Abstract
The gonads form bilaterally as bipotential organs that can develop as testes or ovaries. All secondary sex characteristics that we associate with ‘maleness’ or ‘femaleness’ depend on whether testes or ovaries form. The fate of the gonads depends on a cell fate decision that occurs in a somatic cell referred to as the ‘supporting cell lineage’. Once supporting cell progenitors commit to Sertoli (male) or granulosa (female) fate, they propagate this decision to the other cells within the organ. In this review, we will describe what is known about the bipotential state of somatic and germ cell lineages in the gonad and the transcriptional and antagonistic signaling networks that lead to commitment, propagation, and maintenance of testis or ovary fate.
Formation of the gonad
Gonads form as paired, bilateral organs that are composed of several lineages of somatic cells as well as the population of germ cells. Precursors of many of the somatic cells in the gonad arise from proliferation of the SF1 (steroidogenic factor 1, aka NR5A1)-positive cells in the coelomic epithelium (CE) overlying the region of the intermediate mesoderm called the mesonephros. The CE begins to thicken in this region at approximately embryonic day (E) 10.0 and contributes to at least two distinct somatic precursor lineages that are bipotential: first, supporting cell precursors, which give rise to Sertoli cells in the testis or fetal granulosa cells in the ovary, and second, steroidogenic progenitors, which give rise to Leydig cells in the testis or theca cells in the ovary [1,2]. Genes including Wt1 (Wilms tumor 1 homolog) [3], Lhx9 (LIM homeobox protein 9) [4], Emx2 (empty spiracles homeobox 2) [5], Sf1 [6], M33 (Cbx2, chromobox 2) [7,8], Gata4 [9] and Six1/4 (sine oculis-related homeobox 1/4) [10•] are essential to establish the bipotential population of somatic cells in the gonad.
The bipotential stage
The early somatic progenitors are capable of adopting either male or female fate. In accord with classic theory in the field, the transcriptomes of whole XX and XY gonads are nearly indistinguishable at E10.0 through E11.2 [11••,12]. At this bipotential stage, genes that are later associated with testis fate (i.e. Sox9 (Sry (sex determining region of the Y)-box 9) and Fgf9 (fibroblast growth factor 9)) and ovary fate (i.e. Wnt4 (wingless-type MMTV integration site family, member 4) and Rspo1 (R-spondin homolog 1)) are expressed at similar levels in XX and XY gonads [11••]. This is also true if different cell types in the XX and XY gonad are isolated by flow cytometry and analyzed separately at E11.5 [13]. These results suggest that the bipotential plasticity of the mammalian gonad results from a transient balanced transcriptional state in which many genes later associated with male or female fate are expressed at similar levels in supporting cell precursors of both XX and XY gonads. Although the gonad is poised to follow either pathway at this bipotential stage, the supporting cell lineage expresses more genes later associated with the female than the male pathway, suggesting a female bias in the underlying program [13].
The first steps of male or female fate commitment
Sex determination initiates by tilting the balance in the transcription network toward the male or female fate. The switch to initiate the male pathway in the poised supporting cell progenitors is the Y-linked gene, Sry. An Sry transgene, driven in the XX gonad from its own promoter, caused differentiation of a testis [14]. This experiment showed that first, Sry is the only gene from the Y chromosome that is required for male sex determination, and second, the molecular environment of the XX gonad is fully competent to activate Sry and initiate testis development (for a recent excellent review focused on the regulation of Sry itself, see [15]).
Sry gene expression initiates just after E10.5 (10 tail somites (ts)) based on an RNase protection study [16]. Using in situ hybridization, expression is detectable in the middle of the gonad at ts14 (~E11.0) and expands toward the anterior, then posterior poles [17]. The timing and level of expression of Sry are critical. XY mice carrying a weak allele of Sry that shows a decrease/delay in expression, are susceptible to male-to-female sex reversal [18–20]. Experiments that drive Sry expression in XX gonads using a heat shock promoter, revealed a requirement for Sry in the 6-h time window between E11.0 and E11.25 [21]. If expression is delayed, the testis pathway is aborted and ovarian development ensues. Exactly why the window of opportunity to initiate the male pathway closes at E11.25 remains unclear. Downstream of Sry expression, Sox9 is the earliest gene to be upregulated in the male pathway at E11.2, closely followed by Cited4 (Cbp/p300-interacting transactivator-4, with Glu/Asp-rich carboxy-terminal domain, and Sox13 (SRY-box 13) at E11.4, and a larger group at E11.6 [11••]. Many of these genes are critical to establish male fate [22–24].
Genes associated with the female pathway become dimorphic slightly later, between E11.4 and E11.6, including Wnt4, Rspo1, Irx3 (Iroquois related homeobox 3), Lhx9, Fst (follistatin), and Lef1 (lymphoid enhancer binding factor 1) [11••,13]. The downstream effect of WNT4/RSPO1 signaling is the stabilization of β-catenin [25,26]. β-Catenin accumulates in the nucleus [27,28] where it may interact with the transcription factor LEF1 leading to the activation of downstream genes, as in other systems [29]. Stabilization of β-catenin in the XY gonad leads to down-regulation of SOX9 and male to female sex-reversal [30]. Antagonism between SOX9 and β-catenin may underlie the molecular decision in individual cells. However, loss of Wnt4 and/or Rspo1 and/or β-catenin does not lead to complete female to male sex reversal until perinatal stages [27,31–33].
