Abstract
Chronic pain is a complex disorder with multiple etiologies for which the pathologic mechanisms are still largely unknown, making effective treatment a difficult clinical task. Achieving pain relief along with improved function and quality of life is the primary goal of pain clinicians; however, most patients and healthcare professionals consider 30% pain improvement to be clinically significant—a success level that would be unacceptable in other areas of medicine. Furthermore, patients with chronic pain frequently have multiple comorbidities, including depression and sleep apnea, and most have seen several physicians prior to being seen by a pain specialist, have more than three specific pain generators, and are taking multiple medications. The addition of further oral medications to control pain increases the risk of drug–drug interactions and side effects. However, topical analgesics have the advantage of local application with limited systemic levels of drug. Topical therapies benefit from reduced side effects, lower risk of drug–drug interactions, better patient acceptability/compliance, and improved tolerability. This two-part paper is a review of topical analgesics and their potential role in the treatment of chronic pain.
Keywords: Chronic pain, Neuropathic pain, Rational topical polypharmacy, Skin nociception, Topical analgesics
Introduction
Acute and chronic pain affects millions of Americans. Each year, 25 million people will experience an acute pain event (resolving in <2 weeks), whereas 50 million live with chronic pain [1]; furthermore, as the American population ages, the incidence of chronic pain is expected to only increase. For instance, up to 10% of all American adults report chronic pain; however, this increases to 60% in those older than 65 [2]. Missed work and increased healthcare costs attributed to chronic pain conditions have been estimated to exceed 100 billion dollars annually [3]. Chronic pain manifests with both behavioral and physical components, and it is known that genetics, environment, and diet all impact the generation of pain and analgesic responsiveness. Most often, the clinical presentation and treatment of chronic pain patients is extremely complex, and simple solutions or single modalilty therapy offer limited benefit. Nevertheless, a general lack of acceptance of this axiom may explain why current chronic pain treatments are not more efficacious. Clearly, there is a tremendous need to better understand chronic pain mechanisms and to create novel and effective multimodal treatment options for clinicians and patients.
Topical analgesics (TAs) have been used for centuries in the traditional medical approaches of China and other countries. For example, writings from before 600 BC on the treatment of headache list a plethora of topical options, including botanicals and animal-derived products [4]. The first formal report of the pain-reducing properties of topical capsaicin in the West appeared in 1850 as a recommendation to use an alcoholic hot pepper extract for burning or itching extremities [5]. Unfortunately, despite the long history of TA use, their well-documented benefits, and their current use in traditional types of medicine, TAs are underutilized.
Neuropathic pain is a chronic condition of the somatosensory nervous system that severely impairs the health and quality of life of 5–10% of humans, with a total United States economic impact estimated at more than $600 billion annually [6]. Neuropathic pain, by definition, originates from neural pathology and dysfunctions in the peripheral and/or central nervous system (CNS) resulting in high levels of cortical activity among regions known to influence pain perception. Many types of neuropathic pain originate within peripheral tissues, such as skin, ostensibly driven by hyperexcitability of primary afferents. As such, topical approaches may be effective in alleviating neuropathic pain at the source, and this concept underlies most of the evidence for the function of TAs. TAs can target regional pain through affected skin, where they interfere with the peripheral nociceptive mechanisms directly, by modulating the activity of small nerve fibers, and/or indirectly, via non-neuron interactions (e.g., skin resident cells, keratinocytes, and monocyte-derived infiltrated immune cells). In addition, TAs may reach deeper tissues and act on underlying somatic peripheral nerves.
TAs can be delivered as liquids, ointments, gels, powders, creams, semisolids, emulsions, patches, foams, or aerosols. They are mixed with adjuvants, which enhance viscosity and permeation, and emollients and preservatives. Effective TAs are water soluble and lipophilic, with the delivery goals of reducing drug concentration, increasing local tissue absorption, and holding the drug at the application site. Moreover, TAs avoid standard issues that follow the oral ingestion of medications, such as gastric ulceration, first-pass hepatic metabolism, and problems associated with variable serum concentrations. However, there is distinct individual variation in both skin physiology and metabolism that might affect the absorption and drug distribution of TAs. Ultrasound, electricity, and lasers have all been used to facilitate the permeability and delivery of various topical preparations [7, 8]. This article will not discuss either these delivery approaches or the use of TAs for venipuncture in pediatrics. In this first part of this two-part article on TAs, we discuss basic skin innervation and immunocytochemical characteristics under both normal and neuropathic conditions. Insights into skin function and potential therapies are derived from a discussion of research models using topical capsaicin. We also review other pharmacologic formulations and delivery systems.
This review article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Morphology, Innervation, and Immunocytochemical Characteristics of Skin
The skin is a protective barrier designed to absorb daily physical abuse and environmental extremes. Skin is also the largest sensory organ, providing an essential interface between an organism and external stimuli. However, its exposed location and extreme complexity predisposes the skin, and its innervation, to hundreds of chronic afflictions, many of which are associated with chronic pain and lack effective, safe therapeutic options, including postherpetic neuralgia (PHN), diabetic peripheral polyneuropathy (DPPN), and complex regional pain syndrome (CRPS). In addition, the skin and its innervation are especially vulnerable to pharmacotoxic side effects. It is a highly heterogenous organ that integrates elements of integumentary, nervous, vascular, immune, and endocrine functions as well as self-renewing mechanisms. This complexity provides a wide variety of important functions including physical protection, immunologic defense, extremely sensitive multimodal stimulus detection, thermoregulation, hormonal regulation, pliability, and physical appeal. As such the composition of the skin and the varieties of innervation vary dependent upon different functional demands needed over different parts of the body surface.
