Abstract
Introduction
The fluocinolone acetonide (FA) intravitreal implant 0.59 mg (Retisert®, Bausch + Lomb, Rochester, NY, USA) provides sustained release of FA directly to the vitreous cavity over a prolonged period of time. The purpose of this study was to evaluate the safety and efficacy of a 0.59- and 2.1-mg FA intravitreal implant in patients with noninfectious posterior uveitis.
Methods
A prospective, multicenter, randomized, double-masked, dose-controlled study was performed. Patients were randomized to the 0.59- or 2.1-mg FA implant surgically placed in the vitreous cavity through a pars plana incision and were evaluated at visits through 3 years. Patients with bilateral disease had the more severely affected eye implanted. Outcomes included uveitis recurrence rate, best-corrected visual acuity (BCVA), use of adjunctive therapy, and safety.
Results
A total of 239 patients, predominantly Asian, were implanted (n = 117, 0.59-mg implant; n = 122, 2.1-mg implant). Approximately 80% of patients had bilateral disease. Recurrence rates for implanted eyes decreased from 42.3% during the 1-year pre-implantation period to 25.9% during the 3-year post-implantation period (P = 0.0003) and increased for nonimplanted fellow eyes from 19.8 to 59.7% (P < 0.0001). More implanted eyes gained ≥3 lines of BCVA compared to nonimplanted fellow eyes (P ≤ 0.0046); and implanted eyes required less adjunctive systemic therapy and fewer periocular injections (P < 0.0001). Elevations of intraocular pressure (≥10 mm Hg) were frequent in implanted eyes (67.8%, 0.59-mg implant; 71.3%, 2.1-mg implant); nearly all (94.9%) phakic implanted eyes required cataract surgery.
Conclusion
The FA intravitreal implant significantly reduced uveitis recurrence rates and led to improvements in visual acuity and reductions in adjunctive therapy. Lens clarity and intraocular pressure require monitoring.
Electronic supplementary material
The online version of this article (doi:10.1007/s40123-014-0027-6) contains supplementary material, which is available to authorized users.
Keywords: Adjunctive therapy, Asian population, Fluocinolone acetonide intravitreal implant, Posterior uveitis, Randomized clinical trial, Retisert, Uveitis recurrence
Introduction
The term ‘uveitis’ comprises a group of intraocular inflammatory conditions that directly or indirectly affects the iris, ciliary body, and choroid, collectively known as the uveal tract, as well as the retina, optic nerve, and vitreous [1–4]. In most cases, the etiology of uveitis is unknown; however, it can be associated with autoimmune disease, infection (viral, fungal, or parasitic), or trauma [2–6]. Uveitis results in significant visual impairment and is thought to account for 10–15% of all cases of total blindness in the United States (US) and developed world [1, 4, 6–8]. While posterior uveitis accounts for only 20% of the estimated 1 in 500 people in the US with uveitis [1], it is the more severe form of the disease.
The primary causes of vision loss in patients with uveitis are cystoid macular edema (CME) and/or cataract [1, 4, 7], with CME being the leading cause of vision loss in posterior uveitis. Treatments aimed at reducing CME are therefore effective in the treatment of uveitis. Corticosteroids are considered the mainstay of noninfectious uveitis treatment [4, 9]. However, since the disease is typically chronic in nature, patients often require long-term repeated treatment with either topical or systemic corticosteroids [10, 11]. In severe cases of uveitis, multiple rounds of sub-Tenon or intravitreal corticosteroid injections as well as systemic corticosteroids may be necessary. The potential for complications such as endophthalmitis, vitreous hemorrhage, and retinal detachment following use of repeated intravitreal injections is substantial [12, 13]. Systemic corticosteroids require high dosages to achieve therapeutic concentrations in the eye and are associated with systemic side effects including hypertension, hyperglycemia, and increased susceptibility to infection [14, 15]. Immunosuppressive agents can be an effective treatment option, but are associated with serious and potentially life-threatening systemic adverse events (AEs) such as renal and hepatic failure and bone marrow suppression [9, 16]. Thus, such therapy is usually reserved for patients with severe uveitis who are unresponsive to corticosteroid therapy or with corticosteroid-induced complications.
