Abstract
The pentose phosphate pathway (PPP) is a fundamental component of cellular metabolism. The PPP is important to maintain carbon homoeostasis, to provide precursors for nucleotide and amino acid biosynthesis, to provide reducing molecules for anabolism, and to defeat oxidative stress. The PPP shares reactions with the Entner–Doudoroff pathway and Calvin cycle and divides into an oxidative and non-oxidative branch. The oxidative branch is highly active in most eukaryotes and converts glucose 6-phosphate into carbon dioxide, ribulose 5-phosphate and NADPH. The latter function is critical to maintain redox balance under stress situations, when cells proliferate rapidly, in ageing, and for the ‘Warburg effect’ of cancer cells. The non-oxidative branch instead is virtually ubiquitous, and metabolizes the glycolytic intermediates fructose 6-phosphate and glyceraldehyde 3-phosphate as well as sedoheptulose sugars, yielding ribose 5-phosphate for the synthesis of nucleic acids and sugar phosphate precursors for the synthesis of amino acids. Whereas the oxidative PPP is considered unidirectional, the non-oxidative branch can supply glycolysis with intermediates derived from ribose 5-phosphate and vice versa, depending on the biochemical demand. These functions require dynamic regulation of the PPP pathway that is achieved through hierarchical interactions between transcriptome, proteome and metabolome. Consequently, the biochemistry and regulation of this pathway, while still unresolved in many cases, are archetypal for the dynamics of the metabolic network of the cell. In this comprehensive article we review seminal work that led to the discovery and description of the pathway that date back now for 80 years, and address recent results about genetic and metabolic mechanisms that regulate its activity. These biochemical principles are discussed in the context of PPP deficiencies causing metabolic disease and the role of this pathway in biotechnology, bacterial and parasite infections, neurons, stem cell potency and cancer metabolism.
Keywords: pentose phosphate pathway, glycolysis, glucose 6-phosphate dehydrogenase, NADPH, metabolomics, oxidative stress, cancer, stem cells, host–pathogen interactions, metabolic engineering, inherited metabolic disease, parasitic protozoa, metabolism of infection
I. INTRODUCTION
Next to glycolysis (Embden–Meyerhof–Parnas pathway) and the tricarboxylic acid (Krebs) cycle, the pentose phosphate pathway (PPP) was one of the first metabolic pathways to be discovered. Work on the PPP was stimulated by the famous Otto Warburg laboratory in Berlin-Dahlem. In the 1930s Warburg demonstrated that the pyridine nucleotide diphosphopyridine nucleotide DPN (now known as NAD+) functions as an electron carrier (Warburg, Christian & Griese, 1935; Warburg & Christian, 1936). In addition, this work revealed the existence of a second coenzyme, termed triphosphopyridine nucleotide TPN (now widely known as NADP+), that is required for the oxidation of glucose 6-phosphate to 6-phosphogluconate, by an enzyme which was purified from yeast and erythrocytes and named Zwischenferment [‘intermediate enzyme’ now glucose 6-phosphate dehydrogenase (G6PDH)] (Warburg et al., 1935; Warburg & Christian, 1936; Dickens, 1938). The TPN dependence of the Zwischenferment led to the speculation that there might be a pathway parallel to glycolysis, involved in the direct oxidation of glucose (reviewed by (Horecker, 2002)). Work in the subsequent three decades, driven substantially by Bernard Horecker at Cornell University, but with important contributions by other leading biochemists including Arthur Kornberg, Terry Wood, Frank Dickens, Fritz Lipmann, Severo Ochoa, Hans Klenow and others, yielded a draft version of the pathway that was presented in 1955 (Gunsalus, Horecker & Wood, 1955). However, it took further decades to complete the canonical pathway map as we know it today, with some enzymes being added only recently [i.e. sedoheptulokinase (SHPK) in humans (Wamelink et al., 2008b) and sedoheptulose 1,7 bisphosphatase (SH17BPase) in yeast (Clasquin et al., 2011)]. Meanwhile, the PPP has gained recognition as being a central player in cellular biosynthetic metabolism and in controlling and maintaining the redox homeostasis of cells. As such, it has been implicated in several human diseases including metabolic syndrome, neurodegeneration (Alzheimer’s disease), cardiovascular disease, parasite infections and cancer (Wood, 1985; Zimmer, 1992; Zimmer, 2001; Schaaff-Gerstenschlager & Zimmermann, 1993; Gupte, 2008; Mayr et al., 2008; Orešič et al., 2011; Vander Heiden et al., 2011; Riganti et al., 2012; Wallace, 2012).
II. BIOCHEMISTRY AND EVOLUTIONARY ORIGIN OF THE PENTOSE PHOSPHATE PATHWAY
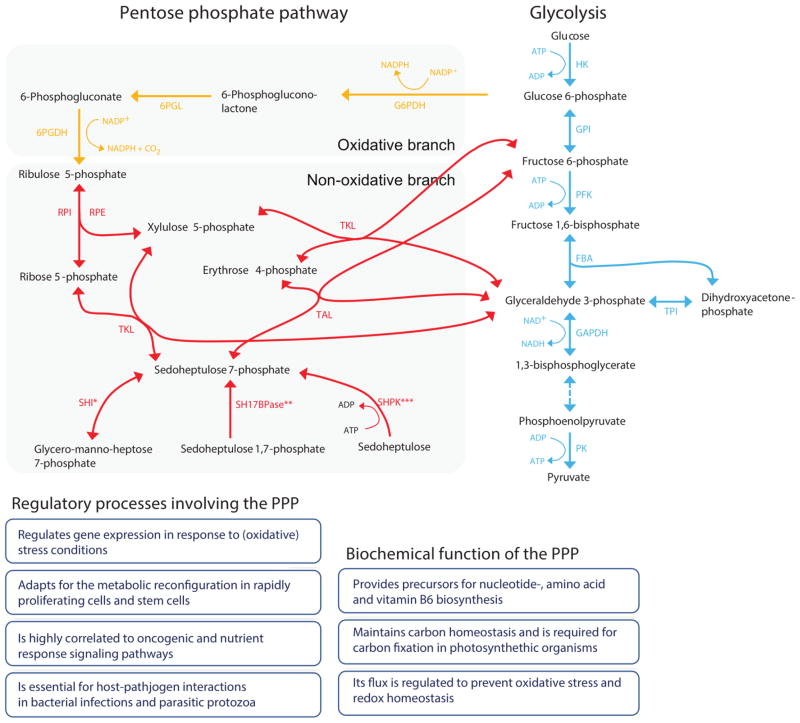
The biochemical reactions that constitute the PPP are, evolutionarily speaking, very old, and seem to accompany life since the earliest steps of evolution. Indeed, metal-catalysed enzyme-free reactions analogous to the PPP are observed in a reconstructed reaction milieu of the prebiotic Archean ocean. This indicates that the basic structure of the PPP is of pre-enzymatic origin and may descend from chemically constraint pre-biotic metal-catalysed sugar phosphate interconversions (Keller, Turchyn & Ralser, 2014). The modern cellular PPP however is catalysed by sophisticated enzymes, except one step, the interconversion of 6-phosphoglucono-δ-lactone to 6-phosphogluconate, which is still considered at least partly spontaneous (Wood, 1985; Horecker, 2002). These enzymatic reactions subdivide the PPP into two biochemical branches, known as the oxidative and non-oxidative PPP (see Fig. 1 for an overview of the pathway, and Table 1 for its enzymes).
Fig. 1.
Schematic representation of the pentose phosphate pathway (PPP, left) and glycolysis (canonical topology of the Embden-Meyerhof-Parnas pathway) (right). The enzymatic reactions constituting both pathways are represented by double or single arrows, according to the reversibility of the reactions. The oxidative and non-oxidative branches of the PPP are highlighted by background coloring. Sedoheptulose conversion enzymes found in *bacteria; **fungi (S. cerevisiae) and plants, ***mammals. Abbreviations are defined in Table 1; FBA, fructose bisphosphate aldolase; HK, hexokinase; PFK, phosphofructokinase; PK, pyruvate kinase; SH17BP, SH17BPase.
Table 1.
Enzymes of the cytosolic pentose phosphate pathway. PPP enzymes, enzyme commission (EC) number and the catalysed reaction
| Enzyme | Abbreviation | EC number | Reaction | References | |
|---|---|---|---|---|---|
| PPP enzymes | Glucose 6-phosphate dehydrogenase | G6PDH | EC1.1.1.49 | Glucose 6-phosphate + NADP+ ↔ 6-phospho-glucono-1,5-lactone + NADPH + H+ | Warburg & Christian (1936) and Glaser & Brown (1955) |
| 6-Phosphogluconolactonase | 6PGL | EC 3.1.1.31 | 6-Phosphoglucono-1,5-lactone + H2O → 6-phosphogluconate | Kawada et al. (1962) and Miclet et al. (2001)) | |
| 6-Phosphogluconate dehydrogenase | 6PGDH | EC 1.1.1.44 | 6-Phosphogluconate + NADP+ → ribulose 5-phosphate + CO2 + NADPH + H+ | Dickens & Glock (1951) | |
| Ribose 5-phosphate isomerase | RPI | EC 5.3.1.6 | Ribulose 5-phosphate ↔ ribose 5-phosphate | Horecker, Smyrniotis & Seegmiller (1951) | |
| Ribulose 5-phosphate epimerase | RPE | EC 5.1.3.1 | Ribulose 5-phosphate ↔ xylulose 5-phosphate | Dickens & Williamson (1956), Horecker & Hurwitz (1956) and Ashwell & Hickman (1957) | |
| Transketolase | TKL | EC 2.2.1.1 | Sedoheptulose 7-phosphate + glyceraldehyde 3-phosphate ↔ ribose 5-phosphate + xylulose 5-phosphate | De La Haba, Leder & Racker (1955) and Horecker, Hurwitz & Smyrniotis (1956) | |
| Transaldolase | TAL | EC 2.2.1.2 | Sedoheptulose 7-phosphate + glyceraldehyde 3-phosphate ↔ erythrose 4-phosphate + fructose 6-phosphate | Horecker & Smyrniotis (1955) | |
| Sedoheptulokinase | SHPK | EC 2.7.1.14 | Sedoheptulose + ATP → sedoheptulose 7-phosphate + ADP | Ebata et al. (1955)) and Wamelink et al. (2008b) | |
| Sedoheptulose 1,7-bisphosphatase | SH17BPase | EC 3.1.3.37 | Sedoheptulose 1,7-bisphosphate + H2O → sedoheptulose 7-phosphate + phosphate | Racker (1962) and Clasquin et al. (2011) | |
| Sedoheptulose 7-phosphate isomerase | SHI | EC 5.3.1.28 | Sedoheptulose 7-phosphate ↔ glycero-manno-heptose 7-phosphate | Kneidinger et al. (2001) and Taylor et al. (2008) | |
| Glycolytic enzymes with PPP substrates (selection) | Glucose phosphate isomerase | GPI | EC 5.3.1.9 | Glucose 6-phosphate ↔ fructose 6-phosphate | Ramasarma & Giri (1956) |
| Triosephosphate isomerase | TPI | EC 5.3.1.1 | Glyceraldehyde 3-phosphate ↔ dihydroxy acetonephosphate (DHAP) | Meyerhof & Beck (1944) | |
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH | EC 1.2.1.12 | Glyceraldehyde 3-phosphate + phosphate + NAD+ ↔ 1,3-bisphosphoglycerate + NADH + H+ | Warburg & Cristian (1939) |
Reactions of the non-oxidative PPP (with the overlapping Calvin cycle and Entner–Doudoroff pathways), occur virtually ubiquitously, and maintain a central metabolic role in providing the RNA backbone precursors ribose 5-phosphate and erythrose 4-phosphate as precursors for aromatic amino acids. By contrast, the oxidative branch of the PPP is not universal and is absent in many aerobic and thermophilic organisms (Grochowski, Xu & White, 2005; Nunoura et al., 2011; Bräsen et al., 2014). While reactions of the non-oxidative branch can also occur non-enzymatically, reactions concerning the interconversion of glucose 6-phosphate to 6-phosphogluconate, defining the oxidative PPP, were not observed in the Archean ocean simulations (Keller et al., 2014). This observation might indicate that the oxidative part of the PPP pathway is evolutionarily newer than the non-oxidative branch. Nonetheless, in the majority of eukaryotes the oxidative branch is highly active and converts the glycolytic/gluconeogenetic metabolite glucose 6-phosphate into ribulose 5-phosphate via the consecutive reactions of G6PDH [in yeast still named Zwf1 (ZWischenFerment) in acknowledgement of Otto Warburg’s original nomenclature], 6-phosphogluconolactonase (6PGL) [catalysing a reaction which can also occur spontaneously but the enzyme increases its specificity (Miclet et al., 2001)] and 6-phosphogluconate dehydrogenase (6PGDH). This metabolic sequence yields two NADPH per metabolized glucose 6-phosphate. Next, the formed ribulose 5-phosphate enters the non-oxidative branch and can be converted either to ribose 5-phosphate by ribose 5-phosphate isomerase (RPI) or to xylulose 5-phosphate by ribulose 5-phosphate epimerase (RPE). While ribose 5-phosphate is required to form the RNA and DNA backbone, erythrose 4-phosphate is required as precursor for the biosynthesis of histidine, for various aromatic metabolites in aromatic amino acid prototrophic organisms and it plays a role in vitamin B6 metabolism (Zimmer, 1992; Wang, Xie & Schultz, 2006; Cadière et al., 2011; Clasquin et al., 2011; Zhao et al., 1995).
The RPI and RPE reactions set the stage for completing the pathway though conversion of ribose 5-phosphate and xylulose 5-phosphate and the glycolytic/gluconeogenetic intermediates glyceraldehyde 3-phosphate and fructose 6-phosphate via reshuffling of the monophosphate sugars. These reactions are catalysed by the two enzymes transketolase (TKL) and transaldolase (TAL), which are responsible for relatively complex (multi-substrate) interconversion reactions at the core of the non-oxidative PPP (Fig. 1).
TKL uses a ketose donor (xylulose 5-phosphate) and aldose acceptors (ribose 5-phosphate or erythrose 4-phosphate) to form aldose and ketose products (glyceraldehyde 3-phosphate and sedoheptulose 7-phosphate or fructose 6-phosphate, respectively), to catalyse the transfer of two-carbon fragments (‘activated glycolaldehyde’) for monosaccharide interconversion (Schenk, Duggleby & Nixon, 1998). Hence, this enzyme is responsible for two distinct reactions within the non-oxidative PPP. TKL activity is dependent on the cofactor thiamine diphosphate (Lindqvist et al., 1992; Schenk et al., 1998; Kochetov & Sevostyanova, 2005). The cofactor is bound at the interface between the two subunits of TKL a homodimer, with two identical catalytic sites (Lindqvist et al., 1992; Kochetov & Sevostyanova, 2005).
TAL instead catalyses the transfer of the three-carbon fragment dihydroxyacetone between sugar phosphates up to eight carbons in length via the formation of a Schiff base at a lysine residue in the active site (Miosga et al., 1993; Banki & Perl, 1996; Samland & Sprenger, 2009). Its donor substrates are ketose sugar phosphates which include fructose 6-phosphate and sedoheptulose 7-phosphate and its acceptor substrates are the aldose sugar phosphates glyceraldehyde 3-phosphate and erythrose 4-phosphate (Samland & Sprenger, 2009).
By sharing these intermediate metabolites with glycolysis (fructose 6-phosphate and glyceraldehyde 3-phosphate), TAL and TKL act as a bridge between glycolysis and the PPP. In addition, they connect to sedoheptulose 7-phosphate which is synthesized also by other sources. These include the recently described enzyme sedoheptulokinase [SHPK, also known under the former systematic name carbohydrate kinase-like (CARKL)] in mammals (Kardon et al., 2008; Wamelink et al., 2008b). SHPK catalyses the phosphorylation of sedoheptulose to sedoheptulose 7-phosphate though ATP consumption in a biochemical reaction first described in 1955 (Ebata, Sato & Bak, 1955). Other novel enzymes that metabolize sedoheptulose 7-phosphate are sedoheptulose 1,7-bisphosphatase (SH17BPase) in yeast (Clasquin et al., 2011) and sedoheptulose 7-phosphate isomerase (SHI) in bacteria (Kneidinger et al., 2001; Valvano, Messner & Kosma, 2002; Taylor et al., 2008). Hence, sedoheptulose 7-phosphate represents a glycolysis-independent entry and exit point into/from the non-oxidative PPP. The formation of this metabolite connects the PPP with open chain and polyol sugar metabolism and bacterial lipopolysaccharide biosynthesis. These connections with both glycolysis, amino acid biosynthesis and open-chain sugar metabolism place the PPP central to the metabolic network. Moreover, its flux and regulation not only depend on, but also influence its neighbouring metabolic routes, which might explain in part the extensive regulation of this biochemical route, as detailed in later sections of this article.
Analysis in yeast and mammalian cells has shown that with the exception of RPI, most of the PPP enzymes are not essential for survival at the cellular level. At the organism level in mammals, at least partial deficiencies of PPP enzymes G6PDH, 6PGDH, TAL and RPI are viable as well, but lead to severe genetic disease (see Section VI). However, no disease phenotypes or deficiencies have been reported for the other PPP enzymes; most likely their deficiency is embryonically lethal to mammalian organisms, indicating that the PPP as pathway is essential. Indeed, double gene deletions that affect both the oxidative and the non-oxidative PPP are also lethal down to the cellular level (Schaaff-Gerstenschlager & Zimmermann, 1993; Juhnke et al., 1996; Krüger et al., 2011). The viability of the partial PPP deficiencies and several null alleles therefore indicates that the oxidative and non-oxidative branches of the PPP can work independently; each of the two parts can compensate and provide sufficient sugar phosphate precursors required for cellular survival.
(1) The L-type PPP and alternative or extended reaction sequences of the PPP
The PPP might also exist in alternative reaction sequences. Named the L-type PPP, a reaction sequence was proposed in liver cells that involves flux over alternative metabolites such as arabinose 5-phosphate and glycero-ido-octulose phosphate (Williams et al., 1984). Also, alternative seven-carbon phosphates and their diphosphates have been associated with the PPP (Wood, 1985). (Longenecker & Williams, 1980) suggested that up to 30% of the PPP flux in hepatocytes could be attributable to these alternative PPP forms. Nevertheless, there is only limited confirmation of these PPP alternatives, indeed biochemical evidence for them has been questioned (Landau & Wood, 1983). Therefore, these alternatives are not addressed in detail herein. The recent discovery of the PPP enzymes SHPK and the SH17BPase however indicates that the full biochemical spectra of the PPP could exceed the core reactivity of the canonical pathway (Fig. 1), and hence that additional discoveries might still be made.
(2) The subcellular localization of the PPP and its enzymes
In most organisms, including fungi and metazoa, the PPP is localized in the cytosol, and contributes both to the cytoplasmic metabolite as well as redox cofactor pool. However, important exceptions do exist. The pathway is split between the cytosol and other organelles such as the plastid, peroxisomes or glycosomes in plants and parasitic protozoa, respectively (Zimmer, 2001; Hannaert et al., 2003; Kruger & von Schaewen, 2003). Part of the PPP might occur in the endoplasmic reticulum (ER) too. Microsomes, vesicles formed from the ER when cells are mechanically homogenized, contain at least five PPP enzymes. These include hexose 6-phosphate dehydrogenase (H6PDH), an enzyme similar to G6PDH (Bublitz & Steavenson, 1988; Nelson, Lehninger & Cox, 2008; Senesi et al., 2010) that is required to provide NADPH to the luminal reductases (Beutler & Morrison, 1967; Takahashi & Hori, 1978; Senesi et al., 2010). H6PDH has a broader range of substrates than G6PDH and it was described as being non-selective regarding the nucleotide cofactor (NAD+ and NADP+). The concentration of reduced NADP(H) in the endoplasmic lumen suggested that under physiological conditions glucose 6-phosphate and NADP+ are preferred. Hence, while the PPP is largely a cytosolic pathway, alternative organelle localisations do exist and are of significant importance.
(3) Glucose 6-phosphate dehydrogenase (G6PDH) and the role of the oxidative PPP in NADPH synthesis
The most intensively studied enzyme of the PPP is G6PDH, an NADP+-dependent oxidoreductase. This enzyme has often been quoted as being rate limiting for the oxidative branch of the PPP. Although the classic concept of ‘rate limitation’ has its limitations (Kacser, 1995), the first enzymatic step involving G6PDH is certainly of central importance as the oxidative PPP is largely considered unidirectional. Eukaryotic G6PDH was first discovered in different strains of brewery yeast (Dickens, 1938), and to date this model organism has served for dissecting most of the functionality of the PPP. Budding yeast G6PDH is encoded by a single gene YNL241C (Nogae & Johnston, 1990; Thomas, Cherest & Surdin-Kerjan, 1991). Deletion of this gene retains viability, but zwf1 cells are unable to synthesize methionine. It is assumed that this methionine auxotrophy is a consequence of the insufficient production of NADPH to sustain methionine biosynthesis, and requires yeast to assimilate ‘inorganic sulphur’ in order to form ‘organic sulphur’ (methionine or cysteine) to grow (Masselot & De Robichon-Szulmajster, 1975; Nogae & Johnston, 1990; Thomas et al., 1991). This notion of NADPH shortage in zwf1Δ cells is supported by the observations that (i) when supplying NADPH from a different source, i.e. through alcohol dehydrogenase (Ald6), the methionine prototrophy is restored (Grabowska & Chelstowska, 2003). Moreover (ii), also yeast cells deleted for cytoplasmic superoxide dismutase (SOD1) become methionine auxotrophs (Slekar, Kosman & Culotta, 1996). These results indicate that G6PDH, and the oxidative PPP in general, play a quantitative role in NADP+ to NADPH recycling and redox balancing.
The importance of the NADPH-producing function of the PPP has been corroborated in several studies mainly addressing the antioxidant function of this coenzyme in yeast and mammalian cells. As NADPH is required as a redox equivalent in the antioxidant machinery, involving the thioredoxin/peroxiredoxin and glutathione systems (Pollak, Dölle & Ziegler, 2007a; Grant, 2008), yeast and mammalian cells deficient for G6PDH become hypersensitive to several oxidants (Juhnke et al., 1996; Gorsich et al., 2006; Krüger et al., 2011).
Which proportion of the cytoplasmic NADPH pool is derived from the PPP? It varies, as the activity of the oxidative PPP is flexibly regulated, and as discussed in Section III, is actively increased during stress situations. A flexible flux of the PPP is supported from studies of NADPH-consuming enzymes, metabolic flux analysis, but in particular by investigations on the oxidative stress response. An illustrative example concerns the yeast NADPH oxidase YNO1, a recently discovered enzyme that similar to mammalian NADPH oxidases, oxidizes NADPH to produce superoxide. When YNO1 is overexpressed in wild-type cells, superoxide levels increase 10-fold. An increase in superoxide levels is however no longer observed upon deletion of zwf1, indicating that the oxidative PPP compensates for the increased NADPH consumption caused by the YNO1 overexpression (Rinnerthaler et al., 2012).
Yeast cells deficient in NADPH production due to zwf1 deletion have an almost normal NADPH/NADP+ ratio when growing exponentially and in glucose media. Their NADPH/NADP+ ratio however collapses when exposed to oxidants (Castegna et al., 2011). Thus, the contribution of the oxidative PPP to the cellular NADPH pool is dynamic and context dependent, and essential for most cell types only when the NADPH requirement is increased. In Section IV we discuss mechanisms that facilitate a dynamic control of PPP activity under different physiological conditions, which is achieved through cooperation of transcriptional regulation, post-translational modifications, and allosteric control (feedback and feedforward regulation) of the involved enzymes.
In mammalian cells, G6PDH was intensively studied because partial deficiency in this enzyme represents the most common human enzyme defect, and as described in Section VI, has severe haematological consequences (haemolytic anaemia). A full depletion of G6PDH in mammals and nematodes is however lethal at the organism level (embryonic lethality) (Longo et al., 2002; Ying, 2007) while the same mutation is tolerated at the cellular level (Pandolfi et al., 1995). Similar to yeast cells, mouse embryonic stem cells possessing a mutation leading to a strong reduction in G6PDH activity are able to grow but are sensitive to externally applied oxidative stress (Pandolfi et al., 1995; Filosa et al., 2003). Also, mouse fibroblasts carrying a permanent deletion of the G6PDH exon are viable, despite their low clonogenicity (Filosa et al., 2003).
(a) Non-PPP sources of NADP(H)
The role of the PPP in providing NADPH has to be seen in the context of other NADPH oxidoreductases, cellular compartmentalisation and the NAD(H)/NADP(H) de novo synthesis pathways. In many cell types and most conditions, NADP(H) is present mostly in its reduced form (Ying, 2007; Pollak et al., 2007a). However, this assumption has a degree of uncertainty.
As membranes are considered to be NADPH impermeable, the NADPH recycling process and de novo biosynthesis is compartment-specific (Ying, 2007; Pollak, Niere & Ziegler, 2007b). Hence, in most organisms the PPP contributes mainly to the cytoplasmic NADPH pool. In mammalian mature erythrocytes which have no nucleus and no mitochondria, the PPP is generally assumed to be the dominating source of this coenzyme. In other cell types, there are important additional cytoplasmic enzymes that contribute to the NADPH pool, including the cytosolic isoforms of isocitrate dehydrogenase, glutamate dehydrogenase, methylene-tetrahydrofolate dehydrogenase, formyl-tetrahydrofolate dehydrogenase, aldehyde dehydrogenase and malic enzyme (Bernt & Bergmeyer, 1974; Wermuth, Münch & von Wartburg, 1977; Scheibe, 1987; Lee et al., 2002; Fan et al., 2014). Another source influencing the NADPH level in mammalian cells, for instance in mitochondria, appears to be trans-hydrogenation between NADH and NADP+, forming NAD+ and NADPH (Jackson, 2003; Venditti, Napolitano & Di Meo, 2013). The enzyme catalysing this reaction, nicotinamide nucleotide trans-hydrogenase, is an energy-driven integral protein of the inner mitochondrial membrane, and required in mitochondria to maintain their high NADPH/NADP+ ratio (Ronchi et al., 2013).
Finally, in the debate about NADPH sources its de novo synthesis is less often taken into account. The synthesis of NADPH de novo is achieved by phosphorylation of NAD(H) by NAD kinase enzymes (Bieganowski et al., 2006; Pollak et al., 2007a). The lack of an NADP(H) phosphatase in many organisms implies that the de novo synthesis might primarily be used for the initial synthesis of the NADP(H) molecules, and not necessarily for controlling the NADP+/NADPH balance. Nonetheless it remains plausible that certain cells might be able to compensate for a lack of NADPH by de novo synthesis of the reduced form by phosphorylation of NADH.
(b) The synthesis of ribulose 5-phosphate in the non-oxidative PPP
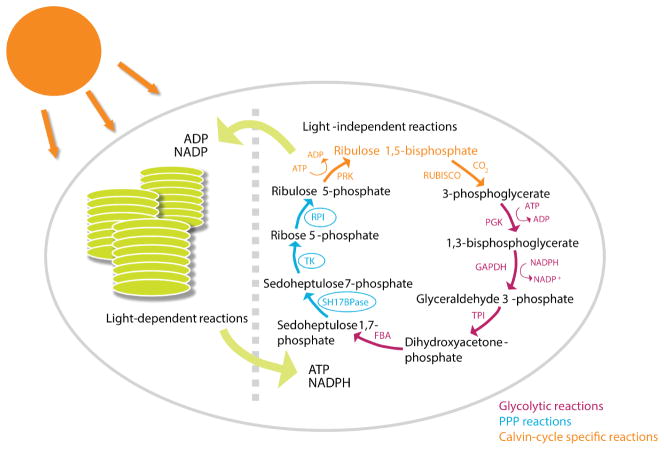
The pentose phosphate pathway in yeast and mammals shares much with the most important carbon assimilatory pathway in plants, the Calvin cycle. Reverse flux through the complete PPP could in theory assimilate carbon in a cyclic manner. The problem is that certain reactions of the oxidative PPP are not readily reversible. Accordingly, the Calvin cycle bypasses these reactions via ribulose 1,5-bisphosphate carboxylase oxygenase (Rubisco), apparently the most abundant metabolic enzyme in the biosphere (Raines, 2003). Rubisco converts ribulose 1,5-bisphosphate plus carbon dioxide into two molecules of 3-phosphoglycerate. While this enzyme is not shared with the PPP, other Calvin cycle reactions are (Fig. 2).
Fig. 2.
The light-independent reactions of carbon fixation in the Calvin cycle share enzymes and reactions with the pentose phosphate pathway (PPP) and glycolysis. Abbreviations are defined in Table 1; PGK, phosphoglycerate kinase; PRK, phosphoribulokinase, TK, transketolase; FBA, fructose-bisphosphate aldolase.
