Abstract
Context
Approximately 30% of women report pain in the affected breast prior to breast cancer surgery.
Objectives
The purpose of this secondary analysis of our prospective study was to determine how women who experienced both preoperative and persistent postsurgical breast pain (n=107) differed from women who did not report preoperative breast pain and did (n=158) or did not (n=122) experience persistent postsurgical breast pain.
Methods
Differences in demographic and clinical characteristics were evaluated. Linear mixed effects (LME) modeling was used to evaluate for group differences in symptom severity, function, sensation, and quality of life (QOL) over time.
Results
Between-group differences in demographic and clinical characteristics as well as trajectories of shoulder function and QOL were identified. Women with both preoperative and persistent postsurgical breast pain were younger; were more likely to report swelling, strange sensations, hardness, and numbness in the affected breast prior to surgery; and were more likely to have reconstruction at the time of surgery. Women with both preoperative and persistent postsurgical breast pain had more biopsies in the prior year, more lymph nodes removed, and reported more severe acute postsurgical pain than women without preoperative breast pain. LME modeling revealed significant group effects for the majority of outcomes evaluated. Over the six months of the study, women with both preoperative and persistent postsurgical pain had persistently poorer shoulder flexion and physical well-being than women without preoperative breast pain.
Conclusion
Investigations of the etiology and molecular mechanisms of preoperative breast pain, as well as interventions for this high risk group, are needed.
Keywords: breast cancer, preoperative pain, persistent postsurgical pain, linear modeling
Introduction
In patients with breast cancer, the prevalence of pain in the affected breast prior to surgery is approximately 30%.1-3 However, little is known about how the occurrence of preoperative breast pain influences postoperative and longer-term outcomes in these patients. Preexisting or preoperative pain was identified as an important predictor of both acute and persistent pain following various types of surgery, including breast cancer surgery.4 Indeed, as noted by Katz and Seltzer,4 it seems quite logical that “no other patient factor is as consistently related to the development of future pain problems as is pain itself” (p. 728). The determination of how patients with preoperative pain differ from their pain-free counterparts in terms of preoperative demographic and clinical characteristics, as well as postsurgical outcomes (e.g., other symptoms, physical function, sensations, quality of life [QOL]) would assist with the identification of potentially modifiable risk factors and potential targets for preoperative interventions and rehabilitation outcomes.
A number of studies evaluated preoperative pain as a predictor of persistent postsurgical pain following breast cancer surgery.1,5-9 Most of these studies demonstrated a significant association between preoperative pain and the development or severity of persistent pain several months to a year following surgery.5-9 However, the main objective of these studies was to identify risk factors for the development of persistent postsurgical pain, rather than to describe the association between preoperative breast pain and other clinically relevant patient outcomes in the first six months following breast cancer surgery.
Recently, our group found that 28% of women reported pain in their affected breast prior to breast cancer surgery.2 This pain, although generally mild, interfered with daily activities approximately three days a week. Pain was described as “tender,” “dull,” and “aching,” and interfered with patients' sleep and mood.2 Preoperative breast pain was associated with higher depressive symptom scores and poorer physical well-being.10 The occurrence of preoperative breast pain was associated with more severe acute postoperative pain (in preparation) and severe persistent postsurgical pain.8
In this same sample of patients, growth mixture modeling (GMM) was used to identify subgroups (i.e., latent classes) of women with distinct trajectories of worst breast pain ratings from prior to through six months following breast cancer surgery.8 After exclusion of women who reported no breast pain at any of the assessments (No Pain = 31.7%), three distinct latent classes were identified (i.e., Mild Pain = 43.4%; Moderate Pain = 13.3%; Severe Pain = 11.6%).8 The results of this longitudinal analysis provided an opportunity to determine how women who experienced preoperative breast pain differed from those who did not experience this pain condition, and who did or did not go on to develop persistent postsurgical breast pain (i.e., Mild, Moderate, Severe latent classes).
Therefore, the purpose of this study, in a sample of women who underwent unilateral breast cancer surgery, was to identify differences in demographic and clinical characteristics among women who: 1) experienced neither preoperative breast pain nor persistent postsurgical breast pain; 2) did not experience preoperative breast pain, but did experience persistent postsurgical breast pain; and 3) experienced both preoperative breast pain and persistent postsurgical breast pain. Additional objectives were to determine whether differences existed over time among the three pain groups in symptom severity (i.e., depressive symptoms, state anxiety, sleep disturbance, fatigue, energy), physical function (i.e., grip strength, shoulder mobility), sensations in the breast scar site, and QOL (i.e., overall QOL and specific dimensions of QOL [physical, psychological, social, spiritual]).
Methods
Patients and Settings
This is a secondary analysis of a larger prospective, longitudinal study that evaluated neuropathic pain and lymphedema in women undergoing breast cancer surgery, which is described in detail elsewhere.2,8,11 These methods are abbreviated and include only those measures that are pertinent to this analysis. Patients were recruited from seven breast care centers including a Comprehensive Cancer Center, two public hospitals, and four community practices.
Women were eligible to participate if they: were 18 years of age or older; were scheduled to undergo unilateral breast cancer surgery; were able to read, write, and understand English; agreed to participate; and gave written informed consent. Patients were excluded if they were having breast cancer surgery on both breasts and/or had distant metastasis. A total of 516 patients met these criteria and were approached to participate; 410 were enrolled (response rate 79.5%) and 398 completed the study.
Measures
Demographic and Clinical Measures
The demographic questionnaire obtained information on age, marital status, education, ethnicity, employment status, living situation, and financial status. Patients completed the Karnofsky Performance Status (KPS) scale, which is widely used to evaluate functional status in patients with cancer and has well-established validity and reliability.12,13
The Self-Administered Comorbidity Questionnaire (SCQ) comprises 13 common medical conditions.14 Patients were asked to indicate if they had the condition; if they received treatment for it; and whether the condition limited their activities. The SCQ has well-established validity and reliability.14,15
Breast Pain Measures
Preoperative and persistent postsurgical breast pain was evaluated using the Breast Symptoms Questionnaire (BSQ). The BSQ consists of two parts. Part 1 obtained information on the occurrence of pain and the occurrence of other symptoms in the affected breast (i.e., swelling, numbness, strange sensations, hardness). In Part 2, patients were asked to rate the intensity of their average and worst pain, in the past week, using a numeric rating scale (NRS) that ranged from 0 (no pain) to 10 (worst imaginable pain). The NRS is a valid and reliable measure of pain intensity.16 Postsurgical pain was evaluated using the Postsurgical Pain Questionnaire. Patients were asked to rate average and worst pain intensity, using a 0 to 10 NRS, in the first 24 to 48 hours after surgery. This questionnaire was completed during the month 1 study visit.
