage maximum score, P=0.06), medial meniscus (2.9±0.4 versus 2.0±0.4, P=0.01; medial meniscus score, P=0.01) and total WORMS scores (28.1±2.4 versus 21.3±2.4, P=0.008; replacing the sum score with the maximum score of bone marrow lesions in the total WORMS score, P=0.006; total maxWORMS score, P=0.008) and compared to control knees for cartilage and lateral meniscus (P<0.05; Table 3). In analyses adjusting for baseline K-L grade, results for worsening of WORMS scores were largely unchanged (total WORMS score, P=0.02), although no longer significant for contralateral versus ipsilateral ligaments (P=0.07) nor for contralateral versus control knee total score worsening (P=0.07). After K-L adjustment no significant differences in baseline WORMS scores between groups were found (total WORMS score, P=0.71).
Examining WORMS cartilage scores in individual cartilage plates (Table 4), there were no significant differences for change in cartilage score. Trochlea (P=0.02) and medial femoral condyle (P=0.008) cartilage worsened more in contralateral knees than control knees. At baseline contralateral knees had significantly worse scores than ipsilateral knees at the trochlea (2.6±0.3 versus 1.7±0.3, P<0.001), lateral femoral condyle (1.7±0.3 versus 1.0±0.3, P=0.02), and lateral tibia (2.1±0.3 versus 1.4±0.3, P=0.02) and worse scores compared control knees at the lateral femoral condyle (P=0.03). Plate-specific cartilage results were essentially unchanged by additional adjustment for baseline K-L grade (change contralateral versus control: trochlea, P=0.02, medial femoral condyle, P=0.01; baseline contralateral versus ipsilateral: trochlea, P=0.05, lateral femoral condyle, P=0.04, lateral tibia, P=0.10; baseline contralateral versus control: lateral femoral condyle, P=0.04).
Table 4. Baseline MRI findings, and change, by compartment, in knees contralateral and ipsilateral to THA.
Adjusted mean baseline values and change over 4 years ± standard error of the mean (SEM) for WORMS compartment-specific cartilage scores by THA status. Contralateral vs ipsilateral knee comparisons are paired, within subject differences. Contralateral and ipsilateral comparisons to right knees of subjects with No THA and adjusted mean values are from multivariate linear regression models. * = P <0.05 for contralateal or ipsilateral knee vs No THA control knees.
| WORMS BASELINE | Unilateral THA | No THA (Controls) right knee | Contralateral vs ipsilateral | Contralateral vs control | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Contralateral knee | Ipsilateral knee | P-value (paired t-test) # | Paired difference (95% CI) # | P-value ## | Adj mean difference (95% CI) ## | ||
| Patella | 3.1±0.4 | 3.0±0.4 | 2.5±0.4 | 0.80 | 0.1 (−0.6; 0.7) | 0.16 | 0.6 (−3; 1.5) |
| Trochlea | 2.6±0.3 | 1.7±0.3 | 1.6±0.4 | <0.001 | 1.0 (0.5; 1.4) | 0.13 | 0.6 (−0.2; 1.5) |
| MFC | 2.1±0.4 | 1.7±0.4 | 1.5±0.4 | 0.26 | 0.4 (−0.3; 1.1) | 0.65 | 0.2 (−0.7; 1.1) |
| LFC | 1.7±0.3 | 1.0±0.3 | 0.9±0.3 | 0.02 | 0.8 (0.2; 1.3) | 0.03 | 0.7 (0.1; 1.4) |
| MT | 1.8±0.3 | 1.3±0.3 | 1.1±0.3 | 0.21 | 0.5 (−0.3; 1.3) | 0.43 | 0.3 (−0.5; 1.0) |