In particular, both the non-oxidative PPP and the Calvin cycle interconvert a total of 15 pentose carbon atoms (contained in ribulose 5-phosphate) with 15 glycolytic carbon atoms (in the form of fructose 6-phosphate and glyceraldehyde 3-phosphate), sharing some important reactions. However, while the classical non-oxidative PPP uses TAL to make sedoheptulose 7-phosphate, the Calvin cycle uses the glycolytic enzyme fructose-bisphosphate aldolase (FBA) to convert erythrose 4-phosphate plus dihydroxyacetone phosphate into sedoheptulose 1,7-bisphosphate, which in turn is hydrolysed by the enzyme SH17BPase to yield sedoheptulose 7-phosphate. This hydrolysis step provides the thermodynamic driving force, pushing the Calvin cycle towards ribulose 5-phosphate. Thus, while the non-oxidative PPP is reversible, the Calvin cycle is not.
Because FBA is a ubiquitous enzyme (playing an essential role in glycolysis and gluconeogenesis, and also producing sedoheptulose 1,7-bisphosphate), the distinguishing enzyme of the Calvin cycle’s path from triose phosphates to pentose phosphates is SH17BPase. Until recently, this enzymatic activity was thought to be specific to photosynthetic organisms. Metabolomic screening of yeast strains lacking genes of unknown function, however, revealed a strain with elevated sedoheptulose 1,7-bisphosphate. The associated gene was subsequently shown to encode an enzyme with SH17BPase activity involved in a novel variant of the non-oxidative PPP that follows yet more closely the Calvin cycle reaction sequences (Clasquin et al., 2011). This thermodynamically driven variant of the non-oxidative PPP is termed riboneogenesis. Just as gluconeogenesis uses the energy of a sugar phosphate bond to convert trioses into hexoses, riboneogenesis uses one to drive flux from trioses to pentoses.
Ribose 5-phosphate biosynthesis via riboneogenesis is useful when demand for ribose exceeds that for NADPH. In such cases it is presumably advantageous to have a thermodynamically driven alternative to the standard non-oxidative PPP, and to avoid an over-reduction of the NADPH pool. Evidence for this effect was provided by experiments in yeast (Clasquin et al., 2011). The cells were fed with glucose labelled selectively at the 6-position with carbon 13 (6-13C-glucose). Such glucose produces doubly labelled sedoheptulose 7-phosphate selectively via SH17BPase. This labelling pattern was observed preferentially when yeast cells were grown on media that decreased their need for NADPH (e.g. by providing them with lipids). One can envision the possibility that growing mammalian cells, including cancer cells, could also in some circumstances need ribose 5-phosphate in excess of NADPH, i.e. when DNA and RNA nucleotide synthesis is maximized (Ferreira, 2010; Cairns et al., 2011). So far, however, has not been observed in doubly labelled sedoheptulose 7-phosphate 6-13C-glucose in mammalian cells (J. D. Rabinowitz, unpublished results). Thus, SH17BPase activity plays a role in plant and microbial metabolism, but not necessarily in animals.
In mammalian cells, a different additional influx into the sedoheptulose 7-phosphate PPP has been discovered recently: SHPK. This enzyme was identified based on the observation that several patients suffering from nephropathic cystinosis (CTNS) possess elevated urinary concentrations of sedoheptulose. In these patients, the CTNS gene was lost due to a 57 kb deletion, which aside from the CTNS gene also contained a gene encoding for a carbohydrate kinase-like (CARKL) protein. Biochemical assays have then shown that CARKL is in fact a sedoheptulokinase (SHPK) and catalyses the ATP-dependent phosphorylation of sedoheptulose (Kardon et al., 2008; Wamelink et al., 2008b). Apparently, the existence of SHPK implies that mammalian cells are able to convert sedoheptulose, and thus non-phosphorylated sugars, into ribose 5-phosphate and glycolytic intermediates. The role of SHPK could be to prevent an accumulation of sedoheptulose and related sugars in the clearance of polyol metabolites (Kardon et al., 2008; Wamelink, Struys & Jakobs, 2008a). Moreover, expressing this gene in yeast increased H2O2-resistance, indicating that a second biological role of SHPK could consist of providing an increase of the PPP flux during the oxidative stress (Krüger et al., 2011). Finally, as discussed in Section VII, SHPK could also ‘report’ altered metabolism to the immune system; expression of this gene directs macrophage polarization through control of glucose metabolism (Haschemi et al., 2012).
III. THE GLYCOLYSIS/PPP TRANSITION: METABOLIC AND TRANSCRIPTIONAL MECHANISMS THAT CHANGE PPP FLUX UPON DEMAND
The survival of a cell in its ever-changing environment depends on the robustness, interconnection and functionality of its biological networks. These are highly dynamic and respond to changing endogenous and exogenous conditions by interactions of a specific and limited set of components (Ihmels, Levy & Barkai, 2004; Ralser et al., 2007; Chechik et al., 2008; Buescher et al., 2010; Fendt et al., 2010; Grüning, Lehrach & Ralser, 2010). Such dynamic activity is particularly relevant for the metabolic network, where a few hundred metabolites are interconnected through biochemical reactions within metabolic modules, providing energy and biomolecules depending on substrate availabilities, enzyme activities and cellular demands. Therefore, to ensure proper functionality of the metabolic network upon environmental changes, metabolism is adapted. These adaptations involve the production of increased amounts of components needed and decreased concentrations of those unneeded, to save resources and energy simultaneously, and importantly, maintain homeostasis and prevent a collapse of the metabolic network. Moreover, these reconfigurations are highly regulated ensuring that concentrations of general cofactor metabolites, such as NAD(H), NADP(H) and A(T)P are not falling to fatal levels, the flux of the metabolic network is stabilized, and enzyme activity and abundance of the metabolic module is adjusted (Ihmels et al., 2004; Patil & Nielsen, 2005; Cakir et al., 2006; Ralser et al., 2009; Grüning et al., 2010; Heinemann & Sauer, 2010).
(1) Regulation of the PPP during the oxidative stress response
A paradigm example to study the rapid metabolic as well as transcriptional regulation of the metabolic network is the response of the PPP to oxidative stress. As aforementioned, in yeast the NADPH-producing role of G6PDH is compensated by other NADP-oxidizing enzymes under normal growth conditions. However the NADP+/NADPH ratio collapses upon a hydrogen peroxide (H2O2) exposure, rendering G6PDH null cells highly oxidant sensitive (Nogae & Johnston, 1990; Todisco et al., 2006; Castegna et al., 2010). Indeed, the activity of the PPP is rapidly augmented when cells are exposed to the oxidant. To induce this metabolic transition, metabolic and gene regulatory mechanisms cooperate (Fig. 3). In the first seconds upon an oxidative burst, enzymes of glycolysis, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Ralser et al., 2007) and pyruvate kinase (PK) (Anastasiou et al., 2011; Grüning et al., 2011) are inactivated causing a block in glycolysis, while the flux of the PPP continues (Shenton & Grant, 2003; Ralser et al., 2007; Ralser et al., 2009). This rapid response lasts a few seconds to minutes, then transcriptional responses take over and maintain higher PPP activity through up-regulation of enzymes and post-translational modifications, including those which increase the activity of G6PDH (Chechik et al., 2008; Ralser et al., 2009; Cosentino, Grieco & Costanzo, 2011; Wang et al., 2014). This tight regulation seems to have a dual role. During normal growth, it prevents an overproduction of NADPH and PPP intermediates, and minimizes carbon depletion due to CO2 production. At the same time, it facilitates a rapid cellular response when stress conditions apply (Shenton & Grant, 2003; Ralser et al., 2007; Ralser et al., 2009; Grant, 2008).
Fig. 3.
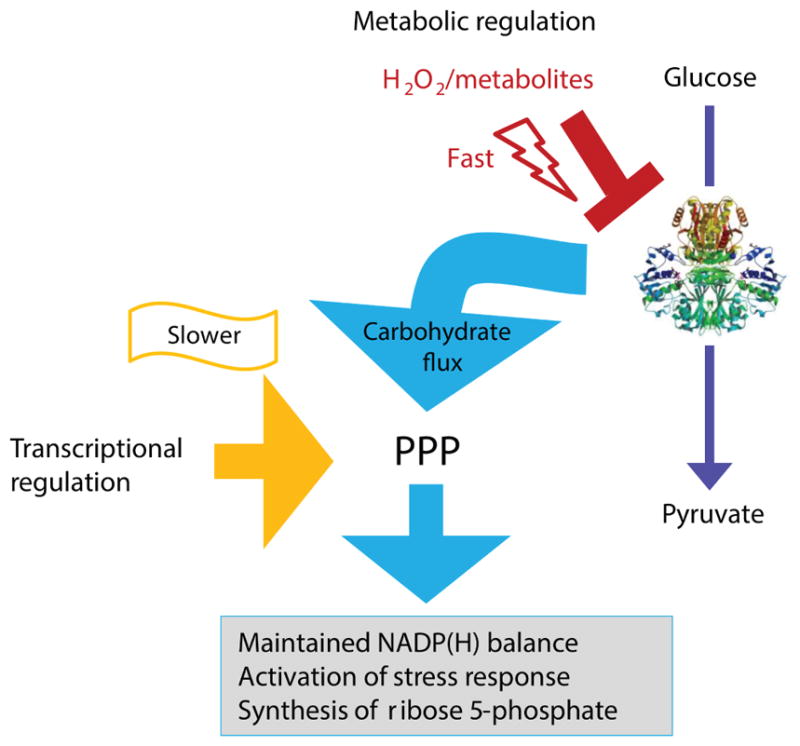
Induction of the glycolysis/pentose phosphate pathway (PPP) transition during oxidative stress. The PPP plays a pivotal role in counteracting oxidative stress and is implicated in (i) maintaining metabolic and redox homeostasis via NADP+ to NADPH reduction, (ii) by synthesizing ribose 5-phosphate used in nucleotide biosynthesis (increased synthesis is required upon DNA damage stress), and (iii) an important role in activating stress-responsive gene expression. In a stress situation, activity of the PPP is increased through orchestrated allosteric/post-translational (=metabolic) and transcriptional regulation, but these are not necessarily acting at the same time. The fastest response (~seconds timescale) is made possible through oxidative inhibition of glycolytic enzymes represented by the arrow moving from glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (illustrated as a crystallographic structure), which acts as one of the metabolic switches, while the PPP remains active. This process is supported by post-translational modifications that increase glucose 6-phosphate dehydrogenase (G6PDH) activity. The comparatively slower (=minutes) process of altering transcript and protein levels allows for cellular adaptation to stress in the long(er)-term response. The GAPDH crystallographic structure was obtained from RCSB-PDB (www.rcsb.org). PDB ID 3PYM: (DOI:10.2210/pdb3pym/pdb).
The temporal inhibition of glycolysis to the benefit of the PPP flux appears to be dependent on different mechanisms. GAPDH for instance is rapidly inactivated by chemical oxidation which correlates well with a boost in PPP metabolite concentrations observed within a few seconds (Ralser et al., 2007; Ralser et al., 2009). Other mechanisms that support the inhibition of glycolytic enzymes concerns allosteric control. A higher activity of the PPP is maintained by feedback inhibition of triosephosphate isomerase (TPI) by the glycolytic intermediate phosphoenolpyruvate (PEP) (Grüning et al., 2014). PEP is the substrate of pyruvate kinase, that itself is controlled allosterically (Lyssiotis et al., 2012; Morgan et al., 2013). A third strategy that facilitates rapid PPP activation appear to be post-translational modifications which affect the activity of G6PDH. In mammalian and Xenopus laevis cells phosphorylation and acetylation increase G6PDH activity during the stress response, so that this enzyme does not become rate limiting (Cosentino et al., 2011; Wang et al., 2014).
The glycolytic/PPP transition during oxidative stress is mechanistically related to steady-state adaptation to physiological conditions that are associated with increased reactive oxygen species (ROS) production. Here, PK and its feedback regulatory function on TPI and other metabolic enzymes play a crucial regulatory role. In budding yeast, the activity of PK is reduced when cells respire at high rate, and less active isoforms (i.e. PKM2 in mammals, PYK2 in yeast) are expressed. The resultant accumulation of PEP causes feedback inhibition of several glycolytic enzymes, including the redox regulator TPI, and flux in the PPP increases (Grüning et al., 2011; Grüning et al., 2014). TPI inhibition by PEP was required to prevent oxidative stress and oxidative damage, and led to protein oxidation and mitochondrial damage in respiring cells when interrupted (Grüning et al., 2011; Grüning et al., 2014). As described in Section VIII, a similar mechanism appears to be used by cancer cells to maintain their metabolic redox balance as well.
(2) Transcriptional regulators of the PPP
The concerted allosteric/post-translational response is followed by transcriptional events and transcripts and proteins of the PPP increase in concentration (Chechik et al., 2008). The transcriptional changes occur in a fully coordinated manner, and enzymes are subsequently induced depending on their molecular function (Ihmels et al., 2004; Chechik et al., 2008). Therefore, the strictly timed program facilitates the cell’s reaction against minatory redox collapse immediately via the metabolome and the proteome, then later via the transcriptome, to adapt to the cellular responsibilities (Fig. 3). Such transcriptional patterns shape metabolic network gene regulation in response to changing conditions due to co-expression of enzymes that catalyse connected reactions.
The details of transcriptional regulation of PPP enzymes varies strongly among organisms; therefore, only principal mechanisms will be discussed here. Both in mouse and yeast, G6PDH is transcriptionally induced upon oxidative stress, and by the need for NADPH and PPP intermediates for anabolic reactions such as lipid synthesis and nucleotide synthesis (Kletzien, Harris & Foellmi, 1994; Lee et al., 1999; Stanton, 2012). These effects are not specific to G6PDH, other PPP enzymes are dependent on transcriptional mechanisms as well (Kletzien et al., 1994; Lee et al., 1999; Stanton, 2012). This transcriptional regulators differs according to the specific demands of the cell or tissue. For instance, PPP regulation for lipid synthesis is achieved by the sterol regulatory element-binding proteins (SREBPs) class transcription factors, whereas the regulation during oxidative stress is mediated by nuclear respiratory factor 2 (Nrf 2)-family and other transcription factors. The latter also govern synthesis of many enzymes directly involved in oxidative stress defence (Stanton, 2012). In budding yeast, PPP gene expression control during oxidative stress is also exerted by basic leucine zipper (bZIP, Yap1) transcription factors and the nuclear response regulator Skn7. These factors, acting either in concert or as single regulators, govern the cellular response not only to oxidative stress, but also when anabolic intermediates are needed (Lee et al., 1999). During oxidative stress, another regulatory role has been attributed to the transcription factor Sin Three Binding protein 5 (Stb5), which activates PPP enzymes in response to exposure to the thiol oxidizing agent diamide (Akache, Wu & Turcotte, 2001; Larochelle et al., 2006; Hector et al., 2009).
(3) Feedforward regulation of the metabolome to the transcriptome: PPP metabolites as regulators of the stress response
During stress conditions, the PPP seems to have attained another role: the induction of stress-responsive gene expression. Evidence for an NADPH-independent function of the PPP in the antioxidant response comes from the observation that enzyme deficiencies of both PPP branches are oxidant sensitive (Juhnke et al., 1996; Krüger et al., 2011). Moreover, a yeast double mutant deleted for G6PDH (Zwf1) and the non-oxidative PPP enzyme Tal1 is more H2O2 sensitive than the parent mutants deleted for either Tal1 or Zwf1 alone (Krüger et al., 2011). By contrast, increased oxidant resistance was obtained when the metabolite load of the non-oxidative pathway was augmented due to expression of the mammalian SHPK in yeast (Kardon et al., 2008; Krüger et al., 2011). In addition, stress response genes were induced when the flux of the PPP was stimulated by genetic perturbation of glycolysis (Krüger et al., 2011). Finally, tuning the NADPH demand gradually by overexpression of an engineered NADPH-dependent butanediol dehydrogenase led to a concomitant accumulation of PPP metabolites and also triggered the induction of PPP and stress response genes (Celton et al., 2012). Hence, during the stress response, the PPP appears to play not only the role of a canonical metabolic pathway which responds to oxidant treatments, but also functions as a transcriptional balancer and is involved in inducing components of the oxidative stress response. The exact underlying molecular mechanisms are however yet unknown.
IV. ANALYTICAL METHODS FOR MEASUREMENT OF PPP INTERMEDIATES
Key to finding new PPP reactions, as well as elucidating regulation of the pathway, are reliable methods to quantify PPP flux and intermediate concentrations. Major challenges of studying the PPP include the high turnover rates of its intermediates in the second or sub-second time range (Weibel, Mor & Fiechter, 1974; De Koning & van Dam, 1992; Douma et al., 2010) and low abundances of these compounds (Casazza & Veech, 1986). Rapid sampling techniques such as cold methanol quenching are typically employed to arrest metabolism immediately in cells (De Koning & van Dam, 1992). A further difficulty in sugar phosphate analytics is the proper separation of pentose isomers (ribose 5-phosphate, ribulose 5-phosphate and xylulose-5-phosphate) and hexose isomers (glucose 6-phosphate, fructose 6-phosphate and other relevant hexose monophosphates).
(1) From historical techniques to LC-MS/MS
A series of different methods have been developed to study PPP metabolites and enzymes, including colorimetric assays (Sable, 1952; Novello & McLean, 1968) and the use of thin layer chromatography in combination with 14C-labelled substrates (Becker, 1976). Another widely used quantification approach is to couple the enzymatic interconversion of specific sugar phosphate substrates to the NAD-dependent oxidation of glyceraldehyde 3-phosphate by GAPDH, or other reactions catalysed by NADP(H)- or NAD(H)-dependent enzymes, and to monitor the consumption of NAD(P)H by spectrophotometric or fluorometric methods (Sable, 1952; Kauffman et al., 1969; Casazza & Veech, 1986; King, Passonneau & Veech, 1990). However, these procedures are limited to measuring one component at a time and are critically dependent on the specificity of purified enzymes and optimal assay conditions. Detailed studies of the PPP were therefore accompanied by long, cumbersome analytical methods with relatively low sensitivity and virtually no dynamic over time (Casazza & Veech, 1986).
With the appearance of high-performance liquid chromatography (HPLC) these extensive measurement times could be drastically reduced to 30–180 min (Giersch, 1979; Smrcka & Jensen, 1988; Swezey, 1995), giving rise to major advances in the field of PPP research. A combination of chromatographic methods with mass spectrometry eventually facilitated the routine separation and analysis of sugar phosphates. Several capillary electrophoresis-MS (Soga, 2007) and gas chromatography-MS methods (Koek et al., 2006; Cipollina et al., 2009) have been developed; however, they have already been relatively outnumbered by a number of liquid chromatography tandem mass spectrometry (LC-MS/MS) techniques for sugar phosphate measurement. In a targeted LC-MS/MS approach, Wamelink et al., 2005 determined absolute concentrations of a series of sugar phosphate intermediates by means of HPLC and tandem mass spectrometry. Later, analogous methods were used to measure extended sets of metabolites (Luo et al., 2007; Buescher et al., 2010; Jannasch, Sedlak & Adamec, 2011; Rühl et al., 2012; Lu et al., 2010; Bajad et al., 2006). Without doubt, mass spectrometry as the detection system has strongly enhanced sugar phosphate analysis; but difficulties in separating structural isomers still need to be overcome. The development therefore of further powerful separation procedures will be of high importance to allow for a more reliable and robust quantification of PPP metabolites.
In addition to measuring metabolite levels, there has been long-standing interest in measuring PPP flux. One classical and reliable approach to measuring absolute oxPPP flux in cells involves feeding, in separate experiments, 1-14C-glucose and 6-14C-glucose and measuring radioactive CO2 release (Katz & Wood, 1963). As carbon 1 of glucose is selectively released by the oxPPP whereas other pathways metabolize carbon 1 and 6 identically, the difference in radioactive CO2 release from these two tracers provides direct quantitation of the oxPPP flux. Kinetic analysis of PPP intermediate labeling from 13C-glucose by LC–MS can also be used to calculate absolute oxPPP flux and gives similar estimates to the 14C-CO2-release approach, but the 14C-approach remains more precise (Fan et al., 2014).
A strategy based on the cleavage of carbon 1 of glucose by the oxPPP has also been employed to measure oxPPP relative to non-oxPPP flux into ribose-5-phosphate. One method involves feeding, separate experiments, 1-13C-glucose and 6-13C-glucose and measuring ribose-5-phosphate labeling by mass spectrometry, with ribose-5-phosphate produced via oxPPP labeled by 6-13C but not 1-13C-glucose. More conveniently, one can feed 1,2-13C-glucose and to look for singly versus doubly labeled ribose-5-phosphate, with the former made by the oxPPP and the latter by the non-oxPPP (Lee et al., 1998). A limitation of these methods is that they do not distinguish between net ribose production by the non-oxPPP versus exchange flux (which can impact ribose-5-phosphate labeling even if net non-oxPPP flux is away from ribose). Thus, definitive methods for understanding non-oxPPP flux are still needed, and additional tracers and measurement of more metabolites’ labeling may, with proper computational deconvolution, provide further insights (Brekke et al., 2012; Tang et al., 2012; Crown et al., 2012).
In this vein, recent work has provided a new tracer method for the PPP: Deuterium-labeled glucose (1-2H or 3-2H-glucose) to track specifically oxPPP-produced NADPH and its subsequent utilization for reductive biosynthesis (Fan et al., 2014; Lewis et al., 2014). Initial data show that the oxPPP accounts for about 50% of total NADPH in transformed mammalian cells growing in culture, with most of this NADPH devoted to fatty acid synthesis. These methods are now poised to quantitate variation in oxPPP activity and NADPH usage across conditions, cell types, and compartments.
(2) In vivo PPP measurements using NMR
MS-based methods are sensitive, selective and robust, but are not applicable in vivo. Classic nuclear magnetic resonance (NMR) methods regularly fall short of providing the sensitivity required for studying the PPP. However, recently, a new NMR technique has been introduced, termed hyperpolarization, which can increase the sensitivity of the 13C NMR experiment by more than 104-fold (Ardenkjaer-Larsen et al., 2003). Being dynamic, this method could be used to measure PPP flux in vivo. A 13C-labelled cell substrate is mixed with a stable radical and cooled to temperatures close to absolute zero (~1 K) in a high magnetic field (typically 3.5-5T). At this temperature the electron spins in the radical are almost completely polarized. This polarization is then transferred to the 13C spins by microwave irradiation and the sample is then rapidly warmed to room temperature with substantial retention of the 13C spin polarization. Cells can then be exposed to the hyperpolarized 13C-labelled substrate, or for in vivo studies the tracer can be injected intravenously or added to the growth media of microorganisms. The signal is now boosted as a result of polarization of the 13C spins, so that the position of the molecule and the metabolites formed from it can be imaged (Brindle et al., 2011; Kurhanewicz et al., 2011).
The major limitation of the technique is the relatively short life time of the spin polarization (typically ~30 s in vivo), which means that only relatively rapid metabolic processes can be imaged and the experiment must be accomplished within 2–5 min following injection of the hyperpolarized substrate. Measurements with hyperpolarized [U-2H, U-13C] glucose in E. coli, yeast and breast cancer cells have shown production of hyperpolarized [1-13C] pyruvate or lactate, which allows real-time measurements of glycolytic flux (Meier, Jensen & Duus, 2011a; Meier et al., 2011b; Harris, Degani & Frydman, 2013). The technique was recently translated to a clinical study of prostate cancer (Nelson et al., 2013), and as discussed in Section VIII, is revealing the activity of the PPP in human cancer cell metabolism in vivo.
V. THE PPP IN BIOTECHNOLOGY: METABOLIC ENGINEERING
The PPP is one of the most important targets for metabolic engineering and biotechnology. One way in which this pathway is utilized is as a source of NADPH and pentose sugars for the overproduction of various commercially and medically important compounds such as carotenoids (Schwender et al., 1996; Martínez et al., 2008), polymers (Kabir & Shimizu, 2003; Jung et al., 2004), antibiotics (Jørgensen et al., 1995; Avignone Rossa et al., 2002; Butler et al., 2002; Li & Townsend, 2006; Borodina et al., 2008), alcohols (Jeppsson et al., 2002; Jeffries & Jin, 2004; Hahn-Hägerdal et al., 2007), nucleosides (Kamada et al., 2001) and amino acids (Marx et al., 1997; Herrmann & Weaver, 1999). Additionally, altering the PPP was used to prevent carbon exhaust during pentose fermentation (Verho et al., 2002). Recently, the PPP has been utilized to create a synthetic non-oxidative glycolysis/PPP hybrid pathway able to produce energy significantly more efficiently by precluding carbon loss via carbon dioxide (Bogorad, Lin & Liao, 2013) – a proof of concept that metabolic engineering could contribute to reducing the current exhaust of greenhouse gases.
Focus on producing the biopolymer poly-hydroxybutyrate (PHB), a non-toxic biodegradable and bio-derived ‘green’ plastic (Hankermeyer & Tjeerdema, 1999), has included modification of both the oxidative and non-oxidative enzymes of the PPP. The insertion of gnd and tktA genes (6PGDH and TKL, respectively) from E. coli into the facultative chemolithoautotroph bacterium, Ralstonia eutropha, amplified gnd, which overproduced NADPH, but also suppressed growth as well as PHB production. Conversely, amplification of tktA significantly increased the generation of PHB via efficient conversion of glyceraldehyde 3-phosphate into acetyl-coenzymeA, the precursor for PHB biosynthesis (Lee, Shin & Lee, 2003). Another attempt focused on generating PHB via the PPP targeted the oxidative pathway only. By deleting the pgi gene in E. coli, carbon flux was shown to be redirected through the PPP in turn increasing the production of NADPH, creating a reducing power imbalance and affecting cell growth. The introduction of the NADPH-consuming PHB biosynthetic pathway into the pgi knockout, allowed partial cell growth recovery (Kabir & Shimizu, 2003).
Another genus where modification of the PPP was successful in industrial application is Streptomyces, a workhorse for the generation of various antibiotics (Hopwood, 2007). To overproduce the pigmented antibiotics actinorhodin (ACT) and undecylprodigiosin (RED), the pfkA2 gene was deleted in S. coelicolor A3(2), leading to increased flux through the PPP (Borodina et al., 2008). Similarly, inactivation of the glycolytic genes gap1 and gap2, encoding GAPDH, in S. clavuligerus was exploited to increase production of clavulanic acid, a β-lactamase inhibitor, used alongside penicillin and cephalosporin to combat antibiotic resistance (Li & Townsend, 2006). The overproduction of clavulanic acid was facilitated through increasing the supply of its precursor glyceraldehyde 3-phosphate. A recent study proposed that in order to increase the flux towards the PPP, TAL overexpression would be much more useful than GAPDH inactivation, because of the compromised carbon balance of the PPP (Linck et al., 2014).
Modification of the PPP has also been effective in fungal biotechnology. The fungus Penicillium chrysogenum was exploited by enhancing flux through the PPP to increase NADPH levels, thereby increasing the penicillin yield (Jørgensen et al., 1995). Other applications of the PPP in fungal biotechnology include the optimisation of alcohol, amino acids (e.g. lysine), nucleosides, inosine and 5′-xanthylic acid production (Marx et al., 1997; Kamada et al., 2001; Jeppsson et al., 2002; Overkamp et al., 2002; Verho et al., 2002). Hence, in several instances an altered PPP flux was beneficial for biotechnological production cycles in both bacteria and yeast systems via its NADPH donor function, or inhibited to decrease carbon exhaustion. Thus, altering PPP activity is exploitable in both microbial and eukaryotic biotechnology in order to optimize cofactor- and sugar-phosphate-dependent processes.
