Abstract
Study Objective
To investigate whether endometrial ablation carries an increased risk or delayed diagnosis of endometrial cancer compared to medical management for abnormal uterine bleeding.
Design
A multi-centered retrospective cohort study comparing rates of endometrial cancer in women who underwent treatment for abnormal uterine bleeding.
Design Classification
Canadian Task-Force Classification II-2
Setting
This study was conducted using data from The Health Improvement Network (THIN), a representative population-based cohort of patients in 495 outpatient General Practitioner practices in the UK.
Participants
Women >25 years of age with an abnormal uterine bleeding diagnosis between June 1994 and September 2010.
Interventions
Endometrial ablation, medical management, or both.
Measurements and Main Results
A total of 234,721 women met study inclusion and exclusion criteria, 4,776 of whom underwent endometrial ablation and the remaining 229,945 underwent medical management. Cox models compared endometrial cancer rates between ablation and medical management groups using hazard ratios (HRs). To investigate a possible diagnostic delay, the median time from bleeding diagnosis to endometrial cancer diagnosis among women who developed endometrial cancer was compared using the Mann-Whitney U test. All statistical tests were two-tailed with α=.05. Over a median observation time of 4.07 years (IQR, 1.88-7.17), three and 601 women developed endometrial cancer in the ablation and medical management groups, respectively (ablation HR, 0.45; 95% CI, 0.15-1.40; p=.17). Median time to diagnosis was 237 and 299 days (ablation IQR, 155-1350; medical management IQR, 144-1,133.5; p=.99) in the ablation and medical management groups, respectively. Adjusted and sensitivity analyses did not change the results.
Conclusions
No difference was seen in endometrial cancer rates, nor was there a delay in diagnosis when comparing endometrial ablation versus medical management. Further studies are needed to investigate the impact of prior ablation exposure on histology or cancer stage at presentation of endometrial cancer.
Keywords: endometrial ablation techniques, endometrial neoplasms, menorrhagia, uterine neoplasms
Introduction
Abnormal uterine bleeding (AUB) has nearly a 30% lifetime prevalence in women and is defined as “bleeding from the uterine corpus that is abnormal in volume, regularity, and/or timing” (1). The effectiveness of minimally invasive surgical techniques and devices versus medical management of abnormal uterine bleeding has been well-studied, and summarized in a meta-analysis (2) of ten randomized comparative effectiveness trials (3-12). While this meta-analysis showed an improvement in bleeding after ablation, there are limited data regarding adverse effects and device safety. Only one small (n=79) study assessed adverse events after 1 year, finding one case of infection in the medical management arm (11). None of these randomized studies measured adverse outcomes in real-world clinical use, nor in a population-based cohort.
Endometrial cancer is the fourth most common cancer in women (13) and is a potential adverse outcome of AUB treatments; several case-reports of endometrial cancer following endometrial ablation have been published (14-24). These authors have hypothesized that all forms of ablation may contribute to endometrial cancer by several shared biological mechanisms. AUB itself can be caused by hormonal irregularities such as anovulation, which increase one's endometrial cancer risk due to endogenous unopposed estrogen (25,26). Progestins, present in medical management treatments for abnormal uterine bleeding, have a known protective effect against endometrial cancer (27,28). Women who choose ablation over medical management would miss out on this protective effect from progestins and could suffer an increased relative risk of endometrial cancer. Additionally the intrauterine scarring from ablations may increase endometrial cancer risk due to increasing cell turnover (24). Intrauterine scarring may also mask the presentation of endometrial cancer and delay its diagnosis by preventing the outflow of blood from the uterine cavity (29), a common clinical hallmark of endometrial cancer that prompts biopsy. Further, this intrauterine scarring may make biopsy less sensitive for detecting underlying malignancy. Such a delay may lead to more advanced stage cancers with higher mortality (30). The alternative remains that ablation may destroy pre-malignant cells that are vulnerable to the ablation techniques, and instead reduce a woman's risk of endometrial cancer. These hypotheses have only been investigated in underpowered studies with inadequate or absent controls (31-33).
The objective of this study was to use a large database of real-world clinical data to compare endometrial cancer rates and time to diagnosis between women with AUB treated with endometrial ablation versus medical management. We hypothesized that ablation would carry an increased rate of endometrial cancer, and delay its diagnosis.
Materials and Methods
The Investigational Review Board at the University of Pennsylvania (case 813152) exempted this study due to its usage of an anonymized database, and the UK Scientific Review Committee (reference 11-021) approved this study.
Study Design
This retrospective cohort study investigated women with AUB diagnoses in a UK-based clinical database (The Health Improvement Network, THIN). THIN is a privately owned database by Cegedim Strategic Data (London, UK), a data vendor and electronic medical record software supplier of Vision to UK General Practitioner (GP) practices. Nearly identical to the public General Practitioner Research Database (GPRD), they report data, diagnostic Read codes, and Multilex drug classification on over 9 million patients (34). These hierarchical Read codes are used for clinical rather than billing purposes, and are therefore less susceptible to “rule-out” or “up-diagnosis” seen in studies using ICD-9 codes (34). Like GPRD, THIN has been found to be generalizable to the entire U.K. population (35) and to accurately report data on chronic diseases (36) and diagnoses such as cancer (37).