Female fate is also regulated by the transcription factor Foxl2 (forkhead box L2). FOXL2 co-operates with BMP2 (bone morphogenetic protein 2) to up-regulate the expression of Fst [34]. In goats, loss of function of Foxl2 leads to female to male sex reversal in fetal life [35••]. However, in mice and humans, disruption of the female pathway does not occur until neonatal stages. Although loss of Foxl2 in combination with Rspo1 or Wnt4 slightly accelerates the sex reversal phenotype in mice [36,37], no gene has been discovered whose loss leads to female to male sex reversal at early fetal stages. Since the bipotential gonad is initially biased toward the ovarian fate, a gene with a comparable role to Sry may not be required to initiate the female pathway. It may be sufficient not to initiate the male pathway.
Supporting cell precursors enter a quiescent state by E12.5 in XX gonads [38], consistent with the upregulation of negative regulators of the cell cycle observed in transcriptome studies [13,39]. The quiescent state of progenitor cells in the ovary may protect them from switching fate until proliferation resumes around the time of birth [38,40•]. In contrast, supporting cell progenitors in the XY gonad upregulate proliferation immediately downstream of Sry, and blocking proliferation disrupts the male pathway [2,41]. Whether proliferation is important for intracellular fate commitment or is required to establish a threshold population of Sertoli progenitors is still unclear.
Sexually dimorphic expression can result from activation in one sex, repression in the other sex, or a combination of both mechanisms. All of these patterns were evident in a study in which the gene expression profile for each gene was compared in XX and XY gonads at fine time points between E11.0 and E12.0 [11••]. Enrichment of male pathway genes occurred primarily through activation in the XY gonad with a minor contribution from repression of male genes in the XX gonad. In contrast, about half of the genes that became enriched in the female gonad did so due to repression in the XY gonad (Figure 1). This is a critical feature of the counterbalanced system that controls sex determination and gonadal fate: to establish a fate decision in the gonad, it is not sufficient to activate one of the alternative pathways — it is also necessary to repress the other.
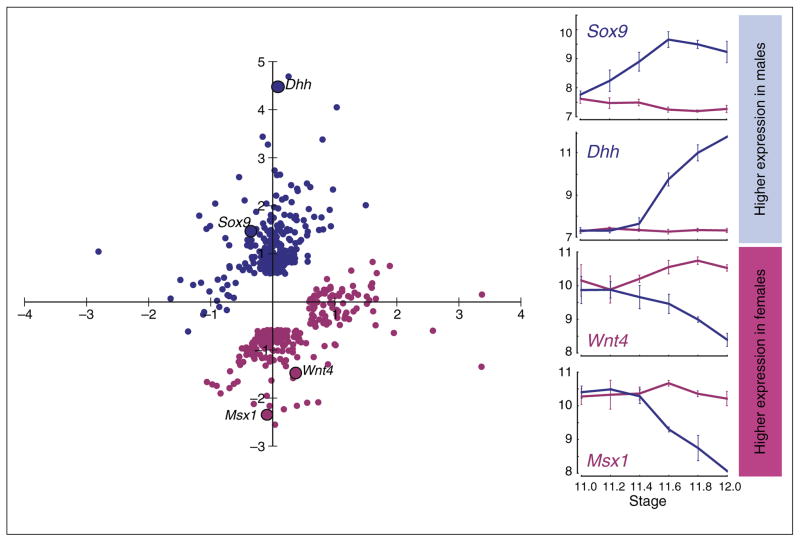
Figure 1.
Commitment to the male pathway involves the down-regulation of many genes associated with female development. Diagram depicting the change in the expression of each gene in the XX gonad (X-axis) and XY gonad (Y-axis) between E11.0 and E12.0. Both of these changes can contribute to sexually dimorphic expression. Line graphs show changes in expression of two representative male genes (Dhh and Sox9) and two representative female genes (Msx1 and Wnt4) in the XY (blue line) and XX (red line) gonads. Higher expression of the male pathway genes, Dhh and Sox9, occur primarily through up-regulation in the XY gonad. Sexually dimorphic expression of many of the genes in the female pathway, Msx1 and Wnt4, occurs through down-regulation in the XY gonad. Adapted from Munger SC et al. [11].
Propagation of fate commitment across the gonad field
Subsequent to the primary fate decision in both differentiation pathways, feedback mechanisms are activated that canalize the chosen sexual fate. This occurs within individual cells and across the gonad field.
Although XX ⇔ XY chimeras typically have a similar number of XX and XY cells in the fetal gonad (as in other organs), more than half develop as males. This result suggests that a threshold number of XY cells can establish the testis pathway throughout the organ, but in cases where this cell threshold is not met, the gonad develops as an ovary. In the adult testes of chimeras, most of the Sertoli cells are XY, however, a small number of XX Sertoli cells are reproducibly found, indicating that XY supporting cells in the gonad can recruit XX cells (that do not have a Y chromosome or Sry gene) to Sertoli fate, presumably through paracrine signals [42,43]. These results indicate that in addition to the fate determination step that occurs in each supporting cell (likely based on antagonism between SOX9 and β-catenin), there is a community decision that takes place across the field of the gonad.
Two paracrine signaling molecules downstream of SOX9, FGF9 and PGD2 (prostaglandin D2 synthase), are known to contribute to propagation and maintenance of the male fate decision [44–50]. Fgf9 is required to signal from the central part of the gonad and establish Sox9 expression in the two ends [51]. Fgf signaling functions to antagonize Wnt signaling [45]. Elimination of the expression of Fgf9 or its receptor Fgfr2 (fibroblast growth factor receptor 2), leads to upregulation of the Wnt pathway and male-to-female sex reversal [44,52–54]. On the other hand, elimination of Wnt4/Rspo1/β-catenin leads to female-to-male sex reversal near birth [27,31–33], suggesting that the Fgf9 and Wnt4 signaling pathways have antagonistic roles in sex determination. Deletion of both Fgf9 and Wnt4 or Fgfr2 and Wnt4 rescues the male-to-female sex reversal phenotype in the XY gonad. In double mutants both somatic cells and germ cells express markers associated with male development, strongly suggesting that the primary role of Fgf signaling in XY gonad development is to repress the Wnt pathway [55]. Similar results were obtained by paired deletion of several other sets of antagonistic factors. For example, deletion of Sox9 leads to male to female sex reversal, but rescue of the male fate occurs when Sox9 and Rspo1 are simultaneously deleted [56]. Similarly, when Wnt4 and ActivinβB were simultaneously deleted, the female fate was rescued [57]. These studies and others indicate a multi-layered input into sex determination and suggest that many coding genes and perhaps microRNAs [58] affect the balance between male and female fate.