A major challenge to the development of more effective therapeutic strategies for treating neuropathic pain originating from the skin and its innervation is to identify the nature of the underlying pathologies and how they may differ across diseases and patients. Multimolecular assessments of skin biopsies are revealing previously unknown pathologies that provide valuable insight into the particular symptoms of individual patients and the most appropriate strategies for treatment. Analyses of skin punch biopsies provide a method for detecting potential pathologies that may provide insight into chronic pain mechanisms and potential therapeutic targets [9–15]. Immunocytochemical analyses of axons and endings has become more selective and reliable as well as increasingly viable for exploring complex pain mechanisms and validating human tissue targets [16–19]. Various receptors are involved in cutaneous nociception, a variety of which are listed in Table 1.
Table 1.
Skin receptors related to pain perception in humans
| ASIC3 |
| PAR2 |
| P2X3 |
| TRAAK |
| TREK1/2 |
| TRPA1 |
| TRPM8 |
| TRPV1 |
| VR1 |
List is not exhaustive. Based on: Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120:3760–3772 and Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248–1257
The Paradoxical Loss of Epidermal Endings
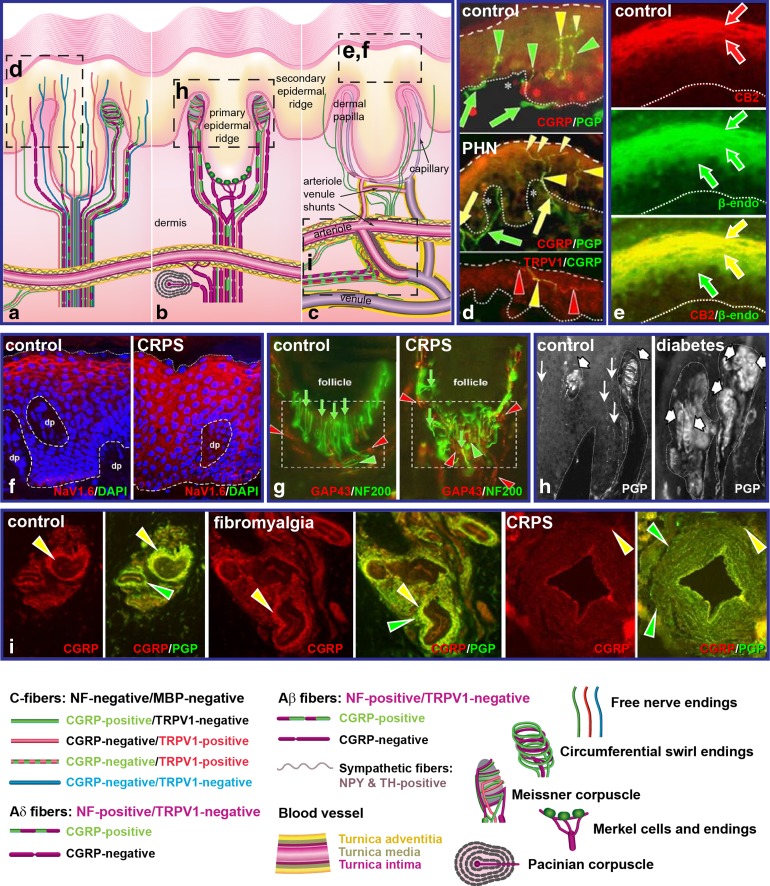
Until the late 1980s, it was widely believed that the epidermis contained few sensory endings, and that most of the small-caliber innervation implicated in cutaneous sensations of pain (i.e., nociceptors) was located in the dermis. However, the development of an antibody against the enzyme ubiquitin C-terminal hydrolase 1 (UCHL-1, also called protein gene product 9.5 [PGP9.5]), which is enriched in neuroendocrine cells, revealed extensive, previously unknown innervation, with the epidermis being a primary site of small-fiber nociceptor endings (Fig. 1a, d) [17, 20–23]. Paradoxically, however, PGP9.5 immunolabeling of punch biopsies from several types of intractable chronic pain afflictions revealed a significant depletion of this intraepidermal nociceptor innervation in these neuropathic pain states (Fig. 1d) [9–15, 18, 19, 24]. Two hypotheses to explain this apparent paradox are: (1) the deafferentation hypothesis, which proposes that CNS neurons may be sensitized by loss of input; and (2) the irritable nociceptors hypothesis, which proposes that the remaining innervation may be hyperactive [25]. Several lines of evidence have revealed that the remaining sensory innervation is, indeed, hyperactive; however, the source of the hyperactivity has yet to be established [26–32]. One potential source may be the selective loss of some innervation types which normally have a regulatory impact, whereas another source is likely to be that the remaining innervation has increased branching and changes in neurochemistry (Fig. 1d)—for example, upregulation of the transient receptor potential cation channel, subfamily V, member 1 (TRPV1) [17–19]. However, such changes have yet to be systematically explored. Although the density of epidermal innervation is generally lower in painful skin, a substantial depletion of innervation can also occur without pain following a herpes zoster outbreak or due to normal aging [33].
Fig. 1.