Symptom Measures
State and trait anxiety were measured using the Spielberger State-Trait Anxiety Inventories (STAI-S and STAI-T) that consist of 20 items, each rated from 1 to 4. Total scores for each scale can range from 20 to 80, with higher scores indicating greater anxiety. The STAI-T measures a person's predisposition to anxiety and estimates how a person generally feels. The STAI-S measures an individual's transitory emotional response, with items assessing worry, nervousness, tension, and apprehension related to how a person feels “right now.” Scores ≥31.8 and ≥32.2 suggest higher levels of trait and state anxiety, respectively.17.18
Depressive symptoms were measured using the Center for Epidemiologic Studies-Depression (CES-D) scale that comprises 20 items representing the major symptoms in the clinical syndrome of depression. Scores can range from 0 to 60, with scores of 16 or greater indicating the need for clinical evaluation for major depression.19,20
The General Sleep Disturbance Scale (GSDS) consists of 21 items that assess the quality of sleep during the previous week.21 Each item is rated on a 0 (never) to 7 (everyday) NRS. The GSDS total score is the sum of the 21 items and ranges from 0 (no disturbance) to 147 (extreme sleep disturbance). A GSDS total score of 43 or greater differentiates poor from good sleepers.21
The Lee Fatigue Scale (LFS) contains 18 items designed to assess physical fatigue and energy.22 Each item is rated on a 0 to 10 NRS. Total fatigue and energy scores were calculated as the mean of the 13 fatigue items and the five energy items, with higher scores indicating greater fatigue severity and higher levels of energy. Scores of ≥4.4 and ≤4.8 indicate clinically meaningful levels of fatigue and decrements in energy, respectively.
Physical Measure
Grip strength in kilograms (kg), in both hands, was measured using a Jamar hydraulic hand dynamometer (Patterson Medical, Bolingbrook, IL). This measure was used to evaluate for changes in muscle strength in women following breast cancer surgery.3 Grip strength was measured three times in each hand and averaged over the three measurements, using the procedure described by Spijkerman and colleagues.23
Shoulder mobility was assessed using goniometric measurements of range of motion (ROM). While the patient was lying supine, ROM was measured, in degrees, twice on each side in four positions (i.e., flexion, abduction, internal rotation, external rotation). For each position, the two measurements were averaged.
Sensitivity in the breast scar area was tested at four to eight sites around the length of the scar, using a 5.07 gram monofilament and compared to the corresponding area on the unaffected side. For each site tested, patients reported whether it was “much less sensitive than the opposite side,” “same as the opposite side,” or “much more sensitive than the opposite side.” The percentages of the total number of sites on the affected side that were classified as “much less,” “same,” and “much more” were calculated.
Quality of Life Measure
The Quality of Life Scale – Patient Version (QOL-PV) is a 41-item instrument that measures four dimensions of QOL in cancer patients (i.e., physical well-being, psychological well-being, spiritual well-being, social well-being) as well as a total QOL score. Each item is rated on a 0 to 10 NRS, with higher scores indicating a better QOL. The QOL-PV has established validity and reliability.24,25
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the Institutional Review Boards at each of the study sites. During the patient's preoperative visit, a clinician explained the study to the patient, determined her willingness to participate, and introduced the patient to the research nurse. After providing written informed consent, patients completed the enrollment questionnaires (Assessment 0). Then, the research nurse performed the following objective measures: height, weight, grip strength, and shoulder mobility.
The research nurse met with the patient either in their home or in the Clinical Research Center at 1, 2, 3, 4, 5, and 6 months after surgery. During each of the study visits, the women completed the study questionnaires, provided information on new and ongoing treatments, and had the objective measures done by the research nurse. Over the course of the study, patients' medical records were reviewed for disease and treatment information. Every six months, the inter-rater reliability among the research nurses for each of the objective measures was evaluated and found to exceed 0.80.
Characterization of the Persistent Breast Pain Classes
Details of the GMM analysis that was used to characterize the persistent breast pain classes were reported previously.8 In brief, at each assessment, patients were asked, “Are you experiencing pain in your affected breast?” If the patient reported pain, she was asked to rate her worst pain during the previous week using a 0 (no pain) to 10 (worst pain imaginable) NRS. Prior to conducting the GMM analysis, patients who reported no pain in their affected breast for all seven assessments (i.e., prior to surgery and at 1, 2, 3, 4, 5, and 6 months) were identified (n=126; 31.7%) and were not included in the GMM analysis. For the remaining 272 women, six ratings of worst breast pain (i.e., prior to surgery, and 2, 3, 4, 5, and 6 months) were used in the GMM analysis to assign each patient into a latent class. Pain ratings obtained at the one-month assessment were excluded from the model because of the high prevalence of pain and reduced variability in pain ratings. The GMM analysis was done using Mplus 6.1.26 In addition to the No Pain group, three persistent pain classes were identified: Mild Pain (n=173; 43.4%; (NRS of ∼3 that remained constant for six months), Moderate Pain (n=53; 13.3%; NRS of ∼2 that increased over six months), and Severe Pain (n=46; 11.6%; NRS of ∼8 that persisted for six months).8
Characterization of Breast Pain Comparison Groups
The breast pain comparison groups analyzed in the current study were characterized by the occurrence of preoperative breast pain in addition to their latent class assignment. Specifically, women who reported no pain in the affected breast prior to surgery and who were excluded from GMM analyses because they did not report pain at any of the six assessments, were characterized as the “ neither preoperative nor persistent postsurgical breast pain” group (Group 0; n=122; 31.5%). Women who reported no pain in the affected breast prior to surgery and who were classified as belonging to one of the breast pain latent classes (i.e., Mild, Moderate, Severe) were characterized as the “no preoperative and persistent postsurgical breast pain” group (Group 1; n=158; 40.8%). Finally, women who experienced preoperative breast pain and were classified as belonging to one of the breast pain latent classes were characterized as the “both preoperative and persistent breast pain” group (Group 2; n=107; 27.6%).
Statistical Analyses
Data were analyzed using SPSS version 21.0 (SPSS, Inc./IBM Corp., Armonk, NY). Descriptive statistics and frequency distributions were calculated for patients' demographic and clinical characteristics and for preoperative symptom severity and QOL scores. One-way analyses of variance (ANOVA) with Bonferroni post hoc comparisons, Kruskal-Wallis (with Mann-Whitney U for pairwise Bonferroni post hoc comparisons), and Chi-square tests (with Fisher's exact tests for pairwise Bonferroni post hoc comparisons) were performed to evaluate for differences among the groups.
Linear mixed effects models fit by restricted maximum likelihood estimation (REML) were evaluated to determine if any differences existed over time among the breast pain groups with respect to: symptom severity (i.e., state anxiety, depressive symptoms, sleep disturbance, fatigue, energy); grip strength and shoulder mobility (i.e., flexion, abduction, internal/external rotation); sensitivity in the breast scar area (i.e., percentage of sites in the breast scar area that were more, the same, or less sensitive than the unaffected breast); and QOL (i.e., total QOL, physical well-being, psychological well-being, spiritual well-being, social well-being). The tests of Group × Time interactions determined whether changes over time in any of these outcomes were significantly different among the breast pain groups. In addition, main effects of group (differences among the groups) and main effects of time (changes over time across the groups) were evaluated for significance using mixed-model tests of main effects.