VI. INBORN ERRORS WITHIN PPP ENZYMES THAT LEAD TO HUMAN METABOLIC DISEASE
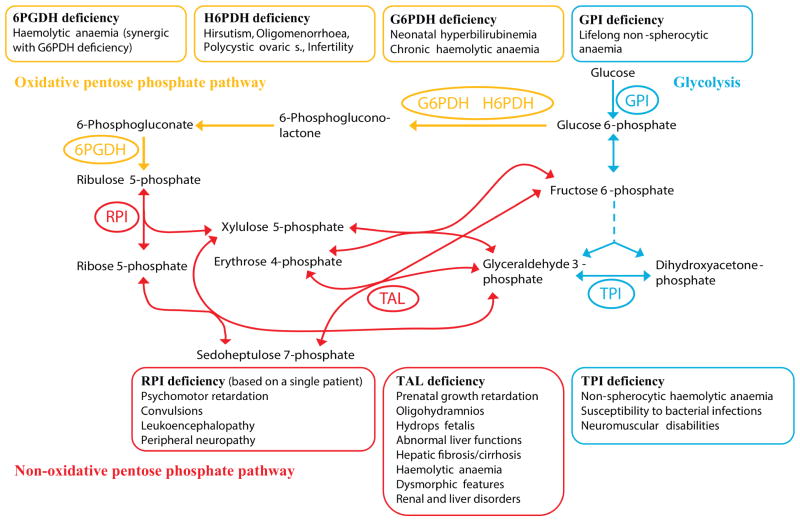
Four known metabolic genetic diseases are the direct consequence of a deficiency in a PPP enzyme; and at least two genetic disorders associated with the PPP are attributed to enzyme mutations in glycolysis via affecting PPP activity. Notably, these PPP disorders encompass both the most frequent human genetic defect (G6PDH deficiency) as well as the so-far rarest human disorder [ribose 5-phosphate isomerase (RPI) deficiency], where only a single patient has been diagnosed to date. The other defects, TAL deficiency, as well as the two glycolytic syndromes TPI and glucose phosphate isomerase (GPI) deficiency, occur at a different frequency but are considered rare disorders as well (Fig. 4).
Fig. 4.
Inherited metabolic disease caused by pentose phosphate pathway (PPP) deficiencies, including two glycolytic enzymopathies with effects on the PPP. PPP enzymopathies are caused either by complete or partial deficiency of PPP and glycolytic enzymes. Abbreviations are defined in Table 1, H6PDH, hexose 6-phosphate dehydrogenase.
(1) G6PDH deficiency, the most common human enzyme defect
G6PDH deficiency (OMIM: 305900) is an X-linked disorder; the gene is located at the telomeric region of the long arm of the X chromosome (band Xq28) (Cappellini & Fiorelli, 2008; Van Zwieten, Verhoeven & Roos, 2014). Prevalent in more than 400 million people worldwide, it represents the most common heritable human enzyme defect (Cappellini & Fiorelli, 2008; Nkhoma et al., 2009). The global occurrence of G6PDH deficiency is geographically correlated with areas inhabited by populations historically exposed to endemic malaria, including Africa, Mediterranean Europe, South-East Asia and Latin America (Ruwende & Hill, 1998).
The most frequent clinical manifestation is neonatal hyperbilirubinaemia and chronic haemolytic anaemia (Luzzatto & Mehta, 1995; Cappellini & Fiorelli, 2008; Van Zwieten et al., 2014). The high frequency of the disorder is likely explained as reduced G6PDH activity appears protective against malaria caused by Plasmodium falciparum (Luzzatto & Bienzle, 1979; Ruwende & Hill, 1998). As the oxidative PPP is the only relevant NADPH source for red blood cells, a decrease in NADPH production is likely associated with the clinical phenotype, but potentially also explains this anti-malaria advantage. As a consequence, however, despite most carriers of mutant G6PDH alleles being asymptomatic, exposure to oxidative stressors such as artemisinin (and other drugs) or infections can elicit acute haemolysis in G6PDH patients. As such, the epidemiology of G6PDH deficiency has been related to the sickle cell anaemia phenotype, caused by Hbs and SS variants of haemoglobin. Sickle cell anaemia is associated with episodes of acute illness and progressive organ damage, but is also associated with heterozygous advantage against malaria (Rees, Williams & Gladwin, 2010).
G6PDH deficiency can be associated with a second, rare defect in the PPP, 6-phosphogluconate dehydrogenase (6PGDH) deficiency (Beutler, Kuhl & Gelbart, 1985). The first evidence of the enzyme deficiency was reported in 1963, when a female patient presenting G6PDH deficiency exhibited reduced activity of 6PGD as well (Brewer & Dern, 1964). More recently, also G6PDH independent incidences of this defect have been reported, and lead to reduced redox tolerance of erythrocytes (Caprari et al., 2001).
(2) RPI deficiency, the currently rarest human disorder
By contrast with G6PDH deficiency, other PPP disorders are exceptionally rare. Huck et al., 2004 described a patient with a deficiency of RPI (OMIM: 608611) who suffered from leukoencephalopathy and peripheral neuropathy. This patient had psychomotor retardation from early in childhood and developed epilepsy at the age of four. From the age of 7 the patient experienced neurological regression, with deterioration of vision, speech, hand coordination, walking, and seizures. Neurological examination at the age of 14 years showed spasticity, bilateral optic atrophy, and nystagmus on lateral gaze, an increased masseter reflex and mixed cerebellar/pseudobulbar dysarthria. He had prominent cerebellar ataxia and mild peripheral neuropathy and displayed severe mental retardation. The patient is now (2014) in his twenties, and so far a unique case, as since the original report no further cases of RPI deficiency have been described.
The molecular diagnosis of the rare case of RPI deficiency was facilitated through a combination of metabolic profiling and candidate gene re-sequencing. Magnetic resonance imaging (MRI) is able to identify brain abnormalities in children with neurological deficits (Watkins, Gadian & Vargha-Khadem, 1999; Huck et al., 2004). MRI of the patient showed extensive anomalies of the cerebral white matter with prominent involvement of the short association fibres (U-fibres), relative sparing of periventricular white matter, and complete sparing of corpus callosum and internal capsule (Van der Knaap et al., 1999). Extremely high concentrations of pentitol metabolites (arabitol and ribitol) were found in the brain by magnetic resonance spectroscopy (MRS), and in cerebrospinal fluid, plasma and urine, as well as xylulose in the urine as tested by mass spectrometry (Van der Knaap et al., 1999). These metabolites can derive from PPP intermediates xylulose, ribose 5-phosphate and ribulose 5-phosphate, which guided to the identification of the candidate gene by targeted re-sequencing of PPP enzymes. Two mutant alleles in the RPI encoding RPIA gene were demonstrated: a 1 bp deletion (540delG) resulting in a frameshift at codon 181 and a predicted truncated protein of 196 amino acids, and a missense mutation C182T, resulting in an Ala-to-Val substitution (A61V). The finding of two mutant alleles in the patient with apparently healthy parents suggests autosomal recessive inheritance. Genetic and biochemical evidence suggests an explanation for the rareness of the case: full RPI deficiency appears to be lethal. Studies of patient-derived cell lines and transgenic yeast models however revealed that the patient carried an uncommon allelic combination: he is heterozygous for a catalytically inactive RPI allele, whereas the second allele encodes a partially catalytically functional enzyme that exhibits a cell-type-dependent expression deficit in addition (Wamelink et al., 2010).
(3) TAL deficiency
Transaldolase deficiency (TAL or TALDO deficiency, OMIM: 606003) is caused by autosomal recessive deficiency in the human TAL-encoding gene (TALDO1) located on chromosome 11p15.5–p15.4, and has recently been diagnosed in more than 30 patients worldwide (Wamelink et al., 2007; Wamelink et al., 2008a; Tylki-Szymańska et al., 2009; Balasubramaniam et al., 2011; Eyaid et al., 2013). TAL-deficient patients suffer from great phenotypic variability. Most patients display first symptoms in the neonatal or antenatal period, with prenatal intra-uterine growth retardation, oligohydramnios and hydrops foetalis being described (Valayannopoulos et al., 2006). Newborns present with hepatosplenomegaly, bleeding diathesis, abnormal liver function, cholestatic jaundice and elevated liver enzymes, while in older patients, hepatic fibrosis or cirrhosis is the pathological liver hallmark. Most patients show haemolytic anaemia, dysmorphic features, neonatal oedema and congenital heart defects. Moreover, renal manifestations and endocrine disorders have been frequently reported (Loeffen et al., 2012). Mild transient hypotonia was described in several patients but mental and motor development was normal in most patients. Recently, a TAL-deficient patient with early onset hepatocellular carcinoma with an 9 years old asymptomatic older brother were described (Leduc et al., 2013).
TAL deficiency results in the accumulation of seven-carbon sugars (sedoheptulose, mannoheptulose), sedoheptulose 7-phosphate, and open chain sugar-alcohols (polyols) including erythritol, arabitol, ribitol, sedoheptitol and perseitol, and erythronic acid derived from the pathway intermediates (Verhoeven et al., 2001; Wamelink et al., 2005; Engelke et al., 2010) that can help as biomarkers in diagnosis. The clinical picture of TAL deficiency is dominated by liver fibrosis/cirrhosis, resulting in permanent scar tissue. Since TAL has been recognized as a regulator of apoptotic signal-processing (Banki et al., 1996), this might have relevance for the pathogenesis of liver disease, as observed in patients and in TAL-deficient mice (Perl et al., 2011). In addition, accumulation of the metabolite sedoheptulose 7-phosphate has been suggested to be involved in the pathophysiology of liver cirrhosis (Verhoeven et al., 2001), and could be functionally connected to the disease phenotype.
In a mouse model of TAL-deficiency, the accumulation of sedoheptulose 7-phosphate and a failure to recycle ribose 5-phosphate through the non-oxidative branch has been observed. Furthermore, diminished production of NADPH led to secondary depletion of reduced glutathione (GSH) and oxidative stress, as well as loss of the mitochondrial transmembrane potential and mitochondrial mass (Hanczko et al., 2009). A decrease of NADPH was potentially caused by the conversion of five-carbon sugar phosphates to five-carbon polyols by aldose reductase at the expense of NADPH levels (Perl et al., 2011). In some earlier diagnosed TAL-deficient patients, low levels of cholesterol, estradiol, testosterone or vitamin D were detected, indicating decreased NADPH/NADP+ and leading to decreased activity of NADPH-dependent reactions (i.e. cholesterol biosynthesis, hormone metabolism) (Banki et al., 1996). Haemolytic anaemia was also observed in most patients, probably related to decreased NADPH production in erythrocytes as observed in G6PDH deficiency.
(4) GPI deficiency
GPI catalyses the interconversion of glucose 6-phosphate to fructose 6-phosphate. A deficiency in this enzyme (OMIM: 613470) increases the flux in the PPP, as the glycolytic route of carbon metabolism becomes inhibited. Deficiency of erythrocyte GPI was first described in a boy with lifelong nonspherocytic anaemia in 1968 (Baughan et al., 1968). In a patient diagnosed in 1985, the GPI deficiency syndrome was characterized by a deficiency in red cells, granulocytes and muscles (Schröter et al., 1985). In 1993, another case of GPI deficiency was associated with hereditary nonspherocytic haemolytic anaemia (Shalev et al., 1993). Mutations found in GPI deficiency retain residual activity of the enzyme, but the deficient enzymes were characterized by reduced thermostability (Kugler & Lakomek, 2000). The decreased activity of the isomerase causes an increase in glucose 6-phosphate, erythrose 4-phosphate and 6-phosphogluconate, indicating increased metabolite load and flux in the PPP.
In yeast cells grown on glucose, a full deficiency of GPI is lethal, but can be complemented by the overexpression of NADPH-oxidising enzymes. This indicates that the fatality of a full GPI deficiency results from redox cofactor imbalance due to NADPH overproduction in the PPP (Verho et al., 2002).
(5) TPI deficiency
TPI deficiency (OMIM: 615512) was one of the first enzymatic defects to be associated with the PPP. Schneider et al., 1965 reported a deficiency of the enzyme in red blood cells referring to the disorder as Dacie’s type II haemolytic anaemia. TPI deficiency is further of historical importance in the treatment of rare diseases, as it was an early case where an enzyme replacement therapy was applied (Ationu et al., 1999).
TPI deficiency is a rare and severe disease involving nonspherocytic haemolytic anaemia, leading to progressive neuronal degeneration, muscle degeneration and is associated with deadly infections and spasticity. In most cases, the affected children die before adulthood (Schneider, 2000; Orosz et al., 2009). Since the discovery of the syndrome less than 100 patients have been diagnosed worldwide (Schneider & Cohen-Solal, 1996). This frequency is lower than the natural mutation rate would predict, but also lower as predicted from the estimated population frequencies of recessive TPI-deficient alleles. This indicates that homozygously defective alleles are embryonically lethal, a notion supported by studies in mice (Merkle & Pretsch, 1989). The substantial frequencies of heterozygote TPI deficiency lead to speculations of a heterozygous advantage of TPI- deficient alleles (Mohrenweiser, 1981; Mohrenweiser, Wurzinger & Neel, 1987; Watanabe, Zingg & Mohrenweiser, 1996). In a more recent study, the entire TPI locus was re-sequenced in 387 centenarians, and single nucleotide polymorphisms (SNPs) were genotyped in an even larger sample of long-lived individuals (N = 1422) and younger controls (N = 967). However, no heterozygous TPI deficient alleles were confirmed (Ralser et al., 2008). The discrepancy could indicate that the observed differences in TPI activity had an epigenetic or post-translational cause, or that high frequencies of heterozygous TPI null alleles are a population-specific phenomenon.
Despite a substantial number of TPI- deficient alleles having been described (Schneider & Cohen-Solal, 1996), a single allele describes the majority of clinical cases. This allele carries a mutation exchanging a glutamic acid residue on position 105 (position 104 when not counting the ATG codon), to an aspartic acid, located in the region of the TPI enzyme responsible for dimer formation (Arya et al., 1997; Schneider, 2000; Rodríguez-Almazán et al., 2008). This allele has been the only one described to cause TPI deficiency in the homozygous state, and it was speculated that the allele may descend from a single individual that may have lived in what is now France or England around 1000 years ago (Arya et al., 1997). Recently, the same allele has also been found in a Turkish family, but it is currently unclear whether it results from a de novo mutation (Sarper et al., 2013). It has been discovered in a transgenic yeast model expressing the human isoform that this residue substantially interferes with the dimerisation of TPI, but does not per se interfere with catalysis (Ralser et al., 2006). The global structure of TPIE104D is similar to that of the wild-type; however, residue 104 is part of a conserved cavity that possesses an elaborate conserved network of buried water molecules at the dimer interface (Rodríguez-Almazán et al., 2008). In the TPIE104D mutant, a disruption of contacts of the amino acid side chains in the conserved cluster leads to a perturbation of the water network in which the water–protein and water–water interactions joining the two monomers are significantly weakened and diminished (Rodríguez-Almazán et al., 2008). Hence, TPI deficiency is primarily caused by a structural defect.
How does TPI deficiency, a disorder caused by a structurally defective glycolytic enzyme, then relate to the PPP? In the course of generating a yeast model for TPI deficiency, it was discovered that TPI alleles with reduced catalytic activity render cells resistant to oxidants (Ralser et al., 2006). In yeast, this was mainly described for thiol-oxidizing reagents such as diamide, however in Caenorhabditis elegans sensitivity was observed also for natural oxidants including juglone (Ralser et al., 2007). Cells expressing the mutant TPI alleles possess increased concentration of PPP metabolites, and the antioxidant effects of TPI mutant alleles are fully dependent on the first enzyme of the oxidative PPP, G6PDH (Ralser et al., 2007; Grüning et al., 2011; Grüning et al., 2014). In Drosophila melanogaster, the situation seems to be more complex and dependent on respiratory activity of the TPI mutant cells. Also Drosophila melanogaster cells’ TPI mutations affect oxidant resistance, however their redox status seem to shift towards oxidation (Hrizo et al., 2013). Moreover, as shown below (Section VIII), TPI mutant alleles were important in understanding the role of the PPP’s antioxidant activity in cancer.
(6) Hexose 6-phosphate dehydrogenase (H6PDH) deficiency
H6PDH is a luminal enzyme analogous to G6PDH responsible for NAD+ and NADP+ reduction in the endoplasmic reticulum. The enzyme oxidizes glucose 6-phosphate, glucose, galactose 6-phosphate and 2-deoxyglucose 6-phosphate (Krczal, Ritter & Kömpf, 1993; Senesi et al., 2010). Several allelic variants of H6PDH mutations are known and result in hirsutism, oligomenorrhoea, obesity, acne and infertility (Jamieson et al., 1999; Draper et al., 2003; Lavery et al., 2008). Draper et al., 2003 hypothesized that mutations in H6PDH could cause an NADPH deficiency in the endoplasmic reticulum (ER), affecting the directionality of the 11-beta-hydroxysteroid dehydrogenase type 1 (HSD11B1) reaction. HSD11B1 is a regulator of the tissue-specific glucocorticoid availability in cortisone reductase deficiency (OMIM: 604931) (Draper et al., 2003). Indeed, in a study conducted on four patients suffering from cortisone reductase deficiency, four novel and one known mutations in the H6PDH gene in homozygous or compound heterozygous state were identified. Expression data on these mutations revealed loss of H6PDH function (Lavery et al., 2008). Mouse models carrying the H6PDH mutations develop fasting hypoglycaemia, increased insulin sensitivity and increased basal and insulin-stimulated glucose uptake (Lavery et al., 2008). It was observed that cortisol reductase deficiency presents a similar phenotype to polycystic ovary syndrome (POS). Furthermore, the H6PDH gene was associated with multiple sclerosis (Alcina et al., 2010).
VII. HOST–PATHOGEN INTERACTIONS: THE ROLE OF THE PPP IN INFECTIOUS DISEASE
(1) The PPP as a target in parasitic protozoa
Protozoan parasites are responsible for a considerable number of debilitating infections that affect a significant number of people around the world, most commonly in developing countries. These protozoa include the kinetoplastids Trypanosoma brucei, Trypanosoma cruzi and Leishmania spp. These parasitic protozoa cause sleeping sickness, Chagas’ disease and leishmaniasis (cutaneous, visceral and mucosal), respectively. Various species of the Aconoidasida genus Plasmodium are responsible for malaria, while the archeamoeban (amitochondriate) parasite Entamoeba histolytica is the causative agent of amoebiasis, a disease characterized by diarrhoea (amoebic colitis) or abscesses principally of the liver. The biology behind the host–parasite relationship, the infection process and in some cases, even to identify drug targets, is highly dependent on parasite metabolism. Parasite metabolic reconfiguration and mutations in their enzymes may contribute towards resistance to drug treatment as well as parasite evasion of the host innate immune response. One important way the host immune system counteracts parasite infection is via the generation of hydrogen peroxide and other oxidants. Due to its function in maintaining the supply of the antioxidant cofactor NADPH, the PPP is therefore of great importance to the pathology of these parasites, becoming an attractive target for drug design.
(a) Kinetoplastids, the trypanothione pathway and the compartmentalization of the PPP in glycosomes
Kinetoplastids have a complete and functional PPP. Studies in both T. cruzi (Igoillo-Esteve et al., 2007) and Leishmania mexicana (Maugeri et al., 2003) have shown that the PPP metabolized 5–10% of total glucose. Most of the canonical PPP enzymes have homologues in these parasites and have been cloned, characterized and crystallized in at least one of the trypanosomatids (Fig. 5A) (Barrett et al., 1994; Phillips et al., 1998; Duffieux et al., 2000; Veitch et al., 2004; Igoillo-Esteve & Cazzulo, 2006; Stern et al., 2007; Stoffel et al., 2011; Kaur et al., 2012). Exceptions include TAL and RPE, for which less information is available (Cronín, Nolan & Voorheis, 1989; Igoillo-Esteve et al., 2007). However, these PPP enzymes could be important for the infection process. It has been observed that bloodstream forms (host stage) in T. brucei have neither TKL nor RPE activities; hence, at this stage, they are not capable of forming glyceraldehyde 3-phosphate from the non-oxidative branch of the PPP (Cronín et al., 1989).
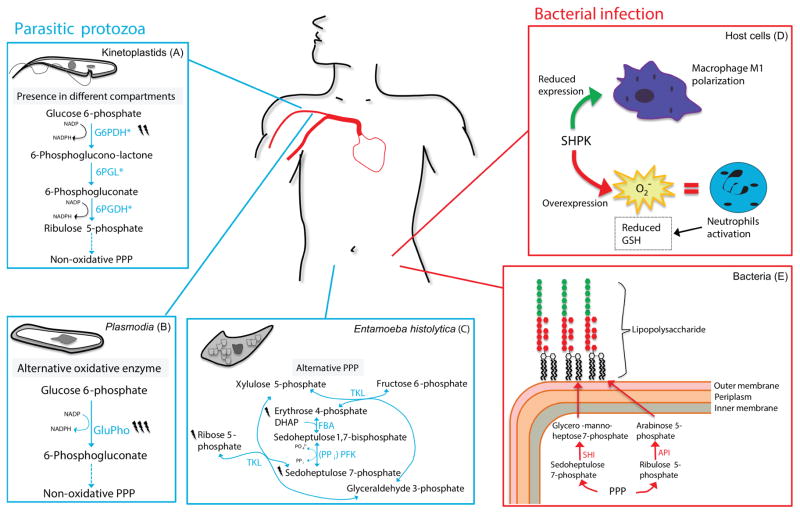
Fig. 5.
The pentose phosphate pathway (PPP) in parasitic protozoa (left) and bacterial infection (right). In kinetoplastids (A), PPP enzymes are localised in the cytosol and glycosomes (*). Plasmodia (B) have a bifunctional enzyme (glucose 6-phosphate dehydrogenase 6-phosphogluconolactonase; GluPho) that has the activity of glucose 6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconolactonase (6PGL). Entamoeba histolytica lacks G6PDH and transaldolase (TAL), however has developed an alternative hexose–pentose interconversion pathway (C) in which the enzymes transketolase (TKL), fructose-bisphopshate aldolase (FBA) and pyrophosphate dependent-phosphofructokinase [(PPi)PFK] are involved. The activity of the PPP pathway is modulated by metabolites that respond to oxidants (i.e. H2O2 or paraquat); oxidant-responsive enzymes; or by the glutathione/glutathione disulfide (GSH/GSSG) ratio. 6PGDH, 6-phosphogluconate dehydrogenase. During bacterial lipopolysaccharide (LPS) infection of the mammalian intestine, sedoheptulokinase (SHPK) is of reduced activity in the host (D) and leads to macrophage M1 polarization. In bacteria (E), sedoheptulose 7-phosphate isomerase (SHI) and arabinose 5-phosphate isomerase (API) enzymes can increase LPS production. O2−: superoxide.
The enzymes of the PPP in these parasites are mainly cytosolic. However, they have also been allocated to the glycosome, the trypanosomatids’ peroxisome, that contains the major part of the glycolytic pathway (Hannaert et al., 2003). The role of the PPP enzymes in the glycosome appears to be: (i) the exchange of intermediates with the glycolytic pathway; (ii) the supply of ribose 5-phosphate for nucleotide biosynthesis, which also occurs in the glycosome; and (iii) the supply of NADPH for the antioxidant system (trypanothione reductase) that has also been detected in the glycosome (Hannaert et al., 2003). In turn, one of the main functions of the PPP in the cytosol is the supply of reducing power (NADPH) to the antioxidant system. In the trypanosome, antioxidant action relies on an alternative molecule, trypanothione [T(SH)2; N1-N8-bisglutathionylspermidine], an analogue of glutathione. Together with its reducing enzyme trypanothione reductase (TryR), trypanothione replaces all the functions that the system glutathione (GSH)/glutathione reductase (GR) has in other cells (Olin-Sandoval, Moreno-Sánchez & Saavedra, 2010). The major part of the antioxidant system in trypanosomatids therefore depends on trypanothione, and its reduction requires NADPH equivalents supplied by the PPP (Barrett, 1997). Due to the central role of the PPP in antioxidant metabolism, several studies have focused on the regulation of PPP enzymes under oxidative stress. It has been demonstrated that G6PDH increases its activity 46-fold in metacyclic trypomastigotes of T. cruzi (infective form) exposed to 70 μM H2O2, an increase related to an increment in protein content. By contrast, epimastigotes (insect form) exposed to 20 μM of H2O2 had decreased G6PDH activity and protein content, an observation which can be explained with the reasoning that under physiological conditions, epimastigotes are not exposed to oxidative stress (Igoillo-Esteve & Cazzulo, 2006). These results demonstrate that oxidative stress not only regulates the activity of G6PDH kinetically but also at the protein level.
RNAi-mediated knock-down of G6PDH in bloodstream forms of T. brucei promoted a negative effect on parasite growth, suggesting an essential function for this enzyme (Cordeiro, Thiemann & Michels, 2009). This PPP enzyme appears to be associated with the antioxidant machinery of the parasite T. brucei partially depleted of G6PDH are sensitive to H2O2 (Gupta et al., 2011). Moreover, PPP flux in Leishmania mexicana increases when the parasites are exposed to methylene blue causing oxidative stress, providing further support that the PPP is highly essential in these parasites’ response to oxidative conditions (Maugeri et al., 2003). The importance of the PPP in the response to oxidative stress in trypanosomes has also been corroborated by dynamic modelling. Recently, a kinetic model of glycolysis in Trypanosoma brucei (Albert et al., 2005; Achcar et al., 2012) was extended with PPP reactions (Kerkhoven et al., 2013). Both models predicted that the flux through the cytosolic PPP was regulated by oxidative stress. Under low-oxidative-stress conditions the flux through this pathway was very low. However, the simulation of oxidative stress promoted an increase in the flux through glucose 6-phosphate dehydrogenase/hexose 6-phosphate dehydrogenase, 6-phosphogluconolactonase (PGL) and ribose 5-phosphate isomerase (RPI) of about seven-, 5.5- and 4-fold, respectively. After the stress, a steady state was predicted to be reached after 1 min (Kerkhoven et al., 2013).
6PGDH in T. brucei is essential (Barrett, 1997; Kerkhoven et al., 2013). The lethal phenotype has been attributed to the accumulation of 6-phosphogluconate inhibiting PGI (Barrett, 1997), a result that has been challenged by (i) the observation that fructose supplementation, which enters glycolysis after PGI, does not rescue the cells from death, and (ii) a lack of support from dynamic modelling (Kerkhoven et al., 2013).
Mutations in TKL (null mutants), by contrast, do not have any effect on cell growth, nor were changes in morphology detected (Stoffel et al., 2011). Metabolite analysis of these mutants showed that the substrates ribulose 5-phosphate, ribose 5-phosphate and xylulose 5-phosphate had accumulated 7.9-fold with a concomitant decline in the product sedoheptulose 7-phosphate. Additionally, intracellular concentration of 2,3-bisphosphoglycerate, phosphoenolpyruvate, fructose 6-phosphate and glyceraldehyde 3-phosphate were reduced 2-, 4.5-, 1.5- and 3.2-fold, respectively (Stoffel et al., 2011). Consequently, taken together, these results indicate that a main role of the PPP in trypanosomatids appears to be in defence against oxidative stress.
(b) Plasmodium spp
Plasmodium spp. parasites possess a complete and functional PPP (Preuss, Jortzik & Becker, 2012). This has been confirmed through in silico analysis, using transcriptome profiles collected hourly during the intra-erythrocytic cycle of the parasite. Both branches of PPP are active at least at 50 hours post-invasion of the erythrocyte and their expression varies most likely due to cell requirements, such as ribonucleotide synthesis or NADPH and ATP demand (Bozdech & Ginsburg, 2005). The canonical PPP enzymes such as TKL and RPI have been cloned and characterized in P. falciparum (Holmes et al., 2006; Joshi et al., 2008). Interestingly, Plasmodium spp. possess fusion enzymes within the PPP. One well-studied example is a protein that combines G6PDH and 6PGL activities in a single enzyme, glucose 6-phosphate dehydrogenase 6-phosphogluconolactonase (GluPho) (Fig. 5B). This enzyme is regulated by S-gluthathionylation, suggesting that the glutathione/glutathione disulfide (GSH/GSSG) ratio regulates GluPho activity (Jortzik et al., 2011). The fusion protein is not the exception, and multiple independent fusions of G6PDH with other PPP enzymes have been found in this plasmodial parasite, indicating that pathway efficiency is potentially increased by channelling metabolites in this manner (Stover, Dixon & Cavalcanti, 2011).
Plasmodial parasites are continuously exposed to oxidative stress during the life-cycle stage in the erythrocyte; as they take haemoglobin into their acid food vacuole, Fe2+ is oxidised to Fe3+ and superoxide anions are produced, which in turn promote the formation of H2O2 and hydroxyl radicals. The reductases involved in these parasite’s antioxidant systems are NADPH-dependent glutathione and thioredoxin reductases (Müller, 2004). Although the parasites have the enzymes isocitrate dehydrogenase and glutamate dehydrogenase, which are also able of supplying NADPH to the cell, the former enzyme generally oxidizes this cofactor and the latter has been demonstrated not to be an NADPH supplier for antioxidant systems (Preuss et al., 2012). Furthermore, ribose 5-phosphate can be obtained from the uptake and degradation of host purines in contrast to being obtained from the PPP. Thus, the main role of the PPP in Plasmodium spp. seems to be to supply NADPH.