Women over age 25 with an abnormal uterine bleeding diagnosis made between June 1994 and September 2010 were included. Abnormal uterine bleeding (AUB) clinical Read codes were generated with widely-accepted code generation methods (38) and were based on ICD-9 and ICD-10 billing codes for menstrual bleeding disorders evaluated in previous investigations in other U.K. databases (35).
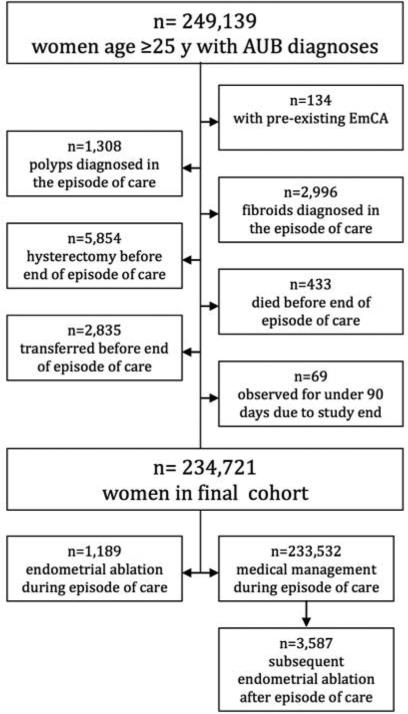
Women were assessed 90 days after the incident diagnosis of an AUB episode of care to assess exclusion criteria. Women with a diagnosis of endometrial cancer, fibroids, or polyps made during the abnormal uterine bleeding episode of care were excluded to achieve a cohort of women with true incident AUB diagnoses who would be equally eligible to undergo either an endometrial ablation or medical management. Women who underwent hysterectomy, were transferred, or died during the episode of care were excluded as they were no longer at risk for endometrial cancer.
Data Collection
All women underwent endometrial ablations or medical management, or both, for AUB. Given the shared biological mechanisms for endometrial cancer risk modification, endometrial ablations were defined to include first-generation resectoscopic transcervical resection of the endometrium, or second-generation endometrial ablation using various devices. Medical management included expectant management, combination estrogen-progestin and progestin-only hormonal medications, or other non-hormonal therapies such as non-steroidal anti-inflammatory or anti-fibrinolytic agents. Treatments were initially assigned during the 90-day AUB episode of care, counting women with an observed ablation Read code as an endometrial ablation subject, and the remaining women as undergoing medical management. An as-treated analysis was performed to account for cross-over in treatment regimens over time. Specifically, if a woman received medical management and an endometrial ablation at a later time, she contributed medical management observation time until the ablation procedure, and then contributed the remainder of the observation time in the endometrial ablation group. Women were observed from the end of the AUB episode of care until the earliest of hysterectomy, death, transfer from practice, or endometrial cancer diagnosis. For the purpose of this study endometrial cancer was defined when a Read code specified either endometrial or uterine cancer, given that 90% of uterine cancers are endometrial (39). Though not validated in THIN, endometrial cancer Read codes have been validated and studied in the nearly identical General Practitioner Research Database (GPRD) (40,41), and other solid tumor diagnostic codes have been validated in THIN (37). Twenty-six baseline demographic and health characteristics were measured including age, socioeconomic status (Townsend score), comorbidities, and medications.
Endometrial Ablation Read Code Validation
To investigate the previously unvalidated Read codes for endometrial ablations, questionnaires were sent to THIN-participating GP practices that agreed to receive questionnaires. A computer-generated random sample of 80 subjects who underwent endometrial ablation were investigated in their respective practices. The GPs then completed the questionnaire based on patient chart review. A positive predictive value (PPV) was calculated by counting the number “yes” responses to the question, “Did this patient receive a first or second generation endometrial ablation?” divided by the number of completed questionnaires, and a 95% CI was generated.
Statistical Methods
Baseline demographic and health characteristics were compared across AUB treatment groups using an independent t-, Mann-Whitney U, χ2, or Fisher's exact tests where appropriate. A hazard ratio (HR) for development of endometrial cancer in the setting of endometrial ablation was calculated using Cox proportional hazards regression models. Adjusted HR was assessed using a multivariable Cox model adjusting for significant confounders using standard defnitions (43). Means and ninety-five percent confidence intervals (95% CI) were reported for normally-distributed data, while medians and inter-quartile ranges (IQR) were reported for skewed data. Multiple imputation (44) was used for missing covariate values of smoking, BMI, and Townsend index when testing for confounding. A priori planned subgroup and sensitivity analyses were performed to assess the potential for bias, unmeasured confounding and confounding by indication (45). To investigate a diagnostic delay, the median time to endometrial cancer diagnosis was compared among women with endometrial cancer using a Mann-Whitney U test. All tests were two-tailed with α=.05, and performed in Stata 12 (College Station, TX).
Power calculations
Preliminary data from THIN was used to calculate a minimum detectable HR for the association between endometrial ablation and cancer. With a known 6,000 ablations, known 165/100,000 prevalence of endometrial cancer in women in THIN, and mean 3.3 years of follow-up in THIN, the study was originally powered to detect a minimum ablation hazard ratio of 1.23 with 90% power.