| LT | 2.1±0.3 | 1.4±0.3 | 1.2±0.3 | 0.02 | 0.7 (0.1; 1.3) | 0.05 | 0.6 (0.0; 1.3) |
|
| |||||||
| CHANGE BASELINE TO 4 YEARS | |||||||
|
| |||||||
| Patella | 0.7±0.3 | 0.7±0.3 | 0.4±0.3 | 0.94 | 0.0 (−0.5; 0.5) | 0.20 | 0.4 (−0.2; 1.0) |
| Trochlea | 0.8±0.2 | 0.5±0.2 | 0.3±0.2 | 0.25 | 0.3 (−0.2; 0.7) | 0.02 | 0.5 (0.1; −1.0) |
| MFC | 0.9±0.2 | 0.6±0.2 | 0.3±0.2 | 0.31 | 0.2 (−0.2; 0.7) | 0.008 | 0.7 (0.2; 1.2) |
| LFC | 0.5±0.2 | 0.5±0.2 | 0.7±0.3 | 0.94 | 0.0 (−0.4; 0.5) | 0.32 | −0.3 (−0.9; 0.3) |
| MT | 0.7±0.2 | 0.4±0.2 | 0.3±0.2 | 0.32 | 0.3 (−0.3; 0.9) | 0.05 | 0.5 (0.0; 1.0) |
| LT | 0.4±0.2 | 0.4±0.2 | 0.4±0.2 | 0.87 | 0.0 (−0.4; 0.4) | 0.82 | −0.0 (−0.4; 0.4) |
paired difference = contralateral – ipsilateral; P-value and 95% from paired t-test
P-value and adjusted mean difference and 95% CI from the multivariate regression model, contralateral vs control knees. Covariates were OA risk factors including: self-reported history of knee injury or surgery, familial predisposition of OA, defined as a total knee replacement for OA in a biological parent or sibling, Heberden’s nodes, age, gender BMI and PASE score.
Cartilage T2 relaxation time measurements
T2 relaxation time measurements were only available in right knees so all comparisons are non-paired between different subjects, adjusting for covariates in a multivariate regression model (contralateral, n=14; ipsilateral, n=16; controls, n=30; Figure 1; Table 5). The increase in T2 of the whole knee over four years was significantly greater in contralateral compared to ipsilateral knees (2.9±0.9ms versus 0.4±0.8ms, P=0.02) and compared to control knees (2.9±0.9ms versus − 0.3±0.7ms, P<0.001). T2 in contralateral medial femoral condyles increased more compared to ipsilateral (3.7±1.0ms versus 0.5±0.8ms, P=0.004) and compared to control knees (3.7±1.0ms versus 0.2±0.7ms, P=0.001). At baseline, cartilage T2 relaxation times were non-significantly higher in contralateral knees (all P>0.05; Table 5). Results for T2 were essentially unchanged by additional adjustment for baseline K-L grade (Contralateral versus ipsilateral whole knee T2; change, P=0.006; baseline, P=0.64).
Figure 1.
T2 relaxation time color maps of right knees of individuals with ipsilateral and contralateral total hip arthroplasty (THA), overlaid with the first-echo images of the MSME sequence. Blue color indicates low, red color high cartilage T2 values. The most severe cartilage, medial meniscus and bone marrow pathologies were found in individuals with contralateral THA.
Table 5. Adjusted mean baseline values ± SEM and four year change for the T2 relaxation time (ms).
P-values are from multivariate regression models comparing right contralateral knees with right ipsilateral knees of different subjects with unilateral THA and comparing right contralateral knees of unilateral THA subjects with right knees of control subjects with no THA. MFC = Medial femoral condyle; LFC = Lateral femoral condyle; MT = Medial tibia; LT = Lateral tibia. * = P <0.05 for contralateal or ipsilateral knee vs No THA control knees.