The role of the PPP in P. falciparum is indispensable during parasite infection. The parasite’s pathway contributes to 82% of total PPP activity in infected red blood cells (IRBCs) and 72% in G6PDH- deficient IRBCs (Atamna, Pascarmona & Ginsburg, 1994). The highest NADPH demand appears when the parasite has reached maturation (trophozoite stage) in IRBCs, which is consistent with an increase in G6PDH activity in IRBCs during this period (Atamna et al., 1994). As discussed in Section VI.1, G6PDH- deficient hosts (46% females heterozygous; 58% males hemizygous) have increased resistance to malaria (Cappellini & Fiorelli, 2008). Although this resistance is not fully understood, two hypotheses have been proposed: one suggests that the oxidative stress in G6PDH- deficient erythrocytes may be the cause of impaired infection; the other proposes that these infected erythrocytes are more easily recognized and destroyed by the host’s immune system (Müller, 2004).
(c) The alternative PPP of Entamoeba histolytica
An alternative PPP is found in the parasite, Entamoeba histolytica. This parasite lacks G6PDH, 6PGDH and TAL (Barrett, 1997; Husain et al., 2012) and has developed an alternative hexose–pentose interconversion pathway for the formation of pentose phosphates. This alternative pathway is constituted of several reactions catalysed by TKL, FBA and phosphofructokinase (PPi-dependent) (Susskind, Warren & Reeves, 1982) (Fig. 5C). When Entamoeba histolytica is exposed to oxidative stress, metabolites of the non-oxidative branch of the PPP, glycerol and chitin biosynthesis are increased, a process attributable to an inhibition of glycolytic enzymes which, in turn, promotes redirection of the carbon flux (Husain et al., 2012). Thus, although the oxidative branch is absent from the PPP in this parasite, it seems that the non-oxidative pathway still responds to the presence of oxidative stress. This observation is analogous to budding yeast, where the non-oxidative PPP plays an NADPH-independent role in the stress response too (Krüger et al., 2011).
(2) The PPP in bacterial infection
Similar to eukaryotes, the PPP and glycolysis (together with overlapping reaction sequences such as the Entner–Doudoroff pathway) constitute core carbon metabolism in bacteria (Sprenger, 1995). As in eukaryotes, the PPP is required to provide NADPH equivalents, nucleotides and sugar phosphate precursors. An important additional function however concerns the provision of sedoheptulose 7-phosphate for the initiation of lipopolysaccharide (LPS) biosynthesis (Taylor et al., 2008). Moreover, the PPP appears to be the only pathway allowing bacteria to utilize sugars such as 3-xylose, 3-ribose, and 3-arabinose, which cannot be catabolised by other means (Wood, 1985; Sprenger, 1995; Lin, 1996). Here we briefly introduce the role of the PPP in the bacterial infection process and the importance of this pathway for both host and pathogen.
(a) The PPP is required in the host response to fight microbial infection
The invasion of host cells gives rise to the activation of defence mechanisms required for survival and pathogen expulsion. The host response is characterized by microbial sensors activating signalling pathways and inducing effector mechanisms (Nish & Medzhitov, 2011). Macrophages are responsible for the host immune response by inducing signalling modules and alterations in cell morphology and metabolic function (Martinon, Mayor & Tschopp, 2009; Haschemi et al., 2012). The cellular reprogramming utilizes metabolic adaptation in response to environmental changes. Nevertheless, there is only limited knowledge of the bioenergetic rearrangement that takes place during macrophage activation. Recently SHPK [formerly carbohydrate kinase- like (CARKL)] has been identified as a modulator of macrophage regulation during LPS-induced infection (Haschemi et al., 2012). Reduced SHPK expression was observed in both in vivo and in vitro experiments and associated with macrophage type 1 (M1) polarization. By contrast, SHPK overexpression induced a pro-inflammatory response, characterized by the presence of nuclear factor kappa-B (NF-κB), and an increase in the production of intracellular superoxide radicals (probably due to sustained sedoheptulose 7-phosphate formation), which is similar to the neutrophil-induced response (Haschemi et al., 2012) (Fig. 5D).
Neutrophil activation characterizes the immune response in the Gram-negative bacterium Helicobacter pylori, the agent causing chronic gastritis and peptic ulcer disease (Nielsen et al., 1994; Basso, Plebani & Kusters, 2010). H. pylori was the first microorganism to be directly associated with stomach carcinogenesis (Sipponen & Hyvärinen, 1993). It was suggested that, following H. pylori infection, neutrophil activation rapidly increases ROS production (Obst et al., 2000). Moreover, GSH availability, normally high in the stomach, is rapidly depleted after H. pylori infection of the human gastric mucosa, mainly because GSH is used for repairing oxidative DNA lesions in gastric carcinogenesis (Shirin et al., 2001; Basso et al., 2010). By contrast, GSH levels were increased in mouse model infections, leading to the hypothesis that the GSH (and the oxidative PPP, regulating GSH synthesis) could be directly involved in the response to the oxidative stress induced by H. pylori infection (Matthews & Butler, 2005).
(b) The PPP is a central pathway for bacterial infection and LPS biosynthesis
The infection process requires rapid adaptation of intracellular and extracellular bacteria, involving reconfiguration of their central carbon metabolism. This is caused predominantly by their newly encountered physical conditions and nutrient availability (Eisenreich et al., 2010; Swanepoel & Loots, 2014). More precisely, pathogens need to modulate their metabolism and coordinate their life cycle in order to develop specific virulence factors (Ray et al., 2009; Eisenreich et al., 2010).
Due to the presence of different microenvironments (or niches), the host environment is characterized by the availability of several nutrient sources (Brown, Palmer & Whiteley, 2008). For example, enterohaemorrhagic (EHEC) and uropathogenic (UPEC) Escherichia coli strains (causing haemolytic colitis and urinary tract infection, respectively), differ in their ability to cause infection according to their localization (i.e. nutrient availability) (Alteri & Mobley, 2012). The mammalian urinary tract is characterized by the presence of amino acids and small peptides, therefore mutations in the genes coding for the PPP and glycolytic enzymes do not affect the pathogenicity of the UPEC E. coli. On the other hand, EHEC E. coli needs to up-regulate both glycolysis and PPP in order to colonize the host intestine, due to the high levels of glycogen available which can be used as an external carbon source (Alteri & Mobley, 2012).
Last but not least, the PPP has been found to play an essential role in the biosynthesis of lipopolysaccharides (LPSs). LPS is part of the external layer of Gram-negative bacteria and is involved in not only bacterial protection but also in the activation of the host immune response (Raetz & Whitfield, 2002). The biosynthesis of LPS has been intensively studied in order to provide new therapeutic agents against Gram-negative pathogens (Wang & Quinn, 2010). One of the possible targets for drug discovery is SHI, as characterized in E. coli, Pseudomonas aeruginosa (Taylor et al., 2008) and H. pylori (Sarkar et al., 2012). This enzyme converts sedoheptulose 7-phosphate from the PPP into the lipopolysaccharide precursor glycero-manno-heptose 7-phosphate (Kneidinger et al., 2001; Valvano et al., 2002; Taylor et al., 2008), and has therefore become a central target for the development of new antibiotics and adjuvants. Further investigations on the LPS biosynthetic pathway highlighted another enzyme involved in the pathogenicity of an E. coli UPEC strain, arabinose 5-phosphate isomerase (API), which converts the PPP sugar ribulose 5-phosphate into the LPS precursor arabinose 5-phosphate (Mosberg et al., 2011) (Fig. 5E), emphasizing these PPP enzymes as attractive targets for the development of future antibiotics.
VIII. THE ROLE OF THE PPP IN CELL PROLIFERATION AND STEM CELLS
Cell growth necessitates biosynthesis of the required intermediates, such as nucleotides, amino acids, and lipid precursors. Consequently, when proliferation is induced, cells restructure their central carbon metabolism in order to adapt to the rise in metabolic demands. This metabolic reconfiguration includes the shuffling of the energy flux outside the mitochondria to fuel glycolysis and the PPP (Levine & Puzio-Kuter, 2010; Vander Heiden et al., 2010; Grüning & Ralser, 2011; Yamamoto et al., 2014). The PPP playing a crucial, non-redundant role in the supply of building blocks such as ribose 5-phosphate, the molecular backbone of nucleic acids, is consequently of central importance. Indeed, a key feature of the metabolic transformation events accompanying cellular proliferation is the enhancement of biosynthetic capacity. Hence, diverting the energy flux towards the non-oxidative branch of the PPP has the key advantage of enabling the needed nucleotide biosynthesis through the production of ribose 5-phosphate (Deberardinis et al., 2008). In addition, this metabolic restructuring safeguards the cellular redox balance, by modulating NADPH production in the PPP.
Human pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) (Thomson et al., 1998) and induced pluripotent stem cells (iPSCs) (Takahashi et al., 2007), are of particular interest in biomedicine given their ability to proliferate indefinitely (self-renewal) and to differentiate into virtually any cell type of the body (pluripotency). The central importance of metabolism in reprogramming and self-renewal has recently caught the attention of the stem cell community, with most discoveries dating back to recent years only (reviewed in (Folmes et al., 2012; Zhang et al., 2012; Xu et al., 2013; Bukowiecki, Adjaye & Prigione, 2014) and has revealed that the induction and maintenance of PSCs is associated with a profound change of anabolic demands. PSCs exhibit an elevated rate of proliferation and distinct cell cycle features compared to common somatic cells (Ruiz et al., 2011). Moreover, PSCs are particularly sensitive to redox imbalance (Saretzki et al., 2008) and display low levels of oxidatively modified proteins, lipids, and DNA (Prigione et al., 2010). Indeed, increased ROS levels have been shown to promote differentiation (Yanes et al., 2010).
The establishment of the proliferative PSC state has been found to be coupled with elevated lactate generation and enhanced glycolytic flux (Prigione et al., 2010; Folmes et al., 2011). Moreover, upon glycolysis-activating conditions, such as under hypoxia stimulation or after treatment with small-molecule inducers of the master metabolic regulator hypoxia-inducible transcription factor 1 (HIF-1) (see section VIII-4-d), the efficiency of somatic cell reprogramming is significantly improved (Yoshida et al., 2009; Zhu et al., 2010). Conversely, genetic ablation of HIF-1 hampers the formation of iPSCs (Prigione et al., 2013; Mathieu et al., 2014). Finally, increased expression of the pyruvate kinase isozyme M2 (PKM2), which regulates the flux distribution between glycolysis and the PPP (section VIII-3), has been identified in both ESCs and iPSCs compared to somatic cells (Prigione et al., 2013).
Currently, evidence from altered PPP metabolism in stem cell populations mainly originates from the analysis of their gene expression profiles. Genes regulating the first steps of glycolysis were up-regulated in PSCs compared to somatic cells, including glucose uptake (SLC2A3) and glucose phosphorylation to glucose 6-phosphate (HK3 and GCK) (Prigione et al., 2011; Varum et al., 2011). Enzymes of the final steps of glycolysis (such as PGAM2, ENO, PKLR, and LDH) are up-regulated in PSCs. On the other hand, the expression level of the glycolytic enzymes downstream of glucose 6-phosphate, including GPI, PFK, and ALDO, is reduced in PSCs, while the level of genes involved in the non-oxidative branch of the PPP (RPIA and TKT) is augmented (Folmes et al., 2011; Prigione et al., 2011; Varum et al., 2011). Hence, the transcriptional data of PSCs suggest that glycolytic intermediates may be diverted into the PPP, in order to support both the biomass accumulation and redox homeostasis that are associated with the maintenance and derivation of PSCs. Accordingly, LC-MS/MS-based metabolite quantification detected the accumulation of glucose 6-phosphate and decreased dihydroxyacetone phosphate in PSCs compared to fibroblasts (Prigione et al., 2011), which may be indicative of overall increased metabolic activity due to proliferation and possibly specific PPP activation. Elevated protein expression of hexokinase II (HXK2), which causes a higher glycolytic rate than isoform I, has also been observed in the mitochondria of PSCs (Varum et al., 2011). This is of particular interest since HXK2 activity may be stimulated under hypoxia by the p53-inducible target TP53-induced glycolysis and apoptosis regulator (TIGAR) (see sectionVIII-4-a) to induce the PPP and preserve redox homeostasis (Cheung, Ludwig & Vousden, 2012).
Finally, the importance of the PPP for the maintenance of the pluripotent state is supported by the findings that G6PDH-depleted ESCs proliferate at a reduced rate and, upon oxidant exposure, are incapable of increasing the PPP flux, thus resulting in apoptotic cell death (Filosa et al., 2003; Fico et al., 2004). Furthermore, genetic or small-molecule-based inhibition of the PPP forces PSCs to exit the self-renewal state and start the differentiation process (Manganelli et al., 2012). Overall, it appears that promoting the activation of the PPP is functionally critical to support the establishment and the maintenance of the proliferative conditions associated with the undifferentiated PSC state. It is however not clear to which extent the metabolic reconfiguration has an active role in maintaining pluripotency, or whether changes in energy metabolism are causative in driving differentiation. Evidence for an active role of the PPP in supporting cell proliferation has however been found by studying the metabolism of cancer cells.
(1) The role of the PPP in deregulated cell proliferation and the pathogenesis of cancer: the Warburg effect
Rising attention has recently been paid to deregulated cell proliferation when it has been noticed that malignant transformation and metabolic reprogramming may be intimately intertwined. Despite a vast amount of research, cancer still represents the second most common cause of death in the world, beaten to the top only by cardiovascular diseases. While the last decade has substantially changed the way cancer therapy is performed, the majority of newly approved molecular-targeted drugs (e.g. antibodies against growth factors or their receptors, tyrosine kinase inhibitors and other small molecules) failed to result in significant and long-lasting improvement of therapy efficacy (Fojo & Parkinson, 2010). This is partially explained by the hallmark genomic instability of malignant cells that results in an impressive propensity to adapt to and, ultimately, resist inactivation of ‘cancer-specific’ signalling pathways (Bock & Lengauer, 2008; Gillies, Verduzco & Gatenby, 2012). Inhibition of processes that are absolutely essential and non-redundant for tumour cell proliferation is a promising strategy to improve cancer therapy. Tumour-specific metabolism clearly represents such a process. Despite being recognized nearly a century ago, the fundamental importance of metabolic deregulation for cancer pathogenesis has escaped the appreciation of most cancer researchers for decades. It was not until the post-genome era that metabolic reprogramming was widely accepted as an emerging hallmark of cancer (Hanahan & Weinberg, 2000; Hanahan & Weinberg, 2011). Recently however, characterization of cancer metabolism has become the focus of a rapidly growing research community, taking advantage of the improved analytical and increasingly also computational methodology to identify fascinating and unexpected interactions (Weinberg, 2014). Observed changes in metabolism are in no way ‘trivial’, indeed can be quite specific dependent on both the responsible genetic lesion and tumour tissue type (Yuneva et al., 2012). While the majority of published work analysed the role of glycolysis, glutaminolysis and mitochondrial activity, the importance of the PPP for malignant transformation remained elusive for quite some time.
Otto Warburg was not only able to identify some of the first enzymes and co-enzymes of central metabolism, but at the same time, was one of the first research pioneers that recognized the importance of altered metabolism for tumour growth. He and his co-workers discovered an increase in glucose uptake and lactate production in concert with a decrease in oxygen uptake (known as the ‘Warburg effect’), reviewed in (Warburg, 1956). Intriguingly, the elevation in glycolytic flux also occurred under sufficient oxygen supply (aerobic glycolysis). Based on these results, Warburg concluded that cancer cells must suffer from defects in their respiratory machinery. Today, we know that although many cancers show reduced activity of oxidative phosphorylation (OXPHOS; (Ferreira, 2010; Cairns et al., 2011)), most cancers [with important exceptions, oncocytoma for instance (Mayr et al., 2008)], possess a fully functional respiratory chain and are therefore biochemically fully capable of using the respiratory chain for ATP production.
But why are cancer cells then not fully exploiting this efficient ATP-producing machinery? A cutback in OXPHOS seems counterintuitive since rapid proliferation demands large amounts of energy. This observation implies that other factors than ATP production are more limiting for the cancer cell. Recent observations indicate that a balanced redox state, achieved in part by increased PPP activity, is essential for tumourigenesis (Anastasiou et al., 2011; Grüning & Ralser, 2011; Tosato et al., 2012). A more disputed theory concerns the cross-feeding of cancer cells through lactate, termed the ‘Reverse Warburg effect’ (Pavlides et al., 2009). Although this theory has attractive components, it fails to explain some aspects of the effect; for instance it does not explain why other organisms, like yeast cells, also show Warburg-like metabolic reconfigurations despite not sharing lactate. Importantly, however, all these studies indicate that the often heard hand-waving explanation ‘Respiration is reduced in cancer cells to save carbon equivalents for biosynthesis’ is to be questioned as a cause for the Warburg effect: First, respiratory activity does not compete with aerobic glycolysis for carbons, as aerobic glycolysis is followed by lactate excretion instead of pyruvate decarboxylation. Second, also the PPP contains a CO2-producing reaction. Thus, Warburg like cells have a negative carbon balance.
Oxidative stress is a major cause of damage for macromolecules and can eventually lead to cell death. On the other hand, a certain amount of oxidizing equivalents are certainly necessary for cell physiology, and thus, only situations with constantly or periodically elevated ROS levels can be considered as a risk factor for tumourigenesis. ROS-induced DNA damage can lead to cancerogenic mutations and genomic instability, and ROS also trigger inflammatory pathways and have a stabilizing effect on HIF-1; a transcription factor highly expressed in cancer cells (Gao et al., 2007; Perera & Bardeesy, 2011; Wu & Le, 2013). Therefore, the pool of intracellular ROS must be kept balanced and below a toxic threshold – a drastic shift towards oxidation would cause tumour cell death (Gao et al., 2007; Perera & Bardeesy, 2011). Thus, dynamic tuning of the metabolic network as well as the antioxidant systems, involving glycolytic metabolite accumulation and PPP activation, is of fundamental importance to keep the production of cellular building blocks, energy and reducing equivalents in check. Vice versa however, pro-oxidant therapies could prove helpful in inhibiting tumour cell proliferation.
(2) Evidence for enhanced PPP activity in cancer cells
Gene and protein expression analyses together with immunohistochemistry are widely applied as surrogate methods to assess the role of specific factors for cancer pathogenesis. However, while these methods are certainly useful and have helped to identify numerous molecules important for cancer biology, a valid and detailed characterization of metabolic pathways cannot be achieved by them. Metabolic pathways appear mostly regulated by post-translational mechanisms (Daran-Lapujade et al., 2004; Buescher et al., 2012; Kochanowski, Sauer & Chubukov, 2013). In addition, flux of the PPP is dependent on the level of co-factors (NADP+ for the oxidative PPP), substrate availability (non-oxidative PPP), and the flux through glycolytic enzymes. Hence, information about the abundance of mRNA and also protein levels is limited to pinpoint accurately changes in PPP activity and their potential causal importance for cancer biology, so it is required to determine these values in concert with flux and/or metabolite concentrations. Due to the difficulty in applying these techniques in heterogeneous tumours, it is hence not surprising that published literature is rather scant. Fortunately, G6PDH is an informative exception as its enzyme activity in tumours is well studied and was increased in various human cancer types when compared to the respective benign control tissue, e.g. cervix, uteri, prostate and breast (Pedersen, 1975; Zampella, Bradley & Pretlow, 1982; Bezwoda et al., 1985). To the best of our knowledge, there is less information available about tumour-specific activities of the non-oxidative PPP, especially for its key enzymes TAL and TKL.
Assessment of enzyme activity however points towards modified PPP flux in cancer. Stable isotope resolved metabolomics (SIRM), indicates enhanced PPP activity in the human breast cancer cell line MCF7 when compared to non-transformed mammary epithelial cells (Meadows et al., 2008). Similar results have been obtained in renal cell carcinoma where altered activity of the PPP has been identified as a key metabolic feature of the cancer state (Catchpole et al., 2011). In addition, PPP adaptation could be crucial for cancer cells that use glucose alternatives, such as fructose, for their carbohydrate needs. There is evidence that in pancreatic cancer cells, fructose is preferentially metabolized via the non-oxidative PPP supporting tumour growth (Liu et al., 2010). In this context, a homologue of TKL, TKL-like protein (TKTL1) is detected in tumour tissue and its expression level has been correlated with the progression of cancer (Diaz-Moralli et al., 2011; Kayser et al., 2011). However, due to the difficulty in detecting TKTL1 enzymatic activity it is currently undecided whether TKTL1 participates in the PPP or not (Meshalkina et al., 2013).
As an alternative to the classical biochemical approaches, functional imaging is becoming increasingly sophisticated and has shown promise as another more direct method to assess metabolic changes in vivo. A recent study used intravenous infusion of [1,2-13C2] glucose, followed by 13C NMR analysis of the micro-dissected tumour mass and non tumour-bearing surrounding brain, to assess PPP flux relative to glycolytic flux. The malignant tissue in this study did not show enhanced PPP flux relative to glycolysis, when compared with the surrounding benign brain tissue (Marin-Valencia et al., 2012a; Marin-Valencia et al., 2012b), and it was suggested that damage to the surrounding brain was confounding the measurements. While the latter two studies demonstrate the potential of measurements for the PPP flux, it is clear that additional data are needed from a broad spectrum of tumours for a more comprehensive picture of PPP activity in cancer.
New opportunities arise from the use of hyperpolarized NMR tracers, as these allow non-invasive and real-time assessment of metabolic flux in vivo. The technique has recently been translated to the clinic with a study in prostate cancer (Nelson et al., 2013). Intriguingly, in studies on E. coli, yeast and cancer cells, in vitro signals from glycolytic intermediates have been detected, such as dihydroxyacetone phosphate, and also a signal that has been assigned to 6-phosphogluconate, offering the potential for in vivo measurements of PPP flux as well. Such measurements have recently been reported for tumors in vivo, where hyperpolarized [U-2H, U-13C] glucose and the lactate formed from it were imaged and the resonance previously assigned to 6-phosphogluconate was observed (Rodrigues et al., 2014).
(3) Enzymatic switches enable metabolic adaptation of cancer cells
Different enzymatic switches could be involved in triggering and modulating metabolic reprogramming towards increased PPP flux. Proliferating mammalian cells have increased levels of glycolytic enzymes, one of these is represented by PK isozymes M1/M2 (PKM1/2) (Bock & Lengauer, 2008; Christofk et al., 2008; Hitosugi et al., 2009; Bluemlein et al., 2011). PK catalyses the ‘final’ step in glycolysis and converts PEP into pyruvate; a reaction which yields ATP, and is thus required for the net gain in glycolytic energy production (Fraenkel, 1986).
Most human tissues are dominated by the expression of one of two mutually exclusive spliceforms of the PKM gene: PKM1 and PKM2 (Mazurek et al., 2005; Bluemlein et al., 2011). In most human tissues whether healthy or cancerous, (bladder, liver, colon, lung, kidney, thyroid, fibroblasts, epithelial cells), but not in muscle and potentially neurons, PKM2 is the dominantly expressed isoform over PKM1 (Bluemlein et al., 2011). At least liver and red blood cells express an additional PK isoform, known as PKLR (Zanella & Bianchi, 2000).
Among PKM-expressing tissues, the total PKM expression level and the distribution between the two isoforms varies significantly, and ranges from 55% PKM2 over PKM1 of total PKM in (muscle-rich) bladder tissue, to up to 96% PKM2 of total PKM in colon tissue. At the same time, in absolute values, the PKM content ranged from ~10 fmol/μg total protein in thyroid to 300 fmol/μg total protein in colorectal carcinoma (Bluemlein et al., 2011). Hence, the expression level of the PKM gene appears to be an important determinant of total pyruvate kinase activity and is highly dependent on the tissue. In many cancer cells PKM2 expression is increased when compared to tissue-matched controls (Ashrafian et al., 2010; Bluemlein et al., 2011; Zhou et al., 2012). Despite the total protein amount being up-regulated, overall PK activity however does not increase accordingly, or it is inhibited, suggesting that the specific PKM activity is lowered in the tumour cells (Hitosugi et al., 2009; Vander Heiden et al., 2010). This modulation of PK activity creates the opportunity to switch between high glycolytic flux, or induce a metabolic reconfiguration towards OXPHOS and an activated PPP. In contrast to PKM1 that is a tetramer with high constitutive activity (Imamura & Tanaka, 1972; Wu & Le, 2013), PKM2 can be flexibly tuned and allosterically modulated in activity and switches between monomeric, dimeric and tetrameric states (Morgan et al., 2013). Allosteric activation of PKM2 can be achieved by interaction with fructose 1,6-bisphosphate, succinylaminoimidazolecarboxamide ribose 5′-phosphate (SAICAR) and serine (Chaneton et al., 2012; Keller, Tan & Lee, 2012). In addition, post-translational modifications can modulate PKM2 activity. For example, phosphorylation at tyrosine 105 prevents the formation of the more active tetrameric form of PKM2 (Hitosugi et al., 2009), and P300/CBP-associated factor (PCAF)-mediated acetylation on lysine 305 reduces activity (Wang et al., 2010). During high ROS levels, lower PK activity could be essential to maintain cell survival. Similarly to respiring yeast (section III), cancer cells accumulate PEP because of reduced PK activity (Anastasiou et al., 2011; Grüning et al., 2011). Oxidation of PKM2 at cysteine 358 can decrease its activity and redirect glycolytic intermediates towards the PPP for the production of NADPH. Disruption of this mechanism can exacerbate oxidative stress and subsequently decrease proliferation in cancer cells (Anastasiou et al., 2011).
What does link decreased PK activity and increased cellular oxidative capacity? The mechanism seems to depend on the glycolytic block and PEP accumulation. PEP interferes with other glycolytic reactions, such as phosphoglycerate mutase, glucokinase, GPI, PFK, FBA and TPI (Ogawa et al., 2007; Fenton & Reinhart, 2009; Grüning et al., 2011; Grüning et al., 2014). The latter can directly lead to increased PPP activity and stress protection. Despite in vitro kinetics indicating a low flux control coefficient of TPI (Knowles & John, 1977), in vivo experiments detect increased PPP activity when TPI activity is only slightly compromised (Ralser et al., 2007). TPI inhibition is sufficient to cause a block in upper glycolysis and an increase in PPP metabolites (Grüning et al., 2011; Grüning et al., 2014). This redirection of metabolites by the PK-PEP-TPI feedback loop enables cells to adapt to a higher level of ROS and protect from oxidative damage (Ralser et al., 2007; Grüning et al., 2014). TPI thus might represent a key enzymatic switch for metabolic reprogramming. Although low PK and TPI activity limit the ATP yield from energy metabolism, the cell’s redox balance is maintained which could be more important for cancer survival (Cairns et al., 2011; Grüning & Ralser, 2011).
(4) Interaction of the PPP with oncogenic pathways
(a) p53
The transcription factor p53 represents a tumour suppressor with well-established functions on genomic integrity, apoptosis and cell cycle control (Vazquez et al., 2008). p53 is the most commonly mutated gene in human cancers and its loss constitutes a pivotal mechanism of therapy failure (Bensaad et al., 2006; Rohwer et al., 2010). It became evident in recent years that p53, in addition to the functions outlined above, exerts control over metabolic pathways. The p53 target gene TIGAR was shown to dampen glycolysis by lowering the level of fructose 2,6-bisphosphate which is a powerful allosteric activator of PFK1. As a result the glycolytic intermediates can be diverted to the oxidative or non-oxidative branch of the PPP (Fig. 6). This leads to decreased cellular levels of ROS due to the action of NADPH, ultimately resulting in enhanced cell survival and growth (Bensaad et al., 2006). In highly proliferative tissues such as the intestine, a lack of TIGAR in vivo leads to decreased regeneration after acute stresses such as ulcerative colitis and irradiation, indicating an important role of TIGAR in proliferation (Cheung et al., 2013). Consistently, overexpression of TIGAR has been observed in a number of tumour types, and also in invasive cancer cells compared to normal tissues (Wanka, Steinbach & Rieger, 2012; Won et al., 2012; Cheung et al., 2013). In an in vivo model of intestinal adenoma where adenomatous polyposis coli (APC) is deleted in LGR5+ intestinal stem cells, TIGAR deficiency decreases tumour burden and average tumour size, which results in increased disease-free survival in these mice. Tumour intestinal crypts isolated from these mice showed that the in vitro growth of the TIGAR- deficient tissues can be rescued by the addition of antioxidants and nucleosides, again indicating an important role of TIGAR in increasing PPP during proliferation. While the role of TIGAR in promoting tumour growth seems to be counterintuitive to p53 as a tumour suppressor, TIGAR expression is uncoupled from p53 expression in various cell lines (Cheung et al., 2013). Hence, it is possible that a p53 target protein such as TIGAR can become oncogenic when it is not properly regulated by p53. Recently, it has been shown that TIGAR predominantly functions as phosphoglycolate-independent 2,3-bisphos phoglycerate phosphatase (Gerin et al., 2014). Another interpretation is thus that p53 could stimulate the non-oxidative PPP via TIGAR by directly targeting lower glycolysis. In contrast to this activity of a p53-target gene, p53 itself can inhibit G6PDH and regulate its activity; this results in reduced PPP activity and a redirection of the central carbon flux towards increased glycolytic activity (Jiang et al., 2011). As a result, p53-deficient cells display enhanced lipid synthesis as well as reduced sensitivity towards oxidative stress-induced cell death as a functional consequence of higher oxidative PPP activity (Jiang et al., 2011). Taken together, p53 seems to influence the PPP antithetically, both as an inhibitor (via direct influence on G6PDH) and as an activator (via TIGAR). These opposing functions of p53 may reflect the different roles of p53 depending on the severity of the damage to the cell. During transient and mild stress, p53 may act as a pro-survival mediator for repair and regeneration. However, if the damage is too high and persistent, p53 may switch off the pro-survival mode for the proper elimination of irreversibly damaged cells. In some cases, as a result of p53 activity, the homeostasis and integrity of the tissue as a whole is preserved. Interestingly, p73 (a p53 relative) was shown to enhance the PPP by activating the expression of G6PDH under conditions where p73 showed tumour-promoting activities (Du et al., 2013). While illustrating the complexity of function of the p53 family of proteins, these studies support the general notion that flux through the PPP supports cancer cell growth.
