Abstract
Importance
A number of interventions for at-risk children have shown benefits immediately after treatment. However, the present study shows persistent long-term effects of a parenting intervention on children's hypothalamic-pituitary-adrenal (HPA) activity, a physiological stress system that is implicated in numerous psychological and physical health problems across the lifespan.
Objective
To examine whether differences in diurnal cortisol production between children receiving the active parenting intervention and children in the control group persisted at a preschool follow-up (approximately 3 years post-intervention).
Design
Between-subject comparison of cortisol patterns among 2 groups of children (experimental and control groups).
Setting
Children involved with Child Protective Services following allegations of neglect.
Participants
A sample of 115 children (43.5% female) between 46.5 and 69.6 months of age (M = 50.73, SD = 4.98), who had been previously randomly assigned to either the ABC intervention (n = 54) or the control intervention (n = 61).
Intervention
The Attachment and Biobehavioral Catch-up Intervention (ABC) was the experimental intervention and it focused on three aims: increasing parental nurturance to child distress, increasing synchronous interactions, and decreasing frightening parental behavior. The control intervention provided educational information about child development to parents. Both interventions were manualized and involved 10 sessions implemented by a trained parent coach in the families' homes or other places of residence.
Main Outcome Measure
Salivary cortisol samples collected at waking and bedtime for children on 3 separate days.
Results
Analyses revealed significant differences in cortisol production at the preschool follow-up, such that children in the ABC intervention group exhibited a typical pattern with higher morning levels and a steep decline across the day, whereas the control group showed a flatter cortisol rhythm with blunted morning levels.
Conclusions and Relevance
Differences in cortisol production between the experimental and control group persisted at the preschool follow-up and resembled differences initially observed 3 months post-intervention. This is encouraging evidence that the ABC intervention for CPS-referred children may have long-lasting effects on a physiological stress system critical for health and adjustment.
Trial Registration
“Intervening Early with Neglected Children”
Children experiencing parental maltreatment face dual harm due to frequent stressful interactions with their family1 and lacking access to the stress-reducing benefits of high-quality parental care.2 It is not surprising then that exposure to parental maltreatment disrupts normative developmental processes and is a major risk factor for a wide range of later psychological and physical health problems.1,3 Chronic activation of physiological stress systems, such as the hypothalamic-pituitary-adrenal (HPA) axis, is considered one of the mediating mechanisms for the unfolding of some of these disease processes.4 Indeed, there is accumulating evidence that the functioning of the HPA axis is altered in children experiencing adverse care.5–9 Disrupted patterns of HPA activity are associated with numerous behavioral and emotional problems in children, including both internalizing and externalizing symptoms.10–13
In humans, the end-product of the HPA axis is cortisol, a steroid hormone which follows a diurnal rhythm that typically rises early in the morning, peaks approximately 30 minutes after wake-up, and declines throughout the day, reaching near-zero levels at night.14 This diurnal pattern is not present at birth, but begins to emerge around three months of age15,16 and is fully entrained to day-light cycles by age two.17 Children experiencing social deprivation or maltreatment show departures from this typical profile of diurnal HPA activity, which are suggestive of chronic stress. For instance, a flattened diurnal cortisol slope with blunted morning cortisol levels is increasingly recognized as a hallmark signature of chronic stress in children as well as adults experiencing adversity.18 Importantly, this has been noted across a wide range of adverse early-life exposures, including child maltreatment, foster care placement, and institutional (i.e., orphanage) rearing.5,6,8,13 Meta-analytic reviews of the literature suggest that the initial response to severe, acute stress is often heightened cortisol18; however, as adversity becomes more chronic, negative feedback mechanisms can lead to down-regulation at various levels of the HPA axis (e.g., reduced synthesis of one of its secretagogues, or decreasing number of receptors reading their signal19), which manifests as blunted cortisol levels and flattened diurnal slopes.18–20 This phenomenon has been referred to as hypocortisolism13,19,20 and is a potential marker of developmental risk.13
There is emerging evidence that a number of interventions with at-risk children and their parents have successfully normalized children's diurnal cortisol that initially had a flat, blunted profile.7,10,21–23 Parenting interventions that aim to enhance caregiving have also been found to result in improved child socio-emotional outcomes and reduced parental stress.21,24,25 In other studies, early parenting interventions have prevented the progressive blunting of morning cortisol observed over time in the untreated or control group.26,27 Across intervention studies that show effects on cortisol regulation, the experimental intervention group often shows cortisol patterns that approximate a low-risk comparison group, suggesting that interventions can support the normalizing of cortisol regulation in children (for a review, see22).