Relatively little is known about how other somatic lineages across the gonad field commit to a testis or ovary fate. However, it is clear that this occurs downstream of the fate commitment step in the supporting cell precursors. The steroidogenic precursor lineage in XX and XY gonads shares an indistinguishable transcriptional profile at E11.5 [13]. At E12.5, XX and XY steroidogenic progenitors begin to show divergent expression patterns downstream of morphological changes that occur in the XY gonad including the formation of the male-specific vasculature. Disruption of Dhh [23,24] or Pdgfrα [59,60] leads to defects in Leydig cell development in the testis, but it is somewhat unclear whether these effects are direct, or are indirectly mediated by effects on the vasculature. Disruption of the male-specific vasculature also disrupts Leydig cell development, likely through Notch/Jag1 signaling [61•]. In the fetal mouse ovary, NR2f2 and MafB label the progenitors of a cell type distinct from the supporting cell lineage [62•,63]. Steroidogenic cells in the ovary begin to produce hormones around birth. Like the steroidogenic lineage in the testis, they likely have a mixed origin, and differentiate in response to induction signals in their testis or ovary environment [64,65].
Germ cell fate commitment
Germ cells arise at the base of the allantois in the E6.25 embryo, migrate within the gut epithelium, and arrive in the gonad during the early stages of gonadogenesis. During these stages, the germ cell genome is stripped of most of its methylation [66]. Like ES cells, germ cells carry bivalent histone marks on many developmentally regulated genes [67•], are highly pluripotent, and can readily give rise to embryonic germ cells (EG cells) [68]. Fate determination in the germ cell lineage involves a repression of pluripotency and a commitment to differentiate as one of two specialized gamete precursors: spermatogonia or oogonia.
Germ cells differentiate as spermatogonia or oogonia irrespective of their genetic sex, but based on cues from their somatic environment. Unlike the supporting cell lineage, germ cells at the bipotential stage express more genes associated with a male fate, than with a female fate [13], suggesting that female germ cells take the more divergent path at this stage of development. At E11.5, XX and XY germ cells have very similar transcriptomes, The only differences are detected in levels of a few Y-linked and X-linked genes [11••]. Regardless of these differences, both XX and XY germ cells are capable of responding to either male or female gonadal cues up until E12.5, after which their fate is fixed [69].
XX and XY germ cells enter meiosis in an ovarian environment, or in cases where they are lost in the mesonephros or adrenal. In contrast, in a testis environment germ cells arrest in G0 of the mitotic cell cycle [70]. Recently a molecular explanation for this behavior has emerged that provides both a repressor of meiosis in the XY gonad, as predicted by McLaren and Southee [69], and an activator of meiosis in the XX gonad, as proposed by Byskov et al. [71].
In XX gonads, retinoic acid (RA) signaling initiates meiotic entry via activation of Stra8 (stimulated by RA gene 8). RA is synthesized in the mesonephros, and may be transported by the mesonephric tubules that are physically connected with the anterior end of the gonad. This could explain the anterior-to-posterior wave of Stra8 expression that triggers meiotic entry between E13.5 and E15.5 [72–74]. The timing of meiotic entry is controlled by polycomb complex 1 (PRC1), which represses expression of Stra8 prior to E13.5 in XX germ cells [75•]. Germ cells in the female gonad progress through leptotene, zygotene and pachytene, and arrest in diplotene near the time of birth.
RA is also produced from the mesonephros in XY embryos (as in XX), however, the P450 enzyme CYP26B1 (cytochrome P450, family 26, subfamily b, polypeptide 1) is synthesized early in the male pathway by somatic cells in the testis, and functions to degrade RA and block activation of Stra8 and meiosis [76,77]. In mouse genetic models where Cyp26b1 is disrupted, germ cells enter meiosis in the testis [77,78].
Additional factors regulate the divergence of male and female germ cell fates. In addition to their role in somatic sex determination, the antagonistic signaling pathways, Wnt/Rspo and Fgf, also affect germ cell fate. Wnt/Rspo signaling acts through β-catenin to regulate proliferation of XX germ cells and promote their entry into meiosis [79]; whereas, loss of Wnt4 leads to an anterior to posterior loss of female germ cells [40•]. In contrast, loss of Fgf9, which is upregulated early in the Sertoli cell differentiation pathway leads to sex-specific apoptosis of germ cells in the testis and up-regulation of meiotic markers [80,81].
FGF9 is required for the up-regulation of Nanos2 [81,82], a key RNA-binding protein (RBP) that is critical for initiating the male pathway in germ cells. RBPs, including NANOS2/3 [83,84•], TDRD1, a tudor domain containing RBP [85,86], DND1 (dead end homolog 1) [87,88], and PUMILIO [89] play a prominent role in male germ cells. In the absence of Nanos2, male germ cells enter meiosis and/or undergo apoptosis [90]. NANOS2 and NANOS3 associate in the CNOT complex to control adenylation of multiple mRNA targets and promote degradation of mRNAs encoding meiotic genes [83,84•]. Dnd1 also promotes translation of several negative regulators of the cell cycle, including P21 (Cdkn1a, cyclin-dependent kinase inhibitor 1A) and P27 (Cdkn1b, cyclin-dependent kinase inhibitor 1B) [87,91]. Accumulation of negative cell cycle regulators is believed to bring male germ cells into mitotic arrest in G0, where they remain until perinatal stages when mitosis resumes prior to the establishment of the spermatogonial stem cell population [92,93]. Some evidence suggests that cell cycle arrest in male germ cells is critical to repress pluripotency and establish spermatogonial fate. Germ cells that fail to enter G0 by E15.5 in the male gonad are susceptible to teratoma formation, a strong indication that germ cells have not repressed their pluripotent state [87,94,95].
