Abstract
Purpose
The conventional spectral-editing experiment used to measure GABA in the human brain also contains a contribution from macromolecules (MM), and the combined GABA plus MM signal is often referred to as “GABA+”. More recently, methods have been developed to estimate GABA free from MM contamination. In this study, the relationship between GABA acquired with MM suppression and conventional GABA+ measurements was examined.
Methods
GABA-edited MEGA-PRESS experiments with and without MM suppression were performed in the sensorimotor and occipital cortex of 12 healthy subjects at 3 Tesla. The correlation between GABA+ and MM-suppressed GABA measures was then determined.
Results
Across all data, a significant correlation between GABA+ and MM-suppressed GABA was found (r = 0.48; P = 0.02). Regionally, the sensorimotor voxel showed a trend toward a correlation of r = 0.53, P = 0.07 and the occipital voxel did not show a correlation, r = 0.058, P = 0.9.
Conclusion
GABA+ and MM-suppressed GABA are moderately correlated, but statistical power to reveal this relationship may vary regionally. The MM signal, while often assumed to be functionally irrelevant, appears to show inter-individual and inter-regional variance that impacts the correlation of GABA+ and MM-suppressed GABA.
Keywords: brain, edited MRS, GABA, macromolecules GABA+
1 INTRODUCTION
The inhibitory neurotransmitter γ-aminobutyric acid (GABA) has an important role in shaping and regulating neuronal activity. In vivo GABA concentrations can be estimated using 1H magnetic resonance (MR) spectroscopy, which has been applied to study disease (1–5) and healthy brain function (6–10). GABA is typically measured using a J-difference spectral-editing approach, because the GABA resonance signals (at 3 ppm, 2.3 ppm, and 1.9 ppm) are overlapped by those of more abundant metabolites and, therefore, are difficult to quantify directly. J-difference editing involves acquiring two subspectra: in the first (ON) subspectrum an editing pulse is applied at 1.9 ppm to refocus the evolution of the coupled signal at 3.0 ppm; in the second (OFF) subspectrum the editing pulse is not applied (or, more often, is applied at a frequency that does not impact GABA signals) and thus, coupling evolves. The difference between the two subspectra resolves the 3.0 ppm GABA resonance from the overlying Creatine (Cr) signal.
One major limitation of this approach is that the edited GABA signal is contaminated by a macromolecular signal, due to finite selectivity of editing pulses. In a typical GABA-edited experiment (refer to the pulse sequence diagram and inversion profile in Figure 1), the editing pulses applied at 1.9 ppm partially invert the macromolecule (MM) resonance at 1.7 ppm (3), which is coupled to an MM signal also at 3 ppm (11). As a result, there is MM contamination of the edited GABA signal, which makes up an estimated 45% of the total signal (12) and the edited signal is, therefore, often referred to as “GABA+” (= GABA+MM).
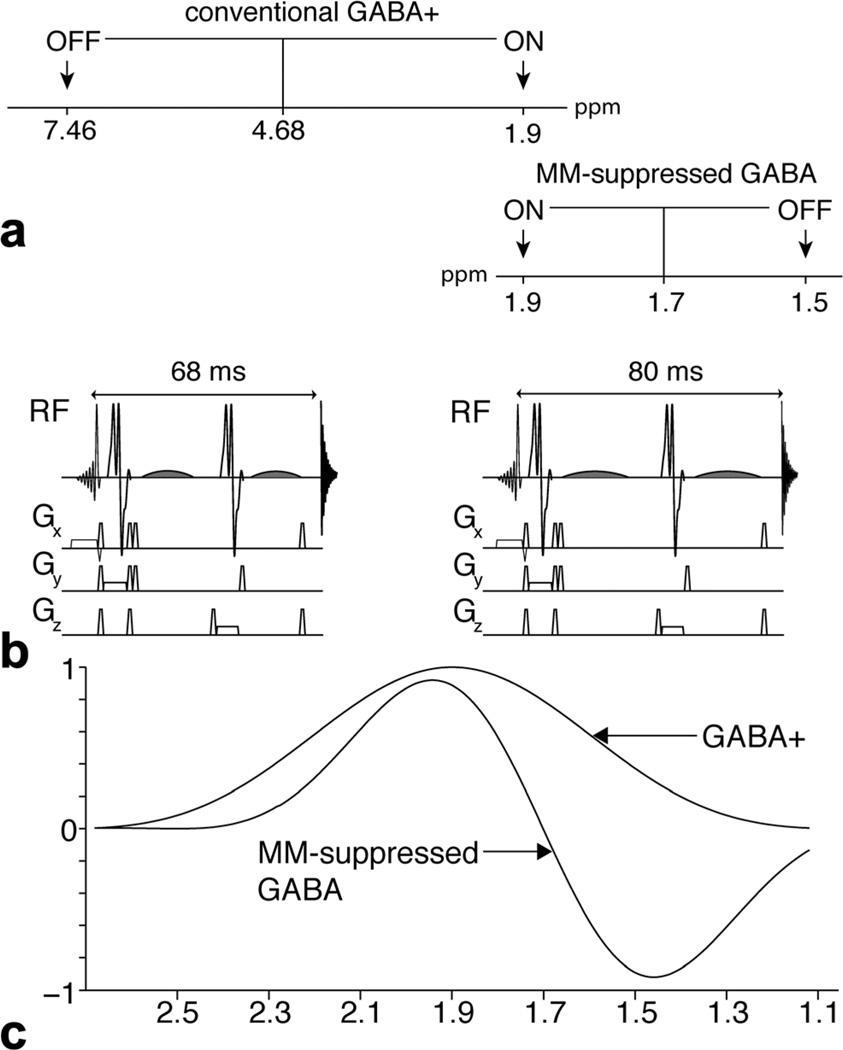
FIG. 1.
