Abstract
To date, brain structure and function changes in children with complex regional pain syndrome (CRPS) as a result of disease and treatment remain unknown. Here, we investigated (a) gray matter (GM) differences between patients with CRPS and healthy controls and (b) GM and functional connectivity (FC) changes in patients following intensive interdisciplinary psychophysical pain treatment. Twenty-three patients (13 females, 9 males; average age ± SD = 13.3 ± 2.5 years) and 21 healthy sex-and age-matched controls underwent magnetic resonance imaging. Compared to controls, patients had reduced GM in the primary motor cortex, premotor cortex, supplementary motor area, midcingulate cortex, orbitofrontal cortex, dorsolateral prefrontal cortex (dlPFC), posterior cingulate cortex, precuneus, basal ganglia, thalamus, and hippocampus. Following treatment, patients had increased GM in the dlPFC, thalamus, basal ganglia, amygdala, and hippocampus, and enhanced FC between the dlPFC and the periaqueductal gray (PAG), two regions involved in descending pain modulation. Accordingly, our results provide novel evidence for GM abnormalities in sensory, motor, emotional, cognitive, and pain modulatory regions in children with CRPS. Furthermore, this is the first study to demonstrate rapid treatment-induced GM and FC changes in areas implicated in sensation, emotion, cognition, and pain modulation.
Keywords: Cortical thickness, voxel-based morphometry, functional connectivity, chronic pain, neuropathic pain, children, hippocampus, prefrontal cortex, periaqueductal gray
Introduction
The brain undergoes morphological changes in chronic pain. Alterations in brain gray matter (GM) have been demonstrated in various chronic pain conditions including complex regional pain syndrome (CRPS) (Borsook et al. 2013; Smallwood et al. 2013). CRPS is a chronic neuropathic pain disorder, which usually develops following an injury to an extremity (Bruehl 2010) and is characterized by both peripheral and central symptoms. Peripheral symptoms include spontaneous ongoing pain, abnormal responses to innocuous and noxious stimuli, swelling, and autonomic changes (i.e., altered sweating, change in skin color, and heightened temperature) in the affected region (Harden et al. 2013). Central CRPS symptoms involve allodynia, movement disorders (van Hilten 2010), altered autonomic function (i.e., resting sweat, whole body warming or cooling) (Smith et al. 2011), changes in space or localization perception (Moseley 2004; Maihöfner et al. 2006; Peltz et al. 2011), and reduced cognition and memory function (Apkarian et al., 2004; Libon et al., 2010).
Adults with CRPS exhibit dynamic changes in brain function (Apkarian et al. 2001; Maihöfner et al. 2004, 2005, 2006, 2007; Pleger et al. 2006; Gieteling et al. 2008; Becerra et al. 2009; Freund et al. 2010; Bolwerk et al. 2013), brain chemistry (Fukumoto et al. 1999; Grachev et al. 2000, 2002; Klega et al. 2010), and brain structure (Geha et al. 2008; Baliki et al. 2011; Barad et al. 2014). In children with CRPS, we have previously demonstrated alterations in brain function (Lebel et al. 2008; Erpelding et al. 2014; Simons et al. 2014). To date, however, there is no evidence for structural brain changes in pediatric CRPS and its plasticity related to treatment (i.e., reversal of CRPS-induced changes).
Pediatric patients with CRPS are particularly interesting to study, as, contrary to adult patients, CRPS symptoms in children often resolve relatively rapidly with treatment (Low et al. 2007; Harris et al. 2012), possibly due to enhanced responses and/or adaptation processes that may take place in children (Anderson et al. 2011; Kolb et al. 2011, 2013). Thus, pediatric patients with CRPS represent a useful model to investigate longitudinal brain changes. The etiology of treatment-induced brain changes in chronic pain however remains largely unknown. Recently, Seminowicz et al. (2011) reported in patients with chronic low back pain that effective treatment is able to restore normal brain structure and function in the dorsolateral prefrontal cortex (dlPFC). The dlPFC exerts pain modulatory functions (Lorenz et al. 2003) and is connected to other descending pain inhibitory regions such as the periaqueductal gray (PAG) (Bragin et al. 1984; An et al. 1998; Lim et al. 2009). However, it remains unknown if effective treatment induces changes in functional connectivity (FC) between pain modulatory brain regions such as the dlPFC and PAG in CRPS.
Thus, the aims of the present study were to investigate (a) GM alterations in children with CRPS compared to healthy matched controls (disease effect); and (b) intensive interdisciplinary psychophysical pain treatment (see Logan et al., 2012) effects on GM and dlPFC-PAG FC changes in patients with CRPS that are otherwise unresponsive to standard medical care (treatment effect).
Methods
Subjects
Patients with CRPS were recruited from the Chronic Pain Clinic in the Pain Treatment Service at Boston Children’s Hospital (BCH) for this BCH institutional review board approved study. Patients who subsequently enrolled in the Pediatric Pain Rehabilitation Center (PPRC) underwent interdisciplinary pediatric pain rehabilitative treatment that entails intensive physical, occupational, and psychological therapy components 8 hours/day, 5 days/week for 3–4 weeks (see Logan et al., 2012). Both the patient and parent were consented for the study. Parents were present during the study visits. Patients were included in the study if (1) they refrained from using analgesic medication > 4 hours prior to the study session, (2) they experienced unilateral lower extremity CRPS (based on Budapest criteria; Harden et al., 2010), and (3) their pain intensity was > 5 on a 11-point numerical rating scale (NRS). They were excluded if they had (1) claustrophobia, (2) significant medical problems (e.g., uncontrollable asthma and seizures, cardiac diseases, severe psychiatric disorders, and neurological disorders other than CRPS), (3) pregnancy, (4) medical implants and/or devices, and (5) weight > 285 pounds which corresponded to the weight limit of the MRI table.
Sex- and age-matched healthy controls were recruited in the greater Boston area through advertisements. Patients participated in two study sessions before and after treatment; a matched time interval was chosen for healthy controls. Each study session consisted of a neurological exam with a study physician, questionnaires, and a magnetic resonance imaging (MRI) scan. No new medications were prescribed during the treatment (i.e., each patient continued the same pharmacological treatment for the duration of the program).
MRI Acquisition
Subjects underwent MRI on a 3 T (Siemens Medical Solutions, Erlangen, Germany) scanner using a 12-channel head coil. For each participant, we collected a 3D T1-weighted anatomical scan using a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence (128 slices; TR = 2100 ms; TE = 2.74 ms; TI = 1100 ms; 256 × 256 matrix; FOV = 200 mm; 1.33 × 1.0 × 1.0 mm voxels). A 6-minute resting-state fMRI scan was acquired using a T2*-weighted echo-planar pulse imaging (EPI) sequence (41 slices; TR = 2500 ms; TE = 30 ms; 64 × 64 matrix; FOV = 1680 mm; 3 × 3 × 3 mm voxels). Subjects were instructed to relax with their eyes open looking at a blank screen.
MRI Preprocessing and Data Analysis
Cortical Thickness
To analyze whether patients exhibited cortical GM alterations before and after treatment, as well as GM differences compared to healthy control subjects, we performed cortical thickness analysis using FreeSurfer (http://surfer.nmr.mgh.harvard.edu). MPRAGE preprocessing steps included (1) intensity normalization, (2) skull stripping, (3) Talairach transformation, (4) hemispheric separation, (5) tissue segmentation, (6) identification of white surface and pial surface, (7) cortical parcellation, and (8) registration to the average surface map. Finally, a 7-mm full-width half-maximum (FWHM) Gaussian smoothing kernel was applied.
Cortical thickness analysis was performed at the whole brain level. To control for variability in head size, the total intracranial volume (TIV) was extracted for each subject and entered as a variable of no interest. To control for possible laterality effects in patients, we flipped the brains of patients with their right lower extremity affected to match them with the remaining patients affected on the left extremity. Accordingly, the reported results will be interpreted as affected-unaffected rather than left-right. Results were corrected for multiple comparisons based on Monte Carlo permutations with 5,000 iterations using AlphaSim (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim). Using an image-wide threshold of p < .01 and a Bonferroni-corrected p < 0.025 (to correct for each hemisphere), AlphaSim simulations revealed that 76 contiguous vertices were required for clusters to be significant.
Subcortical Volume
To investigate subcortical GM volume differences between patients and controls, and treatment-induced changes in patients, we performed voxel-based morphometry (VBM) using FSL-VBM (Douaud et al. 2007). Preprocessing steps included (1) brain extraction, (2) tissue-type segmentation into GM, white matter (WM), and cerebrospinal fluid (CSF), (3) nonlinear registration of GM partial volume maps to MNI152 standard space, (4) creation of a study-specific GM template, (5) nonlinear registration of GM images to this study-specific GM template, and (6) modulation to correct for local expansion or contraction (i.e., by dividing them by the Jacobian of the warp field). Finally, the modulated registered GM images were smoothed with a FHWM kernel of 7.05 mm (i.e., sigma = 3).
Subcortical GM volume analysis was restricted to a subcortical mask that contained thalamus (Thal), caudate (Cau), putamen (Put), hippocampus (Hippo), nucleus accumbens (NAc), amygdala (Amyg), and hypothalamus (Hypo). This mask was created using the Harvard–Oxford Subcortical Structural Atlas in FSL (http://www.cma.mgh.harvard.edu/). Similarly to the cortical thickness analysis, we controlled for possible laterality effects by matching patients’ side of pain/injury. The distribution of z-statistics was not centered around zero, hence we used Gaussian mixture modeling (GMM) to correct for multiple comparisons, a standard technique to classify data into multiple categories similar to other dynamic statistical thresholding techniques such as false discovery rates (Pendse et al. 2009). Additionally, a minimum cluster criterion of 16 voxels in standard space was implemented to identify significant subcortical GM clusters.
Strength of dlPFC-PAG FC
To investigate whether patients exhibit FC changes as a result of treatment, we measured FC changes from the dlPFC, which was shown to have greater cortical GM in patients after treatment (see Figure 3), to the PAG. The PAG is a key structure in the pain modulatory system with extensive connections to the prefrontal cortex (PFC). To measure the dlPFC-PAG FC in patients, fMRI scans underwent standard preprocessing steps that included (1) filtering of 0.01–0.1 Hz, (2) removal of first 4 volumes, (3) brain extraction, (4) motion-correction, (5) linear registration to anatomical space, and (6) nonlinear registration to MNI152 standard space. Scans were smoothed using a 5-mm FWHM kernel (i.e., sigma = 2) and were excluded if motion > 3 mm was detected.