Although FGF9 also has been assigned a role in up-regulation of p15INK4B (Cdkn2b cyclin-dependent kinase inhibitor 2B) [81], if both Fgf9 and Wnt4 are deleted, the XY gonad embarks on the testis pathway, and XY germ cells fail to enter meiosis and express normal markers of the male pathway [55]. This has been interpreted to mean that other Fgfs can compensate for this function of Fgf9, or that there are layers of antagonistic signals that control germ cell fate similar to those that control somatic cell fate.
Maintaining Sertoli or granulosa cell fate in adult life
Transdifferentiation from one committed fate to another is an unusual phenomenon that occurs naturally when germ cells are depleted from the adult ovary: in this situation granulosa cells transdifferentiate to Sertoli cell fate [96,97]. While transdifferentiation was regarded as an indirect effect of germ cell loss, in the past decade, several genes have been identified that are directly involved in active maintenance of supporting cell fate in adult life.
In the female gonad, FoxL2 plays a key role in maintaining ovarian fate postnatally. In cooperation with the estrogen receptors 1 and 2 (ESR1/2), FOXL2 antagonizes the male pathway by direct binding to the TESCO regulatory region of SOX9 to repress its expression. When FOXL2 is lost in adult life, granulosa cells transdifferentiate prior to germ cell loss, and theca cells also begin to produce testosterone [98]. Null mutations in Esr1/Esr2, or in P450 aromatase (Cyp19a1) also result in transdifferentiation in the postnatal ovary accompanied by germ cell loss [99,100]. While it is difficult in these instances of transdifferentiation to disentangle the effects of germ cell loss, it is likely that they are due at least in part to direct effects of estrogen on maintenance of granulosa cell fate.
In the adult testis, DMRT1 (doublesex and mab-3 related transcription factor 1) binds to regulatory regions of testis-promoting and ovary-promoting genes. DMRT1 activates testis-promoting genes such as Sox8, Sox9 and Ptgdr (prostaglandin D receptor), and represses ovary-promoting genes such as FoxL2, Wnt4, and Rspo1. DMRT1 may be important to antagonize the influence of RA, which is produced in the adult testis to drive entry of spermatogonia into meiosis, and may have a feminizing influence on Sertoli cells [101••]. These results suggest that maintenance of testis or ovary fate is an active process in adult life.
Summary
Commitment of the bipotential gonad to testis or ovary fate is the result of antagonistic male and female pathways that compete to control the differentiation of supporting cell precursors, likely through regulation of SOX9 and β-catenin (Figure 2). Sertoli or granulosa cell fate commitment involves the activation of one program and the repression of the alternative pathway of development. The decision is then propagated across the gonad field, and controls the fate of other somatic lineages as well as the germ cell lineage. In the adult testis and ovary, transdifferentiation can occur between Sertoli and granulosa cell fates. Many differentiated cells in other organs share a common bipotential progenitor, however, it is unclear whether the phenomenon of transdifferentiation between differentiated fates occurs naturally in other systems, or whether it is specific to gonadal cells. It could be an evolutionary remnant of the ability of some fish to switch sex in adult life [102] or of some vertebrates’ ability to function as ‘natural hermaphrodites’ by maintaining a gonad with seasonally expanding ovarian and testicular regions such as the mole and alligator [103,104]. It will be interesting to see how this plasticity of cell fate is related to the epigenetic landscape at the bipotential stage, and in Sertoli and granulosa cells during fetal and adult life.
Figure 2.
Commitment and maintenance of gonadal cell fate. In XX and XY gonads at the bipotential stage, supporting cell precursors are exposed to male and female promoting signals that antagonize each other. In the XY gonad, expression of Sry triggers up-regulation of Sox9 and Fgf9, which activate the male pathway and repress signals that promote the female pathway (WNT4/RSPO1 and β-catenin). Supporting cell precursors commit to Sertoli cell fate and orchestrate testis development by promoting Leydig cell differentiation from steroidogenic progenitors, and regulating mitotic arrest in germ cells. In the XX gonad, in the absence of Sry to initiate the male pathway, Wnt4 and Rspo1 maintain β-catenin signaling to promote the female pathway. Supporting cell precursors commit to granulosa cell fate and orchestrate ovary development by promoting Theca cell differentiation from steroidogenic progenitors, and regulating meiotic entry in germ cells. Reinforcing signals (dotted lines) exist between somatic and germ cells in the developing testis and ovary. In the adult testis, Dmrt1 is required for maintaining Sertoli cell fate by repressing female promoting signals, while in the adult ovary, abolishing female promoting signals leads to loss of granulosa cell fate and up-regulation of Sox9 and other markers of Sertoli fate.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- 2.Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127:65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- 3.Hammes A, Guo JK, Lutsch G, Leheste JR, Landrock D, Ziegler U, Gubler MC, Schedl A. Two splice variants of the Wilms’ tumor 1 gene have distinct functions during sex determination and nephron formation. Cell. 2001;106:319–329. doi: 10.1016/s0092-8674(01)00453-6. [DOI] [PubMed] [Google Scholar]