Results
Cohort
A total of 249,139 eligible women were considered for analysis. After application of exclusion criteria (see Figure 1) 234,721 eligible women with an incident AUB diagnosis contributed a median follow-up time of 4.08 years (IQR, 1.88-7.17), yielding a total of 1,015,252.4 person-years. Demographic, health and outcome data are reported in Table 1. Among these, 4,776 women underwent ablations and the remaining 229,945 underwent medical management. Within the medical management arm 30,731 were prescribed a combination estrogen-progestin oral contraceptive, 40,457 were prescribed a progestin-only medication, and 3,588 received a levonorgestrel intrauterine system.
Figure 1. Abnormal uterine bleeding cohort generation flowchart.
Legend. Abbreviations: AUB, abnormal uterine bleeding; y, years. Subjects were excluded step-wise in the order depicted.
Table 1.
Study characteristics of cohort and across exposure groups
| Characteristic | Endometrial ablation n=4,776 | Medical management n=229,945 | All subjects n=234,721 | p a |
|---|---|---|---|---|
| Demographic data | ||||
| Median follow-up time (IQR), years | 5.55 (3.15-8.04) | 4.04 (1.86-7.14) | 4.08 (1.88-7.17) | <.001b |
| Median age (IQR), years | 41.55 (37.08-45.61) | 39.51 (32.32-47.21) | 39.58 (32.41-47.15) | <.001b |
| Median year at abnormal uterine bleeding diagnosis (IQR) | 2004 (2002-2007) | 2005 (2002-2007) | 2005 (2002-2007) | <.001b |
| Median Townsend index of socioeconomic deprivation (IQR)d | 2 (1-3) | 2 (1-4) | 2 (1-4) | <.001b |
| Baseline health data | ||||
| Anemia, n (%) | 1,365 (28.58) | 59,974 (23.47) | 55,339 (23.58) | <.001 |
| Median BMI (IQR) e | 25.8 (22.8-30.6) | 24.7 (21.9-28.8) | 24.7 (22-28.8) | <.001b |
| Obese, n (%) | 2,254 (55.29) | 92,092 (46.8) | 94,346 (46.97) | <.001 |
| Diabetes mellitus, n (%) | 146 (3.06) | 7,355 (3.20) | 7,501 (3.20) | .58 |
| Personal cancer history, n (%) | 118 (2.47) | 6,690 (2.91) | 6,808 (2.90) | .08 |
| Myocardial infarction history n (%) | 1 (0.02) | 840 (0.37) | 841 (0.36) | <.001c |
| Coronary artery disease, n (%) | 4 (0.08) | 696 (0.30) | 700 (0.30) | <.01c |
| Hypertension, n (%) | 336 (7.04) | 17.511 (7.62) | 17,847 (7.60) | .13 |
| Endometrial hyperplasia, n (%) | 1 (0.02) | 76 (0.03) | 77 (0.03) | 1.00 c |
| Gallbladder disease, n (%) | 192 (4.02) | 6,391 (2.78) | 6,583 (2.80) | <.001 |
| Post bilateral oophorectomy, n (%) | 0 (0.00) | 103 (0.04) | 103 (0.04) | .28 |
| Late menopause ≥55 years, n (%) | 15 (0.31) | 2,942 (1.28) | 2,957 (1.26) | <.001 |
| Premature ovarian failure, n (%) | 38 (0.8) | 1,384 (0.60) | 1,422 (0.61) | .09 |
| Polycystic ovarian syndrome, n (%) | 81 (1.70) | 4,125 (1.79) | 4,206 (1.79) | .61 |
| ≥1 observed pregnancy, n (%) | 1,152 (24.12) | 71,616 (31.14) | 72,768 (31.00) | <.001 |
| Present or past smoker, n (%)f | 1,807 (40.95) | 86,753 (40.14) | 88,560 (40.16) | .28 |
| Tamoxifen use, n (%) | 23 (0.48) | 2,028 (0.88) | 2,051 (0.87) | <.01 |
| SERM use, n (%) | 43 (0.90) | 2,250 (0.98) | 2,293 (0.98) | .59 |
| Combined oral contraceptive use, n (%) | 1,695 (35.49) | 97,348 (42.34) | 99,043 (42.20) | <.001 |
| Unopposed estrogen use, n (%) | 222 (4.65) | 16,122 (7.01) | 16,344 (6.96) | <.001 |
| Progestin-only use, n (%) | 1,865 (39.05) | 70,608 (30.71) | 72,473 (30.88) | <.001 |
| Episode of care characteristics | ||||
| Hysteroscopy, n (%) | 280 (5.86) | 6,706 (2.92) | 6,986(2.98) | <.001 |
| Iron supplementation prescribed, n (%) | 470 (9.84) | 13,430 (5.84) | 13,900(5.92) | <.001 |
| Study course outcomes | <.001 | |||
| No event, n (%) | 3,565 (74.64) | 167,965 (73.05) | 171,530 (73.08) | |
| Death, n (%) | 14 (0.29) | 3,556 (1.55) | 3,570 (1.52) | |
| Transfer, n (%) | 474 (9.92) | 48,268 (20.99) | 48,742 (20.77) | |
| Hysterectomy, n (%) | 720 (15.08) | 9.555 (4.16) | 10,275 (4.38) | |
| Endometrial cancer, n (%) | 3 (0.06) | 601 (0.26) | 604 (0.26) |
Abbreviations: IQR, inter-quartile range; SERM, selective estrogen-receptor modifier.