One of the interventions designed to enhance behavioral and biological regulation in young children at-risk for parental neglect is the Attachment and Biobehavioral Catch-up (ABC) intervention.21,23 This 10-week program helps parents become more synchronous and nurturing, as well as less frightening, in interactions with their children. Recent randomized clinical trials have shown that this intervention improves child attachment security25 and normalizes diurnal cortisol production in children at risk for parental neglect when assessed within a few months of the intervention.23 However, the extent to which this normalizing effect persists later in development is currently unknown and represents a major gap in this intervention literature.
A critical question about any intervention's success is whether its effects are long-lasting or simply transient. In addition to answering this question, there are two other reasons to examine long-term effects of the ABC intervention on children's diurnal cortisol production. First, positive findings would reinforce inferences regarding the effects of parenting on child stress physiology, given that correlational studies cannot disentangle effects of parenting from gene-environment correlations (i.e., maltreating parents may also transmit genes predisposing to abnormal HPA functioning to their children). Secondly, some studies with institutionalized children experiencing neglect and transitioning into nurturing homes through adoption show an initial normalization of diurnal cortisol slopes10, but other studies show that years later dysregulated cortisol patterns are present again when compared to non-adopted children.28 This raises the possibility that early adversity may have programming effects on the HPA axis that become apparent with time and development, similar to what has been observed in experimental studies in primates and rodents.29 The follow-up assessment in the present study is ideally suited for testing the possibility of long-lasting reversals in HPA functioning.
For these reasons, the goal of the present study was to test the lasting effects of the ABC intervention by conducting a preschool follow-up. This assessment compared diurnal cortisol rhythms in children who had been randomly assigned to receive the 10-week ABC intervention with those of children in the control intervention condition.
Method
Participants
Primary analyses included 115 children with a history of Child Protective Services (CPS) involvement in infancy. All families had been reported to CPS due to allegations of neglect, and were referred to receive services as part of a city-level program designed to divert children from foster care. Children were between 46.5 and 69.6 months of age at the time of the preschool follow-up of cortisol regulation (M = 50.73, SD = 4.98). See Table 1 for demographic information about the sample. The sample included 3 sets of siblings, with both children within the targeted age range at the time of referral.
Table 1. Child Demographic Characteristics.
| Variable | ABC Intervention (n = 54) | DEF Control Intervention (n = 61) | ||
|---|---|---|---|---|
|
| ||||
| n | % | n | % | |
| Gender | ||||
| Male | 32 | 59% | 33 | 54% |
| Female | 22 | 41% | 28 | 46% |
| Ethnicity | ||||
| White | 4 | 7% | 6 | 10% |
| African American | 37 | 69% | 38 | 62% |
| Hispanic | 1 | 2% | 11 | 18% |
| Bi-racial | 12 | 22% | 6 | 10% |
| Mean (SD) | Min – Max | Mean (SD) | Min – Max | |
|
|
||||
| Child age (months) | 51.5 (5.4) | 46.5 – 66.9 | 50.1 (4.5) | 46.6 – 69.6 |
| Months post intervention | 35.4 (8.7) | 15.9 – 53.9 | 36.1 (6.66) | 20.9 – 46.7 |
Procedures
Agency workers referred parents to the research study if children met inclusion criteria at the time of CPS involvement: (1) younger than 2 years old, and (2) living with birth parent. Following referral, parents were contacted and invited to participate. Upon enrollment, a project coordinator randomly assigned participants to the experimental or control intervention condition using a randomly generated number sequence (with group assignment based on even vs. odd digits). Following pre-intervention research visits, families completed the intervention, and then participated in yearly follow-up visits. Recruitment began in 2006, and preschool follow-up visits were conducted through 2012.