- 4.Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg JA, Parker KL, et al. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature. 2000;403:909–913. doi: 10.1038/35002622. [DOI] [PubMed] [Google Scholar]
- 5.Kusaka M, Katoh-Fukui Y, Ogawa H, Miyabayashi K, Baba T, Shima Y, Sugiyama N, Sugimoto Y, Okuno Y, Kodama R, et al. Abnormal epithelial cell polarity and ectopic epidermal growth factor receptor (EGFR) expression induced in Emx2 KO embryonic gonads. Endocrinology. 2010;151:5893–5904. doi: 10.1210/en.2010-0915. [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 7.Katoh-Fukui Y, Tsuchiya R, Shiroishi T, Nakahara Y, Hashimoto N, Noguchi K, Higashinakagawa T. Male-to-female sex reversal in M33 mutant mice. Nature. 1998;393:688–692. doi: 10.1038/31482. [DOI] [PubMed] [Google Scholar]
- 8.Katoh-Fukui Y, Miyabayashi K, Komatsu T, Owaki A, Baba T, Shima Y, Kidokoro T, Kanai Y, Schedl A, Wilhelm D, et al. Cbx2, a polycomb group gene, is required for Sry gene expression in mice. Endocrinology. 2012;153:913–924. doi: 10.1210/en.2011-1055. [DOI] [PubMed] [Google Scholar]
- 9.Hu YC, Okumura LM, Page DC. Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 2013;9:e1003629. doi: 10.1371/journal.pgen.1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Fujimoto Y, Tanaka SS, Yamaguchi YL, Kobayashi H, Kuroki S, Tachibana M, Shinomura M, Kanai Y, Morohashi K, Kawakami K, et al. Homeoproteins Six1 and Six4 regulate male sex determination and mouse gonadal development. Dev Cell. 2013;26:416–430. doi: 10.1016/j.devcel.2013.06.018. By characterizing the Six1/Six4 double knockout mice, the authors demonstrated that SIX1/SIX4 control Sry expression via FOG2 and regulate gonadal size via NR5A1. [DOI] [PubMed] [Google Scholar]
- 11••.Munger SC, Natarajan A, Looger LL, Ohler U, Capel B. Fine time course expression analysis identifies cascades of activation and repression and maps a putative regulator of mammalian sex determination. PLoS Genet. 2013;9:e1003630. doi: 10.1371/journal.pgen.1003630. An analysis of the transcriptomes from XX and XY gonads at 4-h intervals during sex determination (E11.0–E12.0) elucidated the first steps of sexual fate specification and revealed a strong program of repression of female genes in the XY gonad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Jameson SA, Natarajan A, Cool J, DeFalco T, Maatouk DM, Mork L, Munger SC, Capel B. Temporal transcriptional profiling of somatic and germ cells reveals biased lineage priming of sexual fate in the fetal mouse gonad. PLoS Genet. 2012;8:e1002575. doi: 10.1371/journal.pgen.1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 15.Larney C, Bailey TL, Koopman P. Switching on sex: transcriptional regulation of the testis-determining gene Sry. Development. 2014;141:2195–2205. doi: 10.1242/dev.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- 17.Bullejos M, Koopman P. Spatially dynamic expression of Sry in mouse genital ridges. Dev Dyn. 2001;221:201–205. doi: 10.1002/dvdy.1134. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht KH, Young M, Washburn LL, Eicher EM. Sry expression level and protein isoform differences play a role in abnormal testis development in C57BL/6J mice carrying certain Sry alleles. Genetics. 2003;164:277–288. doi: 10.1093/genetics/164.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagamine CM, Morohashi K, Carlisle C, Chang DK. Sex reversal caused by Mus musculus domesticus Y chromosomes linked to variant expression of the testis-determining gene Sry. Dev Biol. 1999;216:182–194. doi: 10.1006/dbio.1999.9436. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm D, Washburn LL, Truong V, Fellous M, Eicher EM, Koopman P. Antagonism of the testis- and ovary-determining pathways during ovotestis development in mice. Mech Dev. 2009;126:324–336. doi: 10.1016/j.mod.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiramatsu R, Matoba S, Kanai-Azuma M, Tsunekawa N, Katoh-Fukui Y, Kurohmaru M, Morohashi K, Wilhelm D, Koopman P, Kanai Y. A critical time window of Sry action in gonadal sex determination in mice. Development. 2009;136:129–138. doi: 10.1242/dev.029587. [DOI] [PubMed] [Google Scholar]
- 22.Cui S, Ross A, Stallings N, Parker KL, Capel B, Quaggin SE. Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development. 2004;131:4095–4105. doi: 10.1242/dev.01266. [DOI] [PubMed] [Google Scholar]
- 23.Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod. 2001;65:1392–1402. doi: 10.1095/biolreprod65.5.1392. [DOI] [PubMed] [Google Scholar]
- 24.Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tevosian SG, Manuylov NL. To beta or not to beta: canonical beta-catenin signaling pathway and ovarian development. Dev Dyn. 2008;237:3672–3680. doi: 10.1002/dvdy.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomaselli S, Megiorni F, Lin L, Mazzilli MC, Gerrelli D, Majore S, Grammatico P, Achermann JC. Human RSPO1/R-spondin1 is expressed during early ovary development and augments beta-catenin signaling. PLoS One. 2011;6:e16366. doi: 10.1371/journal.pone.0016366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- 28.Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of beta-catenin in mouse gonadal development. Hum Mol Genet. 2009;18:405–417. doi: 10.1093/hmg/ddn362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 32.Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, et al. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- 33.Manuylov NL, Smagulova FO, Leach L, Tevosian SG. Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development. 2008;135:3731–3743. doi: 10.1242/dev.024653. [DOI] [PubMed] [Google Scholar]