Results
Differences in Demographic and Clinical Characteristics Among the Breast Pain Groups
The only demographic characteristic that differed among the breast pain groups was age (Table 1). Women with both preoperative and persistent breast pain were significantly younger than women without preoperative breast pain who developed persistent postsurgical breast pain. In addition, women without preoperative breast pain who developed persistent postsurgical breast pain were significantly younger than women with no preoperative or persistent postsurgical breast pain.
Table 1.
Differences in Demographic Characteristics Among Women with No Preoperative and No Persistent Breast Pain (Group 0), Women with No Preoperative Breast Pain Who Developed Persistent Breast Pain (Group 1), and Women with Both Preoperative and Persistent Breast Pain (Group 2).
| Characteristic | No Preoperative and No Persistent Breast Pain Group 1 n=122 (31.5%) | No Preoperative Breast Pain and Persistent Breast Pain Group 1 n=158 (40.8%) | Preoperative and Persistent Breast Pain Group 2 n=107 (27.6%) | Statistics |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| ||||
| Age (yrs) | 58.7 (11.2) | 54.8 (11.9) | 50.6 (9.5) | F=15.2, P<0.001 2<1<0 |
|
| ||||
| Education (years) | 15.8 (2.8) | 15.8 (2.6) | 15.4 (2.6) | F=0.9, P=0.426 |
|
| ||||
| % (N) | % (N) | % (N) | ||
|
| ||||
| Ethnicity | ||||
| White | 74.6 (91) | 63.1 (99) | 55.7 (59) | X2=11.8, P=0.066 |
| Black | 5.7 (7) | 8.3 (13) | 15.1 (16) | |
| Asian/Pacific Islander | 9.8 (12) | 13.4 (21) | 15.1 (16) | |
| Hispanic/Mixed/Other | 9.8 (12) | 15.3 (24) | 14.2 (15) | |
|
| ||||
| Lives alone | 20.7 (25) | 26.8 (42) | 25.0 (26) | X2=1.4, P=0.494 |
|
| ||||
| Marital status | ||||
| Married/partnered | 40.2 (49) | 43.3 (68) | 41.3 (43) | X2=0.3, P=0.865 |
| Single, separated, widowed, divorced | 59.8 (73) | 56.7 (89) | 58.7 (61) | |
|
| ||||
| Currently working for pay | 52.9 (64) | 44.9 (70) | 50.5 (54) | X2=1.9, P=0.389 |
|
| ||||
| Total annual household income | ||||
| <$10,000 to $29,999 | 11.8 (12) | 24.2 (31) | 28.4 (25) | X2=9.1, P=0.059 |
| $30,000 to $99,999 | 43.1 (44) | 39.8 (51) | 36.4 (32) | |
| ≥$100,000 45.1 (46) | 45.1 (46) | 35.9 (46) | 35.2 (31) | |
SD = standard deviation; X2 = Chi-square statistic.
A number of preoperative and perioperative characteristics differed among the breast pain groups (Table 2). Women with persistent postsurgical pain, regardless of whether they experienced preoperative breast pain, had significantly lower functional status (KPS) scores than women with neither preoperative nor persistent postsurgical breast pain. In addition, women with both preoperative and persistent postsurgical breast pain were less likely to be postmenopausal; had a higher number of breast biopsies in the past year; had more lymph nodes removed; were significantly more likely to experience swelling, numbness, hardness, and strange sensations in the breast preoperatively; and were more likely to have reconstruction at the time of surgery, compared with women who did not experience preoperative or persistent postsurgical breast pain. Finally, severity of average and worst postoperative pain differed significantly among the three groups (i.e., no preoperative and no persistent postsurgical breast pain < no preoperative and persistent postsurgical breast pain < preoperative and persistent postsurgical breast pain). Women without preoperative breast pain who developed postsurgical breast pain were more likely to have radiation therapy and less likely to receive chemotherapy during the six months following surgery than women with preoperative breast pain.
Table 2.
Differences in Clinical Characteristics Among Women with No Preoperative and No Persistent Breast Pain (Group 0), Women with No Preoperative Breast Pain who Developed Persistent Breast Pain (Group 1), and Women with Both Preoperative and Persistent Breast Pain (Group 2).
| Characteristic | No Preoperative and No Persistent Breast Pain Group 0 n=122 (31.5%) | No Preoperative Breast Pain and Persistent Breast Pain Group 1 n=158 (40.8%) | Preoperative and Persistent Breast Pain Group 2 n=107 (27.6%) | Statistics |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| ||||
| Karnofsky Performance Status score | 96.5 (8.5) | 92.2 (11.2) | 91.2 (10.0) | F=9.4, P<0.001 1 and 2 < 0 |
|
| ||||
| Self-Administered Comorbidity Scale score | 4.7 (2.8) | 5.37 (3.5) | 5.2 (3.4) | F= 1.3, P=0.265 |
|
| ||||
| Body mass index (kg/m2) | 27.2 (7.0) | 26.8 (5.8) | 26.2 (5.6) | F=0.7, P=0.484 |
|
| ||||
| Number of biopsies in past year | 1.4 (0.7) | 1.5 (0.9) | 1.6 (0.8) | KW, p = 005 2 > 0 |
|
| ||||
| Number of lymph nodes removed | 4.2 (4.6) | 5.8 (6.4) | 7.5 (8.4) | F=7.0, P=0.001 2 > 0 |
|
| ||||
| Number of surgical drains | 0.4 (0.7) | 0.5 (0.8) | 0.5 (0.7) | F=1.5, P=0.214 |
|
| ||||
| Severity of average postoperative pain | 2.8 (2.0) | 4.0 (2.4) | 4.9 (2.3) | F=21.6, P<0.001 2 > 1 > 0 |
|
| ||||
| Severity of worst postoperative pain | 4.2 (2.5) | 5.3 (2.7) | 6.4 (2.5) | F=17.5, P<0.001 2 > 1 > 0 |
|
| ||||
| % (N) | % (N) | % (N) | ||
|
| ||||
| AJCC stage of disease at diagnosis | ||||
| 0 | 18.0 (18) | 20.3 (32) | 17.8 (19) | X2=4.2, P =0.645 |
| I | 41.0 (50) | 37.3 (59) | 33.6 (36) | |
| IIA & IIB | 36.1 (44) | 33.5 (53) | 37.4 (40) | |
| IIIA, IIB, IIC, & IV | 4.9 (6) | 8.9 (14) | 11.2 (12) | |
|
| ||||
| Post menopausal | 71.7 (86) | 64.9 (100) | 53.4 (55) | X2=8.1, P=0.017 0 > 2 |
|
| ||||