Comparison of the conventional GABA+ acquisition with the MM-suppressed GABA acquisition. a: Schematic to illustrate the differences in the ON and OFF editing pulses in the GABA+ and MM-suppressed GABA experiments. b: Pulse sequences of the conventional GABA+ and the MM-suppressed GABA acquisitions. In the conventional GABA+ acquisition, the TE = 68 ms and the frequency selective editing pulses (shaded) are limited to 14 ms. By increasing the TE to 80 ms, the editing pulses can be 20 ms in duration, thus increasing their selectivity for the MM-suppressed acquisition. Pulse sequence diagrams in b are reproduced from Edden et al (17). c: Editing efficiency profiles for conventional GABA+ and MM-suppressed GABA. This illustrates the editing selectivity of the GABA at 1.9 ppm for both acquisitions. For the MM-suppressed GABA acquisition, inversion at 1.5 ppm results in the 1.7 ppm MM signal being nulled.
One approach to separate the MM contribution from GABA+ is to measure the MM contribution directly by acquiring an MM-only, metabolite-nulled spectrum (i.e., removing the GABA contribution by preinversion and exploiting the difference in T1 relaxation times between GABA and MM). From this, GABA concentration can be calculated as the difference of the measured MM concentration from the GABA+ concentration (13,14). This approach requires the acquisition of two separate spectra from the same location, which not only substantially increases scan time but reduces signal-to-noise ratio (SNR) and is subject to error due to motion between acquisitions. A more efficient scheme is to eliminate (or at least minimize) the MM contribution within the acquisition. Henry et al (15) proposed an editing scheme in which the editing pulses are placed symmetrically about the 1.7 ppm MM resonance peak (i.e., at 1.9 ppm in ON scans and 1.5 ppm in OFF scans, shown in Figure 1). The MM signal is assumed to be inverted to the same degree in both OFF and ON scans and, therefore, no 3-ppm MM signal appears in the difference spectrum. If insufficiently selective editing pulses are applied, this editing scheme will also partially null the GABA signal. MEGA-SPECIAL (16) and a longer-echo time (TE) MEGA-PRESS, applied in this study, shown in Figure 1b, (17) have been proposed to allow longer, more selective editing pulses to be incorporated in the echo time for symmetric suppression of MM signals without loss of GABA signal.
This study examines the relationship between GABA+ and MM-suppressed GABA measurements. A significant correlation between GABA+ and MM-suppressed GABA measures across the brain is expected, both because the GABA signal contributes to the GABA+ signal, and because inter-individual variance in GABA+ has been related to inhibition-related functional outcomes (6,8,10,18). Indeed, such studies often implicitly assume that inter-individual variance in GABA+ measurements is largely GABA-related (1–8,10). Both GABA+ and MM-suppressed GABA measurements were performed in two brain regions in 12 healthy young adults.