Selected examples of chronic pain-related pathologies detected by multimolecular immunofluorescence assessments of skin biopsies from humans and rhesus monkeys. a–c Schematic illustration of all the types of innervation in normal glabrous fingertip based on a compilation from several studies (from [41]). a C-fiber and Aδ-fiber innervation of the epidermis and arterioles. b Aβ-fiber innervation. c Vascular innervation. Broken-line boxes indicate locations where immunolabelled images are shown in corresponding d–i. d Changes in the immunochemical characteristics in patients with postherpetic neuralgia (from [19]). e Expression of cannabinoid receptor 2 and β-endorphin in normal epidermal keratinocytes, which are part of an analgesic regulatory mechanism (from [34]). f Expression of NaV1.6 on epidermal keratinocytes, which is increased in patients with CRPS type 1 as part of an increased algesic mechanism (from [36]). g Disarray of Aβ-fiber lanceolate endings around hair follicles of patients with CRPS type 1 (from [18]). h Excess disorganized Aβ-fiber Meissner corpuscles in the dermal papillae of rhesus monkeys with type 2 diabetic neuropathy (from [17]). i Excess innervation of arteriole venule shunts of patients with fibromyalgia (from [46]), and loss of arteriole innervation coupled with hypertrophy of the tunica media in patients with CRPS type 1 [5]. CGRP calcitonin gene-related peptide, CPRS chronic regional pain syndrome, MBP maltose binding protein, NPY neuropeptide Y, TH, tyrosine hydroxylase (positive nerve terminals)
Pathologies of Epidermal Keratinocyte Neural Chemistry
A contributing factor to the hyperactivity of epidermal innervation may be pathologies involving the signaling of epidermal keratinocytes. Although keratinocytes are typically regarded as nonexcitable cells, they express numerous neurospecific signaling molecules; however, the function of these molecules is typically viewed in the context of keratinocyte proliferation and differentiation. Substantial recent evidence indicates that keratinocytes have both algesic and analgesic properties that are involved in sensory transduction and the modulation of activity at epidermal sensory endings. For example, analgesic mechanisms involve the expression of β-endorphin among the keratinocytes of the upper stratum which can be released by activation of the endothelin-1 receptor B (ETB) and the cannabinoid 2 receptor (CB2), which are coexpressed in the same keratinocytes of the upper stratum (Fig. 1e). The release of β-endorphin, in turn, may suppress the activity of epidermal endings that express μ-opioid receptors, G-protein-activated inward-rectifying potassium channels (GIRK), and the proinflammatory neuropeptides calcitonin gene-related peptide (CGRP) and substance P (SP) [34, 35]. The algesic mechanisms involve the keratinocyte production of ATP and the β-isoform of CGRP, which may be released through the activation of voltage-gated sodium channels (NaV) expressed on the keratinocytes (Fig. 1f) [36, 37]. The release of ATP may activate purinergic receptors (e.g., P2X3), which are expressed primarily on epidermal endings that lack CGRP and SP [38]. Recent evidence indicates that the neurochemistry of keratinocytes obtained from the painful skin of patients with PHN and CRPS is skewed toward an over-representation of algesic components (Fig. 1f) and an underrepresentation of analgesic components.
Pathologies among Aβ Fibers
The Aβ cutaneous innervation is generally regarded as mediating low-threshold mechanoreceptive sensations, with implications that it may play an inhibitory role in pain modulation as hypothesized by the gate theory. Currently, this innervation type has received little attention in biopsy assessments of skin associated with chronic pain conditions, primarily due to the fact that this innervation is concentrated in the glabrous and hairy skin of the hands and feet, which are rarely biopsied. However, multimolecular labeling of glabrous and hairy skin, primarily in rodents and monkeys and a few cases in humans, have revealed that individual Meissner corpuscles and piloneural complexes normally have a complex highly ordered morphology consisting of endings from multiple Aβ fibers with an intermingling of several types of C-fibers (Fig. 1b, g) [22, 39–41]. Moreover, both the Aβ and C fiber endings normally express immunochemically detected properties typically associated with nociceptors. These features indicate that the Meissner corpuscles and the piloneural complexes are sites of sensory integration that may have nociceptive involvement. Another paradox of chronic pain conditions is the common complaint that the painful sites also manifest numbness, which has been attributed to a likely loss of Aβ fiber innervation. However, assessments of extensive skin samples of rhesus monkeys that have naturally occurring type 2 diabetes and a few humans with unmanageable CRPS that required limb amputation revealed that Meissner corpuscles and piloneural complexes were present, but were profoundly disorganized (Fig. 1g, h) [17, 18]. These data suggest that the numbness might be due to abnormal activity from this innervation rather than a loss of innervation, and aberrant piloneural complexes observed in the patients with CRPS may contribute to the profound mechanical allodynia caused by slight movements of hairs.
Pathologies among Vascular Innervation
Afferents containing CGRP/SP have been implicated in an axon-reflex-mediated increase in vascular dilation and permeability of capillaries and precapillary arterioles in the upper dermis as well as in pain sensation in response to intense heat. However, relatively little research has been directed toward the extensive, dense, sensory innervation to the arterioles and arteriole–venule shunts (AVS) in the deeper dermis [16, 40, 41]. Nearly all of the attention to the innervation of these deeper vessels has been focused on the sympathetic innervation, which is primarily noradrenergic, with a recently identified cholinergic contingent [16]. The sensory innervation of deeper vessels consists of several types of C and Aδ fibers, most of which also express CGRP and SP (Fig. 1c, i) [16, 40, 42–45]. This innervation is presumably involved in monitoring vascular dynamics and likely plays a vasodilatory effector role to counter sympathetically mediated vasoconstriction [44]. However, recent evidence indicates that these arteriole and AVS afferents may also contribute to conscious tactile perceptions, including pain [16]. The convergence of the sensory and sympathetic innervation among cutaneous vasculature may be a specific site of sudomotor disorders and sympathetic involvement in chronic pain conditions such as CRPS and fibromyalgia, where profound pathologies of this innervation have been observed in the skin (Fig. 1i) [18, 46].