| Received neoadjuvant chemotherapy | 16.4 (20) | 24.8 (39) | 15.9 (17) | X2=4.5, P=0.108 |
|
| ||||
| Swelling in breast pre-operatively | 3.3 (4) | 4.4 (7) | 20.6 (22) | X2=27.6, p<.001 2 > 0 and 1 |
|
| ||||
| Strange sensations in breast pre-operatively | 12.3 (15) | 17.7 (28) | 56.1 (60) | X2=66.8, P<0.001 2 > 0 and 1 |
|
| ||||
| Numbness in breast pre-operatively | 1.6 (2) | 0.0 (0) | 15.0 (16) | X2=35.8, P<0.001 2 > 0 and 1 |
|
| ||||
| Hardness in breast pre-operatively | 7.4 (9) | 15.8 (25) | 36.4 (39) | X2=33.1, P<0.001 2 > 0 and 1 |
|
| ||||
| Type of surgery | ||||
| Breast conservation | 85.2 (104) | 81.0 (128) | 72.9 (78) | X2=5.6, P=0.061 |
| Mastectomy | 14.8 (18) | 19.0 (30) | 27.1 (29) | |
|
| ||||
| Axillary lymph node dissection | 27.9 (34) | 39.5 (62) | 41.1 (44) | X2=5.5, P=0.063 |
|
| ||||
| Sentinel lymph node biopsy | 85.2 (104) | 81.0 (128) | 82.2 (88) | X2=0.9, P=0.643 |
|
| ||||
| Surgical drain placement | ||||
| No drain | 70.5 (86) | 58.9 (93) | 55.1 (59) | X2=6.6, P=0.357 |
| Only in the breast | 13.1 (16) | 16.5 (26) | 18.7 (20) | |
| Only in the axilla | 12.3 (15) | 18.4 (29) | 19.6 (21) | |
| Both breast and axilla | 4.1 (5) | 6.3 (10) | 6.5 (7) | |
|
| ||||
| Reconstruction at the time of surgery | 21.7 (18) | 42.2 (35) | 36.1 (30) | X2=6.1, P=0.048 2>0 |
|
| ||||
| Any RT during six months following surgery | 57.4 (70) | 67.1 (106) | 40.2 (43) | X2=18.8, P<0.001 1>2 |
|
| ||||
| Any CTX during six months following surgery | 32.0 (39) | 27.8 (44) | 43.0 (46) | X2=6.7, P=0.035 1 < 2 |
|
| ||||
| Any re-excision/mastectomy following surgery | 18.0 (22) | 34.8 (55) | 28.0 (30) | X2=9.7, P=0.008 1>0 |
|
| ||||
| Any breast reconstruction during six months following surgery | 4.9 (6) | 7.0 (11) | 9.3 (10) | X2=1.7, P=0.423 |
|
| ||||
| Any PT during six months following surgery | 9.8 (12) | 20.3 (32) | 15.9 (17) | X2=5.6, P=0.060 |
AJCC = American Joint Committee on Cancer; CTX = chemotherapy; kg = kilogram; KW = Kruskal-Wallis; m2 = meter squared; PT = physical therapy; RT = radiation therapy; SD = standard deviation; X2 = Chi-square statistic.
Differences in Preoperative Symptom and QOL Scores Among the Breast Pain Groups
Table 3 displays preoperative symptom and QOL scores for each of the pain groups. With the exception of energy and spiritual well-being, all of the symptom and QOL scores differed significantly among the groups. Specifically, women with persistent postsurgical breast pain, with or without preoperative breast pain, had significantly higher symptom severity scores and lower QOL scores than women with neither preoperative nor persistent postsurgical breast pain.
Table 3.
Differences in Symptom and QOL Scores Among Women with No Preoperative and No Persistent Breast Pain (Group 0), Women with No Preoperative Breast Pain who Developed Persistent Breast Pain (Group 1), and Women with Both Preoperative and Persistent Breast Pain (Group 2). at Enrollment
| Characteristic | No Preoperative and No Persistent Breast Pain Group 0 n=122 (31.5%) | No Preoperative Breast Pain and Persistent Breast Pain Group 1 n=158 (40.8%) | Preoperative and Persistent Breast Pain Group 3 n=107 (27.6%) | Statistics |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Symptom Scores | ||||
| State-Trait Anxiety Inventory - State anxiety Score | 34.0 (10.6) | 39.5 (12.5) | 41.5 (12.3) | F=11.8, P<0.001 1 and 2 > 0 |
| State-Trait Anxiety Inventory - Trait anxiety Score | 32.7 (7.8) | 36.3 (9.2) | 37.1 (9.1) | F=8.5, P<0.001 1 and 2 > 0 |
| Center for Epidemiological Studies – Depression Scale score | 11.1 (8.4) | 13.9 (9.6) | 16.6 (10.5) | F = 9.1, P<0.001 1 and 2 > 0 |
| General Sleep Disturbance Scale score | 41.9 (18.3) | 50.1 (22.7) | 53.8 (20.6) | F=9.9, P<0.001 1 and 2 > 0 |
| Lee evening fatigue score | 2.5 (2.2) | 3.4 (2.5) | 3.5 (2.2) | F=6.3, P=0.002 1 and 2 > 0 |
| Lee morning energy score | 5.2 (2.6) | 5.0 (2.6) | 4.5 (2.0) | F=2.2, P=0.117 |
| Quality of Life Scores | ||||
| Total score | 7.0 (1.1) | 6.2 (1.3) | 6.1 (1.3) | F=19.0, P<0.001 1 and 2 < 0 |
| Physical well-being score | 8.6 (1.2) | 7.8 (1.7) | 7.5 (1.7) | F=15.1, P<0.001 1 and 2 < 0 |
| Psychological well-being score | 6.4 (1.7) | 5.5 (1.8) | 5.3 (1.8) | F=13.0, P<0.001 1 and 2 < 0 |
| Social well-being score | 7.8 (1.6) | 6.5 (2.0) | 6.4 (2.1) | F=19.9, P<0.001 1 and 2 < 0 |
| Spiritual well-being score | 5.7 (1.7) | 5.6 (1.9) | 5.8 (1.8) | F=0.4, P=0.664 |
Changes Over Time in Symptom Scores Among the Breast Pain Groups
Figs. 1A through 1E illustrate changes over time among the breast pain groups in state anxiety, depressive symptoms, sleep disturbance, fatigue, and energy scores. No significant Group × Time interactions were found for any of the symptoms. Significant group effects were found for all of the symptoms (all P < 0.005). Post hoc pairwise comparisons, with Bonferroni adjustment, revealed that women with persistent postsurgical breast pain (regardless of the occurrence of preoperative breast pain) had higher anxiety, depressive symptom, sleep disturbance, and fatigue scores, and lower energy scores than women with neither preoperative nor persistent postsurgical breast pain. In addition, regardless of group membership, significant time effects were found for anxiety, depressive symptoms, and sleep disturbance (all P < 0.005). State anxiety scores decreased over time. Depressive symptom and sleep disturbance scores appeared to remain unchanged until three months following surgery and then decreased slightly over the remaining three months.
Fig. 1.