Covariates were measured prior to the heavy menstrual bleeding diagnosis, unless designated “episode of care” characteristics or course outcomes. A woman's final treatment group at time of censorship was used to categorize exposure groups in this table.
X2 test used unless otherwise specified.
Mann-Whitney U test used.
Fisher's exact test used.
231,267 subjects had complete socioeconomic data (1.5% missing). In the 0-10 Townsend index, 10 indicates most socioeconomic depravity.
200,359 subjects had complete BMI data (14.6% missing).
220,544 subjects had complete smoking data (6.0% missing).
Endometrial Ablation Validation
A total of 74/80 questionnaires were completed for a 92.5% response rate. A total of 71/74 GPs responded “yes” to indicate that an endometrial ablation was indeed performed. This yielded a positive predictive value (PPV) of 96.0%, (95% CI, 88.6%-99.2%).
Primary Investigation of Ablation and Endometrial Cancer Rates
A total of 604 cases of endometrial cancer were identified, three in women who underwent ablations, and 601 in women who underwent medical management. This corresponds with a study-specific annual endometrial cancer incidence rate of 59.6/100,000 women, 19.3/100,000 in women who underwent ablations, and 60.3/100,000 in women who underwent medical management.
No increased risk was seen from ablation versus any medical management in unadjusted Cox modeling (ablation HR, 0.45; 95% CI, 0.15-1.40; p=.17) (see Figure 2). In creating a multivariable model, among the 26 tested variables (see Table 1), only age was found as a confounder. Age-adjusted results also showed no difference between groups (ablation HR, 0.61; 95% CI, 0.19-1.89; p=.39).
Figure 2. Kaplan-Meier curve for time to endometrial cancer.
Legend. Abbreviations: CI, confidence interval; EmCa, endometrial cancer. There was no statistically significant difference in hazards (ablation HR=0.45, p=.17). The number of subjects indicates the number of subjects present at the beginning of each indicated year. Of note, the as-treated analysis allows a woman to switch from the medical management group to the ablation group.
Sub-group Analyses
In a priori planned sub-group analyses, there was no difference in endometrial cancer HRs between women who had ablations versus specific medical management therapies (see Figure 3). Comparing women who underwent ablations to women who received the levonorgestrel intrauterine system (LNG-IUS) there was a non-statistically significant but notable change in the HR to greater than 1 (ablation HR, 6.04; 95% CI, 0.61-60.2, p=.13), (see Figure 3). However, this observation was based on endometrial cancer rates of only 3/4,776 and 1/3,558 cases in ablation and LNG-IUS groups, respectively (Fisher's exact p=.64). In a secondary analysis, the LNG-IUS was found to have a lower rate of endometrial cancer versus all other AUB treatments (LNG-IUS HR, 0.12, 95% CI, 0.02-0.83, p=.03).
Figure 3. Sub-group specific ablation hazard ratios.
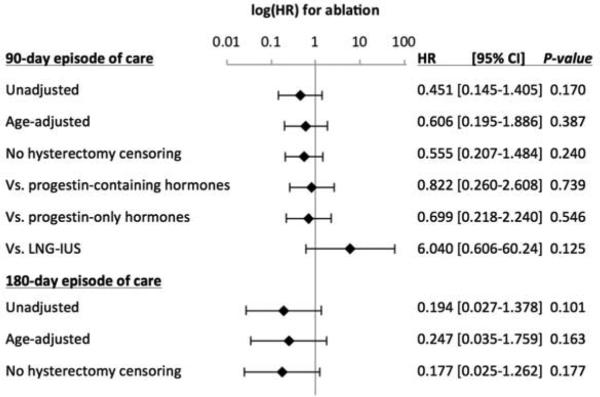
Legend. Abbreviations: HR, hazard ratio; LNG-IUS, levonorgestrel intrauterine system. Displayed are the ablation hazard ratios (HR) vs. all medical management subjects, unless otherwise noted, in various sub-group and sensitivity analyses. Error bars indicate the 95% confidence interval for the ablation HR.
Sensitivity Analyses
In planned sensitivity analyses (see Figure 3), the investigation was repeated without censoring the 10,275 women at time of hysterectomy (ablation HR, 0.56; 95% CI, 0.02-1.3; p=.24), and extending the AUB episode of care to 180 days (ablation HR, 0.19; 95% CI, 0.03-1.4, p=.10).
Further, a sensitivity analysis was performed to document the effect an unmeasured confounding variable or risk factor could have on study results. A hypothetical binary (yes/no) confounding variable was created and assigned at various levels to the ablation and medical management arms (45). At its most extreme imbalance of this hypothetical variable, only a 90% to 0% and 10% to 100% yes/no split yielded ablation HRs above 1, at 3.3 (95% CI, 0.73-15.3; p=.12) and 2.0 (95% CI, 0.39-10.4; p=.41) neither of which demonstrated statistical significance between AUB treatment groups.