| T2 relaxation time BASELINE | Unilateral THA | No THA (Controls) right knee | Contralateral vs ipsilateral | Contralateral vs control | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Contralateral right knee | Ipsilateral right knee | P-value # | Adj mean difference (95% CI) # | P-value # | Adj mean difference (95% CI) # | ||
| Global | 37.0±0.8 | 35.9±0.6 | 36.0±0.6 | 0.42 | 0.7 (−1.0; 2.4) | 0.60 | 0.4 (−1.1; 2.0) |
| Patella | 37.5±1.4 | 36.5±1.1 | 36.3±1.0 | 0.84 | 0.3 (−2.7; 3.2) | 0.51 | 0.9 (−1.8; 3.6) |
| MFC | 39.8±1.2 | 38.5±0.9 | 38.7±0.9 | 0.51 | 0.8 (−1.7; 3.4) | 0.43 | 0.9 (−1.4; 3.3) |
| LFC | 36.3±0.9 | 35.0±0.7 | 34.9±0.7 | 0.08 | 1.7 (−0.2; 3.7) | 0.06 | 1.8 (−0.0; 3.6) |
| MT | 37.7±1.2 | 36.5±0.9 | 36.6±0.8 | 0.74 | 0.4 (−2.1; 2.9) | 0.76 | 0.3 (−1.9; 2.6) |
| LT | 33.5±1.3 | 32.8±1.0 | 32.0±0.9 | 0.43 | 1.1 (−1.7; 3.9) | 0.28 | 1.4 (−1.2; 3.9) |
|
| |||||||
| CHANGE BASELINE TO 4 YEARS | |||||||
|
| |||||||
| Global | 2.9±0.9 | 0.4±0.8 | −0.3±0.7 | 0.02 | 2.5 (0.5; 4.5) | 0.001 | 2.4 (1.6; 5.2) |
| Patella | 5.3±2.1 | 1.3±1.7 | 0.6±1.5 | 0.20 | 2.9 (−1.6; 7.4) | 0.10 | 3.5 (−0.6; 7.6) |
| MFC | 3.7±1.0 | 0.5±0.8 | 0.2±0.7 | 0.004 | 3.2 (1.6; 5.3) | 0.001 | 3.4 (1.4; 5.3) |
| LFC | 2.7±1.1 | 0.7±0.8 | 0.8±0.7 | 0.14 | 1.7 (−0.6; 3–9) | 0.07 | 1.9 (−0.2; 3.9) |
| MT | 0.3±1.4 | −0.7±1.1 | −0.7±1.0 | 0.73 | 0.5 (−2.4; 3.4) | 0.94 | 0.1 (−2.6; 2.8) |
| LT | 2.3±2.0 | −1.4±1.6 | 0.7±1.4 | 0.09 | 3.7 (−0.6; 7.9) | 0.36 | 1.8 (−2.1; 5.7) |
P-value and adjusted mean difference and 95% CI from the multivariate regression model. Covariates were OA risk factors including: self-reported history of knee injury or surgery, familial predisposition of OA, defined as a total knee replacement for OA in a biological parent or sibling, Heberden’s nodes, age, gender BMI and PASE score.
Isometric upper leg strength
Isometric extension strength decreased significantly more in contralateral compared to ipsilateral knees (−51.6±21.9 versus −15.5±22.4, P=0.04) and compared to control knees (P=0.009), but there were no differences in change in flexion strength (P=0.52 and P=0.12; Table 6). There were no differences in baseline strength between groups. These results were essentially unchanged when K-L grade was included as a covariate (Change extension strength; contralateral versus ipsilateral, P=0.03, contralateral versus control, P=0.005).
Table 6. Baseline and change in isometric extension and flexion strength in knees contralateral and ipsilateral to THA.
Adjusted mean baseline isometric values and change over 4 years ± standard error of the mean (SEM) (Newtons) for isometric strength by THA status. Contralateral vs ipsilateral knee comparisons are paired, within subject differences. Contralateral and ipsilateral comparisons to right knees of subjects with No THA and adjusted mean values are from multivariate linear regression models.
| Maximum isometric Strength (Newtons) BASELINE: |
Unilateral THA | No THA (Controls) right knee | Contralateral vs ipsilateral | Contralateral vs control | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Contralateral knee | Ipsilateral knee | P-value (paired t-test) # | Paired difference (95% CI) # | P-value ## | Adj mean difference (95% CI) ## | ||
| Extension | 334.1±26.1 | 327.0±25.9 | 340.9±26.1 | 0.64 | −7.1 (−37.3; 23.2) | 0.90 | −3.6 (−65.7; 33.7) |
| Flexion | 137.7±15.6 | 138.1±15.5 | 131.6±15.6 | 0.94 | 0.4 (−9.7; 10.5) | 0.72 | −0.6 (−39.3; 27.2) |
|
| |||||||
| CHANGE: BASELINE TO 4 YEARS | |||||||
|
| |||||||
| Extension | −51.6±21.9 | −15.5±22.4 | −4.1±21.8 | 0.04 | −42.0 (−2.8; −81.1) | 0.009 | −73-3 (−127.4; −19.3) |
| Flexion | −33.9±16.1 | −44.7±16.3 | −58.7±16.0 | 0.52 | −11.3 (−46.8; 24.2) | 0.12 | 30.1 (−67.8; 7.6) |
paired difference = contralateral – ipsilateral; P-value and 95% from paired t-test
P-value and adjusted mean difference and 95% CI from the multivariate regression model, contralateral vs control knees. Covariates were OA risk factors including: self-reported history of knee injury or surgery, familial predisposition of OA, defined as a total knee replacement for OA in a biological parent or sibling, Heberden’s nodes, age, gender BMI and PASE score.