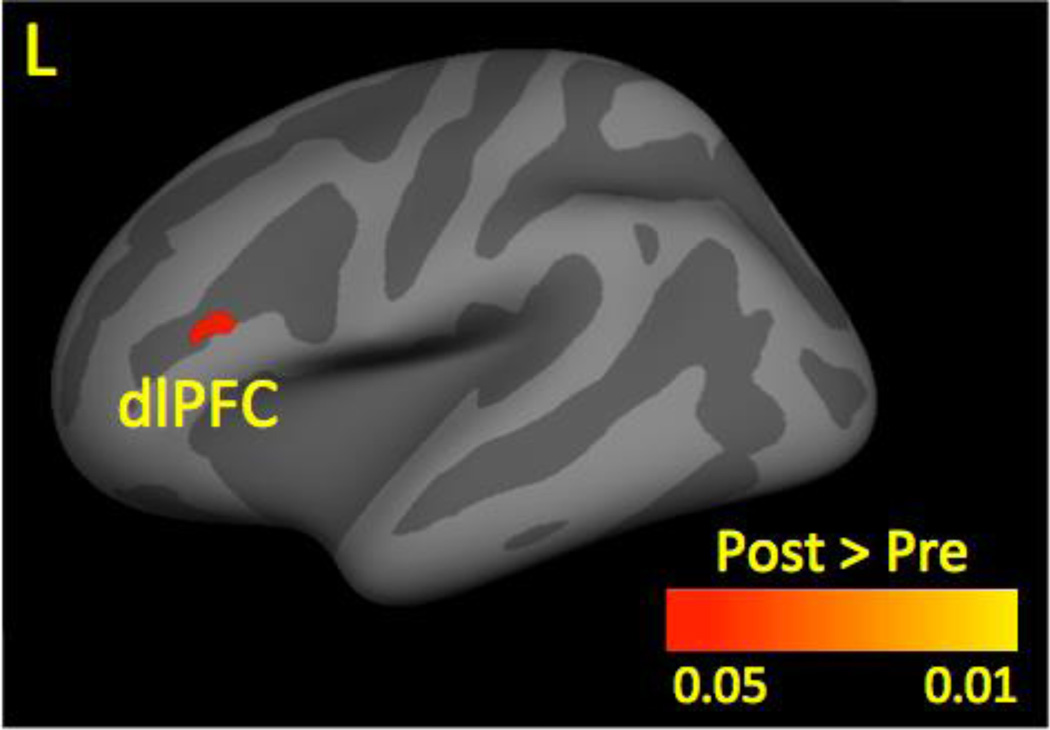
Figure 3.
Cortical Gray Matter Thickness Before and After Treatment. At posttreatment, patients had increased gray matter thickness (shown in red-yellow) in the dlPFC compared to pretreatment.
Result was corrected for multiple comparison using p < 0.025. Left refers to the affected side.
Key: dlPFC, dorsolateral prefrontal cortex; L, left.
To control for motion, we entered the 6 motion parameters (i.e., 3 rotation and 3 translation) that were generated from motion correction as variables of no interest. Furthermore, we included WM and CSF into our model. To do so, the segmented and brain-extracted MPRAGE WM and CSF maps were registered to each subject’s functional space and thresholded at 80%. Average time courses were then extracted and entered into the first-level analysis as variables of no interest. Similarly to the structural analysis, we matched the injury/pain site in all patients by flipping the brains of patients with their right side affected.
Seeds for FC analysis were defined in MNI152 2 mm standard space. The dlPFC seed was placed around the peak voxel coordinate that resulted from cortical thickness analysis between pretreatment and posttreatment from the treatment in patients (xyz MNI coordinates −46, 26, 12); the PAG seed was positioned around coordinates 0, −32, −12. Spheres of 6 mm were drawn around each seed, which were then registered to each patient’s functional space. Finally, average time series for each seed were extracted from the preprocessed EPI scans.
Values for each time course were normalized using z-scores and the FC strength was calculated using Pearson product-moment correlations between dlPFC and PAG in patients before and after treatment.
Pain Intensity and Psychological Assessment
Patients reported their overall pain intensity on an 11-point NRS (0 = no pain; 10 = worst pain imaginable) at each study visit. Patients also completed a battery of psychological measures, including the Child Depression Inventory (CDI) (Kovacs 1985), Multidimensional Anxiety Scale for Children (MASC) (March et al. 1997), Pain Catastrophizing Scale – Child Version (PCS-C) (Crombez et al. 2003), Fear of Pain Questionnaire (FOPQ) (Simons et al. 2011), and Functional Disability Inventory (FDI) (Walker and Greene 1991).
Changes in pain intensity between study visits were calculated using paired t-tests in SPSS 21 (IBM Corp., Armonk, NY). Similarly, changes in CDI, MASC, PCS, FOPQ, and FDI between study time points were analyzed with paired t-tests.
We investigated whether depression, anxiety, pain catastrophizing, fear of pain, and functional disability were related to cortical and subcortical GM correlates. To do so, we computed Pearson product-moment correlations between questionnaire scores and significant GM regions. Pain intensity ratings were available for all patients. CDI, MASC, FOPQ, and FDI scores were available in 15 patients, whereas only 9 patients completed the PCS (see Table 3). Thus, findings involving the PCS need to be interpreted with caution.
Table 3.
Pain intensity and questionnaire scores at pretreatment and posttreatment in patients.
| Scales and Subscales | N | Mean ± SE Pretreatment |
Mean ± SE Posttreatment |
T | P |
|---|---|---|---|---|---|
| Pain Intensity | 20 | 7.0 ± 0.4 | 3.3 ± 0.6 | 6.0 | < 0.001 |
| CDI Total | 15 | 54.8 ± 2.4 | 46.1 ± 2.1 | 3.7 | 0.002 |
| CDI Negative Mood | 15 | 52.9 ± 3.1 | 45.8 ± 2.3 | 2.4 | 0.032 |
| CDI Interpersonal Problems | 15 | 50.6 ± 2.3 | 48.0 ± 1.8 | 1.2 | 0.263 |
| CDI Ineffectiveness | 15 | 51.8 ± 2.4 | 45.1 ± 1.9 | 3.2 | 0.007 |
| CDI Anhedonia | 15 | 58.9 ± 2.2 | 49.9 ± 2.2 | 5.1 | < 0.001 |
| CDI Self-Esteem | 15 | 49.6 ± 2.6 | 44.4 ± 2.4 | 1.9 | 0.084 |
| MASC Total | 15 | 47.7 ± 3.4 | 44.2 ± 3.2 | 1.3 | 0.223 |
| MASC Tense/Restless | 15 | 48.1 ± 2.5 | 45.0 ± 2.0 | 1.3 | 0.225 |
| MASC Somatic/Autonomic | 15 | 45.6 ± 2.3 | 42.3 ± 1.8 | 1.6 | 0.124 |
| MASC Physical Symptoms | 15 | 46.4 ± 2.5 | 43.1 ± 2.1 | 1.4 | 0.181 |
| MASC Perfectionism | 15 | 50.3 ± 2.6 | 49.6 ± 3.0 | 0.2 | 0.813 |
| MASC Anxious Coping | 15 | 45.1 ± 2.8 | 42.9 ± 3.1 | 1.5 | 0.152 |
| MASC Harm Avoidance | 15 | 46.7 ± 2.9 | 44.9 ± 3.2 | 1.0 | 0.342 |
| MASC Humiliation Rejection | 15 | 49.6 ± 3.1 | 45.4 ± 2.5 | 1.5 | 0.153 |
| MASC Performance Fears | 15 | 53.3 ± 3.4 | 50.3 ± 3.7 | 1.0 | 0.345 |
| MASC Social Anxiety | 15 | 51.5 ± 2.8 | 47.3 ± 2.6 | 1.7 | 0.114 |
| MASC Separation/Panic | 15 | 52.2 ± 4.0 | 49.5 ± 3.4 | 0.9 | 0.395 |
| MASC Anxiety Disorder | 15 | 50.1 ± 2.6 | 44.6 ± 2.0 | 2.5 | 0.026 |
| PCS Total | 9 | 22.7 ± 4.7 | 19.0 ± 4.7 | 1.0 | 0.326 |
| PCS Rumination | 9 | 9.9 ± 1.3 | 9.1 ± 1.2 | 0.9 | 0.402 |
| PCS Magnification | 9 | 3.6 ± 1.2 | 3.4 ± 1.2 | 0.1 | 0.902 |
| PCS Helplessness | 9 | 9.2 ± 2.4 | 6.4 ± 2.5 | 1.4 | 0.213 |
| FDI Total | 15 | 30.5 ± 2.4 | 8.3 ± 1.6 | 11.0 | <0.001 |
| FOPQ Total | 15 | 46.4 ± 4.6 | 23.3 ± 3.8 | 4.5 | < 0.001 |
| FOPQ Avoidance | 15 | 20.5 ± 2.6 | 8.5 ± 1.6 | 4.8 | < 0.001 |
| FOPQ Fear | 15 | 25.9 ± 2.7 | 14.7 ± 2.5 | 3.7 | 0.002 |
Key: CDI, Children Depression Inventory;FDI, Functional Disability Inventory; FOPQ, Fear of Pain Questionnaire; MASC, Multidimensional Anxiety Scale for Children; N, number of patients; PCS, Pain Catastrophizing Scale;SE, standard error.
We also explored whether changes in depression, anxiety, pain catastrophizing, fear of pain, and functional disability after treatment were related to volumetric changes of the entire brain structures or associated with regional changes within a specific brain structure (i.e., entire hippocampal volume versus volume of the anterior part of the hippocampus). Hence, for each patient and each time point, we extracted the volume of the Hippo, Amyg, Cau, Put, and Thal (i.e., brain areas with significant regional changes; see Figure 4 and Table 2) from FreeSurfer’s automated subcortical segmentation. We computed Pearson product-moment correlations between the volume of each of those structures and CDI, MASC, PCS, FOPQ, and FDI scores. Finally, we also tested for overall volumetric differences in those structures before and after treatment using paired t-tests.