Clinical Phase Studies of Human Experimental Pain
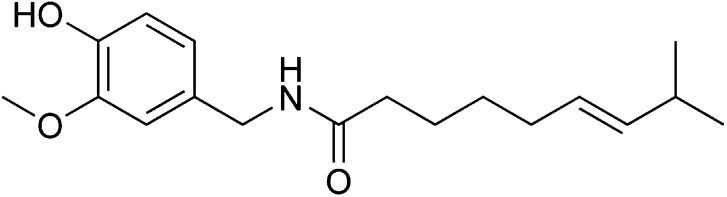
Capsaicin, 8-methyl-N-vanillyl-6-nonenamide, is the active ingredient in chili peppers which provokes a burning sensation by binding to a heat-activated calcium channel, TRPV1. These channels normally open between 37 and 45 °C, but, when bound, the threshold decreases to less than 37 °C or the physiologic body temperature [47]. This stimulates the exocytosis of SP, leading to mast cell degranulation of histamine and serotonin release from platelets. A flare and weal response occurs rapidly while a poorly localized, protracted dull pain lingers from slow-conducting C nerve fibers. Mechanical and heat hyperalgesia ensue as pain thresholds decrease for central and peripheral nociceptors. An area of mechanical allodynia and secondary hyperalgesia occurs due to the activation of adjacent dermatomes. When capsaicin is given intradermally, acute pain is elicited by the activation of fast A fibers and as a direct effect of capsaicin. Elements of chronic neuropathic pain such as mechanothermal hyperalgesia and allodynia are mediated through activation of C fibers. These pain pathways are naturally activated by tissue abrasions, burns, and incisions. The chemical structure of capsaicin is shown in Fig. 2.
Fig. 2.
Capsaicin
There are two capsaicin research models—the intradermal capsaicin and the heat/capsaicin sensitization model. Both models have been well validated as reliable means of producing painful stimuli that include acute sharp pain, burning sensation, mechanical and heat hyperalgesia, and tactile allodynia. Intradermal capsaicin-induced pain is dose dependent, with a minimum dose requirement of 10 µg to elicit a measurable response and ≥100 µg for a robust reaction [48]. Both models have been extensively studied with different classes of pain medications that include opioids, N-methyl-d-aspartic acid (NMDA) antagonists, cannabinoids, sodium and/or calcium channel antagonists, tricyclic antidepressants (TCAs), and cyclooxygenase inhibitors. The pharmacology of the two models appears to be similar. Opioids, cannabinoids, and NMDA antagonists significantly decrease pain and allodynia in this human model. Other nonopioid analgesics (intravenous lidocaine, TCAs, and nonsteroidal anti-inflammatory drugs [NSAIDs]) have minimal effect. Gabapentin, a calcium channel modulator, has shown equivocal results under this model, but its analog, pregabalin, decreased capsaicin-induced pain and hyperalgesia [49]. However, if one looks closely at the studies, the differences appear attributable to the different drug doses and dose scheduling [50–52]. Table 2 shows properties of different classes of topical drugs studied using capsaicin models.
Table 2.
Studies of topical compounds using capsaicin models
| Compound class | Drug | Route | Dose | Experimental pain effect | Neuropathic pain effect | Postoperative pain effect | Reference(s) |
|---|---|---|---|---|---|---|---|
| Opioid | Alfentanil | IV | 75 ng/mL | Positive | Positive as a class | Positive as a class | [50, 51, 61–64] |
| Remifentanil | IV | 0.1 μg/kg/min | Positive | ||||
| Hydromorphone | PO | 8 mg | Positive | ||||
| Morphine | PO | 30 mg | Positive | ||||
| Morphine | IV | 10 mg | Positive | ||||
| Alfentanil | IV | 50 ng/mL | Negative | ||||
| Alfentanil | IV | 200 ng/mL | Positive | ||||
| NMDA antagonist | Ketamine | IV | 150 ng/mL | Positive | Disappointing as a class | Positive only in combination with an opioid | [49, 50, 52, 61] |
| Dextromethorphan | PO | 30 mg | Negative | ||||
| Ketamine | IV | 0.1 mg/kg then 7 μg/kg/min | Positive | ||||
| Magnesium | IV | 0.2 mmol/kg then 0.2 mmol/kg/min for 90 min | Negative | ||||
| Alpha 2 agonist | Clonidine | Intrathecal | 150 μg | Positive | Positive | Positive only in combination with an opioid | [65] |
| Clonidine | IV | 150 μg | Negative | ||||
| Alpha 2 delta ligand | Gabapentin | PO | 1,200 mg | Positive | Positive as a class | Positive only in combination with an opioid | [49–52, 66–69] |
| Gabapentin | PO | 2,400 mg/day | Positive | ||||
| Gabapentin | PO | 1,800 mg/day | Negative | ||||
| Pregabalin | PO | 300 mg | Positive | ||||
| Antihistamine | Diphenhydramine | PO | 50 mg | Negative | Negative | Negative | [49] |
| Diphenhydramine | IV | 25 mg | Negative | [61, 65] | |||
| Tricyclic antidepressant | Desipramine | PO | 225 mg/day | Negative | Positive | Negative | [65–67] |
| Amitriptyline | IM | 25 mg | Negative | ||||
| GABA-A agonist | Midazolam | IM | 4 mg | Negative | Negative as a class | Negative as a class | [65] |
| Na-channel block | Lidocaine | IV | 3 μg/mL | Negative | Disappointing as a class | Disappointing as a class | [65] |
| Lidocaine | IV | 2 mg/kg then 3 μg/kg/h | Positive (but limited) | [68] | |||
| Lidocaine | IV | 5 mg/kg over 50 min | Positive | [69] | |||
| Mexiletine | PO | 859 mg/day | Positive (but limited) | [70] | |||
| Lamotrigine | PO | 300 mg | Negative | [53] | |||
| Lamotrigine | PO | 400 mg | Negative | [63] | |||
| Adenosine agonist | Adenosine | IV | 65 μg/kg/min for 85 min | Negative | Negative | Positive (spinal) | [72] |
| Cannabinoids | Cannabis | Inhaled | 3 doses (2%, 4%, 6%) | Positive 4%, negative 2 % and 6% | Positive 6%, negative 2 and 4% | Modest benefit | [55] |
| Cannabis | Inhaled | 4% | Positive | Positive (low dose) | [73] | ||
| THC/Cannabidiol | Inhaled | 3.56% THC | Equivocal | Negative (high dose) (sativex) | |||
| TRPV1 antagonist | REN-1654 | PO | Oral 100 mg | Negative | Negative | N/aa | [74] |
| AMPA antagonist | NGX426 | PO | 10 mg/cc | Positive | N/ab | N/ab | [75] |
| Magnesium | Magnesium | IV | 0.2 mmol/kg−1 | Negative | N/ab | N/ab | [76] |
AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, GABA γ-aminobutyric acid, IM intramuscularly, IV intravenously, NMDA N-methyl-d-aspartic acid, PO orally, THC tetrahydrocannabinol, TRPV1 transient receptor potential cation channel, subfamily V, member 1
a The patients in this study had a neuropathic component to their pain, but not post-surgical
b This study was done in healthy subjects without neuropathic or postoperative pain
A sodium channel antagonist (4030W92) was shown to be ineffective in an intradermal capsaicin model and had negative effect in a multicenter trial on neuropathic pain with mappable allodynia [53, 54]. Cannabis was studied in this model, but the results showed that a low dose was no different from placebo, and a medium dose reduced, whereas a high dose increased, pain [55].