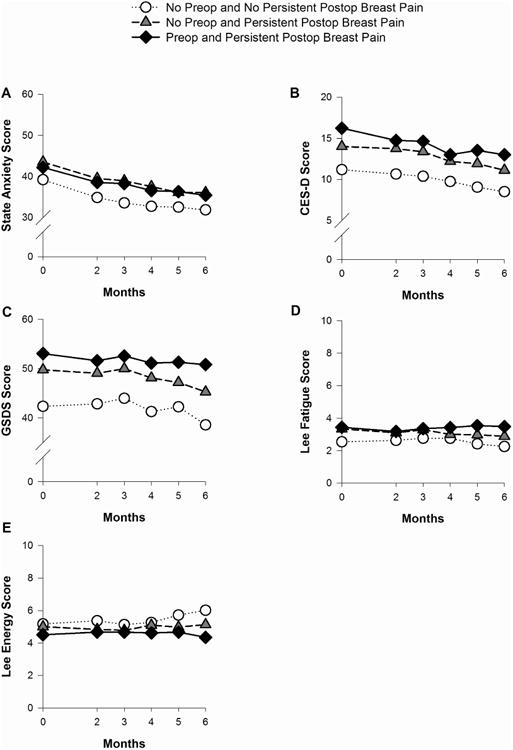
Plots of the estimated marginal means over time among the pain groups for the mixed effects models for state anxiety (A); depressive symptoms (B); sleep disturbance (C); fatigue (D); and energy (E). Statistically significant findings: State anxiety – group effect: P < 0.001; time effect: P < 0.001; Depressive symptoms - group effect: P < 0.001 and time effect: P < 0.001; Sleep disturbance – group effect: P < 0.001 and time effect: P = 0.004; Fatigue – group effect: P = 0.002; Energy – group effect: P = 0.003. CES-D = Center for Epidemiological Studies – Depression Scale; GSDS = General Sleep Disturbance Scale.
Changes Over Time in Grip Strength and Shoulder Mobility Among the Breast Pain Groups
Figs. 2A through 2E illustrate changes over time among the breast pain groups in grip strength and shoulder mobility (i.e., flexion, abduction, internal, external rotation). Significant Group × Time interaction effects were observed for flexion, abduction, and external rotation (all P < 0.05). As displayed in Figs. 2B and 2C, flexion and abduction remain relatively stable across time for women with neither preoperative nor persistent postsurgical breast pain. However, for both groups of women with persistent postsurgical pain, flexion and abduction showed a quadratic trend such that degrees of movement decreased from preoperative levels to two months following surgery, increased from two to four months following surgery, and then stabilized for the remaining two months. For external rotation, women with neither preoperative nor persistent postsurgical pain showed an increase in rotation from preoperative levels to two months following surgery and a decrease from two to three months following surgery, which then stabilized for the remaining three months. In contrast, women with persistent postsurgical breast pain (with or without preoperative breast pain) showed a decrease in external rotation from preoperative levels to two months following surgery. These values remained stable for the remaining four months.
Fig. 2.
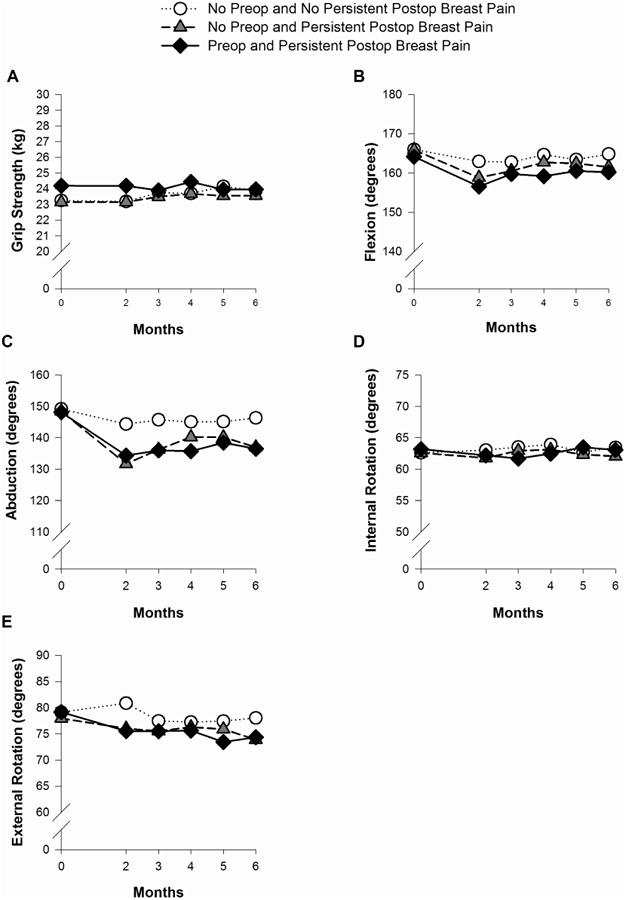
Plots of the estimated marginal means over time among the pain groups for the mixed effects model for grip strength (A), flexion (B), abduction (C), internal rotation (D), and external rotation (E). Statistically significant findings: Flexion - group effect: P = 0.03; time effect: P < 0.001; Group × Time interaction: P = 0.02; Abduction - group effect: P = 0.007; time effect: P < 0.001; Group × Time interaction: P < 0.001; External rotation - time effect: P < 0.001; Group × Time effect: P = 0.03. kg = kilograms.
Significant group effects were observed for flexion and abduction (both P < 0.05). Post-hoc pairwise comparisons found that women with both preoperative and persistent postsurgical breast pain had significantly lower degrees of flexion than women with neither preoperative nor persistent postsurgical breast pain. For abduction, women with persistent postsurgical pain (with or without preoperative breast pain) had significantly lower degrees of abduction than women with neither preoperative nor persistent postsurgical breast pain. No significant group, time, or Group × Time interaction effects were found for internal rotation.
Changes Over Time in Sensitivity in the Breast Scar Area Among the Breast Pain Groups
Fig. 3 illustrates changes over time, among the breast pain groups, in the percentage of breast scar sites that were reported as less, the same, or more sensitive than the unaffected breast. Across pain groups and time, between 50% and 75% of breast scar sites were reported to be less sensitive than the unaffected breast. Between 20% and 50% of breast scar sites were reported to have the same sensitivity as the unaffected breast, and less than 10% of breast scar sites were reported to be more sensitive than the unaffected breast.
Fig. 3.