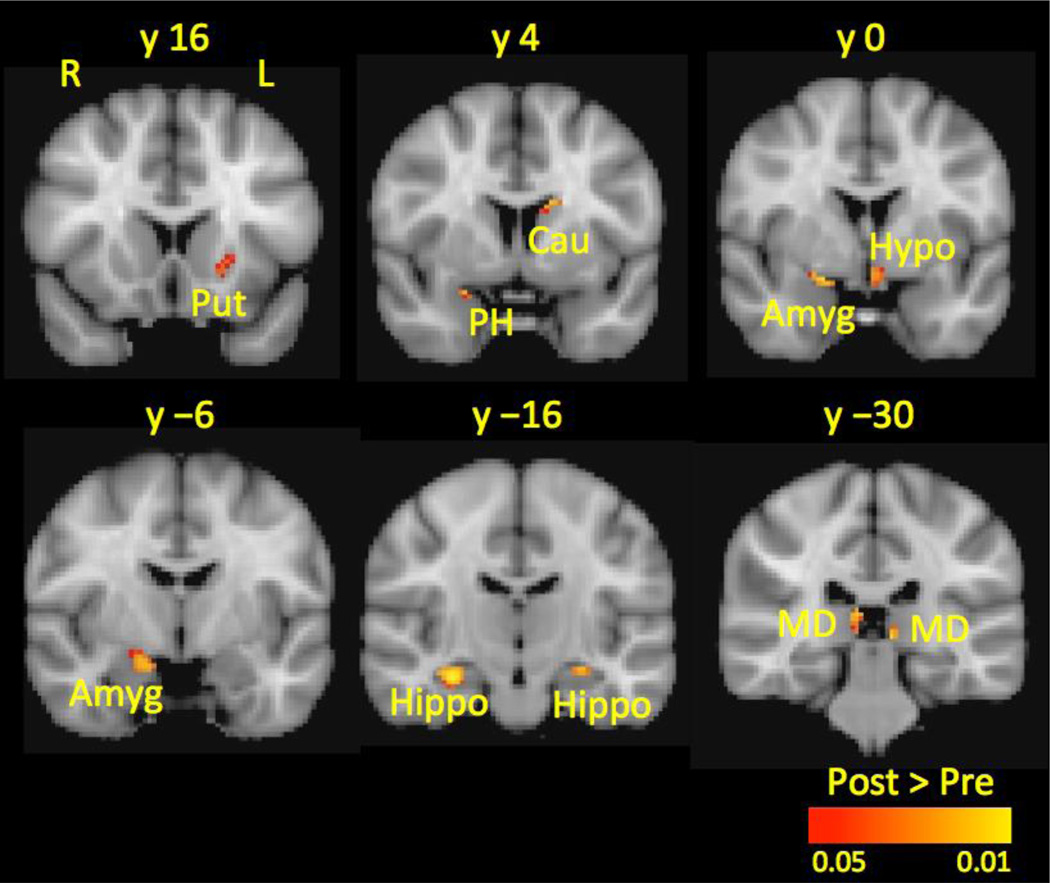
Figure 4.
Subcortical Gray Matter Volume Before and After Treatment. At posttreatment, patients had increased gray matter volume (shown in red-yellow) in the Cau, Put, PH, Hypo, MD Thal, Amyg, and Hippo compared to pretreatment.
All results were corrected for multiple comparisons using Gaussian mixture modeling. Left refers to the affected side and right refers to the unaffected side.
Key: Amyg, amygdala; Cau, caudate; Hippo, hippocampus; Hypo, hypothalamus; L, left; MD Thal, mediodorsal thalamus; PH, parahippocampal gyrus; Put, putamen; R, right.
Table 2.
Cortical and subcortical gray matter increases at posttreatment compared to pretreatment.
| Brain Region | Vertices/ Voxels |
MNI Coordinates | T | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Cortical Thickness | |||||
| Dorsolateral prefrontal cortex | 158 | −46 | 26 | 12 | 2.82 |
| Subcortical Volume | |||||
| Hippocampus | 244 | 30 | −14 | −16 | 2.86 |
| Mediodorsal thalamus | 112 | 8 | −32 | 10 | 1.97 |
| Hippocampus | 35 | −26 | −18 | −14 | 2.11 |
| Caudate | 31 | −14 | 6 | 22 | 2.40 |
| Hypothalamus | 29 | −2 | 2 | −10 | 1.84 |
| Mediodorsal thalamus | 25 | −8 | −32 | 0 | 2.31 |
| Putamen | 22 | −24 | 18 | 0 | 1.43 |
| Hippocampus | 16 | −24 | −12 | −26 | 1.80 |
Key: MNI, Montreal Neurological Institute.
Results
Participants
Supplementary Figure 1 summarizes the patient samples and analyses strategies. Disease effects were studied in 21 patients (12 females, 9 males; average age ± SD = 13.3 ± 2.5 years; 11 left affected, 10 right affected) and compared with age- and sex-matched healthy controls. Treatment effects were studied in a cohort of 20 patients (13 females, 7 males; average age = 13.5 ± 2.4 years; 12 left affected, 10 right affected) before and after treatment. Eighteen patients were used to study both disease and treatment effects. Data of 3 additional patients with pretreatment visits only (i.e., these patients did not enter PPRC) were added to the sample to study disease effects. Data of 2 additional patients with both pretreatment and posttreatment visits but no healthy control match were added to the sample to analyze treatment effects. Accordingly, a total of 23 right-handed patients (14 females, 9 males) with unilateral lower-extremity CRPS (13 left affected, 10 right affected) aged 9–17 years (average age = 13.2 ± 2.4 years) provided informed consent to participate in the study. A subsample of 12 patients with CRPS has been previously used to investigate FC changes of specific brain regions (i.e., habenula, amygdala) to the rest of the brain in patients with CRPS (Erpelding et al. 2014; Simons, et al. 2014). All study participants were right-handed.
Disease effects: Patients versus Controls
There were numerous cortical and subcortical GM differences between patients and healthy controls.
Cortical Thickness
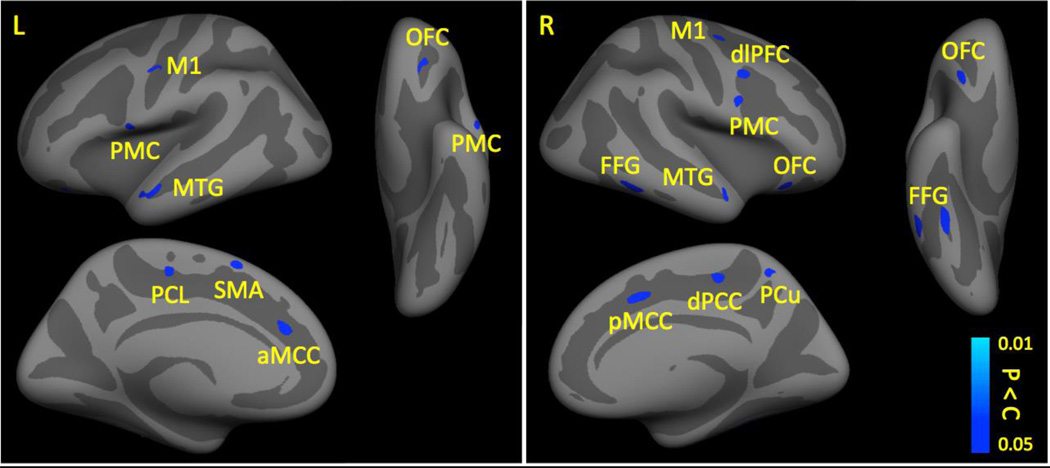
Compared to healthy controls, patients exhibited less cortical GM in pain-related brain regions such as the primary motor cortex (M1), premotor cortex (PMC), supplementary motor area (SMA), paracentral lobule (PCL), middle temporal gyrus (MTG), orbitofrontal cortex (OFC), dlPFC, anterior midcingulate cortex (aMCC), posterior midcingulate cortex (pMCC), dorsal posterior cingulate cortex (dPCC), fusiform gyrus (FFG), and precuneus (PCu) (see Table 1 and Figure 1). Patients did not have any brain areas with increased cortical GM thickness compared to healthy controls.
Table 1.
Cortical and subcortical gray matter differences between patients and healthy controls.
| Brain Region | Vertices/ Voxels |
MNI Coordinates | T | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Cortical Thickness | |||||
| Patients < Controls | |||||
| Occipital cortex | 608 | 41 | −89 | −5 | 3.64 |
| Temporal cortex/Occipital | 266 | 59 | −23 | −2 | 3.65 |
| Perirhinal cortex | 254 | 34 | −4 | −29 | 3.26 |
| Fusiform gyrus | 212 | 41 | −49 | −16 | 3.65 |
| Middle temporal gyrus | 198 | −50 | −9 | −21 | 3.40 |
| Anterior midcingulate cortex | 184 | 16 | 14 | 38 | 3.99 |
| Fusiform gyrus | 170 | 56 | −51 | −14 | 3.18 |
| Occipital cortex | 161 | 44 | −76 | 24 | 3.38 |
| Temporal cortex | 158 | 41 | −6 | −42 | 3.46 |
| Occipital cortex | 142 | 9 | −66 | 10 | 2.84 |
| Premotor cortex | 138 | −57 | 5 | 2 | 3.34 |
| Dorsolateral prefrontal cortex | 135 | 43 | 7 | 36 | 3.01 |
| Orbitofrontal cortex | 124 | 35 | 27 | −23 | 2.85 |
| Premotor cortex | 120 | 55 | 10 | 23 | 3.22 |
| Dorsal posterior cingulate cortex | 111 | 17 | −24 | 47 | 2.84 |
| Precuneus | 108 | 11 | −48 | 57 | 2.22 |
| Primary motor cortex | 104 | 30 | −6 | 49 | 2.65 |
| Inferior temporal gyrus | 100 | 57 | −10 | −24 | 2.70 |
| Entorhinal cortex | 99 | 25 | −26 | −19 | 2.66 |
| Primary motor cortex | 95 | −47 | −5 | 38 | 2.40 |
| Anterior midcingulate cortex | 94 | −14 | 36 | 16 | 2.79 |
| Middle temporal gyrus | 90 | 55 | 1 | −23 | 2.29 |
| Supplementary motor marea | 87 | −7 | 20 | 55 | 2.09 |
| Inferior temporal gyrus | 79 | 38 | −34 | −21 | 2.71 |
| Paracentral lobule | 76 | −10 | −19 | 50 | 2.52 |
| Entorhinal cortex | 76 | 26 | −9 | −33 | 2.62 |
| Subcortical Volume | |||||
| Patients < Controls | |||||
| Putamen | 1113 | 16 | 12 | −10 | 2.62 |
| Caudate | 651 | −8 | 14 | −4 | 2.67 |
| Hippocampus | 260 | −30 | −12 | −26 | 2.66 |
| Anterior thalamus | 140 | 2 | −8 | 4 | 2.10 |
| Hippocampus | 100 | 18 | −10 | −24 | 2.19 |
| Amygdala | 19 | 20 | −10 | −10 | 1.96 |
| Patients > Controls | |||||
| Mediodorsal thalamus | 95 | 20 | −24 | 14 | 2.83 |
| Hippocampus | 48 | 24 | −26 | −12 | 2.27 |
Key: MNI, Montreal Neurological Institute.