The identification of the nature of the underlying pathologies and how they may differ from disease to disease and patient to patient pose a major challenge to the development of more effective strategies and therapeutics for treating neuropathic pain originating from the skin and its innervation. Multimolecular assessments of skin biopsies are just beginning to reveal previously unknown pathologies that may provide valuable insight into the particular symptoms of individual patients and the most appropriate strategies for their treatment. The density of epidermal innervation has received major emphasis in using skin biopsies as an analytical and diagnostic tool for neuropathic pain. However, these same skin biopsies also provide insight into other potential contributors and therapeutic targets for chronic pain such as pathologies among neurochemical properties of epidermal keratinocytes, perturbed Aβ fiber innervation, and aberrations among the converging sensory and sympathetic innervation on arterioles and AVS.
Formulations and Applications
When considering the use of a TA, the risk and severity of adverse effects and drug–drug interactions are less than for the same analgesic administered systemically [56]. Obviously, this is clinically relevant when managing a patient who has been prescribed multiple concurrent systemic medications. Recent guidelines regarding the pharmacologic management of pain in older adults emphasize this point [57]. Furthermore, as TAs typically do not involve dose titration, whereas many systemic agents do, this may provide an additional benefit. Choosing which TA to use depends upon the clinical setting in which the medication is being used. For example, application of the 5% lidocaine patch, by protecting allodynic skin from being stimulated, may reduce allodynia in PHN [58].
Recently, three topical NSAIDs and the capsaicin 8% patch (C8P) have been approved by the Food and Drug Administration. Topical preparations containing opioids, local anesthetics (LAs), antidepressants, glutamate receptor antagonists, α-adrenergic receptor agonists, adenosine, cannabinoids, cholinergic receptor agonists, gabapentinoids, prostanoids, bradykinin, ATP, biogenic amines, and nerve growth factor are each at various developmental stages [59]. Considering using TAs that have more than one mechanism of action might be more efficacious than use of a single TA; for example, the antinociceptive effects of topical morphine may be enhanced by a topical cannabinoid as suggested in a rat study [60]. This is intuitive for those who treat chronic pain and could be considered “rational topical polypharmacy”.
Conclusion
TAs provide a therapeutic option with decreased side effects and decreased drug–drug interactions for patients with neuropathic and other disabling chronic pain syndromes. The range of potential targets that can be used as TAs is expanding. The pharmaceutical industry is taking interest in developing some of these options into marketable products, all of which should help patients with pain. In Part Two of this article, we discuss specific drugs that can be used as TAs as well as the potential to determine through physical examination who would benefit most from specific agents.
Acknowledgments
All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. This work did not receive funding from any source. The authors thank Steven Tresker and Dr. Smitha Mathews of Cactus Communications for editing assistance. Funding for the editing assistance was provided by Mallinckrodt Pharmaceuticals. The authors received no remuneration for this work and the paper was written before Dr. Peppin entered the employ of Mallinckrodt. Dr. Peppin was not with industry when this article was written and accepted.
Conflict of interest
J. Peppin holds consulting and advising roles with AIT Laboratories, Ameritox, Endo Pharmaceuticals, INSYS Therapeutics, Salix Pharmaceuticals, and Zogenix Pharmaceuticals. P.J. Albrecht has received investigator-initiated grants from Endo Pharmaceuticals, Forest Laboratories, and Eli Lilly, and has ownership equity in Integrated Tissue Dynamics, LLC. B. Gustorff received unrestricted commercial research grants from Astellas Pharma Europe Ltd. and Grünenthal, Germany, and is a consultant/advisor for Grünenthal, Pfizer, Mundipharma, Janssen-Cilag, Bayer, Astellas Pharma Europe Ltd., and Newron. F.L. Rice has received investigator-initiated grants from Endo Pharmaceuticals, Forest Laboratories, and Eli Lilly. C. Argoff, M. Pappagallo, and M. Wallace declare they have no potential conflicts of interest.
Compliance with ethics guidelines
This review article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Institute of Medicine. Reliving pain in America. Washington DC; 2011. p. 1. http://books.nap.edu/openbook.php?record_id=13172&page=1. Accessed March 2, 2013.
- 2.National Center for Health Statistics New report finds pain affects millions of Americans. Press release issued by CDC; 2014. http://www.cdc.gov/nchs/pressroom/06facts/hus06.htm. Accessed July 11, 2014.
- 3.National Institutes of Health. The NIH guide: new directions in pain research 1. Washington, DC; 1996. http://grants.nih.gov/grants/guide/pa-files/PA-98-102.html. Accessed June 2013.
- 4.Gorji A, Khaleghi Ghadiri M. History of headache in medieval Persian medicine. Lancet Neurol. 2002;1:510–515. doi: 10.1016/S1474-4422(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 5.Turnbull A. Tincture of capsaicin as a remedy for chilblains and toothache. Dublin Free Press, vol. 1; 1850. pp. 95–96.