Main knee outcome measures at the year 4 follow-up
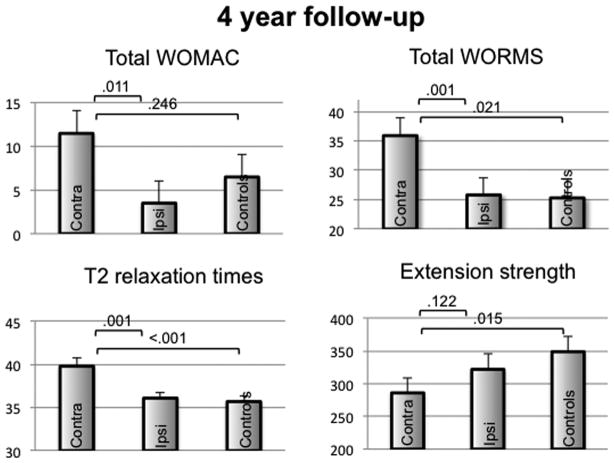
At the 4 year follow-up (Figure 2), compared to ipsilateral knees the contralateral knees had significantly higher WOMAC total scores (11.5±2.6 versus 3.5±2.5, P=0.01); higher WORMS total scores (35.9±3.0 versus 25.8±3.0, P=0.001; total maximum WORMS scores, P=0.001) and higher whole knee T2 values (39.8±0.9 versus 36.1±0.7, P<0.001), while extension and flexion strength did not differ between groups (286±23.4 versus 332±23.9, P=0.12 and 144±12 versus 131±12, P=0.43). At the 4 year follow-up, contralateral knees also had significantly (all P<0.05) worse scores for WOMAC pain, stiffness and function subscale scores, worse scores for WORMS cartilage, medial meniscus and bone marrow lesion subscale scores, and higher T2 values in both medial and lateral femoral condyles and lateral tibia (data not shown). Results were essentially unchanged in models that included baseline K-L grade.
Figure 2.
Mean values at 4-year follow-up ± SEM for the parameters total WOMAC score, total WORMS score, global T2 relaxation time (ms), and isometric strength in knee extension (Force; N) in knees contralateral and ipsilateral to THA. *P<0.05 in a multivariate regression model, adjusted for age, gender, PASE, BMI and OA risk factors.
DISCUSSION
In individuals with a prevalent THA and two native knees, we found that some, but not all, measures of degenerative morphological and T2 cartilage abnormalities assessed by knee MRI and knee strength showed greater worsening over 4 years in the knee contralateral to the THA compared to these individuals’ knees ipsilateral to the THA and compared to control knees of individuals without a THA. These findings are generally consistent with the hypothesis that prevalent unilateral THA, increases the risk of developing OA in the knee contralateral to the THA.