Figure 1 displays the Consolidated Standards of Reporting Trials (CONSORT) flow diagram. As shown in Figure 1, 183 participants (86% of those enrolled in the intervention phase) were retained during the post-intervention phase of the study. Cortisol data were collected from 125 children at the time of the preschool follow-up, reported on here. Of these 125 children, 10 provided samples that were not useable (7 had insufficient volumes of saliva for all samples, 1 had insufficient volumes of saliva for five samples and the other sample was excluded as an outlier, and 2 had all samples excluded as outliers), resulting in a sample size for the present study of 115 children. For the remaining 58 children (of the 183 retained for follow-up), cortisol data were not available because parents did not return the samples (n = 21), or because parents could not be reached to schedule a follow-up visit at that time (n = 37).
Figure 1.
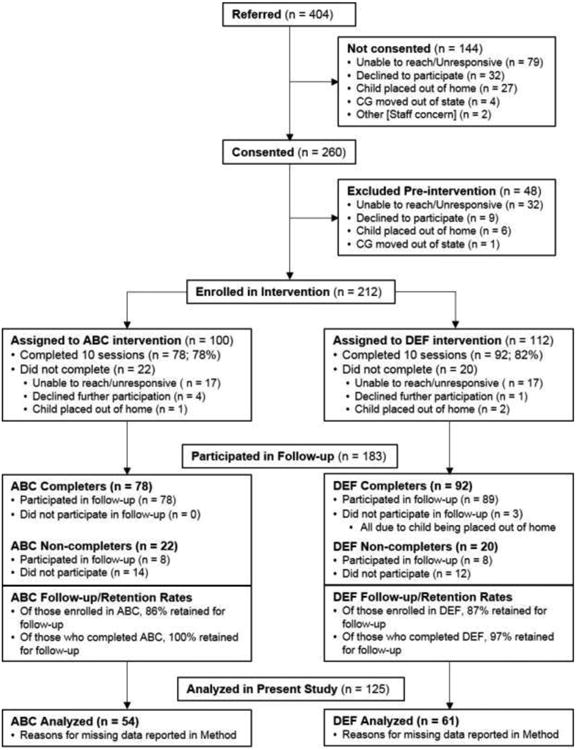
Consolidated Standards of Reporting Trials flow diagram. *We report numbers of children enrolled in ABC (n = 100) and DEF (n = 112) following completion of pre-intervention baseline visits. However, participants were randomly assigned to group upon consenting (N = 260; ABC n = 129, DEF n = 131), at which time the intervention group sample sizes were more similar. Follow-up numbers include participants seen for any post-intervention visits. More specific information is provided in Method section.
Interventions
Both interventions were manualized and involved 10 sessions implemented by trained parent coaches in families' homes or other places of residence.
Experimental intervention
Attachment and Biobehavioral Catch-up Intervention (ABC)
The ABC intervention had three primary aims: increasing nurturance to distress, increasing synchronous interactions, and decreasing frightening parental behavior. Sessions were guided by a manual with each focusing on one of the three ABC targets. Specifically, sessions 1 and 2 focused on nurturance, 3 and 4 on synchrony (or “following the lead”), and 5 and 6 on intrusive and frightening behavior. Sessions 7 through 10 were tailored to address parents' individual strengths and weaknesses and incorporated a focus on how parents' own histories of care influenced their parenting behaviors. Besides guiding discussions about the session topic, parent coaches provided feedback to parents about their interactions during the sessions both in the moment (i.e., live coaching) and using videos. This feedback served to focus attention on the intervention targets and support parents in practicing those behaviors. In recent studies, this in the moment feedback has emerged as a key component of intervention effectiveness.30
Control intervention
Developmental Education for Families (DEF)
The DEF intervention was adapted from a home-visiting program31–33, and focuses on parent education about children's motor, cognitive, and language development. DEF parent coaches provided general information about developmental milestones and suggested developmentally-appropriate activities for parents to engage in with their child.