- 6.O’Reilly KB. Chronic pain costs 635 billion per year. 2013. http://www.ama-assn.org/amednews/2011/07/04/prsr0708.htm. Accessed March 2, 2013.
- 7.Charoo NA, Rahman Z, Repka MA, Murthy SN. Electroporation: an avenue for transdermal drug delivery. Curr Drug Deliv. 2010;7:125–136. doi: 10.2174/156720110791011765. [DOI] [PubMed] [Google Scholar]
- 8.Gratieri T, Kalaria D, Kalia YN. Non-invasive iontophoretic delivery of peptides and proteins across the skin. Exp Opin Drug Deliv. 2011;8:645–663. doi: 10.1517/17425247.2011.566265. [DOI] [PubMed] [Google Scholar]
- 9.Periquet MI, Novak V, Collins MP, et al. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology. 1999;53:1641–1647. doi: 10.1212/WNL.53.8.1641. [DOI] [PubMed] [Google Scholar]
- 10.Barohn RJ. Intraepidermal nerve fiber assessment: a new window on peripheral neuropathy. Arch Neurol. 1998;55:1505–1506. doi: 10.1001/archneur.55.12.1505. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy WR, Wendelschafer-Crabb G. Utility of skin biopsy in diabetic neuropathy. Semin Neurol. 1996;16:163–171. doi: 10.1055/s-2008-1040972. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy BG, Hsieh ST, Stocks A, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/WNL.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 13.Oaklander AL, Romans K, Horasek S, et al. Unilateral postherpetic neuralgia is associated with bilateral sensory neuron damage. Ann Neurol. 1998;44:789–795. doi: 10.1002/ana.410440513. [DOI] [PubMed] [Google Scholar]
- 14.Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem. 2008;110:351–362. doi: 10.1016/j.acthis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauria G, Lombardi R, Camozzi F, Devigili G. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology. 2009;54:273–285. doi: 10.1111/j.1365-2559.2008.03096.x. [DOI] [PubMed] [Google Scholar]
- 16.Bowsher D, Geoffrey Woods C, Nicholas AK, et al. Absence of pain with hyperhidrosis: a new syndrome where vascular afferents may mediate cutaneous sensation. Pain. 2009;147:287–298. doi: 10.1016/j.pain.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Pare M, Albrecht PJ, Noto CJ, et al. Differential hypertrophy and atrophy among all types of cutaneous innervation in the glabrous skin of the monkey hand during aging and naturally occurring type 2 diabetes. J Comp Neurol. 2007;501:543–567. doi: 10.1002/cne.21262. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht PJ, Hines S, Eisenberg E, et al. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120:244–266. doi: 10.1016/j.pain.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 19.Petersen KL, Rice FL, Suess F, Berro M, Rowbotham MC. Relief of post-herpetic neuralgia by surgical removal of painful skin. Pain. 2002;98:119–126. doi: 10.1016/S0304-3959(02)00029-5. [DOI] [PubMed] [Google Scholar]
- 20.Dalsgaard CJ, Rydh M, Haegerstrand A. Cutaneous innervation in man visualized with protein gene product 9.5 (PGP 9.5) antibodies. Histochemistry. 1989;92:385–390. doi: 10.1007/BF00492495. [DOI] [PubMed] [Google Scholar]
- 21.Karanth SS, Springall RR, Kuhn DM, Levene MM, Polak JM. An immunocytochemical study of cutaneous innervation and the distribution of neuropeptides and protein gene product 9.5 in man and commonly employed laboratory animals. Am J Anat. 1991;191:369–383. doi: 10.1002/aja.1001910404. [DOI] [PubMed] [Google Scholar]
- 22.Rice FL, Fundin BT, Arvidsson J, Aldskoqius H, Johansson O. Comprehensive immunofluorescence and lectin binding analysis of vibrissal follicle sinus complex innervation in the mystacial pad of the rat. J Comp Neurol. 1997;385:149–184. doi: 10.1002/(SICI)1096-9861(19970825)385:2<149::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Fundin BT, Arvidsson J, Aldskoqius H, Johansson O, Rice SN, Rice FL. Comprehensive immunofluorescence and lectin binding analysis of intervibrissal fur innervation in the mystacial pad of the rat. J Comp Neurol. 1997;385:185–206. doi: 10.1002/(SICI)1096-9861(19970825)385:2<185::AID-CNE2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 24.Petersen KL, Rice FL, Farhadi M, Reda H, Rowbotham MC. Natural history of cutaneous innervation following herpes zoster. Pain. 2010;150:75–82. doi: 10.1016/j.pain.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis. 1998;5:209–227. doi: 10.1006/nbdi.1998.0204. [DOI] [PubMed] [Google Scholar]
- 26.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moalem-Taylor G, Allbutt HN, Iordanova MD, Tracey DJ. Pain hypersensitivity in rats with experimental autoimmune neuritis, an animal model of human inflammatory demyelinating neuropathy. Brain Behav Immun. 2007;21:699–710. doi: 10.1016/j.bbi.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Ochoa JL. The irritable human nociceptor under microneurography: from skin to brain. Suppl Clin Neurophysiol. 2004;57:15–23. doi: 10.1016/s1567-424x(09)70339-3. [DOI] [PubMed] [Google Scholar]
- 29.Ochoa JL, Campero M, Serra J, Bostock H. Hyperexcitable polymodal and insensitive nociceptors in painful human neuropathy. Muscle Nerve. 2005;32:459–472. doi: 10.1002/mus.20367. [DOI] [PubMed] [Google Scholar]