- 34.Kashimada K, Pelosi E, Chen H, Schlessinger D, Wilhelm D, Koopman P. FOXL2 and BMP2 act cooperatively to regulate follistatin gene expression during ovarian development. Endocrinology. 2011;152:272–280. doi: 10.1210/en.2010-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Boulanger L, Pannetier M, Gall L, Allais-Bonnet A, Elzaiat M, Le Bourhis D, Daniel N, Richard C, Cotinot C, Ghyselinck NB, et al. FOXL2 is a female sex-determining gene in the goat. Curr Biol. 2014;24:404–408. doi: 10.1016/j.cub.2013.12.039. To dissociate Foxl2 loss of function from loss of lncRNA expression in the polled goat interval, the authors used zinc-finger nuclease-directed mutagenesis to generate XX individuals with biallelic mutations of FOXL2. Their study showed that loss of FOXL2 alone is sufficient to cause an XX female-to-male sex reversal in the goat model. [DOI] [PubMed] [Google Scholar]
- 36.Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16:2795–2804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- 37.Auguste A, Chassot AA, Gregoire EP, Renault L, Pannetier M, Treier M, Pailhoux E, Chaboissier MC. Loss of R-spondin1 and Foxl2 amplifies female-to-male sex reversal in XX mice. Sex Dev. 2011;5:304–317. doi: 10.1159/000334517. [DOI] [PubMed] [Google Scholar]
- 38.Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, Capel B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod. 2012;86:37. doi: 10.1095/biolreprod.111.095208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouma GJ, Affourtit JP, Bult CJ, Eicher EM. Transcriptional profile of mouse pre-granulosa and Sertoli cells isolated from early-differentiated fetal gonads. Gene Expr Patterns. 2007;7:113–123. doi: 10.1016/j.modgep.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 40•.Maatouk DM, Mork L, Chassot AA, Chaboissier MC, Capel B. Disruption of mitotic arrest precedes precocious differentiation and transdifferentiation of pregranulosa cells in the perinatal Wnt4 mutant ovary. Dev Biol. 2013;383:295–306. doi: 10.1016/j.ydbio.2013.08.026. The authors showed that loss of Wnt4 or Rspo1 in the fetal ovary leads to a release of cell cycle arrest in pregranulosa cells, and expression of markers associated with growing follicles. They suggest that the maintenance of mitotic arrest in pregranulosa cells precludes upregulation of Sox9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmahl J, Capel B. Cell proliferation is necessary for the determination of male fate in the gonad. Dev Biol. 2003;258:264–276. doi: 10.1016/s0012-1606(03)00122-2. [DOI] [PubMed] [Google Scholar]
- 42.Burgoyne PS, Buehr M, McLaren A. XY follicle cells in ovaries of XX–XY female mouse chimaeras. Development. 1988;104:683–688. doi: 10.1242/dev.104.4.683. [DOI] [PubMed] [Google Scholar]
- 43.Palmer SJ, Burgoyne PS. In situ analysis of fetal, prepuberal and adult XX–XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development. 1991;112:265–268. doi: 10.1242/dev.112.1.265. [DOI] [PubMed] [Google Scholar]
- 44.Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, Capel B. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci U S A. 2007;104:16558–16563. doi: 10.1073/pnas.0702581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Mejean C, Berta P, Poulat F, Boizet-Bonhoure B. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J. 2005;24:1798–1809. doi: 10.1038/sj.emboj.7600660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moniot B, Declosmenil F, Barrionuevo F, Scherer G, Aritake K, Malki S, Marzi L, Cohen-Solal A, Georg I, Klattig J, et al. The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation. Development. 2009;136:1813–1821. doi: 10.1242/dev.032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilhelm D, Hiramatsu R, Mizusaki H, Widjaja L, Combes AN, Kanai Y, Koopman P. SOX9 regulates prostaglandin D synthase gene transcription in vivo to ensure testis development. J Biol Chem. 2007;282:10553–10560. doi: 10.1074/jbc.M609578200. [DOI] [PubMed] [Google Scholar]
- 49.Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol. 2005;287:111–124. doi: 10.1016/j.ydbio.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 50.Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129:1155–1164. doi: 10.1242/dev.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 51.Hiramatsu R, Harikae K, Tsunekawa N, Kurohmaru M, Matsuo I, Kanai Y. FGF signaling directs a center-to-pole expansion of tubulogenesis in mouse testis differentiation. Development. 2010;137:303–312. doi: 10.1242/dev.040519. [DOI] [PubMed] [Google Scholar]
- 52.Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104:875–889. doi: 10.1016/s0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- 53.Bagheri-Fam S, Sim H, Bernard P, Jayakody I, Taketo MM, Scherer G, Harley VR. Loss of Fgfr2 leads to partial XY sex reversal. Dev Biol. 2008;314:71–83. doi: 10.1016/j.ydbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Siggers P, Carre GA, Bogani D, Warr N, Wells S, Hilton H, Esapa C, Hajihosseini MK, Greenfield A. A novel mouse Fgfr2 mutant, hobbyhorse (hob), exhibits complete XY gonadal sex reversal. PLoS One. 2014;9:e100447. doi: 10.1371/journal.pone.0100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jameson SA, Lin YT, Capel B. Testis development requires the repression of Wnt4 by Fgf signaling. Dev Biol. 2012;370:24–32. doi: 10.1016/j.ydbio.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavery R, Chassot AA, Pauper E, Gregoire EP, Klopfenstein M, de Rooij DG, Mark M, Schedl A, Ghyselinck NB, Chaboissier MC. Testicular differentiation occurs in absence of R-spondin1 and Sox9 in mouse sex reversals. PLoS Genet. 2012;8:e1003170. doi: 10.1371/journal.pgen.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu CF, Parker K, Yao HH. WNT4/beta-catenin pathway maintains female germ cell survival by inhibiting activin betaB in the mouse fetal ovary. PLoS One. 2010;5:e10382. doi: 10.1371/journal.pone.0010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wainwright EN, Jorgensen JS, Kim Y, Truong V, Bagheri-Fam S, Davidson T, Svingen T, Fernandez-Valverde SL, McClelland KS, Taft RJ, et al. SOX9 regulates microRNA miR-202-5p/3p expression during mouse testis differentiation. Biol Reprod. 2013;89:34. doi: 10.1095/biolreprod.113.110155. [DOI] [PubMed] [Google Scholar]