Saliva sampling
The procedures used for collecting and assaying cortisol followed established protocols (for description, see5). Parents collected saliva samples from children twice per day (within 30 minutes of wake-up, and right before bedtime) over a 3-day period. Table 2 shows descriptive statistics of sampling times.
Table 2. Descriptive Statistics.
| N | Time of sample | Cortisol value (in ug/dl) | Log-transformed cortisol value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| M (SD) | Min | Max | M (SD) | Min | Max | M (SD) | Min | Max | ||
| ABC Intervention (n = 54) | ||||||||||
| Wake- Day 1 | 42 | 8:12 (1:12) | 5:44 | 11:00 | .21 (.18) | .004 | .89 | -.85 (.44) | -2.40 | -.05 |
| Wake- Day 2 | 43 | 8:25 (1:15) | 6:30 | 11:32 | .19 (.19) | .004 | .96 | -.91 (.44) | -2.40 | -.02 |
| Wake- Day 3 | 43 | 8:12 (1:10) | 6:15 | 11:14 | .19 (.15) | .010 | .73 | -.88 (.41) | -2.00 | -.14 |
| Bed- Day 1 | 47 | 9:14 (1:16) | 7:00 | 12:54 | .11 (.13) | .004 | .67 | -1.24 (.56) | -2.40 | -.18 |
| Bed- Day 2 | 40 | 9:02 (1:01) | 7:00 | 12:00 | .13 (.19) | .004 | 1.00 | -1.21 (.63) | -2.40 | .00 |
| Bed- Day 3 | 42 | 9:10 (1:05) | 7:15 | 12:54 | .13 (.13) | .004 | .47 | -1.13 (.53) | -2.40 | -.33 |
| DEF Control Intervention (n = 61) | ||||||||||
| Wake- Day 1 | 52 | 8:34 (1:20) | 6:18 | 11:45 | .20 (.24) | .004 | 1.04 | -.94 (.50) | -2.40 | .02 |
| Wake- Day 2 | 52 | 8:18 (1:06) | 6:00 | 10:02 | .17 (.16) | .004 | .83 | -1.02 (.58) | -2.40 | -.08 |
| Wake- Day 3 | 54 | 8:33 (1:16) | 6:30 | 11:59 | .14 (.14) | .004 | .68 | -1.10 (.56) | -2.40 | -.17 |
| Bed- Day 1 | 55 | 9:17 (1:08) | 6:30 | 12:00 | .12 (.10) | .004 | .40 | -1.11 (.45) | -2.40 | -.40 |
| Bed- Day 2 | 52 | 9:16 (0:47) | 7:45 | 11:00 | .12 (.12) | .004 | .48 | -1.16 (.55) | -2.40 | -.32 |
| Bed- Day 3 | 52 | 9:17 (1:02) | 7:30 | 12:00 | .12 (.12) | .004 | .58 | -1.16 (.56) | -2.40 | -.23 |
The saliva samples were stored in a freezer at −20°C prior to assay procedures. Samples were assayed using a high-sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics, LLC, State College, Pennsylvania). All samples from a child were assayed in duplicate on the same plate to minimize variability. The intra-assay and interassay coefficients of variation fell below 3.7% and 6.4%, respectively.