Fig. 6.
The pentose phosphate pathway (PPP) is associated with several cancer- and cell-proliferation-related signalling cascades. The p53 pathway can stimulate the PPP by inhibiting phosphofructokinase 1 (PFK1) through TP53-induced glycolysis and apoptosis regulator (TIGAR), and by inhibiting glucose 6-phosphate dehydrogenase (G6PDH). This enzyme is also targeted by the ataxia telangiectasia mutated (ATM) kinase, which increases G6PDH activity by phosphorylating heat shock protein 27 (HSP27), and an NAD-dependent deacetylase (SirT2), which acts on this enzyme directly by de-acetylation. All three mechanisms activate the oxidative branch of the PPP, which is also controlled by the mammalian target of rapamycin complex 1 (mTOR) pathway. Cancer signalling mechanisms operate alongside allosteric and metabolic regulation. For instance, reduced pyruvate kinase PKM2 activity leading to triosephosphate isomerase (TPI) inhibition increases the carbohydrate flux towards the PPP, achieving a metabolic self-regulation that counteracts oxidative stress. The activity of the PPP itself has an influence on cancer signalling pathways. The antioxidant capacity of the PPP modulates proto-oncogene k-ras-driven tumourigenesis; concurrently, reactive oxygen species (ROS) can potentiate the oncogenic activity of k-ras (the two opposing regulations are highlighted). The PPP has also been associated with drug resistance and hypoxia. Depending on the sensitivity to imatinib, chronic myeloid leukaemia (CML) cells can either exhibit reduced (sensitive cells) or increased (resistant cells) transketolase (TKL) activity after hypoxia-inducible transcription factor 1 (HIF-1) activation. F 2,6-BP, fructose 2,6-biphosphatase; P13K (Phosphatidylinositol-3-kinase), Akt (protein kinase B); PEP, phosphoenolpyruvate.
(b) ATM kinase
PPP activity can be also stimulated by the kinase ataxia telangiectasia mutated (ATM). ATM is a serine/threonine kinase which is activated by DNA double-strand breaks and phosphorylates enzymes which are required for DNA checkpoint control and cell cycle arrest (Furgason & Bahassi, 2013). It phosphorylates the heat shock protein 27 (HSP27) which forms a complex with the first enzyme of the oxidative branch of the PPP: G6PDH. This interaction activates G6PDH and increases its activity supporting elevated PPP flux (Cosentino et al., 2011) (Fig. 6). Therefore, by connecting genome stability and cell cycle control to PPP activation and metabolic adaptation, ATM represents another crucial hub for cellular homoeostasis during tumourigenesis (Cosentino et al., 2011; Krüger & Ralser, 2011; Ditch & Paull, 2012).
(c) K-ras
The proto-oncogene K-ras is found activated in a number of human cancers, in particular adenocarcinomas of the pancreas, lung and colon (Downward, 2003). The observation that K-ras-transfected murine fibroblasts display enhanced resistance against oxidative stress via NADPH-mediated glutathione recycling first pointed towards a potential importance of the PPP for K-ras-induced transformation (Recktenwald et al., 2008). Subsequently, it was found that oxidative stress is induced upon matrix detachment of cells and that resistance against detachment-induced cell death (anoikis) largely depends on antioxidant capacity (Schafer et al., 2009). Intriguingly, anoikis resistance, which represents a central hallmark of malignant cells and a fundamental prerequisite for metastatic dissemination, in K-ras-driven human colon and mammary cancer cells, depends on functional integrity of the PPP (Weinberg et al., 2010). While these results point towards a functional importance of PPP-mediated antioxidant capacity for K-ras-driven tumourigenesis, ROS generation has shown to be essential for the full oncogenic potential of K-ras (Weinberg et al., 2010). These apparently contradictory results clearly need further experimental clarification before a clear-cut picture of the interplay between K-ras and the PPP during malignant transformation can be proposed (Fig. 6).
(d) HIF-1
The hypoxia-inducible transcription factor HIF-1 is found overexpressed in the majority of human cancers and regulates pivotal pro-tumourigenic features such as angiogenesis, glucose uptake and glycolysis as well as resistance towards apoptosis and anoikis (Rohwer et al., 2013). Research on the role of HIF-1 for glucose metabolism was long dominated by HIF-1’s robust effect on glucose transport and glycolysis while experimental data supporting a role for HIF-1 in the control of cancer-associated PPP activity is intriguingly meagre. Analyses of the importance of oxidative stress during the pathogenesis of Alzheimer’s disease first reported a functional role of HIF-1 for enhanced PPP activity (Soucek et al., 2003) (Fig. 6). Later, experimental evidence supported a critical role of HIF-1-mediated PPP activation in cellular antioxidant capacity of neuroblastoma cells (Guo et al., 2009). The most compelling experimental evidence in this regard was published by Craig Thompson’s group: analysing chronic myeloid leukemia (CML) cells, they reported robust activation of HIF-1 in cells exhibiting resistance towards the tyrosine-kinase-inhibiting therapeutic imatinib (Zhao et al., 2010). This HIF-1 activation was associated with reduced flux through the oxidative branch of the PPP while the glycolytic rate was significantly enhanced. On the other hand, the non-oxidative PPP branch was found activated in a TKL-dependent manner in cells with stabilized HIF-1, thereby supplying ribose synthesis essential for cellular proliferation (Zhao et al., 2010). Chemical inhibition of TKL resulted in enhanced imatinib sensitivity in vitro and in vivo against CML, pointing towards a functional role of HIF-1-driven non-oxidative PPP in mediating resistance against targeted therapies. These results are especially intriguing as imatinib represents the only targeted therapeutic that was able to result in undisputed and long-lasting clinical benefit of patients with cancer. This work supports the notion of HIF-1 as a pivotal mediator of therapy failure and points towards HIF-1-dependent control of cellular metabolism as an important molecular mechanism (Rohwer & Cramer, 2011).
(e) PI3K-Akt/mTORC1
Aberrant activation of the signalling cascade comprising phosphatidyl inositol kinase (PI3K), protein kinase B (PKB/Akt) and the mammalian target of rapamycin complex 1 (mTORC1) is commonly observed in the majority of human cancers (Laplante & Sabatini, 2012). It became evident in recent years that mTORC1 exerts pro-tumourigenic activity not only via its well-established roles in protein synthesis and autophagy, but also via elaborate control over cellular metabolism. A genomic approach unravelled that mTORC1 induces a variety of genes that encode for specific metabolic pathways, e.g. glycolysis, lipid and sterol biosynthesis as well as both branches of the PPP (Düvel et al., 2010). Despite these encouraging results, the functional importance of the PPP for mTORC1-driven cancers as well as the molecular nature of mTORC1 activation of PPP genes remains elusive. The antioxidant capacity needed to promote survival of tumour cells after detachment from the extracellular matrix depends on PI3K-Akt-induced activation of the oxidative PPP (Schafer et al., 2009) (Fig. 6).
(f) Alternative pathways of PPP activation in cancer
The notion that increased PPP activity is beneficial for cancer cells is also supported by other studies that propose alternative mechanisms of PPP activation in cancer cells. For example, phosphofructokinase 1 (PFK1) is inhibited in cancer cells through glycosylation, drives PPP flux and supports cancer cell growth (Yi et al., 2012). Vice versa, the depletion of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase 4 (PFKFB4) inhibits cancer cell growth by lowering flux through the PPP (Ros et al., 2012). Moreover, a study addressing the plant stilbenoid resveratrol indicates that its suppressive function on human colon cancer cell proliferation is attributable to PPP targeting and talin-focal adhesion kinase (talin-FAK) signalling pathways as well (Vanamala et al., 2011).
(5) Conclusions about the role of the PPP in cancer metabolism
While the prognosis of certain types of cancers (e.g. breast and colon cancer) has improved in recent years, we are still eagerly awaiting successful clinical translation of the billions of funding and an uncountable number of working hours that have been invested in cancer research in the last 40+ years. Otis W. Brawley, chief medical officer of the American Cancer Society, once said ‘One cancer cell is smarter than 100 brilliant cancer scientists’. We still need to unravel the basic principles that enable malignant transformation, unchecked proliferation, systemic spread and therapy resistance. There is good reason to believe that understanding cancer metabolism might provide an important contribution to these attempts. The study of the PPP could be central, as the pathway is at the crossroads of both oncogenic signalling and biosynthetic pathways. In this respect, first results are promising: in a recent study, PPP activity was predictive for the efficacy of cancer therapeutics (Folger et al., 2011).
IX. THE ROLE OF THE PPP IN BRAIN ENERGY METABOLISM
The brain energy demands to maintain its physiological signalling activities are extremely high. Although it represents only 2% of the total body mass, the adult human brain is believed to consume about 20% of oxygen respired at rest (Silver & Erecińska, 1998; Bélanger, Allaman & Magistretti, 2011). A developing brain might have even greater requirements, as estimates suggest that an infant’s brain can utilize more than 40% of basal metabolic rate (Goyal et al., 2014). These large amounts of energy are needed for the maintenance and restoration of ionic gradients and for synaptic transmission (Attwell & Laughlin, 2001). The majority of ATP is generated through OXPHOS, therefore implying the strict reliance of neuronal activity on mitochondria functionality and oxygen supply (Ames, 2000; Erecinska, Cherian & Silver, 2004). Accordingly, mitochondrial impairment has a great impact on neuronal function and survival (Nicholls & Budd, 2000; Kann & Kovács, 2007), and it is considered a key pathogenic player in several neurodegenerative and neurodevelopmental disorders (Fiskum, Murphy & Beal, 1999; Beal, 2005).
Glucose represents the normal obligatory energy substrate of the brain, and 25% of its daily intake is assumed to be dedicated to cerebral functions (Cremer, 1982; Bélanger et al., 2011). A steady glucose supply is necessary since the central nervous system (CNS) is able to store only a limited amount of glycogen within astrocytes (Brown & Ransom, 2007), glial cells outnumbering neurons in the human brain. Interestingly, quantitative measurements of whole-brain metabolism showed that about 10% of consumed glucose is in excess of oxygen utilization (Fox et al., 1988). Therefore, glycolysis-based metabolism appears of fundamental importance for the energetic needs of active neuronal tissue. Studies of primary cultures of glia and neurons helped to demonstrate the physiological metabolic compartmentalization of the CNS. In particular, astrocytes are mainly glycolytic and convert glucose into lactate (Itoh et al., 2003). This lactate can then be transferred to neurons via the so-called ‘astrocyte–neuron lactate shuttle’ and eventually employed by the neurons for OXPHOS-based ATP generation (Pellerin & Magistretti, 1994; Kasischke et al., 2004). This model is supported by a cell-type-specific expression pattern of regulatory members of carbon metabolism (Lovatt et al., 2007). These include glucose transporters (GLUT1 in astrocytes and GLUT3 in neurons), lactate dehydrogenase (LDH1 in astrocytes favouring lactate generation and LDH5 in neurons supporting pyruvate formation from lactate), and lactate transporter (monocarboxylate transporter MCT1/4 in astrocytes promoting lactate release and MCT2 in neurons promoting lactate uptake) (Fig. 7) (Bittar et al., 1996; Ames, 2000; Bélanger et al., 2011).
Fig. 7.
The pentose phosphate pathway (PPP) in neuronal energy metabolism. Schematic representation of glucose metabolism in neurons (left) and astrocytes (right). Metabolic regulators differentially expressed between neurons and astrocytes are highlighted. Summarized is evidence that astrocytes ferment glucose to lactate which is secreted aside GSH into the intracellular space. Neurons then uptake lactate and GSH; lactate is then converted to pyruvate and enters the tricarboxylic acid cycle to generate ATP over the respiratory chain. Abbreviations: as in Table 1; GSH, glutathione; GSSG, glutathione disulfide; GLUT, glucose transporters; GR, glutathione reductase; GPx, glutathione peroxidase; LDH, lactate dehydrogenase; MCT, monocarboxylate transporter.
An interesting consequence of the metabolic coupling between astrocytes and neurons is the peculiar neuronal dependence on PPP activity. Indeed, lactate utilization as an oxidative substrate for energy production in neurons may represent a mechanism for circumventing glycolysis and thus sparing neuronal glucose for the PPP (Bolaños, Almeida & Moncada, 2010). In particular, a fundamental regulatory role has been proposed for the enzyme 6-phosphofructose-2-kinase/fructose-2,6-biphosphatase-3 (PFKFB3), which generates fructose 2,6-bisphosphate (F2,6BP), a potent activator of the rate-limiting glycolytic enzyme phosphofructokinase-1 (PFK1) (Hue & Rider, 1987). Due to constant proteasomal degradation, PFKFB3 is absent in neurons and cannot be activated upon inhibition of mitochondrial respiration (Almeida et al., 2001; Herrero-Mendez et al., 2009). On the contrary, PFKFB3 is expressed in astrocytes was upregulated upon mitochondrial impairment in order to increase the glycolytic rate (Herrero-Mendez et al., 2009). Therefore, the high neuronal sensitivity to mitochondrial dysfunction may be due to their inability to sustain elevated glycolysis because of their dependence on PPP-based utilization of glucose. A similar mechanism may also be present in cancer cells, where PFKFB3 has been reported to display reduced methylation and enhanced degradation in the proteasome, resulting in the shunt of glucose away from glycolysis and towards the PPP (Yamamoto et al., 2014).
The main reason underlying neuronal dependence on glucose metabolism via the PPP may be the maintenance of redox homeostasis (Fernandez-Fernandez, Almeida & Bolaños, 2012). Indeed, to counteract the increase in ROS, common by-products of OXPHOS, neuronal cells would need an antioxidant defence mechanism constantly in place. To this end, the production of NADPH within the oxidative branch of the PPP is critical, as it represents the main electron donor for the generation of reduced glutathione (GSH) through the enzyme glutathione reductase (GR). GSH is in turn employed as electron donor for the reduction of detrimental peroxides (ROOH) by glutathione peroxidase (GPx) (Dringen, 2000). Interestingly, although this process may be essential in neurons, it has been shown that astrocytes are better equipped to stimulate the PPP, and the consequent NADPH generation, in response to oxidative stress (Ben-Yoseph, Boxer & Ross, 1996a; García-Nogales, Almeida & Bolaños, 2003). Neurons are also less capable than astrocytes in utilizing extracellular cysteine, used as precursor of GSH, and thus rely on the uptake of GSH that has been produced and released by the astrocytes (Dringen, 2000). These data emphasize the susceptibility of neuronal cells to redox imbalance and their crucial necessity for PPP-based glucose metabolism (Ben-Yoseph, Boxer & Ross, 1996b). In accordance, brain PPP activity has been found induced upon experimental brain injury in mice and after traumatic brain injury in humans (Bartnik et al., 2005; Dusick et al., 2007). Furthermore, malfunction of the PPP is associated with the appearance of neurological symptoms (Herken, Lange & Kolbe, 1969).
Recent findings suggest a second reason behind the importance of the PPP in brain metabolism. A meta-analysis of glucose and oxygen consumption throughout the human lifespan and among different brain regions suggests that non-oxidative glucose utilization may be important during development to support synaptic remodelling (Vaishnavi et al., 2010; Goyal et al., 2014). This may imply that the nucleotide biosynthesis derived by PPP activity might be crucial in neurons for synaptic plasticity (Magistretti, 2014). Indeed, the PPP/glycolysis ratio has been found to be higher in neonatal brain compared to adult brain (Baquer et al., 1977; Morken et al., 2014).
The current model of neuron/astrocyte bioenergetics has been questioned by some groups (Dienel, 2012), as it has been shown that during network activation neurons may be as capable as astrocytes at employing glucose as an energy substrate (Ivanov et al., 2014). Accordingly, glycolysis-generated ATP appears of fundamental importance for vesicle motility (Zala et al., 2013). Therefore, as mitochondria may be unevenly distributed in the neuronal cells, the glycolytic machinery may provide the constant energy needed for fast axonal transport (Zala et al., 2013).
Overall, our understanding of human brain energy metabolism is still limited. Perhaps, recent advances in stem cells and neuronal differentiation (Jakel, Schneider & Svendsen, 2004; Sandoe & Eggan, 2013) might be helpful in providing human CNS cells for the study of neuroenergetics at the cellular and molecular level. This might potentially clarify the role of the PPP in the CNS and the interplay between different human brain cell types in the basal state and under conditions stimulating remodelling of energy flux.
X. CONCLUSIONS
The PPP is a central component of metabolism in the majority of single- and multicellular organisms. Despite the pathway is central and evolutionary ancient, it possesses a high level of flexibility, which renders it an attractive target for biotechnology and medicine. In summary
The main biochemical function of the PPP is the biosynthesis of nucleic-acid and amino-acid sugar phosphate precursors.
This function of the PPP is bound to the provision of biochemical reducing equivalents in form of NADPH, which renders the PPP an important player in maintaining redox homeostasis.
The PPP is highly flexible, dynamic, and is adapting to varying nutrient supply and stress conditions. This coordinates these functions and is required meet cellular metabolic demands in the constantly changing environment.
The PPP is important for biotechnology, as its flexibility can be exploited to tune NADPH production, and for medical research, as the PPP activity is altered by bacterial and eukaryotic parasites during the infection process, when stem cells differentiate, when cancer cells maintain redox homeostasis, and in neurons to sustain energy metabolism.
Unveiling the complex regulation of the PPP, which despite 80 years of detailed basic and medical research is still not fully understood, appears hence essential for addressing metabolic adaptation and its consequences on cellular and organismic physiology.
Acknowledgments
We thank David Goldfarb (University of Rochester) and Nick Kruger (University of Oxford) for valuable discussions, and Alison Cooper and Aleksej Zelezniak for copyediting the final revision of the manuscript. We acknowledge funding from the European Commission (Brussels) Role of Mitochondria in Conserved Mechanisms of Aging (MIMAGE) Project (Contract 512020, to M.B.), the Cancer Research Programme Grant (C197/A3514 to K.M.B.), Cancer Research UK and ERC Grants 322842-METABOp53 (supporting E.C.), the Wellcome Trust (RG 093735/Z/10/Z to M.R.), the ERC (Starting grant 260809 to M.R.), the German Research Foundation DFG (PR 1527/1-1 to A.P.), and the Austrian Science Fund (FWF) S9302-B05 (to M.B.). V.O.-S. is supported by Consejo Nacional de Ciencia y Tecnologia (CONACyT) Mexico postdoctoral fellowship 203450, M.A.K. by the FWF (Austria) by an Erwin Schroedinger postdoctoral fellowship (J 3341). M.R. is a Wellcome-Trust Research career development and Wellcome-Beit prize fellow.
XII. REFERENCES
- Achcar F, Kerkhoven EJ, Bakker BM, Barrett MP, Breitling R. Dynamic modelling under uncertainty: the case of Trypanosoma brucei energy metabolism. PLoS Computational Biology. 2012;8:e1002352. doi: 10.1371/journal.pcbi.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akache B, Wu K, Turcotte B. Phenotypic analysis of genes encoding yeast zinc cluster proteins. Nucleic Acids Research. 2001;29:2181–2190. doi: 10.1093/nar/29.10.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MA, Haanstra JR, Hannaert V, Van Roy J, Opperdoes FR, Bakker BM, Michels PA. Experimental and in silico analyses of glycolytic flux control in bloodstream form Trypanosoma brucei. The Journal of Biological Chemistry. 2005;280:28306–28315. doi: 10.1074/jbc.M502403200. [DOI] [PubMed] [Google Scholar]
- Alcina A, Ramagopalan SV, Fernández O, Catalá-Rabasa A, Fedetz M, Ndagire D, Leyva L, Arnal C, Delgado C, Lucas M, Izquierdo G, Ebers GC, Matesanz F. Hexose-6-phosphate dehydrogenase: a new risk gene for multiple sclerosis. European Journal of Human Genetics. 2010;18:618–620. doi: 10.1038/ejhg.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Almeida J, Bolaños JP, Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:15294–15299. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alteri CJ, Mobley HLT. Escherichia coli physiology and metabolism dictates adaptation to diverse host microenvironments. Current Opinion in Microbiology. 2012;15:3–9. doi: 10.1016/j.mib.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A. CNS energy metabolism as related to function. Brain Research Brain Research Reviews. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Anastasiou D, Poulogiannis G, Asara JMJ, Boxer MB, Jiang J, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science (New York) 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya R, Lalloz MR, Bellingham AJ, Layton DM. Evidence for founder effect of the Glu104Asp substitution and identification of new mutations in triosephosphate isomerase deficiency. Human Mutation. 1997;10:290–294. doi: 10.1002/(SICI)1098-1004(1997)10:4<290::AID-HUMU4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ashrafian H, O’Flaherty L, Adam J, Steeples V, Chung Y, East P, Vanharanta S, Lehtonen H, Nye E, Hatipoglu E, Miranda M, Howarth K, Shukla D, Troy H, Griffiths J, et al. Expression profiling in progressive stages of fumarate-hydratase deficiency: the contribution of metabolic changes to tumorigenesis. Cancer Research. 2010;70:9153–9165. doi: 10.1158/0008-5472.CAN-10-1949. [DOI] [PubMed] [Google Scholar]
- Ashwell G, Hickman J. Enzymatic formation of xylulose 5-phosphate from ribose 5-phosphate in spleen. The Journal of Biological Chemistry. 1957;226:65–76. [PubMed] [Google Scholar]
- Atamna H, Pascarmona G, Ginsburg H. Hexose-monophosphate shunt activity in intact Plasmodium falciparum-infected erythrocytes and in free parasites. Molecular and Biochemical Parasitology. 1994;67:79–89. doi: 10.1016/0166-6851(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Ationu A, Humphries A, Lalloz MR, Arya R, Wild B, Warrilow J, Morgan J, Bellingham AJ, Layton DM. Reversal of metabolic block in glycolysis by enzyme replacement in triosephosphate isomerase-deficient cells. Blood. 1999;94:3193–3198. [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. Journal of Cerebral Blood Flow and Metabolism. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Avignone Rossa C, White J, Kuiper A, Postma PW, Bibb M, Teixeira de Mattos MJ. Carbon flux distribution in antibiotic-producing chemostat cultures of Streptomyces lividans. Metabolic Engineering. 2002;4:138–150. doi: 10.1006/mben.2001.0217. [DOI] [PubMed] [Google Scholar]
- Bajad SU, Lu W, Kimball EH, Yuan J, Peterson C, Rabinowitz JD. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. Journal of Chromatography A. 2006;1125(1):76–88. doi: 10.1016/j.chroma.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S, Wamelink MMC, Ngu LH, Talib A, Salomons GS, Jakobs C, Keng WT. Novel heterozygous mutations in TALDO1 gene causing transaldolase deficiency and early infantile liver failure. Journal of Pediatric Gastroenterology and Nutrition. 2011;52:113–116. doi: 10.1097/MPG.0b013e3181f50388. [DOI] [PubMed] [Google Scholar]
- Banki K, Hutter E, Colombo E, Gonchoroff NJ, Perl A. Glutathione levels and sensitivity to apoptosis are regulated by changes in transaldolase expression. The Journal of Biological Chemistry. 1996;271:32994–33001. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]
- Banki K, Perl A. Inhibition of the catalytic activity of human transaldolase by antibodies and site-directed mutagenesis. FEBS Letters. 1996;378:161–165. doi: 10.1016/0014-5793(95)01446-2. [DOI] [PubMed] [Google Scholar]
- Baquer NZ, Hothersall JS, McLean P, Greenbaum AL. Aspects of carbohydrate metabolism in developing brain. Developmental Medicine and Child Neurology. 1977;19:81–104. doi: 10.1111/j.1469-8749.1977.tb08027.x. [DOI] [PubMed] [Google Scholar]
- Barrett MP. The pentose phosphate pathway and parasitic protozoa. Parasitology Today (Personal ed) 1997;13:11–16. doi: 10.1016/s0169-4758(96)10075-2. [DOI] [PubMed] [Google Scholar]
- Barrett MP, Phillips C, Adams MJ, Le Page RW. Overexpression in Escherichia coli and purification of the 6-phosphogluconate dehydrogenase of Trypanosoma brucei. Protein Expression and Purification. 1994;5:44–49. doi: 10.1006/prep.1994.1006. [DOI] [PubMed] [Google Scholar]
- Bartnik BL, Sutton RL, Fukushima M, Harris NG, Hovda DA, Lee SM. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. Journal of Neurotrauma. 2005;22:1052–1065. doi: 10.1089/neu.2005.22.1052. [DOI] [PubMed] [Google Scholar]
- Basso D, Plebani M, Kusters JG. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2010;15:14–20. doi: 10.1111/j.1523-5378.2010.00781.x. [DOI] [PubMed] [Google Scholar]
- Baughan MA, Valentine WN, Paglia DE, Ways PO, Simons ER, DeMarsh QB. Hereditary hemolytic anemia associated with glucosephosphate isomerase (GPI). deficiency--a new enzyme defect of human erythrocytes. Blood. 1968;32:236–249. [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Annals of Neurology. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Becker MA. Patterns of phosphoribosylpyrophosphate and ribose-5-phosphate concentration and generation in fibroblasts from patients with gout and purine overproduction. The Journal of Clinical Investigation. 1976;57:308–318. doi: 10.1172/JCI108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabolism. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Bensaad K, Tsuruta A, Selak MA, Vidal MNC, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph O, Boxer PA, Ross BD. Assessment of the role of the glutathione and pentose phosphate pathways in the protection of primary cerebrocortical cultures from oxidative stress. Journal of Neurochemistry. 1996a;66:2329–2337. doi: 10.1046/j.1471-4159.1996.66062329.x. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph O, Boxer PA, Ross BD. Noninvasive assessment of the relative roles of cerebral antioxidant enzymes by quantitation of pentose phosphate pathway activity. Neurochemical Research. 1996b;21:1005–1012. doi: 10.1007/BF02532410. [DOI] [PubMed] [Google Scholar]
- Bernt E, Bergmeyer HU. Methods of Enzymatic Analysis. Vol. 4. Verlag Chemie, Academic Press; Weinhem, New York: 1974. L-Glutamate UV-assay with glutamate dehydrogenase and NAD; pp. 1704–1708. [Google Scholar]
- Beutler E, Kuhl W, Gelbart T. 6-Phosphogluconolactonase deficiency, a hereditary erythrocyte enzyme deficiency: possible interaction with glucose-6-phosphate dehydrogenase deficiency. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:3876–3878. doi: 10.1073/pnas.82.11.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E, Morrison M. Localization and characteristics of hexose 6-phosphate dehydrogenase (glucose dehydrogenase) The Journal of Biological Chemistry. 1967;242:5289–5293. [PubMed] [Google Scholar]
- Bezwoda WR, Derman DP, See N, Mansoor N. Relative value of oestrogen receptor assay, lactoferrin content, and glucose-6-phosphate dehydrogenase activity as prognostic indicators in primary breast cancer. Oncology. 1985;42:7–12. doi: 10.1159/000225992. [DOI] [PubMed] [Google Scholar]