- 59.Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cool J, DeFalco TJ, Capel B. Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci U S A. 2011;108:167–172. doi: 10.1073/pnas.1010299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Defalco T, Saraswathula A, Briot A, Iruela-Arispe ML, Capel B. Testosterone levels influence mouse fetal Leydig cell progenitors through notch signaling. Biol Reprod. 2013;88:91. doi: 10.1095/biolreprod.112.106138. In the fetal gonad, perivascular cells regulate differentiation of Leydig progenitors through Notch/JAG1 signals. In the early postnatal testis, Notch signaling from these cells is modulated by circulating levels of testosterone thus linking Leydig cell differentiation with the physiological environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Rastetter RH, Bernard P, Palmer JS, Chassot AA, Chen H, Western PS, Ramsay RG, Chaboissier MC, Wilhelm D. Marker genes identify three somatic cell types in the fetal mouse ovary. Dev Biol. 2014;394:242–252. doi: 10.1016/j.ydbio.2014.08.013. In this study, the authors identify three distinct somatic cell lineages during fetal ovarian develompment marked by LGR5, FOXL2 and NR2F2. They show that LGR5 cells appear in the CE in fetal life and give rise to granulosa cells of cortical follicles after birth. [DOI] [PubMed] [Google Scholar]
- 63.Maatouk DM, Mork L, Hinson A, Kobayashi A, McMahon AP, Capel B. Germ cells are not required to establish the female pathway in mouse fetal gonads. PLoS One. 2012;7:e47238. doi: 10.1371/journal.pone.0047238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeFalco T, Takahashi S, Capel B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol. 2011;352:14–26. doi: 10.1016/j.ydbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C, Paczkowski M, Othman M, Yao HH. Investigating the origins of somatic cell populations in the perinatal mouse ovaries using genetic lineage tracing and immunohistochemistry. Methods Mol Biol. 2012;825:211–221. doi: 10.1007/978-1-61779-436-0_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maatouk DM, Kellam LD, Mann MR, Lei H, Li E, Bartolomei MS, Resnick JL. DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development. 2006;133:3411–3418. doi: 10.1242/dev.02500. [DOI] [PubMed] [Google Scholar]
- 67•.Lesch BJ, Dokshin GA, Young RA, McCarrey JR, Page DC. A set of genes critical to development is epigenetically poised in mouse germ cells from fetal stages through completion of meiosis. Proc Natl Acad Sci U S A. 2013;110:16061–16066. doi: 10.1073/pnas.1315204110. Using ChIP-seq and RNA-seq to analyze flow-sorted male and female germ cells, the authors identified a set of developmentally critical genes that are epigenetically poised in both sexes from the initiation of sex determination into postmeiotic stages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Labosky PA, Barlow DP, Hogan BL. Embryonic germ cell lines and their derivation from mouse primordial germ cells. Ciba Found Symp. 1994;182:157–168. doi: 10.1002/9780470514573.ch9. discussion 168–178. [DOI] [PubMed] [Google Scholar]
- 69.McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- 70.McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 71.Byskov AG, Fenger M, Westergaard L, Andersen CY. Forskolin and the meiosis inducing substance synergistically initiate meiosis in fetal male germ cells. Mol Reprod Dev. 1993;34:47–52. doi: 10.1002/mrd.1080340108. [DOI] [PubMed] [Google Scholar]
- 72.Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Dev. 2004;68:422–428. doi: 10.1002/mrd.20105. [DOI] [PubMed] [Google Scholar]
- 73.Yao HH, DiNapoli L, Capel B. Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development. 2003;130:5895–5902. doi: 10.1242/dev.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 75•.Yokobayashi S, Liang CY, Kohler H, Nestorov P, Liu Z, Vidal M, van Lohuizen M, Roloff TC, Peters AH. PRC1 coordinates timing of sexual differentiation of female primordial germ cells. Nature. 2013;495:236–240. doi: 10.1038/nature11918. By characterizing the Ring1; Rnf2 double knockout mice, the authors demonstrate that PRC1 regulates the timing of female germ cell differentiation via repression of Stra8 and promotion of Oct4/Nanog expression. [DOI] [PubMed] [Google Scholar]
- 76.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 78.MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology. 2007;148:4560–4567. doi: 10.1210/en.2007-0492. [DOI] [PubMed] [Google Scholar]
- 79.Chassot AA, Gregoire EP, Lavery R, Taketo MM, de Rooij DG, Adams IR, Chaboissier MC. RSPO1/beta-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS One. 2011;6:e25641. doi: 10.1371/journal.pone.0025641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DiNapoli L, Batchvarov J, Capel B. FGF9 promotes survival of germ cells in the fetal testis. Development. 2006;133:1519–1527. doi: 10.1242/dev.02303. [DOI] [PubMed] [Google Scholar]