Cortisol Data Preparation
Following established procedures7, biologically implausible cortisol values (i.e., defined as values greater than 2.0 μg/dl) and cortisol values greater than 3 SDs above the mean were excluded from analyses as outliers. Each child could have up to 6 cortisol values (i.e., 3 wake-up and 3 bedtime samples). Of 690 possible samples, 574 were included in analyses, with 2.2% removed as outliers and 14.6% missing due to an inadequate volume of saliva or because no sample was collected. There were 17 samples (2.5%) that had cortisol levels below the detectable limit of the assay; these samples were replaced with a value of .004 μg/dl. Log10 transformation was used to normalize the distribution of cortisol values due to a positive skew. See Table 2 for descriptive statistics of cortisol values.
Data Analytic Strategy
Hierarchical linear modeling (HLM34) was used to examine intervention group differences in cortisol levels at wake-up and bedtime as well as in slope cortisol levels across the day, following analytic procedures used previously5. HLM accounts for the non-independence of repeated measures by modeling multiple data points as nested within individuals, which further allows for missing data.
The following level 1 within-individual model was specified:
with log-transformed cortisol values as the dependent variable; π0i as the estimated intercept of cortisol at wake-up; π1i as the estimated slope of cortisol from wake-up to bedtime (with SAMPLE representing whether the sample was collected at wake-up [0] or bedtime [1]); π2i as the regression coefficient representing the effect of the time-varying covariate (with TIME representing the sample collection time); and eti as the within-individual error.
Level 2 (i.e., between-subject) variables were included to examine whether there were intervention group effects on cortisol levels at wake-up or bedtime and in change across the day. Child age was included as a covariate given that it was associated with cortisol levels in preliminary analyses. The following level 2 model was specified:
with (β01 and β11 representing the intervention effect (with DEF coded as 0, and ABC coded as 1) on the wake-up log-transformed cortisol value and the cortisol slope, respectively; β02 and β12 representing effect of child age (grand centered at the mean) on the wake-up log-transformed cortisol value and the cortisol slope, respectively.
Results
Wake-up cortisol differed significantly between children in the ABC group and children the DEF group, controlling for time of sample collection and age (β01 = 0.18, p < .05). Specifically, children in the ABC group showed a higher wake-up level of cortisol than children in the DEF group (Table 3). Intervention effects on bedtime cortisol levels were examined by rerunning the model with the bedtime sample as the intercept. Bedtime cortisol levels did not differ significantly between the intervention groups (β01 = -0.01, p = .87). There was a significant intervention effect on the change in cortisol across the day, with children in the ABC group showing a steeper wake-up to bedtime pattern (i.e., more negative slope) than children in the DEF group (β11 = -0.19, p < .05). Thus, children in the DEF group showed a more blunted diurnal cortisol pattern than children in the ABC group (See Figure 2).
Table 3. Multilevel Modeling Coefficients of Intervention Effects on Diurnal Cortisol Production.
| Effect | Log-transformed Cortisol | ||||
|---|---|---|---|---|---|
|
| |||||
| Coefficient | SE | t | df | p | |
| Intercept, β00 | -1.05 | .06 | -18.95 | 112 | .000 |
| ABC, β01 | .18 | .08 | 2.18 | 112 | .032 |
| ChAGE, β02 | -.02 | .01 | -2.46 | 112 | .016 |
| SAMPLE Slope, β10 | -.12 | .06 | -2.13 | 112 | .035 |
| ABC, β11 | -.19 | .08 | -2.33 | 112 | .022 |
| ChAGE, β12 | -.00 | .01 | .58 | 112 | .561 |
| TIME Slope, β20 | -.03 | .03 | -.84 | 112 | .402 |
| ABC, β21 | -.01 | .05 | -.20 | 112 | .839 |
| ChAGE, β22 | -.01 | .01 | -1.49 | 112 | .139 |
Note. β00 and β10 represent the wake-up level of cortisol and the slope of cortisol production across the day, respectively, for DEF (control) children. β01 and β11 represent the difference in the wake-up level of cortisol and slope of cortisol production across the day, respectively, between DEF children and ABC children.
Figure 2.
