Abstract
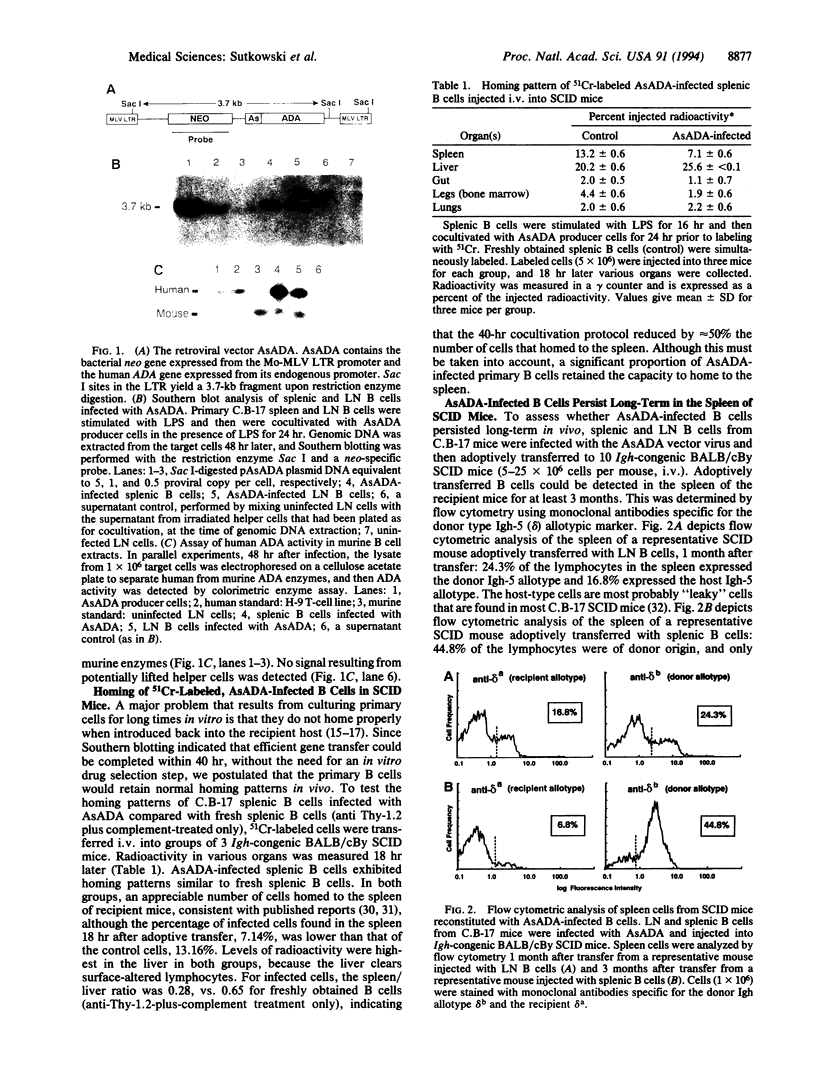
Mature primary B lymphocytes represent a potentially important cellular target for somatic cell gene therapy, which could prove advantageous for the treatment of certain metabolic and immunologic disorders. Their capacity to serve as antigen-presenting cells could be utilized for triggering and/or potentiating immune responses to tumors and viruses. Alternatively, B cells expressing an autoantigen could be manipulated to induce antigen-specific unresponsiveness for treatment of autoimmune diseases. Efficient expression of an exogenous gene product in long-lived B lymphocytes could be particularly useful for providing a corrected gene product in the bloodstream. Despite these advantages, efficient gene transfer into mature primary B cells has not been reported. One reason for this is that current protocols for retroviral vector-mediated gene transfer into lymphocytes rely on in vitro expansion and/or drug selection. This precludes the use of mature primary B cells as targets, since they cannot be readily cultured for long periods of time. In this report, we describe an efficient and rapid protocol for the introduction of exogenous genes into primary B cells without the need for drug selection. We have used retroviral vectors containing the human adenosine deaminase gene as a marker gene, since the biological activity of this enzyme is easy to measure and is readily distinguishable from that of the endogenous mouse adenosine deaminase. Upon adoptive transfer into SCID mice, infected B cells continuously expressing one to three copies of the human adenosine deaminase gene could be found in the spleens of recipient animals for at least 3 months.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Human gene therapy. Science. 1992 May 8;256(5058):808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- Anderson W. F., McGarrity G. J., Moen R. C. Report to the NIH Recombinant DNA Advisory Committee on murine replication-competent retrovirus (RCR) assays (February 17, 1993). Hum Gene Ther. 1993 Jun;4(3):311–321. doi: 10.1089/hum.1993.4.3-311. [DOI] [PubMed] [Google Scholar]
- Auwerx J., Leroy P., Schoonjans K. Lipoprotein lipase: recent contributions from molecular biology. Crit Rev Clin Lab Sci. 1992;29(3-4):243–268. doi: 10.3109/10408369209114602. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Fried M., Custer R. P., Carroll A., Gibson D. M., Bosma M. J. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988 Mar 1;167(3):1016–1033. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman E., Van Beusechem V. W., Van Krimpen B. A., Fischer A., Bolhuis R. L., Valerio D. Genetic correction of cultured T cells from an adenosine deaminase-deficient patient: characteristics of non-transduced and transduced T cells. Eur J Immunol. 1992 Jan;22(1):63–69. doi: 10.1002/eji.1830220111. [DOI] [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Culver K. W., Anderson W. F., Blaese R. M. Lymphocyte gene therapy. Hum Gene Ther. 1991 Summer;2(2):107–109. doi: 10.1089/hum.1991.2.2-107. [DOI] [PubMed] [Google Scholar]