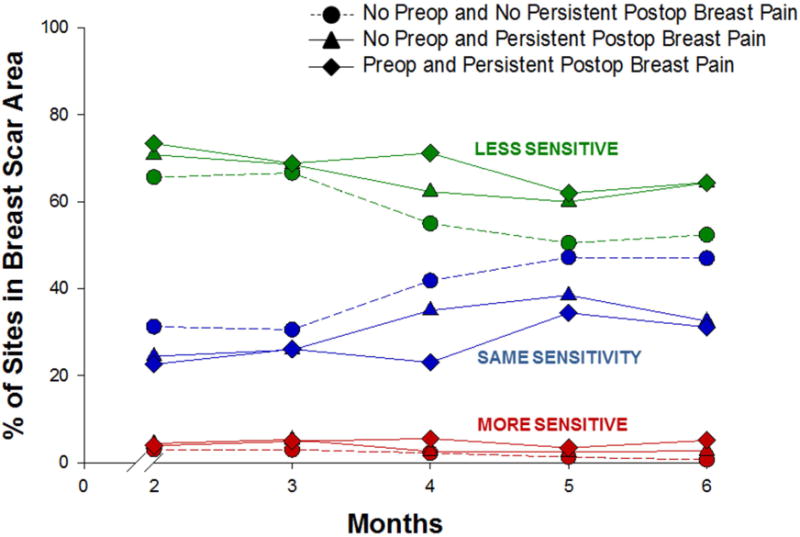
Plots of the estimated marginal means over time among the pain groups for the mixed effects model for the percentage of breast scar sites reported as less sensitive (green), the same (blue), and more sensitive (red) than the unaffected breast. Statistically significant findings: Percentage less sensitive – group effect: P = 0.04; time effect: P < 0.001; Percentage the same – group effect: P = 0.004; time effect: P = 0.001.
Significant group and time effects were found for the sites that were classified as much less sensitive and the same sensitivity as the unaffected breast (P < 0.05 and P < 0.001, respectively). Post hoc pairwise comparisons found that women with both preoperative and persistent postsurgical breast pain reported more breast scar sites as less sensitive than the unaffected breast, compared with women with neither preoperative nor persistent breast pain. In addition, women with persistent postsurgical pain (with or without preoperative breast pain) reported fewer breast scar sites with the same sensitivity as the unaffected breast, compared with women with neither preoperative nor persistent postsurgical breast pain. Across the pain groups, breast scar sites that were classified as less sensitive than the unaffected breast tended to decrease from three to six months following surgery, whereas sites classified as the same sensitivity tended to increase from three to six months following surgery. No significant group, time, or Group × Time interaction effects were found for breast scar sites classified as more sensitive than the unaffected breast.
Changes Over Time in QOL Total and Subscale Scores Among the Breast Pain Groups
Figs. 4A through 4E illustrate changes over time among the breast pain groups in total QOL, as well as physical, psychological, social, and spiritual well-being subscale scores. With the exception of spiritual well-being, a significant group effect was found for all of the QOL scores (all P < 0.001). Post hoc pairwise comparisons found that for QOL total, psychological, and social well-being scores, women with persistent postsurgical pain (with or without preoperative breast pain) had significantly lower QOL scores than women with neither preoperative nor persistent postsurgical breast pain. For physical well-being, post-hoc pairwise comparisons found that all of the pain groups differed from each other in an ordinal manner (i.e., both preoperative and persistent postsurgical breast pain < only persistent postsurgical pain < neither preoperative nor persistent postsurgical pain). In addition, significant time effects were observed for total QOL, as well as, physical, psychological, and social well-being scores (all P < 0.006). For total QOL, physical, and social well-being, the scores appeared to decrease slightly until two to three months following surgery, and then stabilize or increase slightly over the following three to four months. Psychological well-being scores appeared to increase slightly from four to six months following surgery. No group, time, or Group × Time interaction effects were found for spiritual well-being.
Fig. 4.
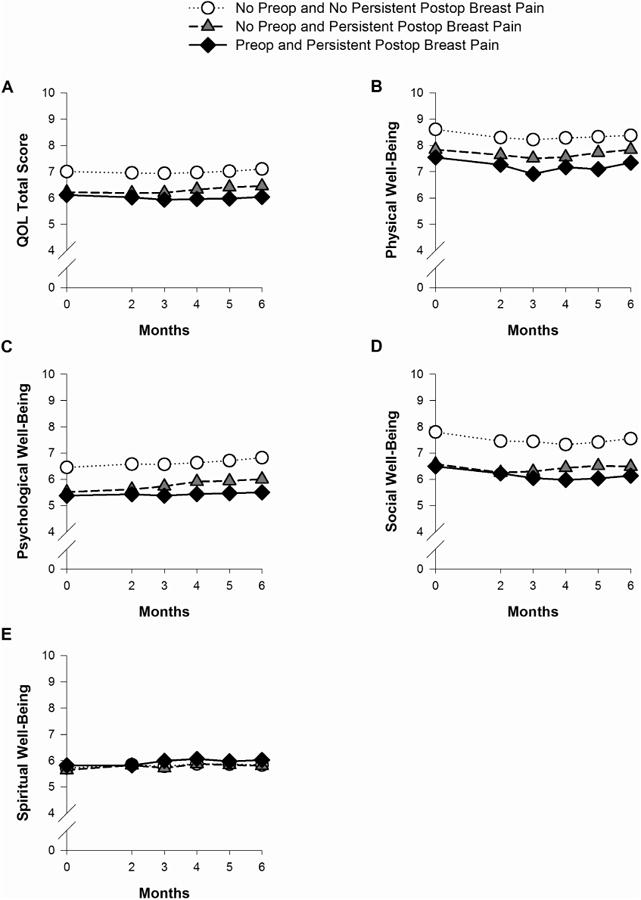
Plots of the estimated marginal means over time among the pain groups for the mixed effects models for total quality of life (QOL) (A); physical well-being (B); psychological well-being (C); social well-being (D); and spiritual well-being (E). Statistically significant findings: Total QOL – group effect: P < 0.001 and time effect: P = 0.005; Physical well-being – group effect: P < 0.001 and time effect: P < 0.001; Psychological well-being – group effect: P < 0.001 and time effect: P < 0.001; Social well-being - group effect: P < 0.001 and time effect: P < 0.001.
Discussion
This study is the first to evaluate whether the occurrence of preoperative breast pain with or without persistent postsurgical pain was associated with changes over time in symptoms, function, sensitivity in the breast scar site, and QOL, following breast cancer surgery. Several similarities were identified between women with persistent postsurgical pain, with or without preoperative breast pain. However, a number of demographic and clinical characteristics differentiated women with preoperative breast pain from the other two groups. Moreover, findings from this study suggest that women with preoperative breast pain are at greater risk for persistent impairments in shoulder flexion and decrements in physical well-being.
Of the 111 women with preoperative breast pain, 96.4% of them developed persistent postsurgical pain. In addition, 55% of the patients without preoperative breast pain developed persistent breast pain. These findings suggest that persistent postsurgical pain is a problem for a high percentage of women. In addition, the mechanisms that underlie the transition from preoperative breast pain to a persistent postsurgical pain problem warrant additional investigation.