METHODS
With local institutional review approval, informed written consent was obtained from 12 healthy males (ages 29.1±5.2 years). GABA+ and MM-suppressed GABA measurements were performed in both the occipital cortex (OCC) and the right sensorimotor cortex (SM), as shown in Figure 2. The OCC voxel was placed on the midline and rotated to align with the cerebellar tentorium. The SM voxel was centered on the hand-knob of the motor cortex (19) and rotated in the coronal and sagittal planes to align with the edge of the brain. The order between OCC and SM acquisitions was counterbalanced and for each region GABA+ measurements were performed before MM-suppressed GABA measurements. Data were collected at 3 Tesla (T) (“Achieva”, Philips Healthcare, Best, The Netherlands) using a 32-channel head coil. Participants were places in the head coil with lateral padding to limit movement. Acquisition parameters common to both MEGA-PRESS acquisitions were: repetition time (TR) = 2 s, 3 × 3 × 3 cm3 voxels for both locations, 40 blocks of an eight-step phase cycle with OFF-ON editing alternating at each phase cycle, 2048 datapoints sampled at a spectral width of 2 kHz, and VAPOR water suppression (20). For GABA+ measurements, TE = 68 ms and 14 ms editing pulses were applied at 1.9 ppm and 7.46 ppm for the ON and OFF experiments, respectively. Unsuppressed water data were also acquired at TE = 68 ms. For MM-suppressed measurements, TE = 80 ms and 20 ms editing pulses were applied at 1.9 and 1.5 ppm and the unsuppressed water data were acquired at TE = 80 ms. The increase in TE from 68 ms to 80 ms moves away from the original theoretical formalism for GABA-edited MEGA-PRESS methods (TE ~ 1/2J (3)), but is necessary to enable longer more selective editing pulses. The effect of extending TE to 80 ms does not cause substantial signal loss (21).
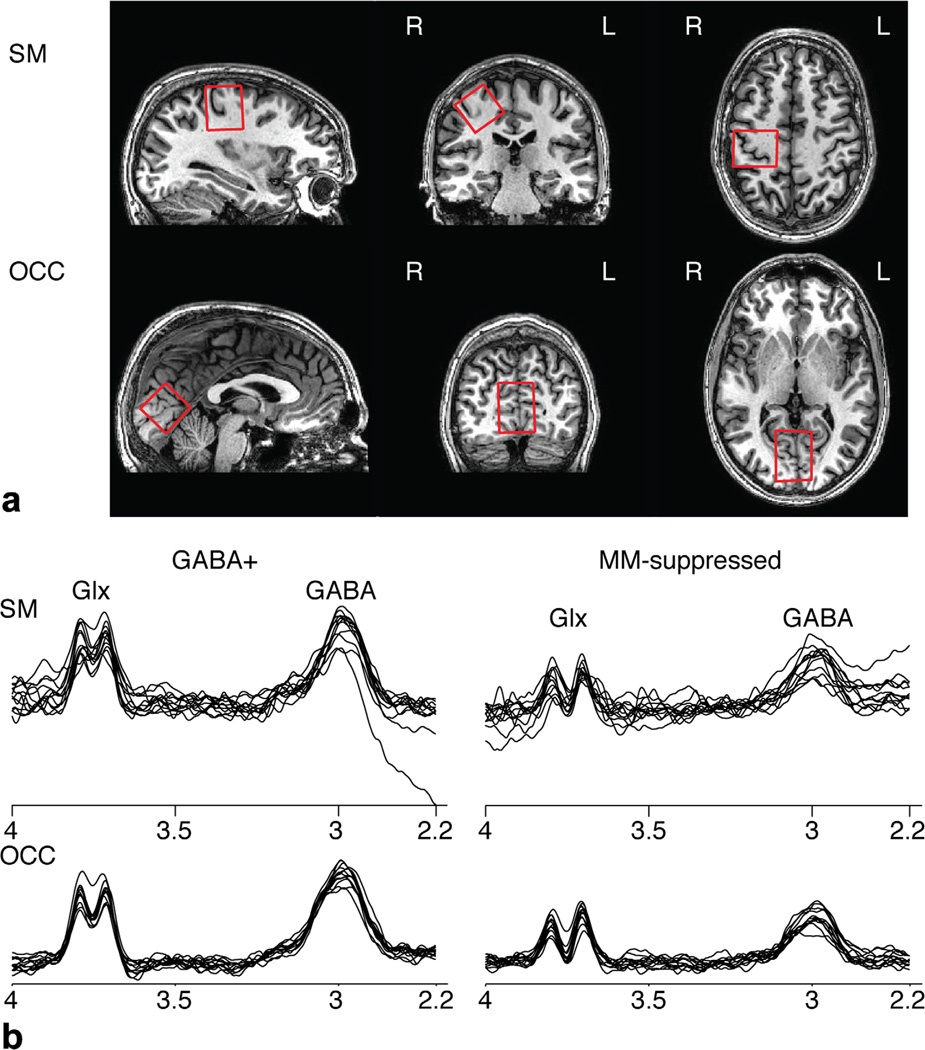
FIG. 2.
a: Voxel locations the sensorimotor (SM) and occipital (OCC) cortex, images are shown according to radiological convention. b: GABA+ and MMsuppressed GABA spectra from all 12 subjects overlapped for the SM and OCC voxels. The GABA and Glx resonances are labeled.