- 30.Schmelz M, Schmidt R. Microneurographic single-unit recordings to assess receptive properties of afferent human C-fibers. Neurosci Lett. 2010;470:158–161. doi: 10.1016/j.neulet.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 31.Xiao WH, Bennett GJ. Persistent low-frequency spontaneous discharge in A-fiber and C-fiber primary afferent neurons during an inflammatory pain condition. Anesthesiology. 2007;107:813–821. doi: 10.1097/01.anes.0000286983.33184.9c. [DOI] [PubMed] [Google Scholar]
- 32.Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-l-carnitine. Pain. 2008;135:262–270. doi: 10.1016/j.pain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen KL, Rowbotham MC. Natural history of sensory function after herpes zoster. Pain. 2010;150:83–92. doi: 10.1016/j.pain.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Khodorova A, Navarro B, Jouaville LS, et al. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim MM, Porreca F, Lai J, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao P, Barr TP, Hou Q, et al. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: evidence for a role in pain. Pain. 2008;139:90–105. doi: 10.1016/j.pain.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Hou Q, Barr T, Gee L, et al. Keratinocyte expression of calcitonin gene-related peptide beta: implications for neuropathic and inflammatory pain mechanisms. Pain. 2011;152:2036–2051. doi: 10.1016/j.pain.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dussor G, Koerber HR, Oaklander AL, Rice FL, Molliver DC. Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain Res Rev. 2009;60:24–35. doi: 10.1016/j.brainresrev.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pare M, Elde R, Mazurkiewicz JE, Smith AM, Rice FL. The Meissner corpuscle revised: a multiafferented mechanoreceptor with nociceptor immunochemical properties. J Neurosci. 2001;21:7236–7246. doi: 10.1523/JNEUROSCI.21-18-07236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fundin BT, Pfaller K, Rice FL. Different distributions of the sensory and autonomic innervation among the microvasculature of the rat mystacial pad. J Comp Neurol. 1997;394:545–568. doi: 10.1002/(SICI)1096-9861(19971229)389:4<545::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Rice FL, Albrecht PJ, et al. Cutaneous Mechanisms of Tactile Perception: Morphological and Chemical Organization of the Innervation to the Skin. In: Basbaum EA, Kaneko A, Shepherd GM, et al., editors. The senses: a comprehensive reference. San Diego: Academic Press; 2008. pp. 1–31. [Google Scholar]
- 42.Cannon KE, Chazot PL, Hann V, Shenton F, Hough LB, Rice FL. Immunohistochemical localization of histamine H3 receptors in rodent skin, dorsal root ganglia, superior cervical ganglia, and spinal cord: potential antinociceptive targets. Pain. 2007;129:76–92. doi: 10.1016/j.pain.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hough LB, Rice FL. H3 receptors and pain modulation: peripheral, spinal, and brain interactions. J Pharmacol Exp Ther. 2011;336:30–37. doi: 10.1124/jpet.110.171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pare M, Smith AM, Rice FL. Distribution and terminal arborizations of cutaneous mechanoreceptors in the glabrous finger pads of the monkey. J Comp Neurol. 2002;445:347–359. doi: 10.1002/cne.10196. [DOI] [PubMed] [Google Scholar]
- 46.Albrecht PJ, Hou Q, Argoff CE, Storey JR, Wymer JP, Rice FL. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: implications for wide-spread deep tissue pain and fatigue. Pain Med. 2013;14:895–915. doi: 10.1111/pme.12139. [DOI] [PubMed] [Google Scholar]
- 47.Spicarová D, Palecek J. The role of spinal cord vanilloid (TRPV1) receptors in pain modulation. Physiol Res. 2008;57(suppl 3):S69–S77. doi: 10.33549/physiolres.931601. [DOI] [PubMed] [Google Scholar]
- 48.Scanlon GC, Wallace MS, Ispirescu JS, Schulteis G. Intradermal capsaicin causes dose-dependent pain, allodynia and hyperalgesia in humans. J Inves Med. 2006;54:1–7. doi: 10.2310/6650.2006.05046. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Bolognese J, Calder N, et al. Effects of morphine and pregabalin compared with diphenhydramine hydrochloride and placebo on hyperalgesia and allodynia induced intradermal capsaicin in healthy male subjects. J Pain. 2008;9:1088–1095. doi: 10.1016/j.jpain.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Dirks J, Peterson KL, Rowbotham MC, Dahl JB. Gabapentin suppresses cutaneous hyperalgesia following heat-capsaicin sensitization. Anesthesiology. 2002;97:102–107. doi: 10.1097/00000542-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Gottrup H, Juhl G, Kristensen AD, et al. Chronic oral gabapentin reduces elements of central sensitization in human experimental hyperalgesia. Anesthesiology. 2004;101:1400–1408. doi: 10.1097/00000542-200412000-00021. [DOI] [PubMed] [Google Scholar]
- 52.Wallace MS, Schulteis G. Effect of chronic oral gabapentin on capsaicin- induced pain and hyperalgesia: a double-blind, placebo-controlled, crossover study. Clin J Pain. 2008;24:544–549. doi: 10.1097/AJP.0b013e3181673b93. [DOI] [PubMed] [Google Scholar]
- 53.Wallace MS, Quessy S, Schulteis G. Lack of effect of two oral sodium channel antagonists, lamotrigine and 4030W92 on intradermal capsaicin-induced hyperalgesia model. Pharmacol Biochem Behav. 2004;78:349–355. doi: 10.1016/j.pbb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Wallace MS, Rowbotham M, Bennett GJ, Jensen TS, Pladna R, Quessy S. A multicenter, double-blind, randomized, placebo-controlled crossover, evaluation of a short course of 4030W92 in patients with chronic neuropathic pain. J Pain. 2002;3:227–233. doi: 10.1054/jpai.2002.123650. [DOI] [PubMed] [Google Scholar]