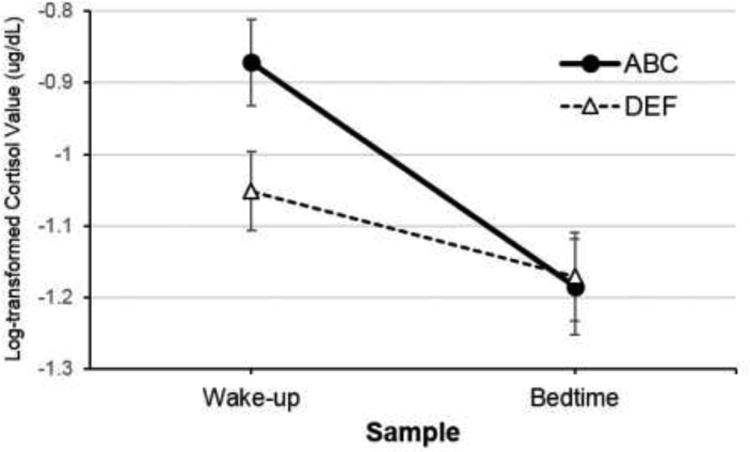
Cortisol levels for neglected children who received the ABC intervention versus neglected children who received the control (DEF) intervention. Error bars represent SE.
In order to estimate effect sizes, Cohen's d was computed by dividing the unstandardized coefficients for intervention effects (accounting for level 1 and level 2 covariates) by the within-group standard deviation.35,36 Estimates of within-group standard deviation were computed using the raw data for wake-up cortisol (to examine the intervention effect on the intercept) and raw data for wake-up to bedtime change in cortisol (to examine the intervention effect on the slope). Based on conventions, the effect sizes for group differences in wake-up cortisol and diurnal slope were approximately medium (d = 0.41 and -0.43, respectively).
Finally, we examined whether findings held if (1) children who did not complete the interventions as intended were excluded, and (2) children who were part of a sibling pair were excluded. Six DEF children and 6 ABC children who provided follow-up cortisol data did not complete the full 10 sessions (considered “non-completers” but retained for follow-up visits). When these 12 non-completers were excluded from analyses, findings held for the effect of the ABC intervention on wake-up cortisol (β01 = 0.20, p < .05), and the diurnal slope (β11 = -0.24, p < 0.05). There were 3 sets of siblings in the sample (2 in ABC, 1 in DEF). When one sibling from each pair was randomly excluded from analyses, the intervention effect held for wake-up cortisol (β01 = 0.20, p < .05) and for the diurnal slope (β11 = -0.20, p < .05).
Discussion
This study examined whether the effects of the ABC intervention on children's diurnal cortisol rhythms are long-lasting and can still be observed during the preschool age range (approximately 3 years post-intervention). Initial assessments conducted roughly 3 months after this 10-week attachment-based parenting program showed a normalization of diurnal cortisol slopes in CPS-referred children who were randomly assigned to the experimental group, but not those in the control group.23 However, the extent to which these positive effects would persist over time was unknown and was the objective of this report. The present analyses revealed a similar pattern of results at the preschool follow-up, such that children in the ABC arm of the randomized clinical trial showed a typical pattern of cortisol production, with higher morning levels and a steep decline across the day, whereas children in the control condition exhibited blunted morning levels and flattened diurnal cortisol slopes that are typical in pediatric samples experiencing neglect7,8 and more generally in groups experiencing ongoing stress.18 The results suggest that the intervention was successful in having persistent, long-term effects on the functioning of the HPA stress system. This may have beneficial implications for preventing child psychological and physical health problems, given previous reports linking cortisol disruptions to these deleterious child outcomes.10–13
Blunted cortisol levels are increasingly recognized as a biomarker of chronic stress, often termed hypocortisolism.18–20 These patterns are thought to be due to down-regulation at some level of the HPA axis subsequent to chronic cortisol elevations.19 Given these suggestions, there are a few possible interpretations for the normalization of these diurnal rhythms observed with the intervention. Given that neglect is chronically stressful for children, the intervention may prevent or minimize exposure to neglectful or insensitive parenting, directly preventing stress. Additionally, the three intervention targets may support children's regulation in a number of ways. First, helping parents respond with nurturance when children were distressed may lead to quicker and more effective soothing following stress responses, thus preventing prolonged exposures to elevated cortisol. Second, synchronous interactions may help children develop a sense of control over their environment, and thus support their independent self-regulatory skills.37,38 Such enhanced biological and behavioral regulation may persist over relatively long time spans. Third, coaching parents to avoid engaging in frightening behaviors towards their children may further serve to prevent dysregulation.