Data were analyzed using the “Gannet” program (22), applying spectral registration phase and frequency correction (23); 3 Hz line broadening; and zero-padding by a factor of 16. The GABA resonance was modeled as a Gaussian with a linear baseline and the water resonance was modeled as a Voigt lineshape with a linear baseline. GABA was then quantified with respect to water according to the equation:
where, SGABA is the integral of the modeled GABA+ or MM-suppressed GABA resonance and SH2O is the integral of the modeled water resonance. The remaining constants are: pure water concentration, [H2O] = 55,000 mmol/dm3, the term visH2O = 0.65 accounts for the concentration and density of MR-visible water (24,25), editing efficiency (κ) = 0.5 (12), T1 and T2 of water were 1.1 s and 0.095 s, respectively (26), T1 and T2 of GABA were 0.80 s [intermediate between MM (11) and GABA (27)] and 0.088 s (21), respectively. Note that an MM correction factor of 0.45 that is typically applied to GABA+ measures (21) has been removed for both the GABA+ and the MM-suppressed GABA quantifications. By not including the MM correction factor, the GABA+ values measured in the current study are expected to be approximately double the value that is typically observed. The creatine resonance was modeled in the OFF spectrum using a two-Lorentzian model for the creatine and choline resonances. The fitting characteristics of the single Lorentzian model for the creatine were then determined. The characteristics of the creatine resonance (full-width, half maximum and fitting error) are included to characterize the spectral quality before subtraction of the ON and OFF spectra.
Voxels were overlaid on each individual’s anatomical image using the SVMask tool (Michael Schär, Philips Healthcare). Tissue segmentation was performed using FAST (FSL, (28)) and all GABA measures were corrected for tissue fraction within the MRS voxel. The Pearson correlation coefficient between the paired GABA+ and MM-suppressed GABA measurements was initially examined across all datasets and subsequently the two locations (OCC and SM) were examined independently. A significance threshold of P = 0.05 was used to test the significance of the correlations. Paired t-tests to test for differences in absolute frequency drift. F-tests were used to test for differences in variance between regions (OCC and SM) for the GABA+ and the MM-suppressed GABA measurements. Follow-up Bayes factor analysis (29) was performed to test the evidence in support of the null hypothesis (i.e., no correlation between GABA+ and MM-suppressed GABA).
RESULTS
All spectra are shown in Figure 2b. By visual inspection, the GABA resonance peak is decreased in the MM-suppressed condition compared with the GABA+ measurement. The Glx resonance peak is also decreased in the MM-suppressed experiment because the more selective editing pulses do not co-edit the 2.1 ppm Glx resonance to the same degree as in the conventional GABA experiment, resulting in a decrease in the observed Glx signal in the difference spectrum. Measured GABA+ and MM-suppressed GABA for all data and OCC and SM voxel locations independently are summarized in Table 1. As expected, the MM-suppressed GABA measurements are 0.49 ± 0.14 that of GABA+.
Table 1.