- 55.Wallace M, Schulteis G, Atkinson JH, et al. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology. 2007;107:785–796. doi: 10.1097/01.anes.0000286986.92475.b7. [DOI] [PubMed] [Google Scholar]
- 56.Argoff CE. Targeted topical peripheral analgesics in the management of pain. Curr Pain Headache Rep. 2002;7:34–38. doi: 10.1007/s11916-003-0007-3. [DOI] [PubMed] [Google Scholar]
- 57.American Geriatrics Society Panel on Pharmacological Management of Persistent Pain In Older Adults Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 58.Argoff CE. New analgesics for neuropathic pain: the lidocaine patch. Clin J Pain 2000; Suppl 16:S62–S65. [DOI] [PubMed]
- 59.Sawynok J. Topical and peripherally acting analgesics. Pharmacol Rev. 2003;55:1–20. doi: 10.1124/pr.55.1.1. [DOI] [PubMed] [Google Scholar]
- 60.Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303–308. doi: 10.1016/S0304-3959(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 61.Wallace MS, Ridgeway B, Leung A, Schulteis G, Yaksh T. Concentration- effect relationships for intravenous alfentanil and ketamine infusions in human volunteers: effects upon acute thresholds and capsaicin-evoked hyperpathia. J Clin Pharm. 2002;42:70–80. doi: 10.1177/0091270002042001008. [DOI] [PubMed] [Google Scholar]
- 62.Petersen KL, Maloney A, Hoke F, Dahl JB, Rowbotham MC. Effect of remifentanil on pain and secondary hyperalgesia associated with the heat-capsaicin sensitization model in healthy volunteers. Anesthesiology. 2001;94:15–20. doi: 10.1097/00000542-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Petersen KL, Maloney A, Hoke F, Dahl JB, Rowbotham MC. A randomized study of the effect of oral lamotrigine and hydromorphone on pain and hyperalgesia following heat/capsaicin sensitization. J Pain. 2003;4:400–406. doi: 10.1016/S1526-5900(03)00718-1. [DOI] [PubMed] [Google Scholar]
- 64.Eisenach JC, Hood DD, Curry R. Intrathecal, but not intravenous, clonidine reduces experimental thermal or capsaicin-induced pain and hyperalgesia in normal volunteers. Anesth Analg. 1998;87:591–596. doi: 10.1097/00000539-199809000-00018. [DOI] [PubMed] [Google Scholar]
- 65.Wallace MS, Laitin S, Licht D, Yaksh TL. Concentration-effect relations for intravenous lidocaine infusions in human volunteers: effect on acute sensory thresholds and capsaicin-evoked hyperpathia. Anesthesiology. 1997;86:1262–1272. doi: 10.1097/00000542-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Wallace MS, Grubbs D. Effects of oral desipramine on capsaicin induced hyperalgesia. Anesth Analg. 2002;95:973–978. doi: 10.1097/00000539-200210000-00034. [DOI] [PubMed] [Google Scholar]
- 67.Eisenach JC, Hood DD, Curry R, Tong C. Alfentanil, but not amitriptyline, reduces pain, hyperalgesia, and allodynia from intradermal injection of capsaicin in humans. Anesthesiology. 1997;86:1279–1287. doi: 10.1097/00000542-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Dirks J, Fabricius P, Petersen KL, Rowbotham MC, Dahl JB. The effect of systemic lidocaine on pain and secondary hyperalgesia associated with the heat/capsaicin sensitization model in healthy volunteers. Anesth Analg. 2000;91:967–972. doi: 10.1097/00000539-200010000-00037. [DOI] [PubMed] [Google Scholar]
- 69.Gottrup H, Hansen PO, Arendt-Nielsen L, Jensen TS. Differential effects of systemically administered ketamine and lidocaine on dynamic and static hyperalgesia induced by intradermal capsaicin in humans. Br J Anaesth. 2000;84:155–162. doi: 10.1093/oxfordjournals.bja.a013396. [DOI] [PubMed] [Google Scholar]
- 70.Ando K, Wallace MS, Braun J, Schulteis G. Neurosensory finding after oral mexiletine in healthy volunteers. Reg Anesth Pain Med. 2000;25:468–474. doi: 10.1053/rapm.2000.8584. [DOI] [PubMed] [Google Scholar]
- 71.Frymoyer AR, Rowbotham MC, Petersen KL. Placebo-controlled comparison of a morphine/dextromethorphan combination with morphine on experimental pain and hyperalgesia in healthy volunteers. J Pain. 2007;8:19–25. doi: 10.1016/j.jpain.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Dirks J, Petersen KL, Rowbotham MC, Dahl JB. Effect of systemic adenosine on pain and secondary hyperalgesia associated with the heat/capsaicin sensitization model in healthy volunteers. Reg Anesth Pain Med. 2001;26:414–419. doi: 10.1097/00115550-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- 74.Carragee EJ, Klapper JA, Schaufele MK, et al. Oral REN-1654 in Sciatica: a phase 2 randomized double-blind, placebo controlled, multicenter study in subjects with pain due to lumbosacral radiculopathy. Spine J. 2006;6:2S. doi: 10.1016/j.spinee.2006.06.004. [DOI] [Google Scholar]
- 75.Wallace MS, Lam V, Schettler J. NGX426, an Oral AMPA-Kainate Antagonist, is effective in human capsaicin-induced pain and hyperalagesia. Pain Med. 2012;13:1601–1610. doi: 10.1111/j.1526-4637.2012.01509.x. [DOI] [PubMed] [Google Scholar]
- 76.Mikkelsen S, Dirks J, Fabricius P, Petersen KL, Rowbotham MC, Dahl JB. Effect of intravenous magnesium on pain and secondary hyperalgesia associated with the heat/capsaicin sensitization model in healthy volunteers. Br J Anaesth. 2001;86:871–873. doi: 10.1093/bja/86.6.871. [DOI] [PubMed] [Google Scholar]