These results are consistent with a few other reports of normalized diurnal cortisol rhythms observed soon after parenting interventions.22 To date, most of these projects have not yet reported long-term follow-up assessments22, with a recent review of intervention effects on cortisol levels in children experiencing adversity concluding that long-term follow-ups are greatly needed in this literature.22 One study with post-institutionalized children conducted roughly 6.5 years after international adoption into welcoming homes did provide evidence of elevated morning cortisol in these children compared to a non-adopted comparison group28, even though the typical pattern of blunted morning cortisol observed within a month of adoption normalized by 6 months later in a comparable sample of children.10 This suggests that it would be important to include a low-risk comparison group in later work with the ABC intervention, to assess whether the steeper slopes observed during follow-up are within the normative range for this age. It will also be important to conduct a longer-term follow-up of this sample, given the results observed more than 6.5 years after adoption in post-institutionalized children. However, it is also possible that children reared in orphanages may differ in important ways from samples like ours, or that the ABC parenting intervention may have more powerful effects than adoption, which would argue in favor of the stability of these results in the future.
One limitation of the present study is that the possible mediating pathways for the effects obtained were not directly tested. Future studies should explore both parent and child characteristics as they change throughout the intervention and afterwards, in order to better understand the processes underlying the interventions' effectiveness. Despite this limitation, the present study provides encouraging evidence that the Attachment and Biobehavioral Catch-up intervention has long-lasting implications for enhancing children's cortisol regulation. Future research will have to clarify the extent to which these persistent biological patterns may contribute to or be accompanied by reductions in physical or mental health problems for children experiencing neglect.
Acknowledgments
Funding/Support: The project described was supported in part by seed grant funding from the Early Experience, Stress, and Neurobehavioral Development Center, Award Number P50MH078105 from the National Institute of Mental Health, as well as Award Numbers R01MH052135, R01MH074374, and R01MH084135 from the National Institute of Mental Health to Dozier.
Footnotes
Author Contributions: Design and conduct of study: Dozier and Bernard; Data collection, analysis, and interpretation of data: Bernard, Hostinar, and Dozier; Preparation, review, and approval of manuscript: Bernard, Hostinar, and Dozier. Bernard had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest: None reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
References
- 1.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128(2):330–366. [PubMed] [Google Scholar]
- 2.Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–82. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137(6):959–97. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2-3):174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard K, Butzin-Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of Child Protective Services. Arch Pediatr Adolesc Med. 2010;164(5):438–43. doi: 10.1001/archpediatrics.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson M, Earls E. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann N Y Acad Sci. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- 7.Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8-10):892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Dev Psychobiol. 2009;51(1):14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dozier M, Manni M, Gordon MK, et al. Foster children's diurnal production of cortisol: Anexploratory study. Child Maltreat. 2006;11(2):189–97. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- 10.Kroupina MG, Fuglestad AJ, Iverson SL, et al. Adoption as an intervention for institutionally reared children: HPA functioning and developmental status. Infant Behav Dev. 2012;35(4):829–37. doi: 10.1016/j.infbeh.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 11.McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Arch Gen Psychiatry. 2000;57(1):38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Arch Gen Psychiatry. 2001;58(3):297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- 13.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13(3):515–38. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 14.Cone RD, Low MJ, Elmquist JK, Cameron JL. Williams Textbook of Endocrinology. Philadelphia, PA: Sanders; 2003. Hypothalamus and pituitary: Neuroendocrinology; pp. 81–176. [Google Scholar]