- Bieganowski P, Seidle H, Wojcik M, Brenner C. Synthetic lethal and biochemical analyses of NAD and NADH kinases in Saccharomyces cerevisiae establish separation of cellular functions. The Journal of Biological Chemistry. 2006;281:22439–22445. doi: 10.1074/jbc.M513919200. [DOI] [PubMed] [Google Scholar]
- Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. Journal of Cerebral Blood Flow and Metabolism. 1996;16:1079–1089. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Bluemlein K, Grüning NM, Feichtinger R, Lehrach H, Kofler B, Ralser M. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget. 2011;2:393–400. doi: 10.18632/oncotarget.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C, Lengauer T. Computational epigenetics. Bioinformatics (Oxford, England) 2008;24:1–10. doi: 10.1093/bioinformatics/btm546. [DOI] [PubMed] [Google Scholar]
- Bogorad IW, Lin TS, Liao JC. Synthetic non-oxidative glycolysis enables complete carbon conservation. Nature. 2013;502:693–697. doi: 10.1038/nature12575. [DOI] [PubMed] [Google Scholar]
- Bolaños JP, Almeida A, Moncada S. Glycolysis: a bioenergetic or a survival pathway? Trends in Biochemical Sciences. 2010;35:145–149. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Borodina I, Siebring J, Zhang J, Smith CP, van Keulen G, Dijkhuizen L, Nielsen J. Antibiotic overproduction in Streptomyces coelicolor A3 2 mediated by phosphofructokinase deletion. The Journal of Biological Chemistry. 2008;283:25186–25199. doi: 10.1074/jbc.M803105200. [DOI] [PubMed] [Google Scholar]
- Bozdech Z, Ginsburg H. Data mining of the transcriptome of Plasmodium falciparum: the pentose phosphate pathway and ancillary processes. Malaria Journal. 2005;4:17. doi: 10.1186/1475-2875-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräsen C, Esser D, Rauch B, Siebers B. Carbohydrate metabolism in Archaea: current insights into unusual enzymes and pathways and their regulation. Microbiology and Molecular Biology Reviews. 2014;78:89–175. doi: 10.1128/MMBR.00041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekke EMF, Walls AB, Schousboe A, Waagepetersen HS, Sonnewald U. Quantitative importance of the pentose phosphate pathway determined by incorporation of 13C from [2-13C]- and [3-13C]glucose into TCA cycle intermediates and neurotransmitter amino acids in functionally intact neurons. Journal of Cerebral Blood Flow and Metabolism. 2012;32:1788–1799. doi: 10.1038/jcbfm.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Dern RJ. A new inherited enzymatic deficiency of human erythrocytes: 6-phosphogluconate dehydrogenase deficency. American Journal of Human Genetics. 1964;16:472–476. [PMC free article] [PubMed] [Google Scholar]
- Brindle KM, Bohndiek SE, Gallagher FA, Kettunen MI. Tumor imaging using hyperpolarized 13C magnetic resonance spectroscopy. Magnetic Resonance in Medicine. 2011;66:505–519. doi: 10.1002/mrm.22999. [DOI] [PubMed] [Google Scholar]
- Brown SA, Palmer KL, Whiteley M. Revisiting the host as a growth medium. Nature Reviews Microbiology. 2008;6:657–666. doi: 10.1038/nrmicro1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Bublitz C, Steavenson S. The pentose phosphate pathway in the endoplasmic reticulum. The Journal of Biological Chemistry. 1988;263:12849–12853. [PubMed] [Google Scholar]
- Buescher JM, Moco S, Sauer U, Zamboni N. Ultrahigh performance liquid chromatography-tandem mass spectrometry method for fast and robust quantification of anionic and aromatic metabolites. Analytical Chemistry. 2010;82:4403–4412. doi: 10.1021/ac100101d. [DOI] [PubMed] [Google Scholar]
- Buescher JM, Liebermeister W, Jules M, Uhr M, Muntel J, Botella E, et al. Global network reorganization during dynamic adaptations of Bacillus subtilis metabolism. Science (New York) 2012;335:1099–1103. doi: 10.1126/science.1206871. [DOI] [PubMed] [Google Scholar]
- Bukowiecki R, Adjaye J, Prigione A. Mitochondrial function in pluripotent stem cells and cellular reprogramming. Gerontology. 2014;60:174–182. doi: 10.1159/000355050. [DOI] [PubMed] [Google Scholar]
- Butler MJ, Bruheim P, Jovetic S, Marinelli F, Postma PW, Bibb MJ. Engineering of primary carbon metabolism for improved antibiotic production in Streptomyces lividans. Applied and Environmental Microbiology. 2002;68:4731–4739. doi: 10.1128/AEM.68.10.4731-4739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadière A, Ortiz-Julien A, Camarasa C, Dequin S. Evolutionary engineered Saccharomyces cerevisiae wine yeast strains with increased in vivo flux through the pentose phosphate pathway. Metabolic Engineering. 2011;13:263–271. doi: 10.1016/j.ymben.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris I, McCracken S, Mak TW. Cancer cell metabolism. Nature Reviews Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Cakir T, Patil KR, Onsan ZI, Ulgen KO, Kirdar B, Nielsen J. Integration of metabolome data with metabolic networks reveals reporter reactions. Molecular Systems Biology. 2006;2:50. doi: 10.1038/msb4100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- Caprari P, Caforio MP, Cianciulli P, Maffi D, Pasquino MT, Tarzia A, Amadori S, Salvati AM. 6-Phosphogluconate dehydrogenase deficiency in an Italian family. Annals of Hematology. 2001;80:41–44. doi: 10.1007/s002770000233. [DOI] [PubMed] [Google Scholar]
- Casazza JP, Veech RL. The measurement of xylulose 5-phosphate, ribulose 5-phosphate, and combined sedoheptulose 7-phosphate and ribose 5-phosphate in liver tissue. Analytical Biochemistry. 1986;159:243–248. doi: 10.1016/0003-2697(86)90338-6. [DOI] [PubMed] [Google Scholar]
- Castegna A, Palmieri L, Spera I, Porcelli V, Palmieri F, Fabis-Pedrini MJ, Kean RB, Barkhouse DA, Curtis MT, Hooper DC. Oxidative stress and reduced glutamine synthetase activity in the absence of inflammation in the cortex of mice with experimental allergic encephalomyelitis. Neuroscience. 2011;185:97–105. doi: 10.1016/j.neuroscience.2011.04.041. [DOI] [PubMed] [Google Scholar]
- Castegna A, Scarcia P, Agrimi G, Palmieri L, Rottensteiner H, Spera I, Germinario L, Palmieri F. Identification and functional characterization of a novel mitochondrial carrier for citrate and oxoglutarate in Saccharomyces cerevisiae. The Journal of Biological Chemistry. 2010;285:17359–17370. doi: 10.1074/jbc.M109.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchpole G, Platzer A, Weikert C, Kempkensteffen C, Johannsen M, Krause H, Jung K, Miller K, Willmitzer L, Selbig J, Weikert S. Metabolic profiling reveals key metabolic features of renal cell carcinoma. Journal of Cellular and Molecular Medicine. 2011;15:109–118. doi: 10.1111/j.1582-4934.2009.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celton M, Goelzer A, Camarasa C, Fromion V, Dequin S. A constraint-based model analysis of the metabolic consequences of increased NADPH oxidation in Saccharomyces cerevisiae. Metabolic Engineering. 2012;14:366–379. doi: 10.1016/j.ymben.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Chaneton B, Hillmann P, Zheng L, Martin ACL, Maddocks ODK, Chokkathukalam A, Coyle JE, Jankevics A, Holding FP, Vousden KH, Frezza C, O’Reilly M, Gottlieb E. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechik G, Oh E, Rando O, Weissman J, Regev A, Koller D. Activity motifs reveal principles of timing in transcriptional control of the yeast metabolic network. Nature Biotechnology. 2008;26:1251–1259. doi: 10.1038/nbt.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EC, Athineos D, Lee P, Ridgway RA, Lambie W, Nixon C, Strathdee D, Blyth K, Sansom OJ, Vousden KH. TIGAR is required for efficient intestinal regeneration and tumorigenesis. Developmental Cell. 2013;25:463–477. doi: 10.1016/j.devcel.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EC, Ludwig RL, Vousden KH. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20491–20496. doi: 10.1073/pnas.1206530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Cipollina C, ten Pierick A, Canelas AB, Seifar RM, van Maris AJA, van Dam JC, Heijnen JJ. A comprehensive method for the quantification of the non-oxidative pentose phosphate pathway intermediates in Saccharomyces cerevisiae by GC-IDMS. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2009;877:3231–3236. doi: 10.1016/j.jchromb.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Clasquin MF, Melamud E, Singer A, Gooding JR, Xu X, Dong A, Cui H, Campagna SR, Savchenko A, Yakunin AF, Rabinowitz JD, Caudy AA. Riboneogenesis in yeast. Cell. 2011;145:969–980. doi: 10.1016/j.cell.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro AT, Thiemann OH, Michels PA. Inhibition of Trypanosoma brucei glucose-6-phosphate dehydrogenase by human steroids and their effects on the viability of cultured parasites. Bioorganic and Medicinal Chemistry. 2009;17:2483–2489. doi: 10.1016/j.bmc.2009.01.068. [DOI] [PubMed] [Google Scholar]
- Cosentino C, Grieco D, Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. The EMBO Journal. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer JE. Substrate utilization and brain development. Journal of Cerebral Blood Flow and Metabolism. 1982;2:394–407. doi: 10.1038/jcbfm.1982.45. [DOI] [PubMed] [Google Scholar]
- Cronín CN, Nolan DP, Voorheis HP. The enzymes of the classical pentose phosphate pathway display differential activities in procyclic and bloodstream forms of Trypanosoma brucei. FEBS Letters. 1989;244:26–30. doi: 10.1016/0014-5793(89)81154-8. [DOI] [PubMed] [Google Scholar]
- Crown SB, Ahn WS, Antoniewicz MR. Rational design of 13C-labeling experiments for metabolic flux analysis in mammalian cells. BMC Systems Biology. 2012;6:43. doi: 10.1186/1752-0509-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daran-Lapujade P, Jansen MLA, Daran JM, van Gulik W, de Winde JH, Pronk JT. Role of transcriptional regulation in controlling fluxes in central carbon metabolism of Saccharomyces cerevisiae. A chemostat culture study. The Journal of Biological Chemistry. 2004;279:9125–9138. doi: 10.1074/jbc.M309578200. [DOI] [PubMed] [Google Scholar]
- Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Current Opinion in Genetics & Development. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koning W, van Dam K. A method for the determination of changes of glycolytic metabolites in yeast on a subsecond time scale using extraction at neutral pH. Analytical Biochemistry. 1992;204:118–123. doi: 10.1016/0003-2697(92)90149-2. [DOI] [PubMed] [Google Scholar]
- De La Haba G, Leder IG, Racker E. Crystalline transketolase from bakers’ yeast: isolation and properties. The Journal of Biological Chemistry. 1955;214:409–426. [PubMed] [Google Scholar]
- Diaz-Moralli S, Tarrado-Castellarnau M, Alenda C, Castells A, Cascante M. Transketolase-like 1 expression is modulated during colorectal cancer progression and metastasis formation. PLoS ONE. 2011;6:e25323. doi: 10.1371/journal.pone.0025323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F. Oxidation of phosphohexonate and pentose phosphoric acids by yeast enzymes: oxidation of phosphohexonate. II. Oxidation of pentose phosphoric acids. The Biochemical Journal. 1938;32:1626–1644. doi: 10.1042/bj0321626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F, Glock GE. Direct oxidation of glucose-6-phosphate, 6-phosphogluconate and pentose-5-phosphates by enzymes of animal origin. The Biochemical Journal. 1951;50:81–95. doi: 10.1042/bj0500081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F, Williamson DH. Pentose phosphate isomerase and epimerase from animal tissues. The Biochemical Journal. 1956;64:567–578. doi: 10.1042/bj0640567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. Journal of Cerebral Blood Flow and Metabolism. 2012;32:1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditch S, Paull TT. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends in Biochemical Sciences. 2012;37:15–22. doi: 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma RD, de Jonge LP, Jonker CTH, Seifar RM, Heijnen JJ, van Gulik WM. Intracellular metabolite determination in the presence of extracellular abundance: application to the penicillin biosynthesis pathway in Penicillium chrysogenum. Biotechnology and Bioengineering. 2010;107:105–115. doi: 10.1002/bit.22786. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nature Reviews Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Draper N, Walker EA, Bujalska IJ, Tomlinson JW, Chalder SM, Arlt W, Lavery GG, Bedendo O, Ray DW, Laing I, Malunowicz E, White PC, Hewison M, Mason PJ, Connell JM, et al. Mutations in the genes encoding 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nature Genetics. 2003;34:434–439. doi: 10.1038/ng1214. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Progress in Neurobiology. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Du W, Jiang P, Mancuso A, Stonestrom A, Brewer MD, Minn AJ, Mak TW, Wu M, Yang X. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nature Cell Biology. 2013;15:991–1000. doi: 10.1038/ncb2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffieux F, Van Roy J, Michels PA, Opperdoes FR. Molecular characterization of the first two enzymes of the pentose-phosphate pathway of Trypanosoma brucei. Glucose-6-phosphate dehydrogenase and 6-phosphogluconolactonase. Journal of Biological Chemistry. 2000;275:27559–27565. doi: 10.1074/jbc.M004266200. [DOI] [PubMed] [Google Scholar]
- Dusick JR, Glenn TC, Lee WNP, Vespa PM, Kelly DF, Lee SM, Hovda DA, Martin NA. Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1,2-13C2]glucose labeling study in humans. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1593–1602. doi: 10.1038/sj.jcbfm.9600458. [DOI] [PubMed] [Google Scholar]
- Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebata M, Sato R, Bak T. The enzymatic phosphorylation of sedoheptulose. Journal of Biochemistry. 1955;42:715–725. [Google Scholar]
- Eisenreich W, Dandekar T, Heesemann J, Goebel W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nature Reviews Microbiology. 2010;8:401–412. doi: 10.1038/nrmicro2351. [DOI] [PubMed] [Google Scholar]
- Engelke UFH, Zijlstra FSM, Mochel F, Valayannopoulos V, Rabier D, Kluijtmans LAJ, Perl A, Verhoeven-Duif NM, de Lonlay P, Wamelink MMC, Jakobs C, Morava E, Wevers RA. Mitochondrial involvement and erythronic acid as a novel biomarker in transaldolase deficiency. Biochimica et Biophysica Acta. 2010;1802:1028–1035. doi: 10.1016/j.bbadis.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Cherian S, Silver IA. Energy metabolism in mammalian brain during development. Progress in Neurobiology. 2004;73:397–445. doi: 10.1016/j.pneurobio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Eyaid W, Al Harbi T, Anazi S, Wamelink MMC, Jakobs C, Al Salammah M, Al Balwi M, Alfadhel M, Alkuraya FS. Transaldolase deficiency: report of 12 new cases and further delineation of the phenotype. Journal of Inherited Metabolic Disease. 2013;36:997–1004. doi: 10.1007/s10545-012-9577-8. [DOI] [PubMed] [Google Scholar]
- Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510(7504):298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt SM, Buescher JM, Rudroff F, Picotti P, Zamboni N, Sauer U. Tradeoff between enzyme and metabolite efficiency maintains metabolic homeostasis upon perturbations in enzyme capacity. Molecular Systems Biology. 2010;6:356. doi: 10.1038/msb.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AW, Reinhart GD. Disentangling the web of allosteric communication in a homotetramer: heterotropic inhibition in phosphofructokinase from Escherichia coli. Biochemistry. 2009;48:12323–12328. doi: 10.1021/bi901456p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fernandez S, Almeida A, Bolaños JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochemical Journal. 2012;443:3–11. doi: 10.1042/BJ20111943. [DOI] [PubMed] [Google Scholar]
- Ferreira LMR. Cancer metabolism: the Warburg effect today. Experimental and Molecular Pathology. 2010;89:372–380. doi: 10.1016/j.yexmp.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Fico A, Paglialunga F, Cigliano L, Abrescia P, Verde P, Martini G, Iaccarino I, Filosa S. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death and Differentiation. 2004;11:823–831. doi: 10.1038/sj.cdd.4401420. [DOI] [PubMed] [Google Scholar]
- Filosa S, Fico A, Paglialunga F, Balestrieri M, Crooke A, Verde P, Abrescia P, Bautista JM, Martini G. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochemical Journal. 2003;370:935–943. doi: 10.1042/BJ20021614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. Journal of Cerebral Blood Flow and Metabolism. 1999;19:351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- Fojo T, Parkinson DR. Biologically targeted cancer therapy and marginal benefits: are we making too much of too little or are we achieving too little by giving too much? Clinical Cancer Research. 2010;16:5972–5980. doi: 10.1158/1078-0432.CCR-10-1277. [DOI] [PubMed] [Google Scholar]
- Folger O, Jerby L, Frezza C, Gottlieb E, Ruppin E, Shlomi T. Predicting selective drug targets in cancer through metabolic networks. Molecular Systems Biology. 2011;7:501. doi: 10.1038/msb.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmes CDL, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmes CDL, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metabolism. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science (New York) 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fraenkel DG. Mutants in glucose metabolism. Annual Review of Biochemistry. 1986;55:317–337. doi: 10.1146/annurev.bi.55.070186.001533. [DOI] [PubMed] [Google Scholar]
- Furgason JM, Bahassi EM. Targeting DNA repair mechanisms in cancer. Pharmacology & Therapeutics. 2013;137:298–308. doi: 10.1016/j.pharmthera.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, Lee LA, Semenza GL, Dang CV. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Nogales P, Almeida A, Bolaños JP. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. Journal of Biological Chemistry. 2003;278:864–874. doi: 10.1074/jbc.M206835200. [DOI] [PubMed] [Google Scholar]
- Gerin I, Noël G, Bolsée J, Haumont O, Van Schaftingen E, Bommer GT. Identification of TP53-induced glycolysis and apoptosis regulator (TIGAR) as the phosphoglycolate-independent 2,3-bisphosphoglycerate phosphatase. Biochemical Journal. 2014;458:439–448. doi: 10.1042/BJ20130841. [DOI] [PubMed] [Google Scholar]
- Giersch C. Quantitative high-performance liquid chromatographic analysis of 14C-labelled photosynthetic intermediates in isolated intact chloroplasts. Journal of Chromatography A. 1979;172:153–161. [Google Scholar]
- Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nature Reviews Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L, Brown DH. Purification and properties of d-glucose-6-phosphate dehydrogenase. Journal of Biological Chemistry. 1955;216:67–79. [PubMed] [Google Scholar]
- Gorsich SW, Dien BS, Nichols NN, Slininger PJ, Liu ZL, Skory CD. Tolerance to furfural-induced stress is associated with pentose phosphate pathway genes ZWF1, GND1, RPE1, and TKL1 in Saccharomyces cerevisiae. Applied Microbiology and Biotechnology. 2006;71:339–349. doi: 10.1007/s00253-005-0142-3. [DOI] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metabolism. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska D, Chelstowska A. The ALD6 gene product is indispensable for providing NADPH in yeast cells lacking glucose-6-phosphate dehydrogenase activity. Journal of Biological Chemistry. 2003;278:13984–13988. doi: 10.1074/jbc.M210076200. [DOI] [PubMed] [Google Scholar]
- Grant CM. Metabolic reconfiguration is a regulated response to oxidative stress. Journal of Biology. 2008;7:1. doi: 10.1186/jbiol63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grochowski LL, Xu H, White RH. Ribose-5-phosphate biosynthesis in Methanocaldococcus jannaschii occurs in the absence of a pentose-phosphate pathway. Journal of Bacteriology. 2005;187:7382–7389. doi: 10.1128/JB.187.21.7382-7389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüning NM, Du D, Keller MA, Luisi BF, Ralser M. Inhibition of triosephosphate isomerase by phosphoenolpyruvate in the feedback-regulation of glycolysis. Open Biology. 2014;4:130232. doi: 10.1098/rsob.130232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüning NM, Lehrach H, Ralser M. Regulatory crosstalk of the metabolic network. Trends in Biochemical Sciences. 2010;35:220–227. doi: 10.1016/j.tibs.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Grüning NM, Ralser M. Cancer: sacrifice for survival. Nature. 2011;480:190–191. doi: 10.1038/480190a. [DOI] [PubMed] [Google Scholar]
- Grüning NM, Rinnerthaler M, Bluemlein K, Mülleder M, Wamelink MMC, Lehrach H, Jakobs C, Breitenbach M, Ralser M. Pyruvate kinase triggers a metabolic feedback loop that controls redox metabolism in respiring cells. Cell Metabolism. 2011;14:415–427. doi: 10.1016/j.cmet.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus IC, Horecker BL, Wood WA. Pathways of carbohydrate metabolism in microorganisms. Bacteriological Reviews. 1955;19:79–128. doi: 10.1128/br.19.2.79-128.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Miyake M, Liu KJ, Shi H. Specific inhibition of hypoxia inducible factor 1 exaggerates cell injury induced by in vitro ischemia through deteriorating cellular redox environment. Journal of Neurochemistry. 2009;108:1309–1321. doi: 10.1111/j.1471-4159.2009.05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Igoillo-Esteve M, Michels PA, Cordeiro AT. Glucose-6-phosphate dehydrogenase of trypanosomatids: characterization, target validation, and drug discovery. Molecular Biology International. 2011;2011:135701. doi: 10.4061/2011/135701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte SA. Glucose-6-phosphate dehydrogenase: a novel therapeutic target in cardiovascular diseases. Current Opinion in Investigational Drugs. 2008;9:993–1000. [PubMed] [Google Scholar]
- Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF. Towards industrial pentose-fermenting yeast strains. Applied Microbiology and Biotechnology. 2007;74:937–953. doi: 10.1007/s00253-006-0827-2. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hanczko R, Fernandez DR, Doherty E, Qian Y, Vas G, Niland B, Telarico T, Garba A, Banerjee S, Middleton FA, Barrett D, Barcza M, Banki K, Landas SK, Perl A. Prevention of hepatocarcinogenesis and increased susceptibility to acetaminophen-induced liver failure in transaldolase-deficient mice by N-acetylcysteine. Journal of Clinical Investigation. 2009;119:1546–1557. doi: 10.1172/JCI35722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankermeyer CR, Tjeerdema RS. Polyhydroxybutyrate: plastic made and degraded by microorganisms. Reviews of Environmental Contamination and Toxicology. 1999;159:1–24. doi: 10.1007/978-1-4612-1496-0_1. [DOI] [PubMed] [Google Scholar]
- Hannaert V, Bringaud F, Opperdoes FR, Michels PA. Evolution of energy metabolism and its compartmentation in Kinetoplastida. Kinetoplastid Biology and Disease. 2003;2:11. doi: 10.1186/1475-9292-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T, Degani H, Frydman L. Hyperpolarized (13) C NMR studies of glucose metabolism in living breast cancer cell cultures. NMR in Biomedicine. 2013;26:1831–1843. doi: 10.1002/nbm.3024. [DOI] [PubMed] [Google Scholar]
- Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, Amir S, Lubec G, Park J, Esterbauer H, Bilban M, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metabolism. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector RE, Bowman M, Skory CD, Cotta MA. The Saccharomyces cerevisiae YMR315W gene encodes an NADP(H)-specific oxidoreductase regulated by the transcription factor Stb5p in response to NADPH limitation. New Biotechnology. 2009;26:171–180. doi: 10.1016/j.nbt.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Heinemann M, Sauer U. Systems biology of microbial metabolism. Current Opinion in Microbiology. 2010;13:337–343. doi: 10.1016/j.mib.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Herken H, Lange K, Kolbe H. Brain disorders induced by pharmacological blockade of the pentose phosphate pathway. Biochemical and Biophysical Research Communications. 1969;36:93–100. doi: 10.1016/0006-291x(69)90654-8. [DOI] [PubMed] [Google Scholar]
- Herrero-Mendez A, Almeida A, Fernández E, Maestre C, Moncada S, Bolaños JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nature Cell Biology. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Herrmann KM, Weaver LM. The shikimate pathway. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:473–503. doi: 10.1146/annurev.arplant.50.1.473. [DOI] [PubMed] [Google Scholar]
- Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Science Signaling. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MA, Buckner FS, Van Voorhis WC, Verlinde CLMJ, Mehlin C, Boni E, DeTitta G, Luft J, Lauricella A, Anderson L, Kalyuzhniy O, Zucker F, Schoenfeld LW, Earnest TN, Hol WGJ, Merritt EA. Structure of ribose 5-phosphate isomerase from Plasmodium falciparum. Acta Crystallographica Section F: Structural Biology and Crystallization Communications. 2006;62:427–431. doi: 10.1107/S1744309106010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood DA. Streptomyces in Nature and Medicine: The Antibiotic Makers. Oxford University Press; New York: 2007. [Google Scholar]
- Horecker BL. The pentose phosphate pathway. Journal of Biological Chemistry. 2002;277:47965–47971. doi: 10.1074/jbc.X200007200. [DOI] [PubMed] [Google Scholar]
- Horecker BL, Hurwitz J. The purification of phosphoketopentoepimerase from Lactobacillus pentosus and the preparation of xylulose 5-phosphate. Journal of Biological Chemistry. 1956;223:993–1008. [PubMed] [Google Scholar]
- Horecker BL, Hurwitz J, Smyrniotis PZ. The role of xylulose 5-phosphate in the transketolase reaction. Journal of Biological Chemistry. 1956;223:1009–1019. [PubMed] [Google Scholar]
- Horecker BL, Smyrniotis PZ. Purification and properties of yeast transaldolase. Journal of Biological Chemistry. 1955;212:811–825. [PubMed] [Google Scholar]
- Horecker BL, Smyrniotis PZ, Seegmiller JE. The enzymatic conversion of 6-phosphogluconate to ribulose-5-phosphate and ribose-5-phosphate. Journal of Biological Chemistry. 1951;193:383–396. [PubMed] [Google Scholar]
- Hrizo SL, Fisher IJ, Long DR, Hutton JA, Liu Z, Palladino MJ. Early mitochondrial dysfunction leads to altered redox chemistry underlying pathogenesis of TPI deficiency. Neurobiology of Disease. 2013;54:289–296. doi: 10.1016/j.nbd.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck JHJ, Verhoeven NM, Struys EA, Salomons GS, Jakobs C, van der Knaap MS. Ribose-5-phosphate isomerase deficiency: new inborn error in the pentose phosphate pathway associated with a slowly progressive leukoencephalopathy. American Journal of Human Genetics. 2004;74:745–751. doi: 10.1086/383204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L, Rider MH. Role of fructose 2,6-bisphosphate in the control of glycolysis in mammalian tissues. Biochemical Journal. 1987;245:313–324. doi: 10.1042/bj2450313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain A, Sato D, Jeelani G, Soga T, Nozaki T. Dramatic increase in glycerol biosynthesis upon oxidative stress in the anaerobic protozoan parasite Entamoeba histolytica. PLoS Neglected Tropical Diseases. 2012;6:e1831. doi: 10.1371/journal.pntd.0001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igoillo-Esteve M, Cazzulo JJ. The glucose-6-phosphate dehydrogenase from Trypanosoma cruzi: its role in the defense of the parasite against oxidative stress. Molecular and Biochemical Parasitology. 2006;149:170–181. doi: 10.1016/j.molbiopara.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Igoillo-Esteve M, Maugeri D, Stern AL, Beluardi P, Cazzulo JJ. The pentose phosphate pathway in Trypanosoma cruzi: a potential target for the chemotherapy of Chagas disease. Anais da Academia Brasileira de Ciências. 2007;79:649–663. doi: 10.1590/s0001-37652007000400007. [DOI] [PubMed] [Google Scholar]
- Ihmels J, Levy R, Barkai N. Principles of transcriptional control in the metabolic network of Saccharomyces cerevisiae. Nature Biotechnology. 2004;22:86–92. doi: 10.1038/nbt918. [DOI] [PubMed] [Google Scholar]