Figure 1.
Cortical Gray Matter in Patients and Healthy Controls. Compared to controls, patients had decreased cortical gray matter (shown in blue-lightblue) in the M1, PMC, SMA, PCL, MTG, PCL, aMCC, pMCC, OFC, dlPFC, dPCC, PCu, and FFG.
All results were corrected for multiple comparisons at p < 0.025. Left refers to the affected side and right refers to the unaffected side.
Key: aMCC, anterior midcingulate cortex; dlPFC, dorsolateral prefrontal cortex; dPCC, dorsal posterior cingulate cortex; FFG, fusiform gyrus; L, left; M1, primary motor cortex; MTG, middle temporal gyrus; OFC, orbitofrontal cortex; PCL, paracentral lobule; PMC, premotor cortex; pMCC, posterior midcingulate cortex; PCu, precuneus; R, right; SMA, supplementary motor area.
Subcortical Volume
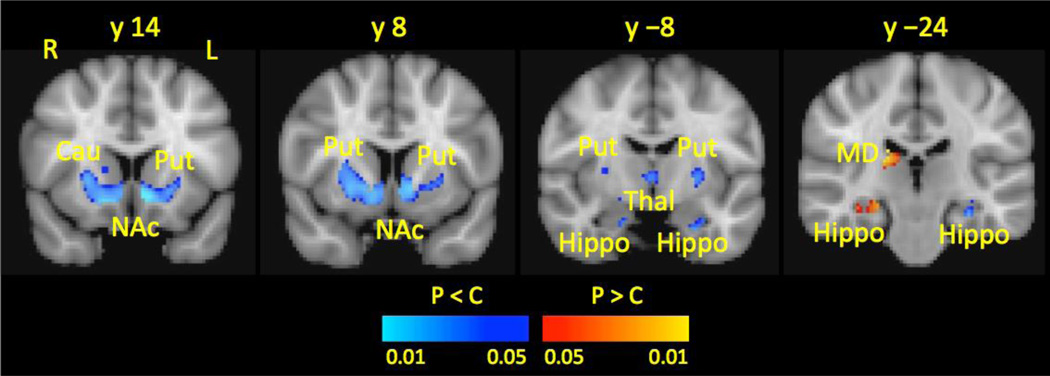
Compared to healthy controls, patients had reduced GM volume in a cluster that included the Cau, Put, and NAc. Furthermore, patients had reduced GM volume in the anterior Thal, Amyg, and anterior Hippo. Conversely, patients had increased GM in the mediodorsal (MD) Thal and the posterior Hippo compared to healthy controls (see Table 1 and Figure 2).
Figure 2.
Subcortical Gray Matter in Patients Compared to Healthy Controls. Compared to healthy controls, patients had reduced gray matter volume (shown in blue-lightblue) in the Cau, Put, NAc, anterior Thal, and anterior Hippo and increased gray matter (shown in red-yellow) in the MD Thal and posterior Hippo.
All results were corrected for multiple comparisons using Gaussian mixture modeling. Left refers to the affected side and right refers to the unaffected side.
Key: Cau, caudate; Hippo, hippocampus; L, left; MD, mediodorsal thalamus; NAc, nucleus accumbens; Put, putamen; R, right; Thal, thalamus.
Treatment effects: Pretreatment versus Posttreatment
We found several prominent changes in patients between study visits.
Treatment-Induced Cortical Thickness Changes
Compared to pretreatment, patients had greater cortical GM in the dlPFC (see Table 2 and Figure 3) at posttreatment. Patients did not exhibit brain regions with significantly reduced cortical GM at posttreatment compared to pretreatment.
Treatment-Induced Subcortical Volume Changes
Patients exhibited increased GM volume in the Put, Cau, parahippocampal gyrus (PH), Hypo, and MD Thal at posttreatment compared to pretreatment. Following treatment, patients also had more GM in a cluster spanning over the Hippo and Amyg (see Table 2 and Figure 4). There were no brain regions with decreased GM volume at posttreatment compared to pretreatment.
Treatment-Induced Whole Structure Volume Changes
Since our results indicated that there are significant treatment-induced changes in GM thickness and volume, we investigated if volumetric changes occur throughout entire GM structures or if they occur in subregions of a given brain area. To do this, we assessed overall GM volumes for Cau, Put, Thal, Hippo, and Amyg (i.e., regions that yieled significant regional GM changes, see Figure 4 and Table 2) before and after treatment. Our results revealed that patients did not exhibit any treatment-related overall volumetric GM changes in those regions (all p > 0.05).
Treatment-Induced FC Changes between the dlPFC and the PAG
We investigated whether structural GM changes were related to functional changes in the brain. Accordingly, we measured the FC strength between the dlPFC, which was found to have thicker cortical GM after treatment (see Figure 3), and the PAG, one of the key structure of the descending pain modulatory system with extensive projections to the PFC. Data of 2 patients had to be excluded: one because of excessive motion (> 3 mm) and one because of signal noise in the EPI scan, so that the FC analysis was performed with data from 18 patients.
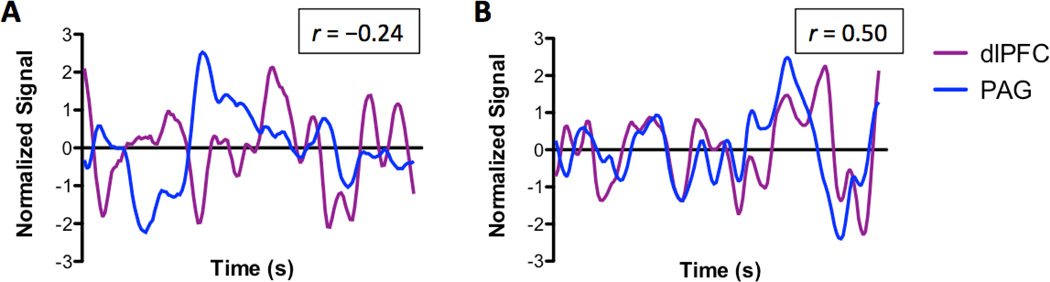
Before treatment, we found a significant negative correlation (r = −0.24; p < 0.01) between the functional activity of the dlPFC and the PAG (see Figure 5A). Intriguingly, after treatment, the FC between the dlPFC and PAG resulted in a significant positive correlation (r = 0.50; p < 0.01) (see Figure 5B), indicating that succesful treatment may enhance the synchronicity between the dlPFC and PAG.
Figure 5.
Normalized Resting State Activity in the dlPFC and PAG at Pretreatment (panel A) and Posttreatment (panel B). Before treatment, the resting (i.e., spontaneous) activity between the dlPFC (shown in purple) and the PAG (shown in blue) was negatively correlated (r = −0.24, p < 0.01). Contrary, at posttreatment, the neural activity between the dlPFC and the PAG was positively correlated (r = 0.50; p < 0.01).
Key: dlPFC, dorsolateral prefrontal cortex; PAG, periaqueductal gray.
Pain Intensity and Psychological Measures
Patients’ pain intensity and CDI, MASC, PCS, FOPQ, and FDI scores at each study visit are summarized in Table 3. Patients reported a clinically significant reduction in pain intensity of 3.7 ± 0.7 points from pretreatment (pain intensity ± SE = 7.0 ± 0.4) to posttreatment (pain intensity = 3.3 ± 0.6) (t (19) = 6.0, p < 0.05). Following treatment, patients also had significantly reduced depression, anxiety, functional disability, and fear of pain (all p < 0.05; see Table 3).
Brain GM and Psychological Measures
We found significant correlations between brain GM and behavioral measures (see Supplementary Figure 2). Our analysis revealed a negative correlation between the GM change in the dlPFC and the overall CDI (r = −0.53; p < 0.05), as well as between dlPFC thickness change and the CDI subscales anhedonia (r = −0.50; p < 0.05) and self–esteem (r = −0.60; p < 0.01), indicating that lower levels of depression were associated with greater changes in the dlPFC (see Supplementary Figure 2A).
Furthermore, we found a significant positive correlation between the GM volume in the Cau and the MASC subscale assessing physical anxiety symptoms (r = 0.53; p < 0.05) at pretreatment, suggesting that patients with more severe restlessness and other anxiety-related physical symptoms before treatment had greater caudal volume (see Supplementary Figure 2B).
Our correlational analyses further showed a link between the GM volume in the Cau and pain catastrophizing. Specifically, we found that the Cau GM volume was positively correlated to overall PCS (r = 0.76; p < 0.01), PCS helplessness (r = 0.75; p < 0.01), and PCS magnification (r = 0.83; p < 0.01) before treatment. At posttreatment, the Cau GM volume correlated positively with overall PCS (r = 0.78; p < 0.01) and PCS helpnessness (r = 0.80; p < 0.01) (see Supplementary Figure 2C). These correlations suggest that patients with higher pain catastrophizing, magnification, and helplessness had greater GM volumes in the Cau.
Our analyses also revealed that changes in GM between study visits were related to pain catastrophizing. For example, we found a positive correlation between the Hippo volume before treatment and overall PCS (r = 0.81; p < 0.01), PCS helplessness (r = 0.74; p < 0.05), PCS magnification (r = 0.83; p < 0.01), and PCS rumination (r = 0.73; p < 0.05) (see Supplementary Figure 2D). Similarly, this same effect was observed between GM volume in the Hippo after treatment and overall PCS (r = 0.71; p < 0.05), PCS helplessness (r = 0.68; p < 0.05), PCS magnification (r = 0.70; p < 0.05), and PCS rumination (r = 0.71; p < 0.05) (see Supplementary Figure 2D). Accordingly, these results suggest that patients with a higher tendency for pain catastrophizing, rumination, magnification, and helplessness exhibted a greater GM volume in the Hippo, both before and after treatment.