- Culver K., Cornetta K., Morgan R., Morecki S., Aebersold P., Kasid A., Lotze M., Rosenberg S. A., Anderson W. F., Blaese R. M. Lymphocytes as cellular vehicles for gene therapy in mouse and man. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3155–3159. doi: 10.1073/pnas.88.8.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey M. O., Gallatin W. M., Weissman I. L. The in vivo behavior of T cell clones: altered migration due to loss of the lymphocyte surface homing receptor. J Mol Cell Immunol. 1985;2(1):27–36. [PubMed] [Google Scholar]
- Ferrari G., Rossini S., Giavazzi R., Maggioni D., Nobili N., Soldati M., Ungers G., Mavilio F., Gilboa E., Bordignon C. An in vivo model of somatic cell gene therapy for human severe combined immunodeficiency. Science. 1991 Mar 15;251(4999):1363–1366. doi: 10.1126/science.1848369. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Rossini S., Nobili N., Maggioni D., Garofalo A., Giavazzi R., Mavilio F., Bordignon C. Transfer of the ADA gene into human ADA-deficient T lymphocytes reconstitutes specific immune functions. Blood. 1992 Sep 1;80(5):1120–1124. [PubMed] [Google Scholar]
- Hayden M. R., Ma Y. Molecular genetics of human lipoprotein lipase deficiency. Mol Cell Biochem. 1992 Aug 18;113(2):171–176. doi: 10.1007/BF00231536. [DOI] [PubMed] [Google Scholar]
- Hoeben R. C., Einerhand M. P., Briët E., van Ormondt H., Valerio D., van der Eb A. J. Toward gene therapy in haemophilia A: retrovirus-mediated transfer of a factor VIII gene into murine haematopoietic progenitor cells. Thromb Haemost. 1992 Mar 2;67(3):341–345. [PubMed] [Google Scholar]
- Hoeben R. C., Fallaux F. J., Van Tilburg N. H., Cramer S. J., Van Ormondt H., Briët E., Van Der Eb A. J. Toward gene therapy for hemophilia A: long-term persistence of factor VIII-secreting fibroblasts after transplantation into immunodeficient mice. Hum Gene Ther. 1993 Apr;4(2):179–186. doi: 10.1089/hum.1993.4.2-179. [DOI] [PubMed] [Google Scholar]
- Kasid A., Morecki S., Aebersold P., Cornetta K., Culver K., Freeman S., Director E., Lotze M. T., Blaese R. M., Anderson W. F. Human gene transfer: characterization of human tumor-infiltrating lymphocytes as vehicles for retroviral-mediated gene transfer in man. Proc Natl Acad Sci U S A. 1990 Jan;87(1):473–477. doi: 10.1073/pnas.87.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Butcher E. C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. L., Sutkowski N., Ron Y., Dougherty J. P. Efficient gene transfer into primary murine lymphocytes obviating the need for drug selection. Blood. 1993 Aug 1;82(3):845–852. [PubMed] [Google Scholar]