OA of one large joint is known to be associated with degenerative changes in other large joints within an individual [2], with the contralateral side of the same joint being at risk for OA and with the contralateral side of the other big joint also having an increased risk for OA [2, 3]. Individuals with a joint replacement are likely to have a subsequent replacement of the same joint on the contralateral side [3][29]. Umeda at al reported increasing radiographic degenerative changes at the contralateral medial knee compartment after unilateral THA, although there was no difference between the ipsilateral and contralateral knee at baseline [5]. Causes for an increased progression of degenerative changes at the knee contralateral to the THA were not investigated in the present study. Despite, potential factors include biomechanical and neuromuscular factors [24], such as muscle weakness [25–27]. Increased peak dynamic knee loads and icreased medial compartment tibial bone mineral density in the knee contralateral to end stage hip OA was decribed previously [28]. Gillam et al stated, that the consequence of gait alterations due to a diseased hip or leg length discrepancy may be responsible for the subsequent development of OA in the contralateral knee [29]. A reduced offset of conventional femoral prostheses may be responsible for shifts in mechanical axes. Increased dynamic loads and elevated knee adduction moments in the knee contralateral to a THA have been found [30]. Foucher et al found that the peak adduction moment was higher on the contralateral versus the ipsilateral knee in patients with unilateral THA [31]. The authors concluded, that implant positioning could influence the biomechanical risk of knee OA progression after THA. On the other hand, Metcalfe et al reported, that also patients with knee OA had significant increases in adduction moment impulse at both knees and the contralateral hip [32]. Alternatively to altered lower limb biomechanics, knee injury, disease and pathomechanics could precede and may lead to altered hip mechanics and hip OA.
Previous studies of this topic however have several limitations. Some focused only on whether replacement of one hip or knee affects the risk of contralateral joint replacement [3, 33]. Other studies have been cross-sectional [2, 33]. Two studies have examined longitudinal changes of hip or knee OA contralateral to the same joint with OA. Spector et al [34] found that over a third of women with unilateral radiographic knee OA will have incident or worsening OA in other knee within 2 years. Vossinakis et al [35] found that patients with idiopathic OA of one hip are at increased risk of developing OA in the other hip. These studies evaluated plain radiographs and did not address hip–knee or knee–hip associations, although MRI can provide substantially more detailed information about joint tissue damage [36, 37]. Additionally, it is important to evaluate both structural changes and clinical symptoms because they often deviate [38].
A strength of our study is that the main analyses examined longitudinal progression over 4 years of knee joint findings that occurred subsequent to unilateral THA. Adjustment for baseline K-L grades had little or no impact on any these associations. Greater increases in T2 values were found for contralateral knees. WOMAC scores also showed consistent but nonsignificantly greater progression in contralaeral knees. Of note, WOMAC scores improved in ipsilateral knees over time. Possibly, this may be due to specific physiotherapy for this extremity or transferred symptoms (hip complaints transferred to knee complaints) may have resolved over time. Also, replacing one major lower extremity joint may improve the overall lower extremity biomechanics, neuromuscular control and may therefore impact function and loading of the entire extremity. Contralateral knees showed greater worsening over four years than ipsilateral knees and than control knees in overall degenerative morphological changes. Surprisingly, 4-year change in cartilage WORMS score and in bone marrow lesion WORMS score was not significant. This may be either due to the small sample size or due to ceiling effects, since cartilage and bone marrow lesion WORMS scores were higher in contralateral knees at baseline (Table 3).
Our findings for contralateral compared to ipsilateral knees differed somewhat for cartilage morphological damage and T2 relaxation times. T2 relaxation time detects early degenerative changes of the biochemical composition of cartilage, mainly collagen disruption and increase of the water content [14, 39, 40]. Therefore is able to detect cartilage changes independently of morphological substance loss [8, 9]. But T2 may be less sensitive than WORMS in assessing progression in more advanced stages when large areas of cartilage are lost [40, 41]. The medial compartments of contralateral knees had consistently greater worsening than the lateral compartment in WORMS cartilage and meniscus scores and T2 values. This pattern is consistent with increases in loading in the medial compartment of contralateral knees associated with end-stage hip OA [30].
We observed a significantly greater decline in knee extension strength in limbs contralateral to THA, which may have several explanations. First, it is consistent with the trends seen for increases in WOMAC scores and significantly greater symptoms at year 4 in contralateral limbs, since knee pain can result in loss of strength [42–44]. Altered gait patterns in persons with endstage hip OA may also contribute to contralateral quadriceps weakeness [43, 45]. Weakness in the contralateral limb may contribute to an increased risk of OA progression and thus a vicious cycle of worsening [46–48].