Average GABA+ and MM-Suppressed GABA Measurements
| GABA+ (i.u.) | MM-suppressed GABA (i.u.) |
MM-suppressed GABA/GABA+ |
Correlation between GABA+ and MM- suppressed GABA |
|
|---|---|---|---|---|
| Pooled data | 6.5 ± 1.3 | 3.2 ± 1.0 | 0.49 ± 0.14 | r = 0.48, p = 0.02 |
| OCC | 7.2 ± 0.9 | 3.5 ± 0.7 | 0.49 ± 0.11 | r = 0.058, p = 0.9 |
| SM | 5.8 ± 1.1 | 2.9 ± 1.1 | 0.50 ± 0.17 | r = 0.53, p = 0.07 |
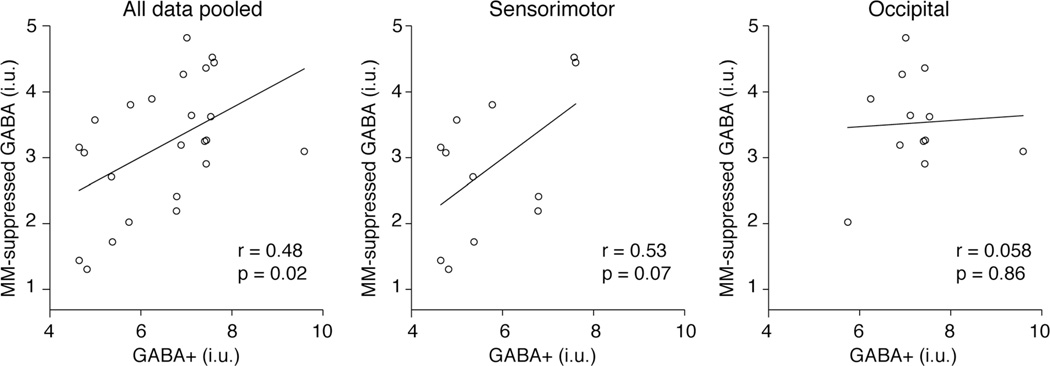
Across all data, a significant correlation between GABA+ and MM-suppressed GABA was observed (r = 0.48; P = 0.02), as shown in Figure 3a and Table 1. When examined in each voxel location independently, this correlation is marginally insignificant in the SM voxel and is lost in the OCC voxel. Bayes factors were 0.22, 1.0, and 2.6 for the OCC, SM, and pooled results, which correspond to substantial evidence for H0 for the OCC result and anecdotal evidence for H1 for the SM and pooled results (29). No differences in variance were detected between the SM and OCC for GABA+ (F-test; P = 0.5) or the MM-suppressed GABA measurements (F-test; P = 0.2).
FIG. 3.
Correlation of MM-suppressed GABA measurement with GABA+ measurement for all data (a), the sensorimotor cortex (b), and the occipital cortex (c).
The tissue segmentation of the OCC voxel was 28% ± 2% white matter, 57% ± 4% gray matter, and 15% ± 4% CSF; the SM voxel was 53% ± 4% white matter, 32% ± 2% gray matter, and 15% ± 3% CSF. No correlation between measured GABA+ or MM-suppressed GABA and tissue fraction was observed.
The average fitting error (defined in Table 2) for the GABA+ measures in the OCC and SM were 4.7 ± 0.7% and 7.0 ± 1.2%, respectively, compared with 6.6 ± 2.3% and 10.3 ± 5.4% for the MM-suppressed data. The relative increase in error with the MM-suppressed data is a result of the smaller MM-suppressed GABA signal compared with the GABA+ signal while the same relative amount of noise is present in both spectra. Frequency drifts were similar between regions, however, the frequency drift of the GABA+ acquisitions were greater than that of the MM-suppressed acquisitions (paired t-test, P < 0.05). Other metrics for data quality are summarized in Table 2.
Table 2.
Metrics of Data Quality for GABA+ and MM-Suppressed GABA Measurements in Both Voxel Locations
| GABA+ | MM-suppressed GABA |
|
|---|---|---|
| OCC | ||
| Cr resonance FWHM (Hz) | 8.9 ± 0.6 | 9.2 ± 0.7 |
| GABA resonance FWHM (Hz) | 18.5 ± 0.9 | 16.7 ± 1.7 |
| Cr fit error (%) | 10.0 ± 1.4 | 8.2 ± 1.7 |
| GABA fit error (%) | 4.7 ± 0.7 | 6.6 ± 2.3 |
| Absolute frequency drift (Hz) | 6.4 ± 5.8 | 3.6 ± 3.7 |
| SM | ||
| Cr resonance FWHM (Hz) | 9.2 ± 3.0 | 9.8 ± 3.0 |
| GABA resonance FWHM (Hz) | 18.9 ± 5.4 | 15.8 ± 2.3 |
| Cr fit error (%) | 15.4 ± 8.7 | 13.1 ± 5.1 |
| GABA fit error (%) | 7.0 ± 1.2 | 10.3 ± 5.4 |
| Absolute frequency drift (Hz) | 6.8 ± 5.3 | 4.2 ± 3.9 |
Fit error is the standard deviation of the residuals normalized by the height of the resonance.