- Lim B., Apperley J. F., Orkin S. H., Williams D. A. Long-term expression of human adenosine deaminase in mice transplanted with retrovirus-infected hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8892–8896. doi: 10.1073/pnas.86.22.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Miller A. D. Progress toward human gene therapy. Blood. 1990 Jul 15;76(2):271–278. [PubMed] [Google Scholar]
- Miller A. R., Skotzko M. J., Rhoades K., Belldegrun A. S., Tso C. L., Kaboo R., McBride W. H., Jacobs E., Kohn D. B., Moen R. Simultaneous use of two retroviral vectors in human gene marking trials: feasibility and potential applications. Hum Gene Ther. 1992 Dec;3(6):619–624. doi: 10.1089/hum.1992.3.6-619. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Reimann J., Heeg K., Wagner H., Keller G., Wagner E. F. Introduction of a selectable gene into murine T-lymphoblasts by a retroviral vector. J Immunol Methods. 1986 May 1;89(1):93–101. doi: 10.1016/0022-1759(86)90036-0. [DOI] [PubMed] [Google Scholar]
- Richter A., Ozer H. L., DesGroseillers L., Jolicoeur P. An X-linked gene affecting mouse cell DNA synthesis also affects production of unintegrated linear and supercoiled DNA of murine leukemia virus. Mol Cell Biol. 1984 Jan;4(1):151–159. doi: 10.1128/mcb.4.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron Y., Sprent J. Prolonged survival in vivo of unprimed B cells responsive to a T-independent antigen. J Exp Med. 1985 Jun 1;161(6):1581–1586. doi: 10.1084/jem.161.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. Circulating T and B lymphocytes of the mouse. I. Migratory properties. Cell Immunol. 1973 Apr;7(1):10–39. doi: 10.1016/0008-8749(73)90180-9. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Hurd M., Surh C. D., Ron Y. Mature murine B and T cells transferred to SCID mice can survive indefinitely and many maintain a virgin phenotype. J Exp Med. 1991 Sep 1;174(3):717–728. doi: 10.1084/jem.174.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S. K., Weissman I. L., Butcher E. C. Differences in the migration of B and T lymphocytes: organ-selective localization in vivo and the role of lymphocyte-endothelial cell recognition. J Immunol. 1982 Feb;128(2):844–851. [PubMed] [Google Scholar]
- Tedder T. F., Penta A. C., Levine H. B., Freedman A. S. Expression of the human leukocyte adhesion molecule, LAM1. Identity with the TQ1 and Leu-8 differentiation antigens. J Immunol. 1990 Jan 15;144(2):532–540. [PubMed] [Google Scholar]
- Tsukada S., Saffran D. C., Rawlings D. J., Parolini O., Allen R. C., Klisak I., Sparkes R. S., Kubagawa H., Mohandas T., Quan S. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993 Jan 29;72(2):279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- Vetrie D., Vorechovský I., Sideras P., Holland J., Davies A., Flinter F., Hammarström L., Kinnon C., Levinsky R., Bobrow M. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993 Jan 21;361(6409):226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]