- Imamura K, Tanaka T. Multimolecular forms of pyruvate kinase from rat and other mammalian tissues. I. Electrophoretic studies. Journal of Biochemistry. 1972;71:1043–1051. doi: 10.1093/oxfordjournals.jbchem.a129852. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Esaki T, Shimoji K, Cook M, Law MJ, Kaufman E, Sokoloff L. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4879–4884. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Malkov AE, Waseem T, Mukhtarov M, Buldakova S, Gubkina O, Zilberter M, Zilberter Y. Glycolysis and oxidative phosphorylation in neurons and astrocytes during network activity in hippocampal slices. Journal of Cerebral Blood Flow and Metabolism. 2014;34:397–407. doi: 10.1038/jcbfm.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JB. Proton translocation by transhydrogenase. FEBS Letters. 2003;545:18–24. doi: 10.1016/s0014-5793(03)00388-0. [DOI] [PubMed] [Google Scholar]
- Jakel RJ, Schneider BL, Svendsen CN. Using human neural stem cells to model neurological disease. Nature Reviews Genetics. 2004;5:136–144. doi: 10.1038/nrg1268. [DOI] [PubMed] [Google Scholar]
- Jamieson A, Wallace AM, Andrew R, Nunez BS, Walker BR, Fraser R, White PC, Connell JM. Apparent cortisone reductase deficiency: a functional defect in 11beta-hydroxysteroid dehydrogenase type 1. Journal of Clinical Endocrinology and Metabolism. 1999;84:3570–3574. doi: 10.1210/jcem.84.10.6031. [DOI] [PubMed] [Google Scholar]
- Jannasch A, Sedlak M, Adamec J. Quantification of pentose phosphate pathway (PPP) metabolites by liquid chromatography-mass spectrometry (LC-MS) Methods in Molecular Biology (Clifton, NJ) 2011;708:159–171. doi: 10.1007/978-1-61737-985-7_9. [DOI] [PubMed] [Google Scholar]
- Jeffries TWW, Jin YS. Metabolic engineering for improved fermentation of pentoses by yeasts. Applied Microbiology and Biotechnology. 2004;63:495–509. doi: 10.1007/s00253-003-1450-0. [DOI] [PubMed] [Google Scholar]
- Jeppsson M, Johansson B, Hahn-Hägerdal B, Gorwa-Grauslund MF. Reduced oxidative pentose phosphate pathway flux in recombinant xylose-utilizing Saccharomyces cerevisiae strains improves the ethanol yield from xylose. Applied and Environmental Microbiology. 2002;68:1604–1609. doi: 10.1128/AEM.68.4.1604-1609.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, Yang X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nature Cell Biology. 2011;13:310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen H, Nielsen J, Villadsen J, Møllgaard H. Metabolic flux distributions in Penicillium chrysogenum during fed-batch cultivations. Biotechnology and Bioengineering. 1995;46:117–131. doi: 10.1002/bit.260460205. [DOI] [PubMed] [Google Scholar]
- Jortzik E, Mailu BM, Preuss J, Fischer M, Bode L, Rahlfs S, Becker K. Glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase: a unique bifunctional enzyme from Plasmodium falciparum. Biochemical Journal. 2011;436:641–650. doi: 10.1042/BJ20110170. [DOI] [PubMed] [Google Scholar]
- Joshi S, Singh AR, Kumar A, Misra PC, Siddiqi MI, Saxena JK. Molecular cloning and characterization of Plasmodium falciparum transketolase. Molecular and Biochemical Parasitology. 2008;160:32–41. doi: 10.1016/j.molbiopara.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Juhnke H, Krems B, Kötter P, Entian KD. Mutants that show increased sensitivity to hydrogen peroxide reveal an important role for the pentose phosphate pathway in protection of yeast against oxidative stress. Molecular & General Genetics. 1996;252:456–464. doi: 10.1007/BF02173011. [DOI] [PubMed] [Google Scholar]
- Jung YM, Lee JN, Shin HD, Lee YH. Role of tktA gene in pentose phosphate pathway on odd-ball biosynthesis of poly-beta-hydroxybutyrate in transformant Escherichia coli harboring phbCAB operon. Journal of Bioscience and Bioengineering. 2004;98:224–227. doi: 10.1016/S1389-1723(04)00272-5. [DOI] [PubMed] [Google Scholar]
- Kabir MM, Shimizu K. Gene expression patterns for metabolic pathway in pgi knockout Escherichia coli with and without phb genes based on RT-PCR. Journal of Biotechnology. 2003;105:11–31. doi: 10.1016/s0168-1656(03)00170-6. [DOI] [PubMed] [Google Scholar]
- Kacser H. Recent developments beyond metabolic control analysis. Biochemical Society Transactions. 1995;23:387–391. doi: 10.1042/bst0230387. [DOI] [PubMed] [Google Scholar]
- Kamada N, Yasuhara A, Takano Y, Nakano T, Ikeda M. Effect of transketolase modifications on carbon flow to the purine-nucleotide pathway in Corynebacterium ammoniagenes. Applied Microbiology and Biotechnology. 2001;56:710–717. doi: 10.1007/s002530100738. [DOI] [PubMed] [Google Scholar]
- Kann O, Kovács R. Mitochondria and neuronal activity. American Journal of Physiology Cell Physiology. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- Kardon T, Stroobant V, Veiga-da-Cunha M, Schaftingen EV. Characterization of mammalian sedoheptulokinase and mechanism of formation of erythritol in sedoheptulokinase deficiency. FEBS Letters. 2008;582:3330–3334. doi: 10.1016/j.febslet.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science (New York) 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Katz J, Wood HG. The use of C14O2 yields from glucose-1- and -6-C14 for the evaluation of the pathways of glucose metabolism. Journal of Biological Chemistry. 1963;238:517–523. [PubMed] [Google Scholar]
- Kauffman FC, Brown JG, Passonneau JV, Lowry OH. Effects of changes in brain metabolism on levels of pentose phosphate pathway intermediates. Journal of Biological Chemistry. 1969;244:3647–3653. [PubMed] [Google Scholar]
- Kaur PK, Dinesh N, Soumya N, Babu NK, Singh S. Identification and characterization of a novel Ribose 5-phosphate isomerase B from Leishmania donovani. Biochemical and Biophysical Research Communications. 2012;421:51–56. doi: 10.1016/j.bbrc.2012.03.107. [DOI] [PubMed] [Google Scholar]
- Kawada M, Kagawa Y, Takiguchi H, Shimazono N. Purification of 6-phosphogluconolactonase from rat liver and yeast; its separation from gluconolactonase. Biochimica et Biophysica Acta. 1962;57:404–407. doi: 10.1016/0006-3002(62)91145-9. [DOI] [PubMed] [Google Scholar]
- Kayser G, Sienel W, Kubitz B, Mattern D, Stickeler E, Passlick B, Werner M, Zur Hausen A. Poor outcome in primary non-small cell lung cancers is predicted by transketolase TKTL1 expression. Pathology. 2011;43:719–724. doi: 10.1097/PAT.0b013e32834c352b. [DOI] [PubMed] [Google Scholar]
- Keller KE, Tan IS, Lee YS. SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science (New York) 2012;338:1069–1072. doi: 10.1126/science.1224409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MA, Turchyn AV, Ralser M. Non-enzymatic glycolysis and pentose phosphate pathway-like reactions in a plausible Archean ocean. Molecular Systems Biology. 2014:10. doi: 10.1002/msb.20145228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoven EJ, Achcar F, Alibu VP, Burchmore RJ, Gilbert IH, Trybiło M, Driessen NN, Gilbert D, Breitling R, Bakker BM, Barrett MP. Handling uncertainty in dynamic models: the pentose phosphate pathway in Trypanosoma brucei. PLoS Computational Biology. 2013;9:e1003371. doi: 10.1371/journal.pcbi.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MT, Passonneau JV, Veech RL. Radiometric measurement of phosphoribosylpyrophosphate and ribose 5-phosphate by enzymatic procedures. Analytical Biochemistry. 1990;187:179–186. doi: 10.1016/0003-2697(90)90438-f. [DOI] [PubMed] [Google Scholar]
- Kletzien RF, Harris PK, Foellmi LA. Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB Journal. 1994;8:174–181. doi: 10.1096/fasebj.8.2.8119488. [DOI] [PubMed] [Google Scholar]
- Kneidinger B, Graninger M, Puchberger M, Kosma P, Messner P. Biosynthesis of nucleotide-activated D-glycero-D-manno-heptose. Journal of Biological Chemistry. 2001;276:20935–20944. doi: 10.1074/jbc.M100378200. [DOI] [PubMed] [Google Scholar]
- Knowles JA, John W. Perfection in enzyme catalysis: the energetics of triosephosphate isomerase. Accounts of Chemical Research. 1977;10:105–111. [Google Scholar]
- Kochanowski K, Sauer U, Chubukov V. Somewhat in control-the role of transcription in regulating microbial metabolic fluxes. Current Opinion in Biotechnology. 2013;24:987–993. doi: 10.1016/j.copbio.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Kochetov GA, Sevostyanova IA. Binding of the coenzyme and formation of the transketolase active center. IUBMB Life. 2005;57:491–497. doi: 10.1080/15216540500167203. [DOI] [PubMed] [Google Scholar]
- Koek MM, Muilwijk B, van der Werf MJ, Hankemeier T. Microbial metabolomics with gas chromatography/mass spectrometry. Analytical Chemistry. 2006;78:1272–1281. doi: 10.1021/ac051683+. [DOI] [PubMed] [Google Scholar]
- Krczal D, Ritter H, Kömpf J. Polymorphism of glucose dehydrogenase (GDH, EC 1.1.1.47): formal and population genetic data. Human Genetics. 1993;91:290–292. doi: 10.1007/BF00218276. [DOI] [PubMed] [Google Scholar]
- Krüger A, Grüning NM, Wamelink MMC, Kerick M, Kirpy A, Parkhomchuk D, Bluemlein K, Schweiger MR, Soldatov A, Lehrach H, Jakobs C, Ralser M. The pentose phosphate pathway is a metabolic redox sensor and regulates transcription during the antioxidant response. Antioxidants & Redox Signaling. 2011;15:311–324. doi: 10.1089/ars.2010.3797. [DOI] [PubMed] [Google Scholar]
- Krüger A, Ralser M. ATM is a redox sensor linking genome stability and carbon metabolism. Science Signaling. 2011;4:pe17. doi: 10.1126/scisignal.2001959. [DOI] [PubMed] [Google Scholar]
- Kruger NJ, von Schaewen A. The oxidative pentose phosphate pathway: structure and organisation. Current Opinion in Plant Biology. 2003;6:236–246. doi: 10.1016/s1369-5266(03)00039-6. [DOI] [PubMed] [Google Scholar]
- Kugler W, Lakomek M. Glucose-6-phosphate isomerase deficiency. Best Practice & Research, Clinical Haematology. 2000;13:89–101. doi: 10.1053/beha.1999.0059. [DOI] [PubMed] [Google Scholar]
- Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, Deberardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia (New York) 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau BR, Wood HG. The pentose cycle in animal tissues: evidence for the classical and against the “L-type” pathway. Trends in Biochemical Sciences. 1983;8:296–297. [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle M, Drouin S, Robert F, Turcotte B. Oxidative stress-activated zinc cluster protein Stb5 has dual activator/repressor functions required for pentose phosphate pathway regulation and NADPH production. Molecular and Cellular Biology. 2006;26:6690–6701. doi: 10.1128/MCB.02450-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery GG, Walker EA, Tiganescu A, Ride JP, Shackleton CHL, Tomlinson JW, Connell JMC, Ray DW, Biason-Lauber A, Malunowicz EM, Arlt W, Stewart PM. Steroid biomarkers and genetic studies reveal inactivating mutations in hexose-6-phosphate dehydrogenase in patients with cortisone reductase deficiency. Journal of Clinical Endocrinology and Metabolism. 2008;93:3827–3832. doi: 10.1210/jc.2008-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc CA, Crouch EE, Wilson A, Lefkowitch J, Wamelink MMC, Jakobs C, Salomons GS, Sun X, Shen Y, Chung WK. Novel association of early onset hepatocellular carcinoma with transaldolase deficiency. JIMD Reports. 2013;12:121–127. doi: 10.1007/8904_2013_254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Godon C, Lagniel G, Spector D, Garin J, Labarre J, Toledano MB. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. Journal of Biological Chemistry. 1999;274:16040–16046. doi: 10.1074/jbc.274.23.16040. [DOI] [PubMed] [Google Scholar]
- Lee SM, Koh HJ, Park DC, Song BJ, Huh TJ, Park JW. Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radical Biology and Medicine. 2002;32:1185–1196. doi: 10.1016/s0891-5849(02)00815-8. [DOI] [PubMed] [Google Scholar]
- Lee JN, Shin HD, Lee YH. Metabolic engineering of pentose phosphate pathway in Ralstonia eutropha for enhanced biosynthesis of poly-beta-hydroxybutyrate. Biotechnology Progress. 2003;19:1444–1449. doi: 10.1021/bp034060v. [DOI] [PubMed] [Google Scholar]
- Lee WN, Boros LG, Puigjaner J, Bassilian S, Lim S, Cascante M. Mass isotopomer study of the nonoxidative pathways of the pentose cycle with [1,2-13C2]glucose. American Journal of Physiology. 1998;274(5 Pt 1):E843–E851. doi: 10.1152/ajpendo.1998.274.5.E843. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science (New York) 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR, Vokes NI, Feist AM, Vander Heiden MG, Metallo CM. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of Mammalian cells. Molecular Cell. 2014;55(2):253–263. doi: 10.1016/j.molcel.2014.05.008. Epub 29 May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Townsend CA. Rational strain improvement for enhanced clavulanic acid production by genetic engineering of the glycolytic pathway in Streptomyces clavuligerus. Metabolic Engineering. 2006;8:240–252. doi: 10.1016/j.ymben.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Lin ECC. Escherichia Coli and Salmonella: Cellular and Molecular Biology. 2. ASM Press; Washington: 1996. Dissimilatory pathways for sugars, polyols, and carboxylates; pp. 307–342. [Google Scholar]
- Linck A, Vu XK, Essl C, Hiesl C, Boles E, Oreb M. On the role of GAPDH isoenzymes during pentose fermentation in engineered. FEMS Yeast Research. 2014;14:389–398. doi: 10.1111/1567-1364.12137. [DOI] [PubMed] [Google Scholar]
- Lindqvist Y, Schneider G, Ermler U, Sundström M. Three-dimensional structure of transketolase, a thiamine diphosphate dependent enzyme, at 2.5 A resolution. EMBO Journal. 1992;11:2373–2379. doi: 10.1002/j.1460-2075.1992.tb05301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Huang D, McArthur DL, Boros LG, Nissen N, Heaney AP. Fructose induces transketolase flux to promote pancreatic cancer growth. Cancer Research. 2010;70:6368–6376. doi: 10.1158/0008-5472.CAN-09-4615. [DOI] [PubMed] [Google Scholar]
- Loeffen YGT, Biebuyck N, Wamelink MMC, Jakobs C, Mulder MF, Tylki-Szymańska A, Fung CW, Valayannopoulos V, Bökenkamp A. Nephrological abnormalities in patients with transaldolase deficiency. Nephrology, Dialysis, Transplantation - European Renal Association. 2012;27:3224–3227. doi: 10.1093/ndt/gfs061. [DOI] [PubMed] [Google Scholar]
- Longenecker JP, Williams JF. Quantitative measurement of the L-type pentose phosphate cycle with [2-14C]glucose and [5-14C]glucose in isolated hepatocytes. Biochemical Journal. 1980;188:859–865. doi: 10.1042/bj1880859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo L, Vanegas OC, Patel M, Rosti V, Li H, Waka J, Merghoub T, Pandolfi PP, Notaro R, Manova K, Luzzatto L. Maternally transmitted severe glucose 6-phosphate dehydrogenase deficiency is an embryonic lethal. EMBO Journal. 2002;21:4229–4239. doi: 10.1093/emboj/cdf426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JHC, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. Journal of Neuroscience. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Clasquin MF, Melamud E, Amador-Noguez D, Caudy AA, Rabinowitz JD. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Analytical Chemistry. 2010;82(8):3212–3221. doi: 10.1021/ac902837x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Groenke K, Takors R, Wandrey C, Oldiges M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography-mass spectrometry. Journal of Chromatography A. 2007;1147:153–164. doi: 10.1016/j.chroma.2007.02.034. [DOI] [PubMed] [Google Scholar]
- Luzzatto L, Bienzle U. The malaria/G.-6-P.D. hypothesis. Lancet. 1979;1:1183–1184. doi: 10.1016/s0140-6736(79)91857-9. [DOI] [PubMed] [Google Scholar]
- Luzzatto L, Mehta A. Glucose-6-phosphate dehydrogenase deficiency. In: Scriver CR, Beaudet AL, Sly WS, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 1995. pp. 3367–3398. [Google Scholar]
- Lyssiotis CA, Anastasiou D, Locasale JW, Vander Heiden MG, Christofk HR, Cantley LC. Cellular control mechanisms that regulate pyruvate kinase M2 activity and promote cancer growth. Biomedical Journal. 2012;23:213–217. [Google Scholar]
- Magistretti PJ. Synaptic plasticity and the Warburg effect. Cell Metabolism. 2014;19:4–5. doi: 10.1016/j.cmet.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Manganelli G, Fico A, Masullo U, Pizzolongo F, Cimmino A, Filosa S. Modulation of the pentose phosphate pathway induces endodermal differentiation in embryonic stem cells. PLoS ONE. 2012;7:e29321. doi: 10.1371/journal.pone.0029321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Marin-Valencia I, Cho SK, Rakheja D, Hatanpaa KJ, Kapur P, Mashimo T, Jindal A, Vemireddy V, Good LB, Raisanen J, Sun X, Mickey B, Choi C, Takahashi M, Togao O, et al. Glucose metabolism via the pentose phosphate pathway, glycolysis and Krebs cycle in an orthotopic mouse model of human brain tumors. NMR in Biomedicine. 2012a;25:1177–1186. doi: 10.1002/nbm.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, Cai L, Good L, Tu BP, Hatanpaa KJ, Mickey BE, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metabolism. 2012b;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I, Zhu J, Lin H, Bennett GN, San KY. Replacing Escherichia coli NAD-dependent glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with a NADP-dependent enzyme from Clostridium acetobutylicum facilitates NADPH dependent pathways. Metabolic Engineering. 2008;10:352–359. doi: 10.1016/j.ymben.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annual Review of Immunology. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- Marx A, Striegel K, de Graaf AA, Sahm H, Eggeling L. Response of the central metabolism of Corynebacterium glutamicum to different flux burdens. Biotechnology and Bioengineering. 1997;56:168–180. doi: 10.1002/(SICI)1097-0290(19971020)56:2<168::AID-BIT6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Masselot M, De Robichon-Szulmajster H. Methionine biosynthesis in Saccharomyces cerevisiae. I. Genetical analysis of auxotrophic mutants. Molecular & General Genetics. 1975;139:121–132. doi: 10.1007/BF00264692. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola-Baker H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014;14(5):592–605. doi: 10.1016/j.stem.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GM, Butler RN. Cellular mucosal defense during Helicobacter pylori infection: a review of the role of glutathione and the oxidative pentose pathway. Helicobacter. 2005;10:298–306. doi: 10.1111/j.1523-5378.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- Maugeri DA, Cazzulo JJ, Burchmore RJS, Barrett MP, Ogbunude POJ. Pentose phosphate metabolism in Leishmania mexicana. Molecular and Biochemical Parasitology. 2003;130:117–125. doi: 10.1016/s0166-6851(03)00173-7. [DOI] [PubMed] [Google Scholar]
- Mayr JA, Meierhofer D, Zimmermann F, Feichtinger R, Kögler C, Ratschek M, Schmeller N, Sperl W, Kofler B. Loss of complex I due to mitochondrial DNA mutations in renal oncocytoma. Clinical Cancer Research. 2008;14:2270–2275. doi: 10.1158/1078-0432.CCR-07-4131. [DOI] [PubMed] [Google Scholar]
- Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Seminars in Cancer Biology. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Meadows AL, Kong B, Berdichevsky M, Roy S, Rosiva R, Blanch HW, Clark DS. Metabolic and morphological differences between rapidly proliferating cancerous and normal breast epithelial cells. Biotechnology Progress. 2008;24:334–341. doi: 10.1021/bp070301d. [DOI] [PubMed] [Google Scholar]
- Meier S, Jensen PR, Duus JØ. Real-time detection of central carbon metabolism in living Escherichia coli and its response to perturbations. FEBS Letters. 2011a;585:3133–3138. doi: 10.1016/j.febslet.2011.08.049. [DOI] [PubMed] [Google Scholar]
- Meier S, Karlsson M, Jensen PR, Lerche MH, Duus JØ. Metabolic pathway visualization in living yeast by DNP-NMR. Molecular Biosystems. 2011b;7:2834–2836. doi: 10.1039/c1mb05202k. [DOI] [PubMed] [Google Scholar]
- Merkle S, Pretsch W. Characterization of triosephosphate isomerase mutants with reduced enzyme activity in Mus musculus. Genetics. 1989;123:837–844. doi: 10.1093/genetics/123.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshalkina LE, Drutsa VL, Koroleva ON, Solovjeva ON, Kochetov GA. Is transketolase-like protein, TKTL1, transketolase? Biochimica et Biophysica Acta. 2013;1832:387–390. doi: 10.1016/j.bbadis.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Meyerhof O, Beck LV. Triose phosphate isomerase. Journal of Biological Chemistry. 1944;156:109–120. [Google Scholar]
- Miclet E, Stoven V, Michels PA, Opperdoes FR, Lallemand JY, Duffieux F. NMR spectroscopic analysis of the first two steps of the pentose-phosphate pathway elucidates the role of 6-phosphogluconolactonase. Journal of Biological Chemistry. 2001;276:34840–34846. doi: 10.1074/jbc.M105174200. [DOI] [PubMed] [Google Scholar]
- Miosga T, Schaaff-Gerstenschlager I, Franken E, Zimmermann FK. Lysine144 is essential for the catalytic activity of Saccharomyces cerevisiae transaldolase. Yeast (Chichester, England) 1993;9:1241–1249. doi: 10.1002/yea.320091111. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser HW. Frequency of enzyme deficiency variants in erythrocytes of newborn infants. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:5046–5050. doi: 10.1073/pnas.78.8.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrenweiser HW, Wurzinger KH, Neel JV. Frequency and distribution of rare electrophoretic mobility variants in a population of human newborns in Ann Arbor, Michigan. Annals of Human Genetics. 1987;51:303–316. doi: 10.1111/j.1469-1809.1987.tb01065.x. [DOI] [PubMed] [Google Scholar]
- Morgan HP, O’Reilly FJ, Wear MA, O’Neill JR, Fothergill-Gilmore LA, Hupp T, Walkinshaw MD. M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5881–5886. doi: 10.1073/pnas.1217157110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morken TS, Brekke E, Håberg A, Widerøe M, Brubakk AM, Sonnewald U. Neuron-astrocyte interactions, pyruvate carboxylation and the pentose phosphate pathway in the neonatal rat brain. Neurochemical Research. 2014;39:556–569. doi: 10.1007/s11064-013-1014-3. [DOI] [PubMed] [Google Scholar]
- Mosberg JA, Yep A, Meredith TC, Smith S, Wang PF, Holler TP, Mobley HLT, Woodard RW. A unique arabinose 5-phosphate isomerase found within a genomic island associated with the uropathogenicity of Escherichia coli CFT073. Journal of Bacteriology. 2011;193:2981–2988. doi: 10.1128/JB.00033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S. Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Molecular Microbiology. 2004;53:1291–1305. doi: 10.1111/j.1365-2958.2004.04257.x. [DOI] [PubMed] [Google Scholar]
- Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Science Translational Medicine. 2013;5:198ra108. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, Lehninger AL, Cox MM. Principles of Biochemistry. London, United Kingdom: Macmillan; 2008. [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiological Reviews. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Birkholz S, Andersen LP, Moran AP. Neutrophil activation by Helicobacter pylori lipopolysaccharides. Journal of Infectious Diseases. 1994;170:135–139. doi: 10.1093/infdis/170.1.135. [DOI] [PubMed] [Google Scholar]
- Nish S, Medzhitov R. Host defense pathways: role of redundancy and compensation in infectious disease phenotypes. Immunity. 2011;34:629–636. doi: 10.1016/j.immuni.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells, Molecules & Diseases. 2009;42:267–278. doi: 10.1016/j.bcmd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Nogae I, Johnston M. Isolation and characterization of the ZWF1 gene of Saccharomyces cerevisiae, encoding glucose-6-phosphate dehydrogenase. Gene. 1990;96:161–169. doi: 10.1016/0378-1119(90)90248-p. [DOI] [PubMed] [Google Scholar]
- Novello F, McLean P. The pentose phosphate pathway of glucose metabolism. Measurement of the non-oxidative reactions of the cycle. Biochemical Journal. 1968;107:775–791. doi: 10.1042/bj1070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoura T, Takaki Y, Kakuta J, Nishi S, Sugahara J, Kazama H, Chee GJ, Hattori M, Kanai A, Atomi H, Takai K, Takami H. Insights into the evolution of Archaea and eukaryotic protein modifier systems revealed by the genome of a novel archaeal group. Nucleic Acids Research. 2011;39:3204–3223. doi: 10.1093/nar/gkq1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obst B, Wagner S, Sewing KF, Beil W. Helicobacter pylori causes DNA damage in gastric epithelial cells. Carcinogenesis. 2000;21:1111–1115. [PubMed] [Google Scholar]
- Ogawa T, Mori H, Tomita M, Yoshino M. Inhibitory effect of phosphoenolpyruvate on glycolytic enzymes in Escherichia coli. Research in Microbiology. 2007;158:159–163. doi: 10.1016/j.resmic.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Olin-Sandoval V, Moreno-Sánchez R, Saavedra E. Targeting trypanothione metabolism in trypanosomatid human parasites. Current Drug Targets. 2010;11:1614–1630. doi: 10.2174/1389450111009011614. [DOI] [PubMed] [Google Scholar]
- Orešič M, Hyötyläinen T, Herukka SK, Sysi-Aho M, Mattila I, Seppänan-Laakso T, Julkunen V, Gopalacharyulu PV, Hallikainen M, Koikkalainen J, Kivipelto M, Helisalmi S, Lötjönen J, Soininen H. Metabolome in progression to Alzheimer’s disease. Translational Psychiatry. 2011;1:e57. doi: 10.1038/tp.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz F, Olah J, Ovadi J, Oláh J, Ovádi J. Triosephosphate isomerase deficiency: new insights into an enigmatic disease. Biochimica et Biophysica Acta. 2009;1792:1168–1174. doi: 10.1016/j.bbadis.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Overkamp KM, Bakker BM, Kötter P, Luttik MAH, Van Dijken JP, Pronk JT. Metabolic engineering of glycerol production in Saccharomyces cerevisiae. Applied and Environmental Microbiology. 2002;68:2814–2821. doi: 10.1128/AEM.68.6.2814-2821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia DE, Valentine WN. Hereditary glucosephosphate isomerase deficiency. A review. American Journal of Clinical Pathology. 1974;62:740–751. doi: 10.1093/ajcp/62.6.740. [DOI] [PubMed] [Google Scholar]
- Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO Journal. 1995;14:5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil KR, Nielsen J. Uncovering transcriptional regulation of metabolism by using metabolic network topology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2685–2689. doi: 10.1073/pnas.0406811102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- Pedersen SN. The glycolytic enzyme activity of the human cervix uteri. Cancer. 1975;35:469–474. doi: 10.1002/1097-0142(197502)35:2<469::aid-cncr2820350226>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RM, Bardeesy N. Cancer: when antioxidants are bad. Nature. 2011;475:43–44. doi: 10.1038/475043a. [DOI] [PubMed] [Google Scholar]
- Perl A, Hanczko R, Telarico T, Oaks Z, Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends in Molecular Medicine. 2011;17:395–403. doi: 10.1016/j.molmed.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C, Dohnalek J, Gover S, Barrett MP, Adams MJ. A 2.8 A resolution structure of 6-phosphogluconate dehydrogenase from the protozoan parasite Trypanosoma brucei: comparison with the sheep enzyme accounts for differences in activity with coenzyme and substrate analogues. Journal of Molecular Biology. 1998;282:667–681. doi: 10.1006/jmbi.1998.2059. [DOI] [PubMed] [Google Scholar]
- Pollak N, Dölle C, Ziegler M. The power to reduce: pyridine nucleotides--small molecules with a multitude of functions. Biochemical Journal. 2007a;402:205–218. doi: 10.1042/BJ20061638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak N, Niere M, Ziegler M. NAD kinase levels control the NADPH concentration in human cells. Journal of Biological Chemistry. 2007b;282:33562–33571. doi: 10.1074/jbc.M704442200. [DOI] [PubMed] [Google Scholar]
- Preuss J, Jortzik E, Becker K. Glucose-6-phosphate metabolism in Plasmodium falciparum. IUBMB Life. 2012;64:603–611. doi: 10.1002/iub.1047. [DOI] [PubMed] [Google Scholar]
- Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721–733. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- Prigione A, Lichtner B, Kuhl H, Struys EA, Wamelink MMC, Lehrach H, Ralser M, Timmermann B, Adjaye J. Human induced pluripotent stem cells harbor homoplasmic and heteroplasmic mitochondrial DNA mutations while maintaining human embryonic stem cell-like metabolic reprogramming. Stem Cells. 2011;29:1338–1348. doi: 10.1002/stem.683. [DOI] [PubMed] [Google Scholar]