Our study has several limitations. Since the time-point of THA implantation was not known for all subjects, results were not adjusted for this parameter. However, the strength of the present study is the longitudinal study design. All observations clearly occured after THA implantation. It is possible that pre-existing knee OA preceded and may be associated with clinical and MRI findings at baseline and with worsening of these outcomes during follow-up. Due to concern about introducing collider selection bias when adjusting for a variable (preexisting knee OA) that may be a result of unilateral hip OA and THA, adjustment for baseline K-L grade was limited to sensitivity analyses. Not surprisingly, in the cross-sectional analyses this adjustment had the effect of attenuating associations of unilateral THA with knee OA outcomes. Importantly, associations of THA with changes in knee OA outcomes in longitudinal analyses were unchanged by adjusting for K-L grade, indicating that the effect of unilateral THA on these outcomes is independent of preexisting knee OA.
Although we included all subjects with a unilateral THA at baseline, the precision of our study is limited by small numbers and this is reflected in wide confidence intervals. While this should not affect statistically significant results, it may have contributed to a lack of statistical significance in some instances, for example, comparison of paired difference changes in WOMAC scores. On the other hand our subjects are from a large community-based cohort and represent a diverse sample of those with unilateral hip OA treated in many different health care settings, which enhances the generalizability of our results.
In conclusion, in individuals with a prevalent unilateral THA and two native knees, we found moderate evidence for worse clinical and structural outcomes assessed by MRI in the knees contralateral to the THA compared to ipsilateral knees in the same individuals and compared to control knees in persons with a THA. These findings are consistent with the hypothesis that endstage unilateral hip OA and consequent altered lower limb biomechanics increase the risk of developing and worsening of OA in the knee contralateral to the THA. They indicate a need for strategies and interventions, that likely need to start early preoperatively and to continue post surgery, to prevent the development and worsening of OA in the contralateral limb. However, additional studies with larger patient populations are needed to confirm the obervation, that THA leads to worsening of knee degeneration in contralateral knees.
Acknowledgments
Role of the funding source:
This study was funded by NIH U01-AR055079 and P50-AR060752, by NIH K24-AR04884 and R01-AR052000 and by the OAI. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. The study was supported by the Commission for Clinical Research, Technische Universitaet Muenchen (TUM), TUM School of Medicine, Munich, Germany (Project No 8762152).
Footnotes
Author contributions:
Drs. Pia M. Jungmann (pia.jungmann@tum.de), Michael Nevitt (mnevitt@psg.ucsf.edu) Thomas M. Link (Thomas.Link@ucsf.edu) take responsibility for the integrity of the work as a whole, from inception to finished article. Conception and design: Link TM, Jungmann PM, Nevitt MC, Lane NE. Acquisition of data: Lynch J, Liu F, Jungmann PM, Nevitt MC, McCulloch CE, Nardo L, Liebl H, Baum T. Analysis and interpretation of the data: Jungmann PM, Baum T, Nevitt MC, McCulloch CE, Link TM. Drafting of the article: Jungmann PM, Nevitt MC, McCulloch CE. Critical revision of the article for important intellectual content: all authors. Final approval of the article: all authors. Final approval of the version to be submitted: all authors.
Competing interest statement:
Nothing to declare for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pia M. Jungmann, Email: pia.jungmann@tum.de.
Michael C. Nevitt, Email: mnevitt@psg.ucsf.edu.
Thomas Baum, Email: thomas.baum@tum.de.
Hans Liebl, Email: lieblhans@gmail.com.
Lorenzo Nardo, Email: lorenzo.nardo@ucsf.edu.
Felix Liu, Email: fliu@psg.ucsf.edu.
Nancy E. Lane, Email: nancy.lane@ucdmc.ucdavis.edu.
Charles E. McCulloch, Email: cmcculloch@psg.ucsf.edu.
Thomas M. Link, Email: thomas.link@ucsf.edu.