DISCUSSION
Studies examining the role of inhibition often implicitly assume a relationship between GABA+ and the underlying true concentration of GABA. While the MM signal is considered to be functionally irrelevant, it may exhibit a relatively high level of variability across individuals (13). This variability will in turn influence GABA+ measurements and could potentially cause misinterpretations regarding the underlying GABA. The contribution of MM to the GABA+ signal is generally considered to be on the order of a half (12,16), consistent with the current results (Table 1). MM-suppression methods (15–17) have been developed to overcome this confound by enabling more specific GABA measurements. To our knowledge no study has examined the relationship between GABA+ measurements and a more specific measure of GABA using MM suppression. In this study, we examine the relationship between GABA+ and MM-suppressed GABA measures in two regions of the brain.
A significant, albeit moderate, correlation between GABA+ and MM-suppressed GABA was observed across all data (both regions pooled). When analyzed regionally, this relationship remained in the SM voxel to approximately the same degree (correlation coefficient across all data = 0.48 compared with SM = 0.53) but was not significant, as a result of insufficient power. However, in the OCC voxel, the correlation between GABA+ and MM-suppressed GABA was lost completely. Bayes Factor analysis is a method to investigate the evidence in favor of the null hypothesis (29). It showed substantial evidence in favor of the null hypothesis for the OCC data, BF = 0.22, providing evidence in support of no correlation, as opposed to a lack of evidence for a correlation. The Bayes factors for the SM and pooled data provided anecdotal evidence in support of the correlation. This is consistent with the Pearson’s correlation result, which for the pooled data was significant (P = 0.02) and for the SM data was marginally nonsignificant (P = 0.07).
The strength of the correlation between GABA+ and MM-suppressed GABA will be affected by inter-individual differences of MM and GABA, as well as the quality of the measurements. Previously, it has been shown that within-individual coefficients of variation (CVs) for GABA+ measurements in OCC and the SM voxel were on average 7% and 9% (30), while between-individual CVs have been shown to be 7% to 22% for GABA+ depending on the quantification and referencing method (30–32). Here, the between-individual CVs were 13% and 19% for the GABA+ measurements in OCC and SM, respectively, which is within the range of previous studies. Thus, the range of values for GABA+ and MM-suppressed GABA in the OCC does appear to be more limited than in the SM, possibly limiting the power to detect a relationship. Conversely, there may be unrecognized noise/artifacts in the SM measurements that is correlated between the GABA+ and MM-suppressed GABA measures which is not present in the OCC data. However, a source of noise that pushes the trend toward a correlation between GABA+ and MM-suppressed GABA in the SM but not in OCC cannot readily be identified. Future research including multiple measurements to assess reproducibility and repeatability of both GABA+ and MM-suppressed GABA measurements will be valuable.
Measurement noise will impact the observed correlations. The MM-suppressed GABA measurement has less signal but will be susceptible to the same experimental and measurement noise as the GABA+ measurements, therefore, it is expected that the fitting error would be increased compared with GABA+ (as seen in Table 2). The fitting errors and linewidths summarized in Table 2 must be interpreted with caution. The MM-suppressed GABA measurements show greater error than the GABA+ as the signal is approximately halved while the errors remain the same. Characteristics of the creatine resonance have also been included to indicate overall spectral quality; however, the creatine resonance is not a well-isolated single resonance peak, thus it has a higher fitting error compared with that of GABA+/GABA. Additionally, the spectrum in the region of the creatine resonance is different between the GABA+ and the MM-suppressed GABA data, resulting in differing performance of the fitting routine and differing fitting residuals. The residual signal from fitting contains information not only about pure thermal noise but also about the baseline signals and is not well described by the simple model. It can, therefore, be used as a metric of fitting quality and consistency, but not measurement accuracy. This is true for both creatine and GABA+/MM-suppressed GABA. The linewidths and the fitting errors are similar between regions (refer to Table 2) indicating that the spectral data quality and fitting performance are similar in the two regions.
Frequency drift can have substantial effects on GABA+ measurements (33). The MM-suppression method is more susceptible to errors due to frequency drift (13,15), both because the editing pulses used are more frequency-selective and because the inversion profiles for OFF and ON experiments overlap. Therefore, even relatively small drifts can have a substantial impact. At 7T, Terpstra et al (13) found that frequency drifts of greater than 5 Hz resulted in MM contamination when applying symmetric pulsing for MM-suppression. While none of the data in this study showed frequency drift of greater than 25 Hz and the absolute frequency drift was on average less than 7 Hz (as shown in Table 2), differing frequency drifts between datasets will contribute to overall variance. The frequency drifts were similar in the two regions and the relatively small frequency drifts indicate high data quality of this data set. The greater frequency drift of the GABA+ acquisitions compared with the MM-suppressed acquisition (P < 0.05, paired t-test, Table 2) is likely a result of the GABA+ measurements being performed before the MM-suppressed measures and thus being more susceptible to gradient-induced frequency drifts associated with prior imaging, even though experiments were generally performed at least 30 min after the previous scanning session in an effort to ensure a relatively “cold” and stable scanner. Improved quality of the MM-suppressed measurements may be required before widespread implementation of MM-suppressed methods over conventional GABA+ measurements. To achieve improved data quality, the use of prospective correction methods to limit frequency drift, and increased SNR by increasing the number of averages or voxel size, may be necessary. Unfortunately, increasing SNR either costs in terms of longer measurement times and/or larger volumes for measurement, which may decrease participant compliance and/or the regional specificity of results. In this study we have chosen common, conservative experimental parameters (27 mm3 volume, 320 averages) (34).