The only demographic characteristic that differentiated women with preoperative and persistent postsurgical pain from the other pain groups was age. Women with preoperative breast pain were significantly younger than women with only persistent postsurgical pain, who in turn were younger than women with neither type of pain. This finding is consistent with previous reports that identified younger age as a risk factor for persistent pain following breast cancer surgery (see Andersen & Kehlet for review27). That women with preoperative and persistent breast pain were almost a decade younger than the no pain group may be related to age-related differences in biological responses (e.g., increase in inflammatory responses in younger individuals28) or differences in life circumstances and responsibilities that might exacerbate symptoms. The hypothesis that an increased inflammatory response occurs in women with both preoperative and persistent breast pain is supported by our findings that a higher proportion of these women reported swelling, strange sensations, numbness, and hardness in the breast prior to surgery compared to the other two groups. Of note, compared to the no pain group, women who had both preoperative and persistent postsurgical breast pain were less likely to be postmenopausal. Additional research is needed on whether cyclic variations in the breast contribute to preoperative breast pain and other changes in breast sensations reported by these women. Finally, given that breast density is increased in younger women,29 the relationship between preoperative and persistent breast pain and breast density warrants investigation in future studies.
The finding that women who experienced both preoperative and persistent postsurgical pain reported the most severe postoperative pain ratings is consistent with previous reports.4,27 This finding suggests that women with preoperative breast pain are at particularly high risk for severe postoperative pain as well as persistent pain. If causal, these women may benefit from presurgical as well as aggressive postoperative pain management to decrease the occurrence of persistent postsurgical pain. It is noteworthy that, compared to women with both preoperative and persistent postsurgical breast pain, women without preoperative breast pain who developed persistent postsurgical breast pain were significantly more likely to receive radiation therapy in the six months following surgery. This finding, coupled with the finding that this group of women was more likely to experience re-excision/mastectomy in the six months following surgery, suggests that the development of persistent breast pain may be a result of postsurgical tissue injury.
In terms of symptoms and QOL experienced by these women prior to surgery, patients with persistent postsurgical pain, regardless of whether they reported preoperative breast pain, reported more severe symptoms and poorer QOL than women without pain. Of note, scores for women with preoperative breast pain exceed clinically meaningful cutoff scores for state anxiety,30 depressive symptoms,31 and sleep disturbance.21 Moreover, a moderately large effect size was found for the difference in overall QOL between women with preoperative and persistent postsurgical pain and women with no pain (d=0.74). Associations between a variety of psychosocial factors and persistent pain following breast cancer surgery are well documented.3,4,6,27 However, our findings suggest that these symptoms precede the development of persistent pain and highlight the need for a preoperative evaluation of the etiology and interventions to decrease these elevated levels of anxiety, depressive symptoms, and sleep disturbance.
These preoperative differences in symptom severity scores among the groups persisted for six months following surgery. Although in general, the severity of symptoms decreased over the six months following surgery, symptom severity scores of women with persistent postsurgical pain never decreased to the levels reported by women without pain. Women with persistent postsurgical breast pain had elevated levels of depressive symptoms (i.e., approached the cut-off that indicates a need for evaluation for a depressive disorder31). Although often clinically undetected, elevated levels of depressive symptoms, even if not meeting criteria for major depression, are associated with poorer functional status and QOL.32
In addition, from the preoperative assessment through to six months following surgery, sleep disturbance scores for both of the persistent postsurgical breast pain groups exceeded the cut-point that distinguishes poor from good sleepers.21 Sleep disturbance appears to be a clinically significant problem for women with persistent pain. In a study of over 11,000 oncology patients, the best fitting structural equation model demonstrated that trouble sleeping led to increased ratings of physical pain.33 These findings suggest that interventions that decrease sleep disturbance may improve pain outcomes in these patients.
In terms of the measures of physical function, decrements in shoulder flexion, abduction, and external rotation were most strongly associated with pain. For all three measures, the preoperative values were equivalent for all of the pain groups. However, women who developed persistent postsurgical breast pain (with or without preoperative pain), showed statistically significant impairments two months after surgery. In contrast, range of motion for women without pain did not appear to be affected by the surgery, with measurements remaining stable over time. Particularly for women with preoperative breast pain, these measures never returned to preoperative levels or to the levels found in women with neither preoperative nor persistent breast pain across the six months of the study.
Of note, these shoulder functions involve the use of the pectoralis major muscle,34,35 located in the chest area, behind the breast, which may be impacted by the surgery. Damage to this muscle and/or the nerves innervating it may cause pain and result in impaired range of motion. However, this muscle is necessary for internal rotation, which showed no group, time, or Group × Time interaction effects.34 Adhesive capsulitis (or frozen shoulder), likely caused by guarding behavior as a result of incisional breast pain, may be associated with persistent postsurgical pain. This condition, defined by restricted range of motion, is prevalent among women following breast cancer surgery.36 Further research is warranted on the association between pain and shoulder function. However, the experience of persistent pain clearly places women at risk for impairments in range of motion following surgery. These women may benefit from increased assessments and physical therapy interventions to improve function and potentially relieve pain. A recent pilot study found that an early physical therapy intervention following breast cancer surgery improved shoulder function.37
In terms of evoked sensations in the breast scar area, across group and time, sites were most frequently classified as less sensitive than the unaffected breast. In particular, women with both preoperative and persistent postsurgical breast pain reported more sites as less sensitive compared to women with neither preoperative nor persistent breast pain. In addition, both persistent pain groups reported that fewer sites had the same sensitivity as the unaffected breast. Interestingly, allodynia was not reported at the breast scar site, despite the occurrence of persistent breast pain. These findings highlight the complexity of this persistent pain condition and suggest that women with preoperative breast pain may be at greater risk for sensory disturbances following surgery.
Regarding changes in QOL over time, both groups of women with persistent postsurgical breast pain had persistently poorer ratings of overall QOL, as well as various aspects of physical, psychological, and social well-being. In addition, despite no differences in preoperative scores, women with preoperative and persistent breast pain reported poorer physical well-being scores than women with only persistent breast pain. The effect size for this between group difference at six months was small (d=0.22). However, when the physical well-being scores of the women with both preoperative and persistent postsurgical breast pain were compared to those of the women with no pain, a medium effect size was found (d=0.63). These findings suggest that women with both preoperative and persistent postsurgical breast pain may have a more difficult physical recovery following surgery.
Limitations of the study should be acknowledged. The current study is a secondary analysis of our larger study, designed to evaluate neuropathic pain and lymphedema in women following breast cancer surgery. As such, a comprehensive evaluation of the preoperative breast pain experience was not conducted. Therefore, important aspects such as etiology, laterality, location, and duration (i.e., acute versus persistent) are not known. In addition, data on the location and specific type of breast cancer were not collected. Therefore, it is not known whether preoperative breast pain occurred at the tumor site and might be the result of changes associated with the tumor microenvironment.38 Moreover, information is not available about which patients had pain as a result of a biopsy and which patients had breast pain prior to the biopsy. Future research that includes an evaluation of biopsy-related breast pain is needed. Patients in the study sample were generally high functioning, predominantly white and highly educated, which limits the generalizability of these findings. Finally, quantitative sensory testing was not performed and may be warranted in future studies.