- Prigione A, Rohwer N, Hoffman S, Mlody B, Drews K, Bukowiecki R, Blümlein K, Wanker EE, Ralser M, Cramer T, Adjaye J. HIF1α modulates reprogramming through early glycolytic shift and up-regulation of PDK1-3 and PKM2. Stem Cells. 2013;32:364–376. doi: 10.1002/stem.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E. [29b] Sedoheptulose-1,7-diphosphatase from yeast. Methods in Enzymology. 1962;5:270–272. [Google Scholar]
- Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annual Review of Biochemistry. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines CA. The Calvin cycle revisited. Photosynthesis Research. 2003;75:1–10. doi: 10.1023/A:1022421515027. [DOI] [PubMed] [Google Scholar]
- Ralser M, Heeren G, Breitenbach M, Lehrach H, Krobitsch S. Triose phosphate isomerase deficiency is caused by altered dimerization--not catalytic inactivity--of the mutant enzymes. PLoS ONE. 2006;1:e30. doi: 10.1371/journal.pone.0000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralser M, Nebel A, Kleindorp R, Krobitsch S, Lehrach H, Schreiber S, Reinhardt R, Timmermann B. Sequencing and genotypic analysis of the triosephosphate isomerase (TPI1) locus in a large sample of long-lived Germans. BMC Genetics. 2008;9:38. doi: 10.1186/1471-2156-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralser M, Wamelink MMC, Kowald A, Gerisch B, Heeren G, Struys EA, Klipp E, Jakobs C, Breitenbach M, Lehrach H, Krobitsch S. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. Journal of Biology. 2007;6:10. doi: 10.1186/jbiol61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralser M, Wamelink MMC, Latkolik S, Jansen EEW, Lehrach H, Jakobs C. Metabolic reconfiguration precedes transcriptional regulation in the antioxidant response. Nature Biotechnology. 2009;27:604–605. doi: 10.1038/nbt0709-604. [DOI] [PubMed] [Google Scholar]
- Ramasarma T, Giri KV. Phosphoglucose isomerase of green gram (Phaseolus radiatus) Archives of Biochemistry and Biophysics. 1956;62:91–96. doi: 10.1016/0003-9861(56)90091-1. [DOI] [PubMed] [Google Scholar]
- Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nature Reviews Microbiology. 2009;7:333–340. doi: 10.1038/nrmicro2112. [DOI] [PubMed] [Google Scholar]
- Recktenwald CV, Kellner R, Lichtenfels R, Seliger B. Altered detoxification status and increased resistance to oxidative stress by K-ras transformation. Cancer Research. 2008;68:10086–10093. doi: 10.1158/0008-5472.CAN-08-0360. [DOI] [PubMed] [Google Scholar]
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radical Biology and Medicine. 2012;53:421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Rinnerthaler M, Büttner S, Laun P, Heeren G, Felder TK, Klinger H, Weinberger M, Stolze K, Grousl T, Hasek J, Benada O, Frydlova I, Klocker A, Simon-Nobbe B, Jansko B, et al. Yno1p/Aim14p, a NADPH-oxidase ortholog, controls extramitochondrial reactive oxygen species generation, apoptosis, and actin cable formation in yeast. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8658–8663. doi: 10.1073/pnas.1201629109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues TB, Serrao EM, Kennedy BWC, Hu DE, Kettunen MI, Brindle KM. Magnetic resonance imaging of tumor glycolysis using hyperpolarized (13)C-labeled glucose. Nature Medicine. 2014;20:93–97. doi: 10.1038/nm.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Almazán C, Arreola R, Rodríguez-Larrea D, Aguirre-López B, de Gómez-Puyou MT, Pérez-Montfort R, Costas M, Gómez-Puyou A, Torres-Larios A. Structural basis of human triosephosphate isomerase deficiency: mutation E104D is related to alterations of a conserved water network at the dimer interface. Journal of Biological Chemistry. 2008;283:23254–23263. doi: 10.1074/jbc.M802145200. [DOI] [PubMed] [Google Scholar]
- Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resistance Updates: Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy. 2011;14:191–201. doi: 10.1016/j.drup.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Rohwer N, Dame C, Haugstetter A, Wiedenmann B, Detjen K, Schmitt CA, Cramer T. Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS ONE. 2010;5:e12038. doi: 10.1371/journal.pone.0012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer N, Zasada C, Kempa S, Cramer T. The growing complexity of HIF-1α’s role in tumorigenesis: DNA repair and beyond. Oncogene. 2013;32:3569–3576. doi: 10.1038/onc.2012.510. [DOI] [PubMed] [Google Scholar]
- Ronchi JA, Figueira TR, Ravagnani FG, Oliveira HCF, Vercesi AE, Castilho RF. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6 J mice results in mitochondrial redox abnormalities. Free Radical Biology and Medicine. 2013;63:446–456. doi: 10.1016/j.freeradbiomed.2013.05.049. [DOI] [PubMed] [Google Scholar]
- Ros S, Santos CR, Moco S, Baenke F, Kelly G, Howell M, Zamboni N, Schulze A. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discovery. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- Rühl M, Rupp B, Nöh K, Wiechert W, Sauer U, Zamboni N. Collisional fragmentation of central carbon metabolites in LC-MS/MS increases precision of 13C metabolic flux analysis. Biotechnology and Bioengineering. 2012;109:763–771. doi: 10.1002/bit.24344. [DOI] [PubMed] [Google Scholar]
- Ruiz S, Panopoulos AD, Herrerías A, Bissig KD, Lutz M, Berggren WT, Verma IM, Izpisua Belmonte JC. A high proliferation rate is required for cell reprogramming and maintenance of human embryonic stem cell identity. Current Biology. 2011;21:45–52. doi: 10.1016/j.cub.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruwende C, Hill A. Glucose-6-phosphate dehydrogenase deficiency and malaria. Journal of Molecular Medicine (Berlin, Germany) 1998;76:581–588. doi: 10.1007/s001090050253. [DOI] [PubMed] [Google Scholar]
- Sable HZ. Pentose metabolism in extracts of yeast and mammalian tissues. Biochimica et Biophysica Acta. 1952;8:687–697. doi: 10.1016/0006-3002(52)90106-6. [DOI] [PubMed] [Google Scholar]
- Samland AK, Sprenger GA. Transaldolase: from biochemistry to human disease. International Journal of Biochemistry & Cell Biology. 2009;41:1482–1494. doi: 10.1016/j.biocel.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Sandoe J, Eggan K. Opportunities and challenges of pluripotent stem cell neurodegenerative disease models. Nature Neuroscience. 2013;16:780–789. doi: 10.1038/nn.3425. [DOI] [PubMed] [Google Scholar]
- Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, Stewart R, Hoare S, Stojkovic M, Armstrong L, von Zglinicki T, Lako M. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26:455–464. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- Sarkar M, Maganti L, Ghoshal N, Dutta C. In silico quest for putative drug targets in Helicobacter pylori HPAG1: molecular modeling of candidate enzymes from lipopolysaccharide biosynthesis pathway. Journal of Molecular Modeling. 2012;18:1855–1866. doi: 10.1007/s00894-011-1204-3. [DOI] [PubMed] [Google Scholar]
- Sarper N, Zengin E, Jakobs C, Salomons GS, Wamelink MMC, Ralser M, Kurt K, Kara B. Mild hemolytic anemia, progressive neuromotor retardation and fatal outcome: a disorder of glycolysis, triosephosphate isomerase deficiency. Turkish Journal of Pediatrics. 2013;55:198–202. [PubMed] [Google Scholar]
- Sauer U. Metabolic networks in motion: 13C-based flux analysis. Molecular Systems Biology. 2006;2:62. doi: 10.1038/msb4100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaff-Gerstenschlager I, Zimmermann FK. Pentose-phosphate pathway in Saccharomyces cerevisiae: analysis of deletion mutants for transketolase, transaldolase, and glucose 6-phosphate dehydrogenase. Current Genetics. 1993;24:373–376. doi: 10.1007/BF00351843. [DOI] [PubMed] [Google Scholar]
- Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R. NADP+–malate dehydrogenase in C3-plants: regulation and role of a light-activated enzyme. Physiologia Plantarum. 1987;71:393–400. [Google Scholar]
- Schenk G, Duggleby RG, Nixon PF. Properties and functions of the thiamin diphosphate dependent enzyme transketolase. International Journal of Biochemistry & Cell Biology. 1998;30:1297–1318. doi: 10.1016/s1357-2725(98)00095-8. [DOI] [PubMed] [Google Scholar]
- Schneider AS. Triosephosphate isomerase deficiency: historical perspectives and molecular aspects. Best Practice & Research, Clinical Haematology. 2000;13:119–140. doi: 10.1053/beha.2000.0061. [DOI] [PubMed] [Google Scholar]
- Schneider AS, Cohen-Solal M. Hematologically important mutations: triosephosphate isomerase. Blood Cells, Molecules & Diseases. 1996;22:82–84. doi: 10.1006/bcmd.1996.0011. [DOI] [PubMed] [Google Scholar]
- Schneider AS, Valentine WN, Hattori M, Heins HL. Hereditary haemolytic anemia with triosephosphate isomerase deficiency. New England Journal of Medicine. 1965;272:229–235. doi: 10.1056/NEJM196502042720503. [DOI] [PubMed] [Google Scholar]
- Schröter W, Eber SW, Bardosi A, Gahr M, Gabriel M, Sitzmann FC. Generalised glucosephosphate isomerase (GPI) deficiency causing haemolytic anaemia, neuromuscular symptoms and impairment of granulocytic function: a new syndrome due to a new stable GPI variant with diminished specific activity (GPI Homburg) European Journal of Pediatrics. 1985;144:301–305. doi: 10.1007/BF00441768. [DOI] [PubMed] [Google Scholar]
- Schwender J, Seemann M, Lichtenthaler HK, Rohmer M. Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green alga Scenedesmus obliquus. Biochemical Journal. 1996;316:73–80. doi: 10.1042/bj3160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senesi S, Csala M, Marcolongo P, Fulceri R, Mandl J, Banhegyi G, Benedetti A. Hexose-6-phosphate dehydrogenase in the endoplasmic reticulum. Biological Chemistry. 2010;391:1–8. doi: 10.1515/BC.2009.146. [DOI] [PubMed] [Google Scholar]
- Shalev O, Shalev RS, Forman L, Beutler E. GPI Mount Scopus--a variant of glucosephosphate isomerase deficiency. Annals of Hematology. 1993;67:197–200. doi: 10.1007/BF01695868. [DOI] [PubMed] [Google Scholar]
- Shenton D, Grant CM. Protein S-thiolation targets glycolysis and protein synthesis in response to oxidative stress in the yeast Saccharomyces cerevisiae. Biochemical Journal. 2003;374:513–519. doi: 10.1042/BJ20030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirin H, Pinto JT, Liu LU, Merzianu M, Sordillo EM, Moss SF. Helicobacter pylori decreases gastric mucosal glutathione. Cancer Letters. 2001;164:127–133. doi: 10.1016/s0304-3835(01)00383-4. [DOI] [PubMed] [Google Scholar]
- Silver I, Erecińska M. Oxygen and ion concentrations in normoxic and hypoxic brain cells. Advances in Experimental Medicine and Biology. 1998;454:7–16. doi: 10.1007/978-1-4615-4863-8_2. [DOI] [PubMed] [Google Scholar]
- Sipponen P, Hyvärinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scandinavian Journal of Gastroenterology Supplement. 1993;196:3–6. doi: 10.3109/00365529309098333. [DOI] [PubMed] [Google Scholar]
- Slekar KH, Kosman DJ, Culotta VC. The yeast copper/zinc superoxide dismutase and the pentose phosphate pathway play overlapping roles in oxidative stress protection. Journal of Biological Chemistry. 1996;271:28831–28836. doi: 10.1074/jbc.271.46.28831. [DOI] [PubMed] [Google Scholar]
- Smrcka AV, Jensen RG. HPLC separation and indirect ultraviolet detection of phosphorylated sugars. Plant Physiology. 1988;86:615–618. doi: 10.1104/pp.86.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga T. Capillary electrophoresis-mass spectrometry for metabolomics. Methods in Molecular Biology (Clifton, NJ) 2007;358:129–137. doi: 10.1007/978-1-59745-244-1_8. [DOI] [PubMed] [Google Scholar]
- Soucek T, Cumming R, Dargusch R, Maher P, Schubert D. The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid beta peptide. Neuron. 2003;39:43–56. doi: 10.1016/s0896-6273(03)00367-2. [DOI] [PubMed] [Google Scholar]
- Sprenger GA. Genetics of pentose-phosphate pathway enzymes of Escherichia coli K-12. Archives of Microbiology. 1995;164:324–330. doi: 10.1007/BF02529978. [DOI] [PubMed] [Google Scholar]
- Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64:362–369. doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern AL, Burgos E, Salmon L, Cazzulo JJ. Ribose 5-phosphate isomerase type B from Trypanosoma cruzi: kinetic properties and site-directed mutagenesis reveal information about the reaction mechanism. Biochemical Journal. 2007;401:279–285. doi: 10.1042/BJ20061049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel SA, Alibu VP, Hubert J, Ebikeme C, Portais JC, Bringaud F, Schweingruber ME, Barrett MP. Transketolase in Trypanosoma brucei. Molecular and Biochemical Parasitology. 2011;179:1–7. doi: 10.1016/j.molbiopara.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Stover NA, Dixon TA, Cavalcanti ARO. Multiple independent fusions of glucose-6-phosphate dehydrogenase with enzymes in the pentose phosphate pathway. PLoS ONE. 2011;6:e22269. doi: 10.1371/journal.pone.0022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susskind BM, Warren LG, Reeves RE. A pathway for the interconversion of hexose and pentose in the parasitic amoeba Entamoeba histolytica. Biochemical Journal. 1982;204:191–196. doi: 10.1042/bj2040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanepoel CC, Loots DT. The use of functional genomics in conjunction with metabolomics for Mycobacterium tuberculosis research. Disease Markers. 2014;2014:124218. doi: 10.1155/2014/124218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swezey RR. High-performance liquid chromatographic system for separating sugar phosphates and other intermediary metabolites. Journal of Chromatography B, Biomedical Applications. 1995;669:171–176. doi: 10.1016/0378-4347(95)00117-2. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Hori SH. Intramembraneous localization of rat liver microsomal hexose-6-phosphate dehydrogenase and membrane permeability to its substrates. Biochimica et Biophysica Acta. 1978;524:262–276. doi: 10.1016/0005-2744(78)90163-8. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tang JKH, You L, Blankenship RE, Tang YJ. Recent advances in mapping environmental microbial metabolisms through 13C isotopic fingerprints. Journal of the Royal Society, Interface/the Royal Society. 2012;9:2767–2780. doi: 10.1098/rsif.2012.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PL, Blakely KM, de Leon GP, Walker JR, McArthur F, Evdokimova E, Zhang K, Valvano MA, Wright GD, Junop MS. Structure and function of sedoheptulose-7-phosphate isomerase, a critical enzyme for lipopolysaccharide biosynthesis and a target for antibiotic adjuvants. Journal of Biological Chemistry. 2008;283:2835–2845. doi: 10.1074/jbc.M706163200. [DOI] [PubMed] [Google Scholar]
- Thomas D, Cherest H, Surdin-Kerjan Y. Identification of the structural gene for glucose-6-phosphate dehydrogenase in yeast. Inactivation leads to a nutritional requirement for organic sulfur. EMBO Journal. 1991;10:547–553. doi: 10.1002/j.1460-2075.1991.tb07981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science (New York, NY) 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Patel AB. Pyruvate carboxylase and pentose phosphate fluxes are reduced in AβPP-PS1 mouse model of Alzheimer’s disease: a 13C NMR study. Journal of Alzheimer’s Disease. 2014 doi: 10.3233/JAD-122449. [DOI] [PubMed] [Google Scholar]
- Todisco S, Agrimi G, Castegna A, Palmieri F. Identification of the mitochondrial NAD + transporter in Saccharomyces cerevisiae. Journal of Biological Chemistry. 2006;281:1524–1531. doi: 10.1074/jbc.M510425200. [DOI] [PubMed] [Google Scholar]
- Tosato V, Grüning NM, Breitenbach M, Arnak R, Ralser M, Bruschi CV. Warburg effect and translocation-induced genomic instability: two yeast models for cancer cells. Frontiers in Oncology. 2012;2:212. doi: 10.3389/fonc.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylki-Szymańska A, Stradomska TJ, Wamelink MMC, Salomons GS, Taybert J, Pawłowska J, Jakobs C. Transaldolase deficiency in two new patients with a relative mild phenotype. Molecular Genetics and Metabolism. 2009;97:15–17. doi: 10.1016/j.ymgme.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valayannopoulos V, Verhoeven NM, Mention K, Salomons GS, Sommelet D, Gonzales M, Touati G, de Lonlay P, Jakobs C, Saudubray JM. Transaldolase deficiency: a new cause of hydrops fetalis and neonatal multi-organ disease. Journal of Pediatrics. 2006;149:713–717. doi: 10.1016/j.jpeds.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Valvano MA, Messner P, Kosma P. Novel pathways for biosynthesis of nucleotide-activated glycero-manno-heptose precursors of bacterial glycoproteins and cell surface polysaccharides. Microbiology. 2002;148:1979–1989. doi: 10.1099/00221287-148-7-1979. [DOI] [PubMed] [Google Scholar]
- Vanamala J, Radhakrishnan S, Reddivari L, Bhat VB, Ptitsyn A. Resveratrol suppresses human colon cancer cell proliferation and induces apoptosis via targeting the pentose phosphate and the talin-FAK signaling pathways-A proteomic approach. Proteome Science. 2011;9:49. doi: 10.1186/1477-5956-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, Cantley LC. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Lunt SY, Dayton TL, Fiske BP, Israelsen WJ, Mattaini KR, Vokes NI, Stephanopoulos G, Cantley LC, Metallo CM, Locasale JW. Metabolic pathway alterations that support cell proliferation. Cold Spring Harbor Symposia on Quantitative Biology. 2011;76:325–334. doi: 10.1101/sqb.2012.76.010900. [DOI] [PubMed] [Google Scholar]
- Van der Knaap MS, Wevers RA, Struys EA, Verhoeven NM, Pouwels PJ, Engelke UF, Feikema W, Valk J, Jakobs C. Leukoencephalopathy associated with a disturbance in the metabolism of polyols. Annals of Neurology. 1999;46:925–928. doi: 10.1002/1531-8249(199912)46:6<925::aid-ana18>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Van Zwieten R, Verhoeven AJ, Roos D. Inborn defects in the antioxidant systems of human red blood cells. Free Radical Biology and Medicine. 2014;67:377–386. doi: 10.1016/j.freeradbiomed.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CA, Ramalho-Santos J, Van Houten B, Schatten G. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE. 2011;6:e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nature Reviews Drug Discovery. 2008;7:979–987. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- Veitch NJ, Maugeri DA, Cazzulo JJ, Lindqvist Y, Barrett MP. Transketolase from Leishmania mexicana has a dual subcellular localization. Biochemical Journal. 2004;382:759–767. doi: 10.1042/BJ20040459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti P, Napolitano G, Di Meo S. Role of enzymatic and non-enzymatic processes in H2O2 removal by rat liver and heart mitochondria. Journal of Bioenergetics and Biomembranes. 2013;46:83–91. doi: 10.1007/s10863-013-9534-8. [DOI] [PubMed] [Google Scholar]
- Verho R, Richard P, Jonson PH, Sundqvist L, Londesborough J, Penttilä M. Identification of the first fungal NADP-GAPDH from Kluyveromyces lactis. Biochemistry. 2002;41:13833–13838. doi: 10.1021/bi0265325. [DOI] [PubMed] [Google Scholar]
- Verhoeven NM, Huck JH, Roos B, Struys EA, Salomons GS, Douwes AC, van der Knaap MS, Jakobs C. Transaldolase deficiency: liver cirrhosis associated with a new inborn error in the pentose phosphate pathway. American Journal of Human Genetics. 2001;68:1086–1092. doi: 10.1086/320108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondria and cancer. Nature Reviews Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamelink MMC, Grüning NM, Jansen EEW, Bluemlein K, Lehrach H, Jakobs C, Ralser M. The difference between rare and exceptionally rare:molecular characterization of ribose 5-phosphate isomerase deficiency. Journal of Molecular Medicine. 2010;88:931–939. doi: 10.1007/s00109-010-0634-1. [DOI] [PubMed] [Google Scholar]
- Wamelink MMC, Smith DE, Jansen EE, Verhoeven NM, Struys EA, Jakobs C. Detection of transaldolase deficiency by quantification of novel seven-carbon chain carbohydrate biomarkers in urine. Journal of Inherited Metabolic Disease. 2007;30:735–742. doi: 10.1007/s10545-007-0590-2. [DOI] [PubMed] [Google Scholar]
- Wamelink MMC, Struys EA, Huck JHJ, Roos B, van der Knaap MS, Jakobs C, Verhoeven NM. Quantification of sugar phosphate intermediates of the pentose phosphate pathway by LC-MS/MS: application to two new inherited defects of metabolism. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2005;823:18–25. doi: 10.1016/j.jchromb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Wamelink MMC, Struys EA, Jakobs C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. Journal of Inherited Metabolic Disease. 2008a;31:703–717. doi: 10.1007/s10545-008-1015-6. [DOI] [PubMed] [Google Scholar]
- Wamelink MMC, Struys EA, Jansen EEW, Levtchenko EN, Zijlstra FSM, Engelke U, Blom HJ, Jakobs C, Wevers RA. Sedoheptulokinase deficiency due to a 57-kb deletion in cystinosis patients causes urinary accumulation of sedoheptulose: elucidation of the CARKL gene. Human Mutation. 2008b;29:532–536. doi: 10.1002/humu.20685. [DOI] [PubMed] [Google Scholar]
- Wang L, Xie J, Schultz PG. Expanding the genetic code. Annual Review of Biophysics and Biomolecular Structure. 2006;35:225–249. doi: 10.1146/annurev.biophys.35.101105.121507. [DOI] [PubMed] [Google Scholar]
- Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio ALB, Dias SMG, Dang CV, Cerione RA. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Quinn PJ. Lipopolysaccharide: biosynthetic pathway and structure modification. Progress in Lipid Research. 2010;49:97–107. doi: 10.1016/j.plipres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Wang YP, Zhou LS, Zhao YZ, Wang SW, Chen LL, Liu LX, Ling ZQ, Hu FJ, Sun YP, Zhang JY, Yang C, Yang Y, Xiong Y, Guan KL, Ye D. Regulation of G6PD acetylation by KAT9/SIRT2 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO Journal. 2014 doi: 10.1002/embj.201387224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanka C, Steinbach JP, Rieger J. Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. Journal of Biological Chemistry. 2012;287:33436–33446. doi: 10.1074/jbc.M112.384578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warburg O, Christian W. Optischer Nachweis der Hydrierung und Dehydrierung des Pyridins im Gärungs-Co-Ferment. Biochemische Zeitschrift. 1936;286:81. [Google Scholar]
- Warburg O, Christian W, Griese A. Wasserstoff{ü}bertragendes Co-Ferment, seine Zusammensetzung und Wirkungsweise. Biochemische Zeitschrift. 1935;282:157–205. [Google Scholar]
- Warburg O, Cristian W. Isolierung u. Kristallisation des Proteins des oxydierenden G{ä}rungsferments. Biochemische Zeitschrift. 1939;303:40. [Google Scholar]
- Watanabe M, Zingg BC, Mohrenweiser HW. Molecular analysis of a series of alleles in humans with reduced activity at the triosephosphate isomerase locus. American Journal of Human Genetics. 1996;58:308–316. [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Gadian DG, Vargha-Khadem F. Functional and structural brain abnormalities associated with a genetic disorder of speech and language. American Journal of Human Genetics. 1999;65:1215–1221. doi: 10.1086/302631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel KE, Mor JR, Fiechter A. Rapid sampling of yeast cells and automated assays of adenylate, citrate, pyruvate and glucose-6-phosphate pools. Analytical Biochemistry. 1974;58:208–216. doi: 10.1016/0003-2697(74)90459-x. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. Coming full circle-from endless complexity to simplicity and back again. Cell. 2014;157:267–271. doi: 10.1016/j.cell.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GRS, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermuth B, Münch JD, von Wartburg JP. Purification and properties of NADPH-dependent aldehyde reductase from human liver. Journal of Biological Chemistry. 1977;252:3821–3828. [PubMed] [Google Scholar]
- Williams JF, Clark MG, Arora KK, Reichstein IC. Glucose 6-phosphate formation by L-type pentose phosphate pathway reactions of rat liver in vitro: further evidence. Hoppe-Seyler’s Zeitschriftfür Physiologische Chemie. 1984;365:1425–1434. doi: 10.1515/bchm2.1984.365.2.1425. [DOI] [PubMed] [Google Scholar]
- Won KY, Lim SJ, Kim GY, Kim YW, Han SA, Song JY, Lee DK. Regulatory role of p53 in cancer metabolism via SCO2 and TIGAR in human breast cancer. Human Pathology. 2012;43:221–228. doi: 10.1016/j.humpath.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Wood T. The Pentose Phosphate Pathway. Academic Press; Orlando, Florida (US): 1985. [Google Scholar]
- Wu S, Le H. Dual roles of PKM2 in cancer metabolism. Acta Biochimica et Biophysica Sinica. 2013;45:27–35. doi: 10.1093/abbs/gms106. [DOI] [PubMed] [Google Scholar]
- Xu X, Duan S, Yi F, Ocampo A, Liu GH, Izpisua Belmonte JC. Mitochondrial regulation in pluripotent stem cells. Cell Metabolism. 2013;18:325–332. doi: 10.1016/j.cmet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Takano N, Ishiwata K, Ohmura M, Nagahata Y, Matsuura T, Kamata A, Sakamoto K, Nakanishi T, Kubo A, Hishiki T, Suematsu M. Reduced methylation of PFKFB3 in cancer cells shunts glucose towards the pentose phosphate pathway. Nature Communications. 2014;5:3480. doi: 10.1038/ncomms4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanes O, Clark J, Wong DM, Patti GJ, Sánchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G. Metabolic oxidation regulates embryonic stem cell differentiation. Nature Chemical Biology. 2010;6:411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Sedlak M, Regnier FE, Mosier N, Ho N, Adamec J. Simultaneous quantification of metabolites involved in central carbon and energy metabolism using reversed-phase liquid chromatography-mass spectrometry and in vitro 13C labeling. Analytical Chemistry. 2008;80:9508–9516. doi: 10.1021/ac801693c. [DOI] [PubMed] [Google Scholar]
- Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, Peters EC, Driggers E, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W. NAD+ and NADH in neuronal death. Journal of Neuroimmune Pharmacology. 2007;2:270–275. doi: 10.1007/s11481-007-9063-5. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Yuneva MO, Fan TWM, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Matés JM, Alonso FJ, Wang C, Seo Y, Chen X, Bishop JM. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metabolism. 2012;15:157–170. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelières FP, Marco S, Saudou F. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Zampella EJ, Bradley EL, Pretlow TG. Glucose-6-phosphate dehydrogenase: a possible clinical indicator for prostatic carcinoma. Cancer. 1982;49:384–387. doi: 10.1002/1097-0142(19820115)49:2<384::aid-cncr2820490229>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Zanella A, Bianchi P. Red cell pyruvate kinase deficiency: from genetics to clinical manifestations. Best Practice&Research, Clinical Haematology. 2000;13:57–81. doi: 10.1053/beha.1999.0057. [DOI] [PubMed] [Google Scholar]
- Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 2012;11:589–595. doi: 10.1016/j.stem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Mancuso A, Bui TV, Tong X, Gruber JJ, Swider CR, Sanchez PV, Lum JJ, Sayed N, Melo JV, Perl AE, Carroll M, Tuttle SW, Thompson CB. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1alpha-induced metabolic reprograming. Oncogene. 2010;29:2962–2972. doi: 10.1038/onc.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Pease AJ, Bharani N, Winkler ME. Biochemical characterization of gapB-encoded erythrose 4-phosphate dehydrogenase of Escherichia coli K-12 and its possible role in pyridoxal 5′-phosphate biosynthesis. Journal of Bacteriology. 1995;177(10):2804–2812. doi: 10.1128/jb.177.10.2804-2812.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CF, Li XB, Sun H, Zhang B, Han YS, Jiang Y, Zhuang QL, Fang J, Wu GH. Pyruvate kinase type M2 is upregulated in colorectal cancer and promotes proliferation and migration of colon cancer cells. IUBMB Life. 2012;64:775–782. doi: 10.1002/iub.1066. [DOI] [PubMed] [Google Scholar]
- Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer HG. The oxidative pentose phosphate pathway in the heart: regulation, physiological significance, and clinical implications. Basic Research in Cardiology. 1992;87:303–316. doi: 10.1007/BF00796517. [DOI] [PubMed] [Google Scholar]
- Zimmer HG. eLS. John Wiley & Sons, Ltd; Hoboken, NJ, US: 2001. Pentose phosphate pathway. [Google Scholar]