- 81.Bowles J, Feng CW, Spiller C, Davidson TL, Jackson A, Koopman P. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell. 2010;19:440–449. doi: 10.1016/j.devcel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 82.Barrios F, Filipponi D, Pellegrini M, Paronetto MP, Di Siena S, Geremia R, Rossi P, De Felici M, Jannini EA, Dolci S. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci. 2010;123:871–880. doi: 10.1242/jcs.057968. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki A, Igarashi K, Aisaki K, Kanno J, Saga Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc Natl Acad Sci U S A. 2010;107:3594–3599. doi: 10.1073/pnas.0908664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84•.Suzuki A, Niimi Y, Saga Y. Interaction of NANOS2 and NANOS3 with different components of the CNOT complex may contribute to the functional differences in mouse male germ cells. Biol Open. 2014;3:1207–1216. doi: 10.1242/bio.20149308. The authors characterize a dominant negative effect of a NANOS2 transgene harboring mutations in the zinc finger domain. This transgene leads to down-regulation of both NANOS2 and NANOS3, and a severe germ cell phenotype resembling double mutants. They show that NANOS2 associates with the CCR4-NOT deadenylation complex via CNOT1, whereas NANOS3 associates via CNOT8, and can partially compensate for NANOS2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hosokawa M, Shoji M, Kitamura K, Tanaka T, Noce T, Chuma S, Nakatsuji N. Tudor-related proteins TDRD1/MTR-1, TDRD6 and TDRD7/TRAP: domain composition, intracellular localization, and function in male germ cells in mice. Dev Biol. 2007;301:38–52. doi: 10.1016/j.ydbio.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 86.Chuma S, Hosokawa M, Kitamura K, Kasai S, Fujioka M, Hiyoshi M, Takamune K, Noce T, Nakatsuji N. Tdrd1/Mtr-1, a tudor-related gene, is essential for male germ-cell differentiation and nuage/germinal granule formation in mice. Proc Natl Acad Sci U S A. 2006;103:15894–15899. doi: 10.1073/pnas.0601878103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cook MS, Munger SC, Nadeau JH, Capel B. Regulation of male germ cell cycle arrest and differentiation by DND1 is modulated by genetic background. Development. 2011;138:23–32. doi: 10.1242/dev.057000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cook MS, Coveney D, Batchvarov I, Nadeau JH, Capel B. BAX-mediated cell death affects early germ cell loss and incidence of testicular teratomas in Dnd1(Ter/Ter) mice. Dev Biol. 2009;328:377–383. doi: 10.1016/j.ydbio.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu EY, Chang R, Salmon NA, Reijo Pera RA. A gene trap mutation of a murine homolog of the Drosophila stem cell factor Pumilio results in smaller testes but does not affect litter size or fertility. Mol Reprod Dev. 2007;74:912–921. doi: 10.1002/mrd.20687. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki A, Saga Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008;22:430–435. doi: 10.1101/gad.1612708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kedde M, Strasser MJ, Boldajipour B, Oude Vrielink JA, Slanchev K, le Sage C, Nagel R, Voorhoeve PM, van Duijse J, Orom UA, et al. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell. 2007;131:1273–1286. doi: 10.1016/j.cell.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 92.Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells. 2008;26:339–347. doi: 10.1634/stemcells.2007-0622. [DOI] [PubMed] [Google Scholar]
- 93.Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 94.Bustamante-Marin X, Garness JA, Capel B. Testicular teratomas: an intersection of pluripotency, differentiation and cancer biology. Int J Dev Biol. 2013;57:201–210. doi: 10.1387/ijdb.130136bc. [DOI] [PubMed] [Google Scholar]
- 95.Heaney JD, Anderson EL, Michelson MV, Zechel JL, Conrad PA, Page DC, Nadeau JH. Germ cell pluripotency, premature differentiation and susceptibility to testicular teratomas in mice. Development. 2012;139:1577–1586. doi: 10.1242/dev.076851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guigon CJ, Magre S. Contribution of germ cells to the differentiation and maturation of the ovary: insights from models of germ cell depletion. Biol Reprod. 2006;74:450–458. doi: 10.1095/biolreprod.105.047134. [DOI] [PubMed] [Google Scholar]
- 97.Merchant H. Rat gonadal and ovarioan organogenesis with and without germ cells. An ultrastructural study. Dev Biol. 1975;44:1–21. doi: 10.1016/0012-1606(75)90372-3. [DOI] [PubMed] [Google Scholar]
- 98.Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 99.Britt KL, Kerr J, O’Donnell L, Jones ME, Drummond AE, Davis SR, Simpson ER, Findlay JK. Estrogen regulates development of the somatic cell phenotype in the eutherian ovary. FASEB J. 2002;16:1389–1397. doi: 10.1096/fj.01-0992com. [DOI] [PubMed] [Google Scholar]
- 100.Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- 101••.Minkina A, Matson CK, Lindeman RE, Ghyselinck NB, Bardwell VJ, Zarkower D. DMRT1 protects male gonadal cells from retinoid-dependent sexual transdifferentiation. Dev Cell. 2014;29:511–520. doi: 10.1016/j.devcel.2014.04.017. Using mouse genetic models, the authors showed that DMRT1 is essential to maintiain male fate of adult Sertoli cells by preventing RA signaling from activating genes involved in female sex determination and ovarian development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu GC, Chang CF. The switch of secondary sex determination in protandrous black porgy, Acanthopagrus schlegeli. Fish Physiol Biochem. 2013;39:33–38. doi: 10.1007/s10695-012-9618-0. [DOI] [PubMed] [Google Scholar]
- 103.Jimenez R, Barrionuevo FJ, Burgos M. Natural exceptions to normal gonad development in mammals. Sex Dev. 2013;7:147–162. doi: 10.1159/000338768. [DOI] [PubMed] [Google Scholar]
- 104.Guillette LJ, Jr, Woodward AR, Crain DA, Masson GR, Palmer BD, Cox MC, You-Xiang Q, Orlando EF. The reproductive cycle of the female American alligator (Alligator mississippiensis) Gen Comp Endocrinol. 1997;108:87–101. doi: 10.1006/gcen.1997.6953. [DOI] [PubMed] [Google Scholar]