We also investigated the relationship between GM and pain intensity due to treatment and did not find any significant correlation (all p > 0.05), indicating that GM plastcity in our identified brain regions may not be related to patients’ sensory changes before and after treatment.
Since our results indicated that there is a significant link between changes in GM and behavioral improvement, we investigated whether behavioral measures were correlated with whole structure volumes (see Treatment-Induced Whole Structure Volume Changes above) but did not find any significant correlation (all p > 0.05).
Discussion
This is the first study to report brain differences compared to controls as well as treatment-induced brain changes in children and adolescents. Compared to controls, patients with CRPS had significant GM differences in pain-related cortical (M1, SMA, PMC, PCL, aMCC, pMCC, dPCC, OFC, dlPFC, and PCu) and subcortical areas (Cau, Put, NAc, Thal, and Hippo). Accordingly, our findings suggest that there are significant underlying morphometric differences between children with CRPS and healthy controls. Following treatment, patients exhibited significant behavioral improvement (i.e., lower scores in depression, anxiety, fear of pain, and functional disability), as well as structural (increased GM in dlPFC, Put, Cau, MD Thal, PH, Hippo and Amyg) and functional brain alterations (e.g., enhanced dlPFC-PAG FC). These results support prior animal data (Metz et al. 2009) indicating that rapid alterations in brain structure and function can occur with targeted psychophysical therapies.
Disease Effect (Patients versus Controls)
Cortical Thickness
Compared to healthy controls, patients had cortical thinning in multiple regions, including the M1, SMA, PMC, PCL, aMCC, pMCC, dPCC, OFC, dlPFC, and PCu. Symptoms such as high levels of spontaneous pain, allodynia, hyperalgesia, changes in motor control (Schilder et al. 2012), and limb protection and/or disuse (Bruehl and Chung 2006) may relate to cortical thinning in the SMA, M1, PMC, and PCL as these brain areas are primarily involved in motor function, motor planning, and chronic pain processing and shown to be altered in CRPS (Juottonen et al. 2002; Maihöfner et al. 2007; Schwenkreis et al. 2009; Kirveskari et al. 2010; Barad et al. 2014; Pleger et al. 2014).
Patients displayed cortical thinning in subregions of the cingulate cortex, notably in the dPCC and aMCC, compared to healthy controls. The aMCC is a key structure involved in controlling pain, cognition, and negative emotions (Vogt 2005; Shackman et al. 2011). Given that the aMCC has extensive connections to limbic brain regions such as the Amyg (Morecraft et al. 2007) and the NAc (Kunishio and Haber 1994), it likely acts as a hub where pain and other negative events are evaluated and integrated. Conversely, the dPCC is thought to be involved in organizing motor responses to pain (Vogt et al. 2006). Taken together, decreased GM in the aMCC and dPCC in patients may thus provide a structural correlate for altered affective and nocifensive processing.
Similarly, we found that patients had less cortical GM in the OFC compared to healthy controls. The OFC is critically involved in the cognitive and affective evaluation of external stimuli (Kringelbach 2005) and the coordination of goal-directed behavior (Schoenbaum et al. 2009). Chronic pain has been shown to impair decision-making processes in rats which coincided with decreased dopamine levels in the OFC, thus providing support for the notion that chronic pain may affect brain regions relevant for cognitive-affective reappraisal and strategic planning (Pais-Vieira et al. 2009). The cortical thinning in the OFC seen in these patients may impact its communication with other brain regions and may therefore result in altered emotional and cognitive regulation processes, which may contribute to increased sensory and affective pain behavior.
Our results also showed cortical thinning in the dlPFC in patients compared to healthy controls. The dlPFC is important for cognition and executive functions. Human imaging studies have repeatedly demonstrated functional and structural alterations in the dlPFC in various chronic pain disorders (Grachev et al. 2000; Apkarian et al. 2004; Kwan et al. 2005; Schmidt-Wilcke et al. 2006; Becerra et al. 2006; DaSilva et al. 2008; Rodriguez-Raecke et al. 2009; Seminowicz et al. 2010; Seo et al. 2012; Bolwerk et al. 2013; Ceko et al. 2013; Jin et al. 2013; Barad et al. 2014). The etiology of dlPFC alterations in chronic pain disorders remains unknown, however, it is possible that the thinning seen in patients may result from the constant sensory input from regions such as the Thal (Mesulam 1998), which may result in comorbid clinical problems such as depression and anxiety (see Brain GM and Psychological Measures below). Moreover, given that the dlPFC has pain modulatory functions, GM thinning may also reflect defective pain inhibitory mechanisms in chronic pain.
Subcortical Volume
We found GM alterations in the basal ganglia (including the NAc), MD Thal, Amyg, and Hippo in patients compared to controls. Basal ganglia structures such as Cau and Put are thought to play a key role in pain and analgesia (reviewed in Borsook et al., 2010). In addition, patients may exhibit movement disorders that likely arise from alterations in basal ganglia networks (Schwartzman and Kerrigan 1990; Birklein et al. 2000; Marinus et al. 2011; Bank et al. 2013). The lower GM in the NAc in patients may reflect the hedonic tone in patients with chronic pain and are consistent with prior findings in adults patients (Seymour et al. 2005; Scott et al. 2006; Becerra and Borsook 2008; Baliki et al. 2010; Navratilova et al. 2012). Given that the functional activity in the NAc is dependent on the signal valence of the painful stimulus (Becerra and Borsook 2008) and that its FC to other mesolimbic structures predicts the transition from acute to chronic pain (Baliki et al. 2012), the NAc atrophy in patients seen in this study may potentially relate to the presence of a permanent low reward/high punishment situation due to the constant nociceptive input.
Treatment Effects (Pretreatment versus Posttreatment)
Cortical Thickness
Following treatment, patients had cortical thickening in the dlPFC, which was also recently shown to change in structure and function with treatment in chronic low back pain (Seminowicz et al. 2011). In the latter study, the authors observed cortical thinning and reduced functional activity in response to an attention-demanding task in the dlPFC before treatment, whereas both dlPFC structure and function were restored following treatment. To date, the causality of chronic pain and associated brain changes remains largely unknown. Previous study findings (Rodriguez-Raecke et al. 2009, 2013; Seminowicz et al. 2011) support the notion that morphometric brain changes occur as a result of the chronic pain. As such, patients may be more or less resilient to a chronic pain state. For example, in a recent study (Brown et al., 2014), patients with fibromyalgia and osteoarthritis showed less activation in the dlPFC compared to healthy controls, thus providing further evidence for the involvement of the dlPFC in pain processing and pain modulation. Accordingly, given that the dlPFC is likely involved in pain modulation processes, it could be used as a potential target for treating chronic pain disorders. Transcranial magnetic stimulation (TMS) in the dlPFC, a technique frequently used to treat depression (reviewed in George, 2010), elicits analgesic effects in both experimental pain (Graff-Guerrero et al. 2005; Boggio et al. 2008; Nahmias et al. 2009; Fierro et al. 2010; Brighina et al. 2011) and clinical pain (Reid and Pridmore 2001; Brighina et al. 2004; Arul-Anandam et al. 2009; Borckardt et al. 2009). Interestingly, Brown and Jones (2013) showed that chronic pain patients who participated in a non-pharmacological (i.e., mindfulness-based) treatment program exhibited less deactivation (i.e., greater activation) in the dlPFC during pain anticipation, which was also related to improvements in psychological variables and enhanced coping strategies. Taken together, our study results corroborate previous study findings that the dlPFC may be an important pain modulatory region and that changes in structure and function are likely related to patients’ improvement following treatment.
Subcortical Volume
Following treatment, patients had significant GM volume increases in the basal ganglia, Hypo, MD Thal, Hippo, PH, and Amyg. These brain regions have been implicated with the manifestation of movement-related symptoms (basal ganglia) (Schwartzman and Kerrigan 1990; Birklein et al. 2000; Marinus et al. 2011; Bank et al. 2013), autonomic manifestations (Hypo) (Lebel et al. 2008; Barad et al. 2014), and sensory changes (MD Thal) (Fukumoto et al. 1999; Fukui 2003; Fukui et al. 2006; Shiraishi et al. 2006; Wu et al. 2006; Ushida et al. 2010; Walton et al. 2010). The Hippo contributes to stress regulation via the hypothalamic-pituitary-adrenal axis (HPA axis) by providing inhibitory feedback (Jacobson and Sapolsky 1991). Additionally, there is repeated evidence of functional and structural changes in the Hippo that occur with chronic pain (Kwan et al. 2005; Schweinhardt et al. 2008; Seminowicz et al. 2010; Younger et al. 2010; Napadow et al. 2010; Baliki et al. 2011; Robinson et al. 2011; As-Sanie et al. 2012; Mutso et al. 2012, 2014; Vachon-Presseau et al. 2013; Maleki et al. 2013; Barad et al. 2014). From a functional perspective, prolonged stressful, uncontrollable, and threatening situations, such as chronic pain, will most likely alter Hippo functioning (Borsook et al. 2012). A recent study supports this hypothesis as patients with chronic pain had higher levels of cortisol, smaller Hippo volume, and greater pain-evoked activity in the PH, a brain area implicated in anticipatory anxiety and associative learning (Vachon-Presseau et al. 2013). Given that smaller Hippo volume may increase the vulnerability to stress (Lyons et al. 2001), anxiety (Karatsoreos and McEwen 2011), and post-traumatic stress disorder (Gilbertson et al. 2002), it is conceivable that Hippo volume increases following treatment may be indicative of an increased resilience due to reductions in anxiety, stress, and pain in our patients.
Following treatment, we also observed volume increases in the Amyg in our patients. The Amyg is primarily known for its involvement in fear regulation (LeDoux 2003). However, there is emerging evidence that the Amyg is also involved in pain processing (Neugebauer et al. 2004; Lumley et al. 2011; Simons, Moulton, et al. 2014; Strobel et al. 2014). Given its anatomical and functional connections with ascending systems and descending pain pathways, and with affective and cognitive regions, GM volume increases within the Amyg could reflect improved integrated information processing in our patients after treatment. Contrary, we did not observe any treatment-induced GM changes in the NAc, may be due to delayed structural changes that may have been outside of our study time frame. Alternatively, the lack of GM alterations in the NAc may reflect ongoing brain alterations, even though patients experienced significant pain relief (Lebel et al. 2008; Linnman et al. 2013; Erpelding et al. 2014).