Delayed Diagnosis Investigation
Among women who underwent endometrial ablation, the three cases of endometrial cancer were diagnosed at 155, 237, and 1,350 days following AUB diagnosis. The remaining 601 cases in women who underwent medical management were diagnosed between 92 and 4,779 days at a median of 299 days (IQR, 144-1,133.5 days). Among women who were diagnosed with endometrial cancer, there was no difference in median time to diagnosis between endometrial ablation and medical management (p=.995). To investigate the possibility that the early diagnosed endometrial cancers were misdiagnosed AUB, a sensitivity analysis expanded the AUB episode of care to 180 days to exclude endometrial cancer cases made fewer than 6 months from the initial AUB episode. Only one endometrial cancer case remained in the ablation group, at 237 days, and 357 cases remained in the medical management group between 182 and 4,779 days (median time to endometrial cancer diagnosis 237 days for the ablation group, 903 days in the medical management group (IQR, 366-1,900 days, p=.165).
Discussion
This study sought to investigate a difference in endometrial cancer rates between endometrial ablation and medical management of AUB, and to determine if there was a delayed diagnosis with endometrial ablation. This investigation did not find the difference in endometrial cancer rates as had been hypothesized. Few cancers were observed in the ablation group; however, the study results exclude an increased risk of 40% or more from ablation. The adjusted, sub-group, and sensitivity analyses showed that the study findings were robust since the results did not change when adjusted for age, or in the secondary analyses addressing concerns for AUB study definitions, inclusion criteria, and comparison groups. Moreover, our sensitivity analyses demonstrate that no degree of imbalance from an unmeasured risk factor would reverse study results to a significantly increased risk, making unmeasured confounding unlikely. This study did not find a difference between endometrial ablation and medical management in time to cancer diagnosis among those who developed endometrial cancer, as hypothesized, and this finding was not reversed by secondary analyses.
Prior to this investigation, no adequately powered study compared endometrial cancer rates between women undergoing endometrial ablation versus medical management of AUB. Neuwirth et. al. (31) assembled a retrospective cohort of 509 women undergoing ablation and found two cases of endometrial cancer. This investigation used historical controls without AUB and was therefore unable to calculate an unbiased relative cancer risk assessment. Gaia et. al. (32) studied a cohort of 3,769 women who underwent ablation and found four cases of endometrial cancer, but did not have any controls to report a relative risk assessment for endometrial ablation. A study by Krogh et. al. (33) suffered the same weakness, and found three cases of endometrial cancer among its cohort of 302 women. Thus, the presented study assembled the largest cohort to date of women who underwent ablations to investigate endometrial cancer, and was appropriately designed to compare them to women who underwent medical management to control for the increased baseline cancer risk among women with AUB. A prospective cohort design or a prospective randomized control trial would have the benefit of more granular data on its participants, and allow for such measurements as endometrial biopsies. This study investigates an outcome that is relatively uncommon on a population level (endometrial cancer), so a prospective study design would require an unfeasibly large number of subjects and long follow-up time to achieve meaningful conclusions in a timely manner. An observational study is also more generalizable than a randomized controlled trial as it studies real-world use and subjects in a full population, rather than subjects who underwent strict enrollment inclusion and exclusion criteria.
This investigation was not without limitations. Despite over one million person-years of observation and nearly 5,000 subjects undergoing endometrial ablation, only three endometrial cancer cases were observed. The only larger retrospective cohort of endometrial ablations was by Cooper et. al. who examined 14,078 women undergoing endometrial ablation and found two cases (46). This 2011 study compared these women to those who underwent hysterectomy and had a zero risk of future endometrial cancer, and therefore was not designed to determine a relative risk assessment of endometrial cancer in the ablation arm. The presented investigation's low number of endometrial cancer cases in the ablation group does not explicitly mean an underpowered study; results were capable of excluding an increased relative risk of 1.4 (95% CI, 0.15-1.40), a clinically relevant cutoff. This tight confidence interval owes to the thousands of observed subjects who did not develop cancer. The calculated annual endometrial cancer incidence rate of 60.3/100,000 women is reassuring in that it is within an order of magnitude of the UK national annual incidence rate for endometrial cancer, 19.4/100,000 women (47). The rate being roughly three times the national rate is consistent with some AUB etiologies increasing one's overall risk of endometrial cancer (25,26). The UK incidence is similar though slightly lower than the US annual endometrial cancer incidence, 24.3/100,000 women (13), though the incidence of endometrial cancer in women with AUB in the US has not been estimated. Additionally, the UK population studied may be different than the US population with regards to healthcare access and BMI, as the median BMI was 24.7 in this study, slightly lower than the published 27.3 in US women (48). BMI and obesity, tested separately, did not appear to affect study results as either a confounder or effect modifier in multivariable analyses, which suggests these factors are unlikely to have a direct impact on the specific biological effect under study.