A strong, highly significant correlation between GABA+ and MM-suppressed GABA would have supported the use of GABA+ as a surrogate for underlying GABA; however, this was not observed in the current experiments. The moderate correlation that was observed may be a result of great inter-individual variability of MM. This would support the use of MM-suppressed GABA methods to measure GABA concentrations. However, a reduced correlation could be the result of variability in both GABA and MM, making the case for MM-suppressed measures less clear. Future GABA+, MM-suppressed GABA and metabolite-suppressed MM experiments could be used differentiate these possibilities.
The difference in correlation between the two regions was not expected. It may indicate the relationship between GABA+ and MM-suppressed GABA changes regionally and/or the power to discriminate this relationship varies regionally. In terms of tissue content, both the OCC and the SM voxel contained ~15% CSF; however, the OCC voxel was on average 28% white matter and 57% gray matter while the SM voxel was 53% white matter and 32% gray matter. GABA concentration differs between white and gray matter (35) and regionally (30) but the regional and tissues differences of MM and the variability across regions and tissue types is less clear. A greater MM content in gray matter compared with white has been shown previously (36); however, this finding is not consistently seen (37,38). Differences in MM between tissues may be specific to particular MM resonances (39) and may vary regionally (40). No correlation between gray or white matter proportion and measured GABA was seen in the current data. However, regional differences in tissue composition may impact the correlation observed. The MM concentration may be more variable between individuals in the OCC, leading to the lack of correlation between GABA+ and MM-suppressed GABA, possibly as a result of the increased proportion of gray matter in the OCC compared with the SM.
In conclusion, a correlation of GABA+ and MM-suppressed GABA measurements using MEGA-PRESS methods was found for data pooled from 2 regions. This supports at least in part the use of GABA+ as a surrogate measure of GABA; however, the MM contribution appears to display heterogeneity as evidenced by the reduced/lost correlation in the regional data analysis. Interpreting the results of experiments measuring GABA+, therefore, requires caution. MM-suppressed GABA measurements are more specific measures of GABA and removes the MM confound for interpreting results. However, MM-suppression methods have lower SNR and are more susceptible to experimental errors such as scanner frequency drift, and may require additional technical development before they can be used on a routine basis to estimate brain GABA levels.
Acknowledgments
Grant sponsor: NIH; Grant numbers: R21 NS077300; R01 EB016089; P41 EB015909; Grant sponsor: The Dr. Milosh and Smiljka C. Perovitch Fund.