Strength of dlPFC-PAG FC
The FC between the dlPFC and the PAG was enhanced following treatment. The dlPFC is believed to be involved in top-down pain modulatory processes (Lorenz et al. 2003; Brighina et al. 2004; Fierro et al. 2010). Furthermore, the dlPFC is connected to other pain modulatory brain areas such as PAG (Bragin et al. 1984; An et al. 1998; Lim et al. 2009). The PAG is located in the midbrain and exerts descending pain modulatory processes. For example, electrical stimulation of the PAG in rodents induced reduced behavioral responses to noxious stimulation by inhibiting nociceptive dorsal horn neurons (Basbaum et al. 1976). Chronic pain populations exhibit brain abnormalities in descending pain modulatory circuits, and most notably in the PAG (Rocca et al. 2006; Mainero et al. 2011; Desouza et al. 2013). In patients with major depressive disorder, evoked pain resulted in reduced activity in both dlPFC and PAG (Strigo et al. 2008). Accordingly, the antagonistic resting state activity pattern between the dlPFC and PAG in patients at pretreatment may indicate a reduced ability to engage pain coping mechanisms due to altered pain inhibitory mechanisms. Following treatment, the FC between the dlPFC and PAG was significantly enhanced, suggesting better functional communication between descending pain inhibitory mechanisms at posttreatment. Alternatively, it is conceivable that changes in dlPFC-PAG FC may indicate a shift from descending facilitation processes, which have been proposed to contribute to chronic pain disorders (Porreca et al. 2002; Gebhart 2004; Suzuki et al. 2004), towards descending inhibitory processes after treatment, however, future studies will be needed to further investigate FC changes and their significance for facilitatory and inhibitory processes.
Brain GM and Psychological Measures
After treatment, patients reported significantly lower pain intensities, as well as lower depression, anxiety, fear of pain, and functional disability. In addition, we found that patients with lower depression scores at the time of posttreatment exhibited greater GM changes in the dlPFC, suggesting that the dlPFC likely has a common neural substrate of depression and pain (Grachev et al. 2003).
Furthermore, our results showed a link between brain structure and pain catastrophizing. Pain catastrophizing has been defined as “an exaggerated negative mental set brought to bear during actual or anticipated painful experience” (Sullivan et al. 2001) and measures the perceived helplessness, magnification, and rumination in the context of pain. Previously, higher pain catastrophizing has been associated with structural and functional alterations in pain-related brain regions in chronic pain (Gracely et al. 2004; Burgmer et al. 2011; Chen et al. 2011; Salomons et al. 2012; Kucyi et al. 2014). Although PCS was not available for all of our patients, the correlation between PCS and GM volume may confirm the notion that pain catastrophizing may predispose individuals to develop chronic pain. In addition, the positive correlation between catastrophizing and helplessness with the Cau, a brain region implicated in motor function and sensorimotor integration, may compound patients’ physical symptoms and disabilities due to CRPS. Furthermore, given that the Hippo is recruited in situations such as uncertainty about upcoming pain (Ploghaus et al. 1999) and exacerbation of pain by anxiety (Ploghaus et al. 2001), Hippo volume correlations with catastrophizing, helplessness, magnification, and rumination may reflect patients’ predisposition for higher expectation and increased anxiety about pain-related cues. Related to pain coping mechanisms, a recent study (Seminowicz et al. 2013) showed that cognitive-behavioral therapy in chronic pain resulted in a reduction of pain catastrophizing scores which also coincided with GM increases in somatosensory and PFC regions.
Adaptive Responses to Treatment and Brain Plasticity
The changes observed in this study suggest that interdisciplinary treatment approaches produce measurable clinical effects that overlap with objective changes in brain structure and function. With the initiation of the pain condition, plasticity from primary afferent systems to brain regions may set off alterations in plasticity in sensory and/or affective regions (Shyu and Vogt 2009), most commonly characterized by GM increases in sensory brain areas and GM decreases in pain modulatory regions. However, the pathophysiology of GM changes due to chronic pain remains poorly understood. Several underlying mechanisms have been proposed such as alterations in the number of neurons, cell volume, blood flow, and/or synaptic connectivity (Gage 2002; May et al. 2007; May 2008). Reversal of these changes due to treatment (both pharmacological and non-pharmacological) may therefore arise from alterations in one or multiple of these underlying systems. For example, the use of ketamine has been shown to induce rapid clinical improvements in CRPS (Correll et al. 2004; Becerra et al. 2009) and may thus have similar underpinnings, which have yet to be evaluated. Taken together, chronic pain states could potentially be undone through directed treatments that target and modify individuals’ structural and functional systems. Unfortunately, other factors such as stress, due to the influence of elevated levels cortisol (Vachon-Presseau et al. 2013) and cytokines (Slade et al. 2011), may induce additional morphological brain changes that may hamper and/or prevent reversal of brain changes. Accordingly, patients with chronic CRPS, which constitute the majority of adult patients with CRPS, may undergo more significant structural and functional changes due to the duration of the pain and associated maladaptive processes, which may underpin treatment resistance.
Pediatric versus Adult CRPS
This is the first study to explore GM alterations in children with CRPS (see Table 4 for comparison with adult CRPS). In adults with CRPS, Geha et al. (2008) have found decreased GM in the insula (Ins), ventromedial PFC, and NAc compared to healthy control subjects. Similarly, another study found decreased GM in the Ins and OFC (Baliki et al. 2011). Recently, GM alterations have been reported in the Put, Hypo, aMCC, pMCC, frontal orbital cortex, and dorsal Ins in adult CRPS (Barad et al. 2014). From a clinical perspective, children with CRPS typically have higher chances of recovery, likely due to enhanced brain plasticity processes during childhood and adolescence. This higher plasticity at a young age may explain GM discrepancies between adults and children, as some GM changes may occur secondary to abnormalities in pain-related regions. Contrary to the studies in adult CRPS patients, we did not find any significant correlations between pain intensity and GM, which could be due to the differences in the duration of pain and/or long-term medication use between pediatric and adult CRPS patients.
Table 4.
Cortical and subcortical brain regions altered in adult patients compared to pediatric patients with CRPS.
| Adult CRPS | Pediatric CRPS |
|---|---|
| Cortical | Cortical |
| ↓ aMCC2 | ↓ aMCC |
| ↓ OFC1,2 | ↓ OFC |
| ↓ aIns1,3 | ↓ M1 |
| ↓ dIns2 | ↓ SMA |
| ↓ vmPFC3 | ↓ PCL |
| ↓ pMCC2 | ↓ dPCC |
| ↓ dlPFC | |
| ↓ Pcu | |
| Subcortical | Subcortical |
| ↓ NAc3 | ↓ NAc |
| ↑ Put2 | ↓ Put |
| ↑ Hypo2 | ↓ Cau |
| ↓ Thal | |
| ↓ Hippo | |
| ↑ | |
| ↑ MD Thal |
Barad et al. (2013);
Key: aIns, anterior insula; aMCC, anterior midcingulate cortex; Cau, caudate; dIns, dorsal insula; dlPFC, dorsolateral prefrontal cortex; dPCC, dorsal posterior cingulate cortex; Hippo, hippocampus; Hypo, hypothalamus; M1, primary motor cortex; MD Thal, mediodorsal thalamus; NAc, nucleus accumbens; OFC, orbitofrontal cortex; PCL, paracentral lobule; PMC, premotor cortex; pMCC, posterior midcingulate cortex; Put, putamen; SMA, supplementary motor area; Thal, thalamus; vmPFC, ventromedial prefrontal cortex.
Study Limitations
There are some study limitations that need to be addressed. Our study sample included more females than males, which is comparable to the sex ratio in adult CRPS (de Mos et al. 2007). However, since most of the females were in their post-pubertal stage, it remains unknown whether sex hormones may constitute a potential confound, although the inclusion of healthy age- and sex-matched controls does mitigate this influence. Additionally, we investigated short-term treatment effects, whereas certain structural and functional changes may occur over a longer period of time. Although patients were kept on the same medications for the duration of the treatment, it is possible that medication may contribute to both disease and treatment effects observed in this study. Another potential confound is the flipping of the brains to match patients’ injury side as there may be laterality effects in brain networks (Caeyenberghs and Leemans 2014). Finally, some of the psychological measures, such as the PCS, were only available in a subgroup of patients and results therefore need to be interpreted with caution.
Conclusions
In summary, our study provides novel evidence for GM abnormalities in pain-related brain regions in pediatric CRPS. Furthermore, this is the first study to show rapid treatment-induced GM increases in brain areas involved in sensation, emotion, cognition, and pain modulation as well as enhanced FC between pain modulatory regions.
Supplementary Material
Acknowledgements
This study was primarily supported by National Institute of Neurological Disorders and Stroke Grants R01-NS-065051 and K24-NS-064050 (to D. Borsook), by National Institute of Child Health and Human Development K23 Career Development Award HD-067202 (to L. Simons), and by a Grant from the Mayday Foundation, New York. We would like to thank Charles Berde, MD PhD for his support through the Sara Page Mayo Endowment for Pediatric Pain Research. We would like to thank Simona Sava, PhD for contributing to the data collection.