This retrospective cohort study, like any observational study, leaves the possibility for error, unmeasured confounding and confounding by indication. Concerns that the study uses a non-specific definition of medical management are addressed in the sub-group analysis where ablated subjects are compared to those placed on progestin-containing oral contraceptive pills (Figure 3), which did not change study results. There may be some concern regarding the overly broad definition of AUB given that the UK National Health Service (NHS) does not differentiate ovulatory from anovulatory women in the diagnosis of AUB as an indication for endometrial ablation (49). As such, we cannot address the role of dysfunctional ovulation with respect to ablation and endometrial cancer risk in this study; however this generalizable study cohort is likely heterogeneous with respect to ovulatory patterns and therefore this variable is unlikely to introduce significant bias in the observed results. Concerns about the miscoding of endometrial ablation Read codes, as a code given to patients undergoing dilation and curettage for instance, are answered by the validation questionnaire study. A positive predictive value (PPV) of 96.0% demonstrates that Read codes in THIN validly code these procedures and is consistent with over a dozen other studies demonstrating the validity of THIN data. There was a higher rate of hysterectomy in the ablation group, which censored women in the primary analysis. The sensitivity analysis not censoring at hysterectomy does not account for the fact that hysterectomy may mask a number of women in the ablation group who would have developed endometrial cancer at a later date. Though concerning from a biological perspective, this investigation studied the real-world use of abnormal uterine bleeding treatments and is capable of informing clinically relevant decisions where patients and clinicians can balance the competing concerns for hysterectomy versus endometrial cancer. Despite the finding of no delayed diagnosis in the three ablation cancer cases, these clearly lacked power to entirely exclude differences in endometrial cancer presentation or diagnostic timing. This is an important possibility to consider, as differences in time to diagnosis could severely bias study results. Nevertheless, the fact that this investigation had over six years of follow-up on almost 1,500 women who underwent endometrial ablation makes a future unmeasured risk of endometrial cancer highly unlikely.
There remains a concern with a diagnostic delay in this and all cancer studies. Because later presentations lead to higher cancer stage, a null or protective effect may be outweighed by increased cancer mortality due to higher stages. Though this study tested for over twenty-six known and possible endometrial cancer risk factors, endometrial biopsies or pathology specimens were not available in THIN., so stage at diagnosis was unknown. A possible difference in stage or cancer mortality is an important question that has bearing on the cancer survival of thousands of women who have already undergone endometrial ablation, and warrants an additional investigation. Repeating this study in the future would allow for more followup time, and would accrue an older population with a higher endometrial cancer incidence. This could address many of the aforementioned limitations.
This study finds that the comparative effectiveness of endometrial ablation and medical management for AUB is similar with regards to endometrial cancer outcomes. In light of a superior effectiveness of ablations with regards to bleeding and AUB symptoms, as indicated by prior studies (2), this study confirms the safe use of endometrial ablation over other treatment options for AUB. Given the population-based nature of the database used, these results are generalizable to the whole UK population, as well as North American and Europe, where women are given similar treatment options for AUB.
PRECÍS.
Endometrial ablations, when compared to medical management for abnormal uterine bleeding, did not demonstrate an increased rate of endometrial cancer, nor delay its diagnosis.
Acknowledgments
Supported by the Agency for Healthcare Research and Quality (AHRQ, R03 HS021336-01, Philadelphia, PA), a National Institutes of Health Clinical and Translational Science Award (NIH, TL1 RR024133, Philadelphia, PA), a National Institutes of Health National Center for Research Resources and the National Center for Advancing Translational Sciences Grant (NIH, UL1TR000003), the Center for Clinical Epidemiology and Biostatistics at the Perelman School of Medicine at the University of Pennsylvania, the American Society for Reproductive Medicine Resident/Fellow-In Training Grant for Heavy Menstrual Bleeding, a Bertha Dagan Berman Award, and the American Medical Association (AMA) Foundation (Seed grant program, Philadelphia, PA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet. 2011;113:3–13. doi: 10.1016/j.ijgo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2006;19:CD003855. doi: 10.1002/14651858.CD003855.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Barringon JW, Arunkalaivanan AS, Abdel-Fattah M. Comparison between the levonorgestrel intrauterine system (LNG-IUS) and thermal balloon ablation in the treatment of menorrhagia. Eur J Obstet Gynecol Reprod Biol. 2003;108:72–4. doi: 10.1016/s0301-2115(02)00408-6. [DOI] [PubMed] [Google Scholar]