REFERENCES
- 1.Edden RA, Crocetti D, Zhu H, Gilbert DL, Mostofsky Sh. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69:750–753. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, Roberts TP. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanacora G, Gueorguieva R, Epperson N, Wu Y-T, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gaminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 5.Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579–583. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao F, Edden RA, Li M, et al. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013;78:75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthukumaraswamy SD, Evans CJ, Edden RA, Wise RG, Singh KD. Individual variability in the shape and amplitude of the BOLD-HRF correlates with endogenous GABAergic inhibition. Hum Brain Mapp. 2012;33:455–465. doi: 10.1002/hbm.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 10.Stagg CJ. Magnetic resonance spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. Neuroimage. 2014;86:19–27. doi: 10.1016/j.neuroimage.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Behar KL, Rothman DL, Spencer DD, Petroff OAC. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 12.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Cardiff Symposium on MRSoG. Edden RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terpstra M, Ugurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn Reson Med. 2002;47:1009–1012. doi: 10.1002/mrm.10146. [DOI] [PubMed] [Google Scholar]
- 14.Hetherington HP, Newcomer BR, Pan JW. Measurements of human cerebral GABA at 4.1 T using numerically optimized editing pulses. Magn Reson Med. 1998;39:6–10. doi: 10.1002/mrm.1910390103. [DOI] [PubMed] [Google Scholar]
- 15.Henry P-G, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45:517–520. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Near J, Simpson R, Cowen P, Jezzard P. Efficient gamma-aminobutyric acid editing at 3T without macromolecule contamination: MEGA-SPECIAL. NMR Biomed. 2011;24:1277–1285. doi: 10.1002/nbm.1688. [DOI] [PubMed] [Google Scholar]
- 17.Edden RA, Puts NA, Barker PB. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn Reson Med. 2012;68:657–661. doi: 10.1002/mrm.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puts NA, Edden RA, Evans CJ, McGlone F, McGonigle DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci. 2011;31:16556–16560. doi: 10.1523/JNEUROSCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousry TA, Schmid UD, Schmidt AD, Peraud A, Buettner A, Winkler P. Localization of hte motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 20.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 21.Edden RA, Intrapiromkul J, Zhu H, Cheng Y, Barker PB. Measuring T2 in vivo with J-difference editing: application to GABA at 3 Tesla. J Magn Reson Imaging. 2012;35:229–234. doi: 10.1002/jmri.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edden RAE, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: a batch-processing tool for the quantitative analysis of GABA-edited MRS spectra. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2014 doi: 10.1002/mrm.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson Ser B. 1993;102 [Google Scholar]
- 25.Helms G. Volume correction for edema in single-volume proton MR spectroscopy of contrast-enhancing multiple sclerosis lesions. Magn Reson Med. 2001;46:256–263. doi: 10.1002/mrm.1186. [DOI] [PubMed] [Google Scholar]
- 26.Wansapura JP, Holland SK, Dunn RS, Ball WS., Jr NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging. 1999;9:531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Puts NA, Barker PB, Edden RA. Measuring the longitudinal relaxation time of GABA in vivo at 3 Tesla. J Magn Reson Imaging. 2013;37:999–1003. doi: 10.1002/jmri.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 29.Wetzels R, Wagenmakers EJ. A default Bayesian hypothesis test for correlations and partial correlations. Psychon Bull Rev. 2012;19:1057–1064. doi: 10.3758/s13423-012-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans CJ, McGonigle DJ, Edden RA. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 2010;31:204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 31.Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, Trattnig S, Doerfler A, Stefan H, Hammen T. In vivo quantification of intracerebral GABA by single-voxel H-MRS-How reproducible are the results? Eur J Radiol. 2010;73:526–531. doi: 10.1016/j.ejrad.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 32.O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J Magn Reson Imaging. 2011;33:1262–1267. doi: 10.1002/jmri.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris AD, Glaubitz B, Near J, John Evans C, Puts NA, Schmidt-Wilcke T, Tegenthoff M, Barker PB, Edden RA. Impact of frequency drift on gamma-aminobutyric acid-edited MR spectroscopy. Magn Reson Med. 2014;72:941–948. doi: 10.1002/mrm.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi IY, Lee SP, Merkle H, Shen J. In vivo detection of gray and white matter differences in GABA concentration in the human brain. Neuroimage. 2006;33:85–93. doi: 10.1016/j.neuroimage.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 36.McLean MA, Barker GJ. Concentrations and magnetization transfer ratios of metabolites in gray and white matter. Magn Reson Med. 2006;56:1365–1370. doi: 10.1002/mrm.21070. [DOI] [PubMed] [Google Scholar]
- 37.Snoussi K, Gillen JS, Horska A, Puts NA, Pradhan S, Edden RAE, Barker PB. Comparison of brain gray and white matter macromolecular resonances at 3 and 7 Tesla. Magn Reson Med. 2014 doi: 10.1002/mrm.25468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaller B, Xin L, Gruetter R. Is the macromolecule signal tissuespecific in healthy human brain? A H MRS study at 7 tesla in the occipital lobe. Magn Reson Med. 2014;72:934–940. doi: 10.1002/mrm.24995. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann L, Slotboom J, Boesch C, Kreis R. Characterization of the macromolecule baseline in localized 1H-MR spectra of human brain. Magn Reson Med. 2001;46:855–863. doi: 10.1002/mrm.1269. [DOI] [PubMed] [Google Scholar]
- 40.Mader I, Seeger U, Karitzky J, Erb M, Schick F, Klose U. Proton magnetic resonance spectroscopy with metabolite nulling reveals regional differences of macromolecules in normal human brain. J Magn Reson Imaging. 2002;16:538–546. doi: 10.1002/jmri.10190. [DOI] [PubMed] [Google Scholar]