References
- An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134:2197–2221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Thomas PS, Krauss BR, Szeverenyi NM. Prefrontal cortical hyperactivity in patients with sympathetically mediated chronic pain. Neurosci Lett. 2001;311:193–197. doi: 10.1016/s0304-3940(01)02122-x. [DOI] [PubMed] [Google Scholar]
- Arul-Anandam AP, Loo C, Martin D, Mitchell PB. Chronic neuropathic pain alleviation after transcranial direct current stimulation to the dorsolateral prefrontal cortex. Brain Stimul. 2009;2:149–151. doi: 10.1016/j.brs.2008.12.003. [DOI] [PubMed] [Google Scholar]
- As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, Kairys A, Williams D, Clauw DJ, Schmidt-Wilcke T. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 2012;153:1006–1014. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS One. 2011;6:e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank PJM, Peper CLE, Marinus J, Beek PJ, van Hilten JJ. Motor dysfunction of complex regional pain syndrome is related to impaired central processing of proprioceptive information. J Pain. 2013;14:1460–1474. doi: 10.1016/j.jpain.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Barad MJ, Ueno T, Younger J, Chatterjee N, Mackey S. Complex Regional Pain Syndrome is associated with structural abnormalities in pain-related regions of the human brain. J Pain. 2014;15:197–203. doi: 10.1016/j.jpain.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Clanton CH, Fields HL. Opiate and stimulus-produced analgesia: functional anatomy of a medullospinal pathway. Proc Natl Acad Sci U S A. 1976;73:4685–4688. doi: 10.1073/pnas.73.12.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain. 2008;12:866–869. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Schwartzman RJ, Kiefer RT, Rohr P, Moulton EA, Wallin D, Pendse G, Morris S, Borsook D. CNS Measures of Pain Responses Pre- and Post-Anesthetic Ketamine in a Patient with Complex Regional Pain Syndrome. Pain Med. 2009 doi: 10.1111/pme.12939. [DOI] [PubMed] [Google Scholar]
- Birklein F, Riedl B, Sieweke N, Weber M, Neundörfer B. Neurological findings in complex regional pain syndromes--analysis of 145 cases. Acta Neurol Scand. 2000;101:262–269. doi: 10.1034/j.1600-0404.2000.101004262x./. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol. 2008;15:1124–1130. doi: 10.1111/j.1468-1331.2008.02270.x. [DOI] [PubMed] [Google Scholar]
- Bolwerk A, Seifert F, Maihöfner C. Altered resting-state functional connectivity in complex regional pain syndrome. J Pain. 2013;14:1107.e8–1115.e8. doi: 10.1016/j.jpain.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Smith AR, Reeves ST, Madan A, Shelley N, Branham R, Nahas Z, George MS. A pilot study investigating the effects of fast left prefrontal rTMS on chronic neuropathic pain. Pain Med. 2009;10:840–849. doi: 10.1111/j.1526-4637.2009.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Erpelding N, Becerra L. Losses and gains: chronic pain and altered brain morphology. Expert Rev Neurother. 2013;13:1221–1234. doi: 10.1586/14737175.2013.846218. [DOI] [PubMed] [Google Scholar]
- Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73:219–234. doi: 10.1016/j.neuron.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia--insights gained through human functional imaging. Mol Pain. 2010;6:27. doi: 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin EO, Yeliseeva ZV, Vasilenko GF, Meizerov EE, Chuvin BT, Durinyan RA. Cortical projections to the periaqueductal grey in the cat: a retrograde horseradish peroxidase study. Neurosci Lett. 1984;51:271–275. doi: 10.1016/0304-3940(84)90563-9. [DOI] [PubMed] [Google Scholar]
- Brighina F, De Tommaso M, Giglia F, Scalia S, Cosentino G, Puma A, Panetta M, Giglia G, Fierro B. Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J Headache Pain. 2011;12:185–191. doi: 10.1007/s10194-011-0322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, Fierro B. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci. 2004;227:67–71. doi: 10.1016/j.jns.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Brown CA, El-Deredy W, Jones AKP. When the brain expects pain: common neural responses to pain anticipation are related to clinical pain and distress in fibromyalgia and osteoarthritis. Eur J Neurosci. 2014;39:663–672. doi: 10.1111/ejn.12420. [DOI] [PubMed] [Google Scholar]
- Brown CA, Jones AKP. Psychobiological correlates of improved mental health in patients with musculoskeletal pain after a mindfulness-based pain management program. Clin J Pain. 2013;29:233–244. doi: 10.1097/AJP.0b013e31824c5d9f. [DOI] [PubMed] [Google Scholar]
- Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113:713–725. doi: 10.1097/ALN.0b013e3181e3db38. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY. Psychological and behavioral aspects of complex regional pain syndrome management. Clin J Pain. 2006;22:430–437. doi: 10.1097/01.ajp.0000194282.82002.79. [DOI] [PubMed] [Google Scholar]
- Burgmer M, Petzke F, Giesecke T, Gaubitz M, Heuft G, Pfleiderer B. Cerebral activation and catastrophizing during pain anticipation in patients with fibromyalgia. Psychosom Med. 2011;73:751–759. doi: 10.1097/PSY.0b013e318236588a. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A. Hemispheric lateralization of topological organization in structural brain networks. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceko M, Bushnell MC, Fitzcharles M-A, Schweinhardt P. Fibromyalgia interacts with age to change the brain. NeuroImage Clin. 2013;3:249–260. doi: 10.1016/j.nicl.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JY-W, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 2011;1392:121–131. doi: 10.1016/j.brainres.2011.03.069. [DOI] [PubMed] [Google Scholar]
- Correll GE, Maleki J, Gracely EJ, Muir JJ, Harbut RE. Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004;5:263–275. doi: 10.1111/j.1526-4637.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104:639–646. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Pendse G, Chizh B, Tully S, Borsook D. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS One. 2008;3:e3396. doi: 10.1371/journal.pone.0003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mos M, de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BHC, Sturkenboom MCJM. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Desouza DD, Moayedi M, Chen DQ, Davis KD, Hodaie M. Sensorimotor and Pain Modulation Brain Abnormalities in Trigeminal Neuralgia: A Paroxysmal, Sensory-Triggered Neuropathic Pain. PLoS One. 2013;8:e66340. doi: 10.1371/journal.pone.0066340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Erpelding N, Sava S, Simons LE, Lebel A, Serrano P, Becerra L, Borsook D. Habenula functional resting-state connectivity in pediatric CRPS. J Neurophysiol. 2014;111:239–247. doi: 10.1152/jn.00405.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro B, De Tommaso M, Giglia F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex. Exp Brain Res. 2010;203:31–38. doi: 10.1007/s00221-010-2206-6. [DOI] [PubMed] [Google Scholar]
- Freund W, Wunderlich AP, Stuber G, Mayer F, Steffen P, Mentzel M, Weber F, Schmitz B. Different activation of opercular and posterior cingulate cortex (PCC) in patients with complex regional pain syndrome (CRPS I) compared with healthy controls during perception of electrically induced pain: a functional MRI study. Clin J Pain. 2010;26:339–347. doi: 10.1097/AJP.0b013e3181cb4055. [DOI] [PubMed] [Google Scholar]
- Fukui S. Evaluation of thalamic neural activity in CRPS type 1 patients by proton MR spectroscopy: a correlative study with rCBF. J Anesth. 2003;17:142–144. doi: 10.1007/s005400300033. [DOI] [PubMed] [Google Scholar]
- Fukui S, Matsuno M, Inubushi T, Nosaka S. N-Acetylaspartate concentrations in the thalami of neuropathic pain patients and healthy comparison subjects measured with (1)H-MRS. Magn Reson Imaging. 2006;24:75–79. doi: 10.1016/j.mri.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Fukumoto M, Ushida T, Zinchuk VS, Yamamoto H, Yoshida S. Contralateral thalamic perfusion in patients with reflex sympathetic dystrophy syndrome. Lancet. 1999;354:1790–1791. doi: 10.1016/S0140-6736(99)03746-0. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27:729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS. Transcranial magnetic stimulation for the treatment of depression. Expert Rev Neurother. 2010;10:1761–1772. doi: 10.1586/ern.10.95. [DOI] [PubMed] [Google Scholar]
- Gieteling EW, van Rijn MA, de Jong BM, Hoogduin JM, Renken R, van Hilten JJ, Leenders KL. Cerebral activation during motor imagery in complex regional pain syndrome type 1 with dystonia. Pain. 2008;134:302–309. doi: 10.1016/j.pain.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Fredrickson BE, Apkarian A V. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89:7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Ramachandran TS, Thomas PS, Szeverenyi NM, Fredrickson BE. Association between dorsolateral prefrontal N-acetyl aspartate and depression in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. J Neural Transm. 2003;110:287–312. doi: 10.1007/s00702-002-0781-9. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Thomas PS, Ramachandran TS. Decreased levels of N-acetylaspartate in dorsolateral prefrontal cortex in a case of intractable severe sympathetically mediated chronic pain (complex regional pain syndrome, type I) Brain Cogn. 2002;49:102–113. doi: 10.1006/brcg.2001.1489. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, González-Olvera J, Fresán A, Gómez-Martín D, Méndez-Núñez JC, Pellicer F. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Brain Res Cogn Brain Res. 2005;25:153–160. doi: 10.1016/j.cogbrainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Harden RN, Bruehl S, Perez RSGM, Birklein F, Marinus J, Maihofner C, Lubenow T, Buvanendran A, Mackey S, Graciosa J, Mogilevski M, Ramsden C, Chont M, Vatine J-J. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain. 2010;150:268–274. doi: 10.1016/j.pain.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden RN, Oaklander AL, Burton AW, Perez RSGM, Richardson K, Swan M, Barthel J, Costa B, Graciosa JR, Bruehl S. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Med. 2013;14:180–229. doi: 10.1111/pme.12033. [DOI] [PubMed] [Google Scholar]
- Harris EJ, Schimka KE, Carlson RM. Complex regional pain syndrome of the pediatric lower extremity: a retrospective review. J Am Podiatr Med Assoc. 2012;102:99–104. doi: 10.7547/1020099. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jin C, Yuan K, Zhao L, Zhao L, Yu D, von Deneen KM, Zhang M, Qin W, Sun W, Tian J. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013;26:58–64. doi: 10.1002/nbm.2819. [DOI] [PubMed] [Google Scholar]
- Juottonen K, Gockel M, Silén T, Hurri H, Hari R, Forss N. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain. 2002;98:315–323. doi: 10.1016/S0304-3959(02)00119-7. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci. 2011;15:576–584. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kirveskari E, Vartiainen NV, Gockel M, Forss N. Motor cortex dysfunction in complex regional pain syndrome. Clin Neurophysiol. 2010;121:1085–1091. doi: 10.1016/j.clinph.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Klega A, Eberle T, Buchholz H-G, Maus S, Maihöfner C, Schreckenberger M, Birklein F. Central opioidergic neurotransmission in complex regional pain syndrome. Neurology. 2010;75:129–136. doi: 10.1212/WNL.0b013e3181e7ca2e. [DOI] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Muhammad A, Gibb R. Brain plasticity in the developing brain. Prog Brain Res. 2013;207:35–64. doi: 10.1016/B978-0-444-63327-9.00005-9. [DOI] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Williams P, Gibb R. Brain plasticity and recovery from early cortical injury. Dev Med Child Neurol. 2011;53(Suppl 4):4–8. doi: 10.1111/j.1469-8749.2011.04054.x. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- Kringelbach M. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. 2014;34:3969–3975. doi: 10.1523/JNEUROSCI.5055-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishio K, Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J Comp Neurol. 1994;350:337–356. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, Davis KD. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology. 2005;65:1268–1277. doi: 10.1212/01.wnl.0000180971.95473.cc. [DOI] [PubMed] [Google Scholar]
- Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, Jasciewicz J, Stein M, Aiello-Lammens M, Grant E, Berde C, Borsook D. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131:1854–1879. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Schwartzman RJ, Eppig J, Wambach D, Brahin E, Peterlin BL, Alexander G, Kalanuria A. Neuropsychological deficits associated with Complex Regional Pain Syndrome. J Int Neuropsychol Soc. 2010;16:566–573. doi: 10.1017/S1355617710000214. [DOI] [PubMed] [Google Scholar]
- Lim LW, Temel Y, Visser-Vandewalle V, Blokland A, Steinbusch H. Fos immunoreactivity in the rat forebrain induced by electrical stimulation of the dorsolateral periaqueductal gray matter. J Chem Neuroanat. 2009;38:83–96. doi: 10.1016/j.jchemneu.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Linnman C, Becerra L, Lebel A, Berde C, Grant PE, Borsook D. Transient and persistent pain induced connectivity alterations in pediatric complex regional pain syndrome. PLoS One. 2013;8:e57205. doi: 10.1371/journal.pone.0057205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DE, Carpino EA, Chiang G, Condon M, Firn E, Gaughan VJ, Hogan M, Leslie DS, Olson K, Sager S, Sethna N, Simons LE, Zurakowski D, Berde CB. A day-hospital approach to treatment of pediatric complex regional pain syndrome: initial functional outcomes. Clin J Pain. 2012;28:766–774. doi: 10.1097/AJP.0b013e3182457619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Low AK, Ward K, Wines AP. Pediatric complex regional pain syndrome. J Pediatr Orthop. 2007;27:567–572. doi: 10.1097/BPO.0b013e318070cc4d. [DOI] [PubMed] [Google Scholar]
- Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol. 2011;67:942–968. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry. 2001;58:1145–1151. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Baron R, DeCol R, Binder A, Birklein F, Deuschl G, Handwerker HO, Schattschneider J. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130:2671–2687. doi: 10.1093/brain/awm131. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Forster C, Birklein F, Neundörfer B, Handwerker HO. Brain processing during mechanical hyperalgesia in complex regional pain syndrome: a functional MRI study. Pain. 2005;114:93–103. doi: 10.1016/j.pain.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Handwerker HO, Neundörfer B, Birklein F. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63:693–701. doi: 10.1212/01.wnl.0000134661.46658.b0. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Neundörfer B, Birklein F, Handwerker HO. Mislocalization of tactile stimulation in patients with complex regional pain syndrome. J Neurol. 2006;253:772–779. doi: 10.1007/s00415-006-0117-z. [DOI] [PubMed] [Google Scholar]
- Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011;70:838–845. doi: 10.1002/ana.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D. Common hippocampal structural and functional changes in migraine. Brain Struct Funct. 2013;218:903–912. doi: 10.1007/s00429-012-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Marinus J, Moseley GL, Birklein F, Baron R, Maihöfner C, Kingery WS, van Hilten JJ. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10:637–648. doi: 10.1016/S1474-4422(11)70106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- May A, Hajak G, Gänssbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex. 2007;17:205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Metz AE, Yau H-JJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, van Hoesen GW. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J Comp Neurol. 2007;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- Moseley GL. Why do people with complex regional pain syndrome take longer to recognize their affected hand? Neurology. 2004;62:2182–2186. doi: 10.1212/01.wnl.0000130156.05828.43. [DOI] [PubMed] [Google Scholar]
- Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann K, Schnitzer TJ, Apkarian AV. Reorganization of Hippocampal Functional Connectivity with Transition to Chronic Back Pain. J Neurophysiol. 2014;111:1065–1076. doi: 10.1152/jn.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias F, Debes C, de Andrade DC, Mhalla A, Bouhassira D. Diffuse analgesic effects of unilateral repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers. Pain. 2009;147:224–232. doi: 10.1016/j.pain.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012;109:20709–20713. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Pais-Vieira M, Mendes-Pinto MM, Lima D, Galhardo V. Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience. 2009;161:671–679. doi: 10.1016/j.neuroscience.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Peltz E, Seifert F, Lanz S, Müller R, Maihöfner C. Impaired hand size estimation in CRPS. J Pain. 2011;12:1095–1101. doi: 10.1016/j.jpain.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Pendse G, Borsook D, Becerra L. Enhanced false discovery rate using Gaussian mixture models for thresholding fMRI statistical maps. Neuroimage. 2009;47:231–261. doi: 10.1016/j.neuroimage.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Draganski B, Schwenkreis P, Lenz M, Nicolas V, Maier C, Tegenthoff M. Complex regional pain syndrome type I affects brain structure in prefrontal and motor cortex. PLoS One. 2014;9:e85372. doi: 10.1371/journal.pone.0085372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Ragert P, Schwenkreis P, Förster A-F, Wilimzig C, Dinse H, Nicolas V, Maier C, Tegenthoff M. Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. Neuroimage. 2006;32:503–510. doi: 10.1016/j.neuroimage.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Reid P, Pridmore S. Improvement in chronic pain with transcranial magnetic stimulation. Aust N Z J Psychiatry. 2001;35:252. doi: 10.1046/j.1440-1614.2001.0884e.x. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain. 2011;12:436–443. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, Comi G, Scotti G, Filippi M. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37:1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Structural brain changes in chronic pain reflect probably neither damage nor atrophy. PLoS One. 2013;8:e54475. doi: 10.1371/journal.pone.0054475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Perceived helplessness is associated with individual differences in the central motor output system. Eur J Neurosci. 2012;35:1481–1487. doi: 10.1111/j.1460-9568.2012.08048.x. [DOI] [PubMed] [Google Scholar]
- Schilder JCM, Schouten AC, Perez RSGM, Huygen FJPM, Dahan A, Noldus LPJJ, van Hilten JJ, Marinus J. Motor control in complex regional pain syndrome: a kinematic analysis. Pain. 2012;153:805–812. doi: 10.1016/j.pain.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Leinisch E, Gänssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman RJ, Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology. 1990;40:57–61. doi: 10.1212/wnl.40.1.57. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140:411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Maier C, Tegenthoff M. Functional imaging of central nervous system involvement in complex regional pain syndrome. AJNR Am J Neuroradiol. 2009;30:1279–1284. doi: 10.3174/ajnr.A1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta J-K. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57. e2. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, Newhouse PA, Filippi CG, Keefe FJ, Naylor MR. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. 2013;14:1573–1584. doi: 10.1016/j.jpain.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Kim S-H, Kim Y-T, Song H, Lee J, Kim S-H, Han SW, Nam EJ, Kim S-K, Lee HJ, Lee S-J, Chang Y. Working memory impairment in fibromyalgia patients associated with altered frontoparietal memory network. PLoS One. 2012;7:e37808. doi: 10.1371/journal.pone.0037808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8:1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi S, Kobayashi H, Nihashi T, Kato K, Iwano S, Nishino M, Ishigaki T, Ikeda M, Kato T, Ito K, Kimura T. Cerebral glucose metabolism change in patients with complex regional pain syndrome: a PET study. Radiat Med. 2006;24:335–344. doi: 10.1007/s11604-006-0035-0. [DOI] [PubMed] [Google Scholar]
- Shyu B-C, Vogt BA. Short-term synaptic plasticity in the nociceptive thalamic-anterior cingulate pathway. Mol Pain. 2009;5:51. doi: 10.1186/1744-8069-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp. 2014;35:527–538. doi: 10.1002/hbm.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons LE, Pielech M, Erpelding N, Linnman C, Moulton E, Sava S, Lebel A, Serrano P, Sethna N, Berde C, Becerra L, Borsook D. The responsive amygdala: Treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. Pain. 2014 May 23; doi: 10.1016/j.pain.2014.05.023. pi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. J Pain. 2011;12:677–686. doi: 10.1016/j.jpain.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Slade GD, Conrad MS, Diatchenko L, Rashid NU, Zhong S, Smith S, Rhodes J, Medvedev A, Makarov S, Maixner W, Nackley AG. Cytokine biomarkers and chronic pain: association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. Pain. 2011;152:2802–2812. doi: 10.1016/j.pain.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain. 2013;14:663–675. doi: 10.1016/j.jpain.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Karalis DG, Rosso AL, Grothusen JR, Hessen SE, Schwartzman RJ. Syncope in complex regional pain syndrome. Clin Cardiol. 2011;34:222–225. doi: 10.1002/clc.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Craig ADB, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008;65:1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel C, Hunt S, Sullivan R, Sun J, Sah P. Emotional regulation of pain: the role of noradrenaline in the amygdala. Sci China Life Sci. 2014 doi: 10.1007/s11427-014-4638-x. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Ushida T, Fukumoto M, Binti C, Ikemoto T, Taniguchi S, Ikeuchi M, Nishihara M, Tani T. Alterations of contralateral thalamic perfusion in neuropathic pain. Open Neuroimag J. 2010;4:182–186. doi: 10.2174/1874440001004010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon-Presseau E, Roy M, Martel M-O, Caron E, Marin M-F, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ, Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136:815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- Van Hilten JJ. Movement disorders in complex regional pain syndrome. Pain Med. 2010;11:1274–1277. doi: 10.1111/j.1526-4637.2010.00916.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- Walton KD, Dubois M, Llinás RR. Abnormal thalamocortical activity in patients with Complex Regional Pain Syndrome (CRPS) type I. Pain. 2010;150:41–51. doi: 10.1016/j.pain.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Wu C-T, Fan Y-M, Sun C-M, Borel CO, Yeh C-C, Yang C-P, Wong C-S. Correlation between changes in regional cerebral blood flow and pain relief in complex regional pain syndrome type 1. Clin Nucl Med. 2006;31:317–320. doi: 10.1097/01.rlu.0000218538.06832.69. [DOI] [PubMed] [Google Scholar]
- Younger JW, Shen YF, Goddard G, Mackey SC. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149:222–228. doi: 10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.