- 4.Cooper KG, Jack SA, Parkin DE, Grant AM. Five-year follow up of women randomised to medical management or transcervical resection of the endometrium for heavy menstrual loss: clinical and quality of life outcomes. BJOG. 2001;108:1222–8. doi: 10.1111/j.1471-0528.2001.00275.x. [DOI] [PubMed] [Google Scholar]
- 5.Crosignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, De Giorgi O. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstet Gynecol. 1997;90:257–63. doi: 10.1016/S0029-7844(97)00226-3. [DOI] [PubMed] [Google Scholar]
- 6.de Souza SS, Camargos AF, de Rezende CP, Pereira FA, Araújo CA, Silva Filho AL. A randomized prospective trial comparing the leveonorgestrel-releasing intrauterine system with thermal balloon ablation for the treatment of heavy menstrual bleeding. Contraception. 2010;81:226–31. doi: 10.1016/j.contraception.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Rauramo I, Elo I, Istre O. Long-term treatment of menorrhagia with levonorgestrel intrauterine system versus endometrial resection. Obstet Gynecol. 2004;104:1314–21. doi: 10.1097/01.AOG.0000143824.16435.91. [DOI] [PubMed] [Google Scholar]
- 8.Malak KA, Shawki O. Management of menorrhagia with the levonorgestrel intrauterine system versus endometrial resection. Gynecol Surg. 2006;3:275–80. [Google Scholar]
- 9.Shaw R, Symonds IM, Tamizian O, Chaplain J, Mukhopadhyay S. Randomised comparison of thermal balloon ablation and levonorgestrel intrauterine system in patients with idiopathic menorrhagia. Aust N Z J Obstet Gynaecol. 2007;47:335–40. doi: 10.1111/j.1479-828X.2007.00747.x. [DOI] [PubMed] [Google Scholar]
- 10.Soysal M, Soysal S, Ozer S. A randomized controlled trial of levonorgestrel releasing IUD and thermal balloon ablation in the treatment of menorrhagia. Zentralbl Gynakol. 2002;124:213–9. doi: 10.1055/s-2002-32434. [DOI] [PubMed] [Google Scholar]
- 11.Busfield RA, Farquhar CM, Sowter MC, et al. A randomised trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113:257–63. doi: 10.1111/j.1471-0528.2006.00863.x. [DOI] [PubMed] [Google Scholar]
- 12.Tam WH, Yuen PM, Shan Ng DP, Leung PL, Lok IH, Rogers MS. Health status function after treatment with thermal balloon endometrial ablation and levonorgestrel intrauterine system for idiopathic menorrhagia: a randomised study. Gynecol Obstet Invest. 2006;62:84–8. doi: 10.1159/000092660. [DOI] [PubMed] [Google Scholar]
- 13.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2008. National Cancer Institute; Bethesda, MD: 2011. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site. [Google Scholar]
- 14.Dwyer NA, Sirrat GM. Early endometrial carcinoma: an incidental finding after endometrial resection. Case report. BJOG. 1991;98:733–4. doi: 10.1111/j.1471-0528.1991.tb13468.x. [DOI] [PubMed] [Google Scholar]
- 15.Copperman AB, DeCherney AH, Olive DL. A case of endometrial cancer following endometrial ablation for dysfunctional uterine bleeding. Obstet Gynecol. 1993;82:640–2. [PubMed] [Google Scholar]
- 16.Ramey JW, Koonings PP, Given FT, Acosta AA. The process of carcinogenesis for endometrial adenocarcinoma could be short: development of a malignancy after endometrial ablation. Am J Obstet Gynecol. 1994;170:1370–1. doi: 10.1016/s0002-9378(94)70158-x. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz IR, Copas PR, Aaronoff M, Spann CO, McGuire WP. Endometrial adenocarcinoma following endometrial ablation for post menopausal bleeding. Gynecol Oncol. 1995;56:460–3. doi: 10.1006/gyno.1995.1083. [DOI] [PubMed] [Google Scholar]
- 18.Margolis MT, Thoen LD, Boike GM, Mercer LJ, Keith LG. Asymptomatic endometrial carcinoma after endometrial ablation. Int J Gynaecol Obstet. 1995;51:255–8. doi: 10.1016/0020-7292(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 19.Baggish MS, Ringgenberg E, Sze EHM. Adenocarcinoma of the corpus uteri following endometrial ablation. J Gynecol Surg. 1995;11:91–4. [Google Scholar]
- 20.Klein Z, Markovitch O, Altaras M, Beyth Y, Fishman A. Advanced endometrial adenocarcinoma following endometrial ablation: a case report and review of the literature. Int J Gynecol Cancer. 1997;7:163–5. [Google Scholar]
- 21.Iqbal PK, Paterson ME. Endometrial carcinoma after endometrial resection for menorrhagia. Br J Obstet Gynaecol. 1997;104:1097–8. doi: 10.1111/j.1471-0528.1997.tb12076.x. [DOI] [PubMed] [Google Scholar]
- 22.Le Marrec A, Lavoue V, Morcel K. Ocurrence of endometrial cancer six years after treatment with thermal balloon ablation (Thermachoice®): first case report. Eur J Obset Gynecol Reprod Biol. 2010;150:219–20. doi: 10.1016/j.ejogrb.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 23.Brooks-Carter GN, Killacky MA, Neuwirth RS. Adenocarcinoma of the endometrium after endometrial ablation. Obstet Gynecol. 2000;96:836–7. [PubMed] [Google Scholar]