The fluocinolone acetonide (FA) intravitreal implant 0.59 mg (Retisert®, Bausch + Lomb, Rochester, NY, USA) was developed to provide sustained release of a corticosteroid directly to the vitreous cavity over a prolonged period of time, thus avoiding complications with systemic therapy as well as those associated with repeated corticosteroid injections. Approved by the US Food and Drug Administration for the treatment of chronic non-infectious posterior uveitis (NIPU), the FA implant is inserted into the posterior segment through a small pars plana incision and sutured to the sclera. The implant releases FA at a nominal initial rate of 0.6 µg/day, decreasing over the first month to a steady state between 0.3 and 0.4 µg/day for approximately 2.5 years. The sustained release of FA was previously reported to result in long-term, continuous control of inflammation [17, 18], and the implant is therefore considered to be particularly suitable for patients with chronic inflammation due to NIPU.
The FA intravitreal implant was evaluated in three large multicenter clinical trials during the course of its development. Thirty-four-week [19] and 3-year results [18] of the first trial and 2-year results [20] of the second trial have been published previously. Both of these trials were conducted in predominantly non-Asian patients. Herein, we report the results of the third trial which evaluated the safety and efficacy of the 0.59-mg implant (marketed formulation) and a 2.1-mg FA implant in a predominantly Asian population with chronic, recurrent, unilateral or bilateral NIPU.
Methods
Study Design
This was a 3-year multicenter, randomized, double-masked, dose-controlled safety and efficacy study of two FA intravitreal implants—one containing 0.59 mg and the other 2.1 mg—in patients with chronic, recurrent, unilateral or bilateral NIPU (ClinicalTrials.gov identifier: NCT0456482). Patient inclusion and exclusion criteria are presented in Table 1. The study was conducted at 19 sites in the following countries: India (3), Canada (5), Australia (4), US (4), Hong Kong (1), and the Philippines (2); the study received Institutional Review Board approval at each center. Before study entry and providing written informed consent, each patient received a full explanation of study procedures. An independent Data Safety Monitoring Board assessed the safety and efficacy data as the study progressed and alerted the sponsor if any issues arose. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Table 1.
Patient inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Males or non-pregnant females ≥6 years | Allergy to FA or any component of the delivery system |
| One or both eyes must have | History of iritis only; no vitreous cells or haze |
| History of recurrent NIPU of ≥1 year requiring either | Infectious uveitis or vitreous hemorrhage |
| Systemic steroid therapy/equivalent for ≥3 months, or | Toxoplasmosis scar/retinal detachment |
| ≥ 2 sub-Tenon steroid injections during the 6 months prior to enrollment, or | Ocular media opacity |
| ≥2 recurrences that require systemic or sub-Tenon injection steroid therapy within the 6 months prior to enrollment | History/presence of uncontrolled IOP while receiving steroid therapy resulting in vision loss, or IOP >25 mm Hg requiring ≥2 anti-glaucoma medications |
| The eye randomized to undergo implantation must have had | Ocular surgery within 3 months of enrollment |
| ≤10 anterior chamber cells and vitreous haze less than grade 2 (treatment to attain these criteria was allowed) | Need for chronic systemic steroids (>15 mg prednisolone/day) or systemic immunosuppressive therapy for nonocular disease |
| Visual acuity of ≥1.4 logMAR in the implanted eye | Positive HIV test |
| Ability to understand and sign the informed consent form | |
| Patients for whom risk outweighs study benefits according to the physician | |
| Current enrollment in another study or participation within 1 month before entry into this study |
FA fluocinolone acetonide, HIV human immunodeficiency virus, IOP intraocular pressure, logMAR logarithm of the minimum angle resolution, NIPU noninfectious posterior segment uveitis
All patients were randomized 1:1 via a computer-generated randomization procedure to receive one of the two FA implant doses (0.59 or 2.1 mg) in their study eye. Patients were stratified according to the investigative site and method of NIPU management history [(1) systemic corticosteroids or immunosuppressive agents and (2) periocular injection of corticosteroids] before treatment group assignment. Patients with unilateral disease received the implant in the affected eye. In patients with bilateral disease, the more severely affected eye underwent implantation. Criteria for determining which eye was more severely affected included (1) an increased number of recurrences of NIPU during the 1-year pre-implantation period or, if both eyes had similar recurrences, (2) an increase in therapy during the 1-year pre-implantation period or, if both eyes had similar therapy, (3) a greater degree of visual acuity impairment or, if both eyes had similar visual acuity, (4) the judgment of the physician. Implanted eyes were designated as ‘study eyes’, while nonimplanted eyes were designated as ‘fellow eyes’. Patients with bilateral disease were considered for inclusion in the study only if the investigator felt that it would be possible to control the fellow-eye ocular inflammation with local therapy.