- 15.Larson MC, White BP, Cochran A, Donzella B, Gunnar M. Dampening of the cortisol response to handling at 3 months in human infants and its relation to sleep, circadian cortisol activity, and behavioral distress. Dev Psychobiol. 1998;33(4):327–37. [PubMed] [Google Scholar]
- 16.Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Arch Dis Child. 1983;58(6):454–6. doi: 10.1136/adc.58.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1-2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 18.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–6. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 21.Dozier M, Peloso E, Lindhiem O, et al. Developing evidence-based interventions for foster children: An example of a randomized clinical trial with infants and toddlers. J Soc Issues. 2006;62(4):767–785. [Google Scholar]
- 22.Slopen N, McLaughlin KA, Shonkoff JP. Interventions to improve cortisol regulation in children: A systematic review. Pediatrics. 2014;133(2):312–26. doi: 10.1542/peds.2013-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernard K, Dozier M, Bick J, Gordon MK. Intervening to enhance cortisol regulation among children at risk for neglect: Results of a randomized clinical trial. Dev Psychopathol. doi: 10.1017/S095457941400073X. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher PA, Stoolmiller M. Intervention effects on foster parent stress: Associations with child cortisol levels. Dev Psychopathol. 2008;20(3):1003–21. doi: 10.1017/S0954579408000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard K, Dozier M, Bick J, Lewis-Morrarty E, Lindhiem O, Carlson E. Enhancing attachment organization among maltreated children: Results of a randomized clinical trial. Child Dev. 2012;83(2):623–36. doi: 10.1111/j.1467-8624.2011.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cicchetti D, Rogosch FA, Toth SL, Sturge-Apple ML. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Dev Psychopathol. 2011;23(3):789–800. doi: 10.1017/S0954579411000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher PA, Van Ryzin MJ, Gunnar MR. Mitigating HPA axis dysregulation associated with placement changes in foster care. Psychoneuroendocrinology. 2011;36(4):531–9. doi: 10.1016/j.psyneuen.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13(3):611–28. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13(3):419–49. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 30.Meade E, Dozier M. “In the moment” commenting: A fidelity measurement and active ingredient in a parent training program. University of Delaware; 2012. [Google Scholar]
- 31.Brooks-Gunn J, Klebanov PK, Liaw F, Spiker D. Enhancing the development of low-birthweight, premature infants: Changes in cognition and behavior over the first three years. Child Dev. 1993;64(3):736–53. doi: 10.1111/j.1467-8624.1993.tb02940.x. [DOI] [PubMed] [Google Scholar]
- 32.Ramey CT, McGinness GD, Cross L, Collier AM, Barrie-Blackley S. The Abecedarian approach to social competence: Cognitive and linguistic intervention for disadvantaged preschoolers. In: Borman K, editor. The Social Life of Children in a Changing Society. Hillsdale, NJ: Erlbaum; 1982. pp. 145–174. [Google Scholar]
- 33.Ramey CT, Yeates KO, Short EJ. The plasticity of intellectual development: Insights from preventive intervention. Child Dev. 1984;55(5):1913. [PubMed] [Google Scholar]
- 34.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 35.Kärnä A, Voeten M, Little TD, Poskiparta E, Kaljonen A, Salmivalli C. A large-scale evaluation of the KiVa Antibullying Program: Grades 4-6. Child Dev. 2011;82(1):311–30. doi: 10.1111/j.1467-8624.2010.01557.x. [DOI] [PubMed] [Google Scholar]
- 36.Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods. 2009;14(1):43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman R, Greenbaum CW, Yirmiya N. Mother-infant affect synchrony as an antecedent of the emergence of self-control. Dev Psychol. 1999;35(1):223–31. doi: 10.1037//0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- 38.Raver CC. Relations between social contingency in mother-child interaction and 2-year-olds' social competence. Dev Psychol. 1996;32(5):850–859. [Google Scholar]