References
- 1.Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2014 doi: 10.1016/j.joca.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Sayre EC, Jordan JM, Cibere J, Murphy L, Schwartz TA, Helmick CG, et al. Quantifying the association of radiographic osteoarthritis in knee or hip joints with other knees or hips: the Johnston County Osteoarthritis Project. J Rheumatol. 2010;37:1260–1265. doi: 10.3899/jrheum.091154. [DOI] [PubMed] [Google Scholar]
- 3.Shakoor N, Block JA, Shott S, Case JP. Nonrandom evolution of end-stage osteoarthritis of the lower limbs. Arthritis Rheum. 2002;46:3185–3189. doi: 10.1002/art.10649. [DOI] [PubMed] [Google Scholar]
- 4.Shakoor N, Hurwitz DE, Block JA, Shott S, Case JP. Asymmetric knee loading in advanced unilateral hip osteoarthritis. Arthritis Rheum. 2003;48:1556–1561. doi: 10.1002/art.11034. [DOI] [PubMed] [Google Scholar]
- 5.Umeda N, Miki H, Nishii T, Yoshikawa H, Sugano N. Progression of osteoarthritis of the knee after unilateral total hip arthroplasty: minimum 10-year follow-up study. Arch Orthop Trauma Surg. 2009;129:149–154. doi: 10.1007/s00402-008-0577-y. [DOI] [PubMed] [Google Scholar]
- 6.Burstein D, Gray M. New MRI techniques for imaging cartilage. J Bone Joint Surg Am. 2003;85-A(Suppl 2):70–77. doi: 10.2106/00004623-200300002-00009. [DOI] [PubMed] [Google Scholar]
- 7.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226:373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 8.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liess C, Lusse S, Karger N, Heller M, Gluer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10:907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 10.Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31:37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18:776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3. 0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254:509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, et al. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19:984–989. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebl H, Joseph G, Nevitt MC, Singh N, Heilmeier U, Subburaj K, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin W, Alizai H, Joseph GB, Srikhum W, Nevitt MC, Lynch JA, et al. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013;21:1558–1566. doi: 10.1016/j.joca.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 18.Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1. 5 T. J Magn Reson Imaging. 2003;17:358–364. doi: 10.1002/jmri.10263. [DOI] [PubMed] [Google Scholar]
- 19.Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C. T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Med. 2010;63:181–193. doi: 10.1002/mrm.22178. [DOI] [PubMed] [Google Scholar]
- 20.Bellamy N. The WOMAC Knee and Hip Osteoarthritis Indices: development, validation, globalization and influence on the development of the AUSCAN Hand Osteoarthritis Indices. Clin Exp Rheumatol. 2005;23:S148–153. [PubMed] [Google Scholar]
- 21.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 22.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein MG, Pinto BM, Marcus BH, Lynn H, Jette AM, Rakowski W, et al. Physician based physical activity counseling for middle-aged and older adults: a randomized trial. Ann Behav Med. 1999;21:40–47. doi: 10.1007/BF02895032. [DOI] [PubMed] [Google Scholar]
- 24.Frost HM. Perspectives: a biomechanical model of the pathogenesis of arthroses. Anat Rec. 1994;240:19–31. doi: 10.1002/ar.1092400103. [DOI] [PubMed] [Google Scholar]
- 25.Bennell KL, Hunt MA, Wrigley TV, Lim BW, Hinman RS. Role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am. 2008;34:731–754. doi: 10.1016/j.rdc.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Jackson BD, Wluka AE, Teichtahl AJ, Morris ME, Cicuttini FM. Reviewing knee osteoarthritis--a biomechanical perspective. J Sci Med Sport. 2004;7:347–357. doi: 10.1016/s1440-2440(04)80030-6. [DOI] [PubMed] [Google Scholar]