In conclusion, women with persistent breast pain following breast cancer surgery, regardless of whether they experience preoperative breast pain may be at risk for a number of negative outcomes, such as persistently elevated levels of psychological symptoms, sleep disturbance, impairments in range of motion, and poorer QOL. Women with preoperative breast pain appear to be particularly at risk for more severe acute postoperative pain; impaired shoulder flexion; sensory loss at the breast scar site; and persistently poorer physical well-being. Based on our previous findings of associations between variations in cytokine2 and potassium channel39 genes and the occurrence of preoperative breast pain in this sample, it is reasonable to suggest that some of these patients are predisposed to experience preoperative breast pain. Future research that includes molecular characterization of both normal and diseased breast tissue from patients at the time of surgery, as well as a detailed phenotypic and sensory evaluation of preoperative breast pain, may provide insights into the mechanisms that underlie preoperative breast pain and suggest avenues for interventions to reduce the occurrence and severity of persistent postsurgical breast pain.
Acknowledgments
This study was funded by grants from the National Cancer Institute (NCI; CA107091 and CA118658). Dr. Christine Miaskowski is an American Cancer Society Clinical Research Professor and has a K05 award from the NCI (CA168960). Dr. Langford is supported by a Department of Defense Breast Cancer Research Program Postdoctoral Fellowship. This project was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosures: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poleshuck EL, Katz J, Andrus CH, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7:626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCann B, Miaskowski C, Koetters T, et al. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. J Pain. 2012;13:425–437. doi: 10.1016/j.jpain.2011.02.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tasmuth T, von Smitten K, Kalso E. Pain and other symptoms during the first year after radical and conservative surgery for breast cancer. Br J Cancer. 1996;74:2024–2031. doi: 10.1038/bjc.1996.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9:723–744. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 5.Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155:232–243. doi: 10.1016/j.pain.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Meretoja TJ, Leidenius MH, Tasmuth T, Sipila R, Kalso E. Pain at 12 months after surgery for breast cancer. JAMA. 2014;311:90–92. doi: 10.1001/jama.2013.278795. [DOI] [PubMed] [Google Scholar]
- 7.Kroner K, Krebs B, Skov J, Jorgensen HS. Immediate and long-term phantom breast syndrome after mastectomy: incidence, clinical characteristics and relationship to pre-mastectomy breast pain. Pain. 1989;36:327–334. doi: 10.1016/0304-3959(89)90092-4. [DOI] [PubMed] [Google Scholar]
- 8.Miaskowski C, Cooper B, Paul SM, et al. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13:1172–1187. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sipila R, Estlander AM, Tasmuth T, Kataja M, Kalso E. Development of a screening instrument for risk factors of persistent pain after breast cancer surgery. Br J Cancer. 2012;107:1459–1466. doi: 10.1038/bjc.2012.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyranou M, Paul SM, Dunn LB, et al. Differences in depression, anxiety, and quality of life between women with and without breast pain prior to breast cancer surgery. Eur J Oncol Nurs. 2012;17:190–195. doi: 10.1016/j.ejon.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miaskowski C, Dodd M, Paul SM, et al. Lymphatic and angiogenic candidate genes predict the development of secondary lymphedema following breast cancer surgery. PLoS One. 2013;8:e60164. doi: 10.1371/journal.pone.0060164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 13.Karnofsky D. Performance Scale. New York: Plenum Press; 1977. [Google Scholar]
- 14.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 15.Brunner F, Bachmann LM, Weber U, et al. Complex regional pain syndrome 1--the Swiss cohort study. BMC Musculoskelet Disord. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher BS, Paul SM, Dodd MJ, et al. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008;26:599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- 18.Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA. Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 19.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 21.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 22.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 23.Spijkerman DC, Snijders CJ, Stijnen T, Lankhorst GJ. Standardization of grip strength measurements. Effects on repeatability and peak force. Scand J Rehabil Med. 1991;23:203–206. [PubMed] [Google Scholar]
- 24.Ferrell BR, Wisdom C, Wenzl C. Quality of life as an outcome variable in the management of cancer pain. Cancer. 1989;63:2321–2327. doi: 10.1002/1097-0142(19890601)63:11<2321::aid-cncr2820631142>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Padilla GV, Presant C, Grant MM, et al. Quality of life index for patients with cancer. Res Nurs Health. 1983;6:117–126. doi: 10.1002/nur.4770060305. [DOI] [PubMed] [Google Scholar]
- 26.Muthen L, Muthen B. Mplus user's guide. 6th. Los Angeles, CA: Muthen & Muthen; 2010. [Google Scholar]
- 27.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–746. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Wu D, Meydani SN. Age-associated changes in immune and inflammatory responses: impact of vitamin E intervention. J Leukoc Biol. 2008;84:900–914. doi: 10.1189/jlb.0108023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Checka CM, Chun JE, Schnabel FR, Lee J, Toth H. The relationship between mammographic density and age: implications for breast cancer screening. Am J Roentgenol. 2012;198:292–295. doi: 10.2214/AJR.10.6049. [DOI] [PubMed] [Google Scholar]
- 30.Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A) Arthritis Care Res. 2011;63(Suppl 11):S467–472. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 32.Dunn LB, Cooper BA, Neuhaus J, et al. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychol. 2011;30:683–692. doi: 10.1037/a0024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stepanski EJ, Walker MS, Schwartzberg LS, et al. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med. 2009;5:132–136. [PMC free article] [PubMed] [Google Scholar]
- 34.Dark A, Ginn KA, Halaki M. Shoulder muscle recruitment patterns during commonly used rotator cuff exercises: an electromyographic study. Phys Ther. 2007;87:1039–1046. doi: 10.2522/ptj.20060068. [DOI] [PubMed] [Google Scholar]
- 35.Wattanaprakornkul D, Halaki M, Boettcher C, Cathers I, Ginn KA. A comprehensive analysis of muscle recruitment patterns during shoulder flexion: an electromyographic study. Clin Anat. 2011;24:619–626. doi: 10.1002/ca.21123. [DOI] [PubMed] [Google Scholar]
- 36.Stubblefield MD, Custodio CM. Upper-extremity pain disorders in breast cancer. Arch Phys Med Rehabil. 2006;87:S96–99. doi: 10.1016/j.apmr.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Singh C, De Vera M, Campbell KL. The effect of prospective monitoring and early physiotherapy intervention on arm morbidity following surgery for breast cancer: a pilot study. Physiother Can. 2013;65:183–191. doi: 10.3138/ptc.2012-23O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt BL, Hamamoto DT, Simone DA, Wilcox GL. Mechanism of cancer pain. Mol Interv. 2010;10:164–178. doi: 10.1124/mi.10.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langford DJ, West C, Elboim C, et al. Variations in potassium channel genes are associated with breast pain in women prior to breast cancer surgery. J Neurogenet. 2014;28:122–135. doi: 10.3109/01677063.2013.856430. [DOI] [PMC free article] [PubMed] [Google Scholar]