- 24.Sandridge DA, Councell RB, Thorp JM. Endometrial carcinoma arising within extensive intrauterine synechiae. Eur J Obstet Gynecol Reprod Biol. 1994;56:147–9. doi: 10.1016/0028-2243(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 25.Pillay OC, Te Fong LF, Crow JC, et al. The association between polycystic ovaries and endometrial cancer. Hum Reprod. 2006;21:924–9. doi: 10.1093/humrep/dei420. [DOI] [PubMed] [Google Scholar]
- 26.Soliman PT, Oh JC, Schmeler KM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol. 2005;105:575–80. doi: 10.1097/01.AOG.0000154151.14516.f7. [DOI] [PubMed] [Google Scholar]
- 27.Strom BL, Schinnar R, Weber AL, et al. Case-control study of postmenopausal hormone replacement therapy and endometrial cancer. Am J Epidemiol. 2006;164:775–86. doi: 10.1093/aje/kwj316. [DOI] [PubMed] [Google Scholar]
- 28.Beral V, Reeves G. Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365:1543–51. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- 29.Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: High-risk factors predicting its occurrence. Am J Obstet Gyncol. 1998;179:569–72. doi: 10.1016/s0002-9378(98)70045-6. [DOI] [PubMed] [Google Scholar]
- 30.Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973-87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994;10:31–46. doi: 10.1002/ssu.2980100107. [DOI] [PubMed] [Google Scholar]
- 31.Neuwirth R, Loffer F, Trenhaile T, Levin B. The incidence of endometrial ablation in a low-risk population. J Am Assoc Gynecol Laparosc. 2004;11:492–2. doi: 10.1016/s1074-3804(05)60081-3. [DOI] [PubMed] [Google Scholar]
- 32.Gaia G, Botchorishvili R, Canis M, et al. Endometrial cancer following endometrial resection. Gynecol Surg. 2007;4:179–185. [Google Scholar]
- 33.Krogh R, Lauszus F, Guttorn E, Rasmussen K. Surgery and cancer after endometrial resection. Long-term follow-up on menstrual bleeding and hormone treatment by questionnaire and registry. Arch Gynecol Obstet. 2009;280:911–6. doi: 10.1007/s00404-009-0989-0. [DOI] [PubMed] [Google Scholar]
- 34.Ogdie A, Langan SM, Parkinson J, et al. Medical Records Databases. In: Strom BL, Kimmel SE, Hennessey S, editors. Pharmacoepidemiology. 5th ed. John Wiley & Sons, Ltd.; Chichester, West Sussex: 2012. pp. 224–243. Chapter 15. [Google Scholar]
- 35.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Pharmacoepidemiol Drug Saf. Vol. 16. 2007; Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. pp. 1195– 2002. [DOI] [PubMed] [Google Scholar]
- 36.Blak BT, Thompson M. How does the health improvement network (thin) data on prevalence of chronic diseases compare with national figures? Value Health. 2009;12:A253. [Google Scholar]
- 37.Haynes K, Forde KA, Schinnar R, et al. Cancer incidence in The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2009;18:730–6. doi: 10.1002/pds.1774. [DOI] [PubMed] [Google Scholar]
- 38.Davé S, Petersen I. Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf. 2009;18:704–707. doi: 10.1002/pds.1770. [DOI] [PubMed] [Google Scholar]
- 39.Reid PC. Endometrial ablation in England-coming of age? An examination of hospital episode statistics 1989/1990 to 2004/2005. Eur J Obstet Gynecol Repr Bio. 2007;135:191–4. doi: 10.1016/j.ejogrb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Linh MD, Wilson RJ, Ajani UA, Singh SD, Eheman CR. Trends in endometrial cancer incidence rates in the United States, 1999-2006. J Womens Health (Larchmt) 2011;20:1157–63. doi: 10.1089/jwh.2010.2529. [DOI] [PubMed] [Google Scholar]
- 41.Schneider C, Jick SS, Meier CR. Risk of gynecologic cancers in users of estradiol/dydrogesterone of other HRT preparations. Climacteric. 2009;12:514–24. doi: 10.3109/13697130903075352. [DOI] [PubMed] [Google Scholar]
- 42.deVries CS, Bromley SE, Thomas H, Farmer RD. Tibolone and endometrial cancer: a cohort and nested case-control study in the UK. Drug Saf. 2005;28:241–9. doi: 10.2165/00002018-200528030-00005. [DOI] [PubMed] [Google Scholar]
- 43.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. Wiley-Interscience; New York, NY: 1999. [Google Scholar]
- 44.Marshal A, Altman DG, Holder RL. Comparison of imputation methods for handling missing covariate data when fitting a Cox proportion hazards model: a resampling study. BMC Res Methodol. 2010;10:112. doi: 10.1186/1471-2288-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin DY, Psaty BM, Kronmal RA. Assessing the Sensitivity of Regression Results to Unmeasured Confounders in Observational Studies. Biometrics. 1998;54:948–63. [PubMed] [Google Scholar]
- 46.Cooper K, Lee A, Chien P, Raja E, Timmaraju V, Bhattacharya S. Outcomes following hysterectomy or endometrial ablation for heavy menstrual bleeding: retrospective analysis of hospital episode statistics in Scotland. BJOG. 2011;118:1171–9. doi: 10.1111/j.1471-0528.2011.03011.x. [DOI] [PubMed] [Google Scholar]
- 47.Office for National Statistics . Cancer Statistics registrations: Registrations of cancer diagnosed in 2008, England. National Statistics; London: 2010. Series MB1 no.39. [Google Scholar]
- 48.Flegal K, Carroll M, Kit B, Ogden C. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 49.Endometrial ablation advisory group Endometrial ablation service. NHS Commissioning Guide. 2007 Dec;:8. [Google Scholar]