- 27.Pan J, Stehling C, Muller-Hocker C, Schwaiger BJ, Lynch J, McCulloch CE, et al. Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T MRI - an analysis from the incidence cohort of the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19:65–73. doi: 10.1016/j.joca.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shakoor N, Dua A, Thorp LE, Mikolaitis RA, Wimmer MA, Foucher KC, et al. Asymmetric loading and bone mineral density at the asymptomatic knees of patients with unilateral hip osteoarthritis. Arthritis Rheum. 2011;63:3853–3858. doi: 10.1002/art.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillam MH, Lie SA, Salter A, Furnes O, Graves SE, Havelin LI, et al. The progression of end-stage osteoarthritis: analysis of data from the Australian and Norwegian joint replacement registries using a multi-state model. Osteoarthritis Cartilage. 2013;21:405–412. doi: 10.1016/j.joca.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Foucher KC, Wimmer MA. Contralateral hip and knee gait biomechanics are unchanged by total hip replacement for unilateral hip osteoarthritis. Gait Posture. 2012;35:61–65. doi: 10.1016/j.gaitpost.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Foucher KC, Wimmer MA. Does hip implant positioning affect the peak external adduction moments of the healthy knees of subjects with total hip replacements? J Orthop Res. 2013;31:1187–1194. doi: 10.1002/jor.22350. [DOI] [PubMed] [Google Scholar]
- 32.Metcalfe AJ, Stewart C, Postans N, Dodds AL, Holt CA, Roberts AP. The effect of osteoarthritis of the knee on the biomechanics of other joints in the lower limbs. Bone Joint J. 2013;95-B:348–353. doi: 10.1302/0301-620X.95B3.30850. [DOI] [PubMed] [Google Scholar]
- 33.Chitnavis J, Sinsheimer JS, Suchard MA, Clipsham K, Carr AJ. End-stage coxarthrosis and gonarthrosis. Aetiology, clinical patterns and radiological features of idiopathic osteoarthritis. Rheumatology (Oxford) 2000;39:612–619. doi: 10.1093/rheumatology/39.6.612. [DOI] [PubMed] [Google Scholar]
- 34.Spector TD, Hart DJ, Doyle DV. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population: the effect of obesity. Ann Rheum Dis. 1994;53:565–568. doi: 10.1136/ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vossinakis IC, Georgiades G, Kafidas D, Hartofilakidis G. Unilateral hip osteoarthritis: can we predict the outcome of the other hip? Skeletal Radiol. 2008;37:911–916. doi: 10.1007/s00256-008-0522-8. [DOI] [PubMed] [Google Scholar]
- 36.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jungmann PM, Liu F, Link TM. What has imaging contributed to the epidemiological understanding of osteoarthritis? Skeletal Radiol. 2014;43:271–275. doi: 10.1007/s00256-013-1783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls - data from the osteoarthritis initiative. Arthritis Res Ther. 2011;13:R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jungmann PM, Kraus MS, Nardo L, Liebl H, Alizai H, Joseph GB, et al. T(2) relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur: longitudinal data from the osteoarthritis initiative. J Magn Reson Imaging. 2013;38:1415–1424. doi: 10.1002/jmri.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koff MF, Amrami KK, Kaufman KR. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15:198–204. doi: 10.1016/j.joca.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Steultjens MP, Dekker J, van Baar ME, Oostendorp RA, Bijlsma JW. Muscle strength, pain and disability in patients with osteoarthritis. Clin Rehabil. 2001;15:331–341. doi: 10.1191/026921501673178408. [DOI] [PubMed] [Google Scholar]
- 43.Lin YC, Davey RC, Cochrane T. Tests for physical function of the elderly with knee and hip osteoarthritis. Scand J Med Sci Sports. 2001;11:280–286. doi: 10.1034/j.1600-0838.2001.110505.x. [DOI] [PubMed] [Google Scholar]
- 44.Costa RA, Oliveira LM, Watanabe SH, Jones A, Natour J. Isokinetic assessment of the hip muscles in patients with osteoarthritis of the knee. Clinics (Sao Paulo) 2010;65:1253–1259. doi: 10.1590/S1807-59322010001200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horstmann T, Listringhaus R, Brauner T, Grau S, Mundermann A. Minimizing preoperative and postoperative limping in patients after total hip arthroplasty: relevance of hip muscle strength and endurance. Am J Phys Med Rehabil. 2013;92:1060–1069. doi: 10.1097/PHM.0b013e3182970fc4. [DOI] [PubMed] [Google Scholar]
- 46.Glass NA, Torner JC, Frey Law LA, Wang K, Yang T, Nevitt MC, et al. The relationship between quadriceps muscle weakness and worsening of knee pain in the MOST cohort: a 5-year longitudinal study. Osteoarthritis Cartilage. 2013;21:1154–1159. doi: 10.1016/j.joca.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennell KL, Wrigley TV, Hunt MA, Lim BW, Hinman RS. Update on the role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am. 2013;39:145–176. doi: 10.1016/j.rdc.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Segal NA, Glass NA. Is quadriceps muscle weakness a risk factor for incident or progressive knee osteoarthritis? Phys Sportsmed. 2011;39:44–50. doi: 10.3810/psm.2011.11.1938. [DOI] [PubMed] [Google Scholar]