The FA implants and surgical implantation procedure have been described in detail elsewhere [11, 18, 19]. Briefly, the polymer-based intravitreal implant contains a sustained-release formulation of a 0.59- or 2.1-mg FA tablet 1.5 mm in thickness encased in a silicone elastomer cup that is attached to a heat-cured polyvinyl alcohol suture tab. The 0.59-mg implant releases FA at approximately 0.4 μg/day, whereas the 2.1-mg implant releases FA at a rate of approximately 2 μg/day initially, decreasing to approximately 1 μg/day over a 3-year period. Surgical implantation was performed under local or general anesthesia with placement of the implant in the inferonasal or inferotemporal quadrant of the posterior segment at the pars plana. A pars plana infusion line was placed to reduce the possibility of globe collapse in vitrectomized eyes. An 8-0 Prolene™ (Ethicon, Inc., Somerville, NJ, USA) suture anchored the implant, such that the top surface of the implant faced the front of the eye.
One week post-implantation, patients discontinued use of existing therapy for ocular inflammation as follows: (1) those receiving oral corticosteroids reduced their use over at least 6 weeks by approximately 30% per week until the dose reached 2.5 mg/day for 1 week before completely discontinuing systemic treatment; (2) those receiving topical corticosteroids gradually tapered the dosage from hourly use to once-per-day use for 1 week before completely discontinuing systemic treatment; and (3) those receiving immunosuppressive therapy gradually tapered the dosage over a 6-week period at the investigator’s discretion. Patients were evaluated on day 2 and at weeks 1, 4, 8, 12, 18, 24, 30, 34, and 52. After the 1-year visit, follow-up evaluation visits were conducted at 3-month intervals for an additional 2 years. Evaluations, also described previously [18], included best-corrected visual acuity (BCVA), applanation tonometry, slitlamp biomicroscopy, indirect ophthalmoscopy, automated visual field testing (Humphrey 24–2), hematology, and serum chemistry testing. Optical coherence tomography was not widely available at the time this study was conducted; consequently, patients were evaluated with fluorescein angiography at screening, at week 8, week 34, and 1, 2, and 3 years using a standardized protocol with macular hyperfluorescence evaluated by masked readers as described previously [19].
Efficacy Outcomes
Three types of comparisons were utilized in the study: (1) a comparison of post-implantation findings to retrospective findings recorded during the 1-year pre-implantation period, (2) a dose comparison in terms of both efficacy and safety, and (3) a within-patient comparison of implanted eyes and nonimplanted fellow eyes in patients with bilateral disease.
The primary efficacy outcome was the change in uveitis recurrence rate in the implanted eye (during the 1-year period pre-implantation versus 3 years post-implantation). Based on medical chart review, investigators recorded whether an episode during the 1-year pre-implantation period met the protocol definition of a post-implantation recurrence. A pre-implantation recurrence with (1) a maximum anterior chamber (AC) cell score <2; (2) a maximum vitreous haze score <2; and (3) a maximum reduction in visual acuity of <0.30 logarithm of the minimum angle resolution (logMAR) or Snellen equivalent was not considered sufficiently severe to be counted in this analysis. If the medical chart lacked sufficient detail to determine whether the pre-implantation recurrence met the protocol definition, no pre-implantation recurrence was recorded for this analysis. Recurrence within the 3-year post-implantation period was defined as follows: (1) a ≥2-step increase compared to baseline in the number of AC cells not attributable to any condition other than NIPU, (2) a ≥2-step increase compared to baseline in vitreous haze not attributable to any condition other than NIPU, or (3) a deterioration in BCVA from baseline of at least 0.30 logMAR not attributable to any condition other than NIPU. Recurrences were considered ‘observed’ when they were seen and recorded by study investigators, whereas they were considered ‘imputed’ when a subject was not seen within 10 weeks of the final scheduled visit.
Secondary efficacy outcomes were evaluated using the fellow nonimplanted eye as a control for the study eye and included: rate of and time to post-implantation recurrence of uveitis; change in BCVA; and area of CME using a 300-s fluorescein angiogram, and the proportion of eyes requiring systemic therapy or periocular injections to control inflammation using the pre-implantation comparison group data. Secondary efficacy outcomes included observed and imputed recurrence data where applicable.
Safety Outcomes
Safety outcomes included intraocular pressure (IOP), lens opacity estimated using the lens opacity classification system (LOCS) II, visual field, ocular and nonocular AEs, visual acuity, and ophthalmoscopic examination findings.
Ocular AEs were defined as any unexpected ocular condition that was considered by the investigator to be clinically significant including but not limited to: (1) any IOP increase requiring medication or increased dosage/frequency, (2) any IOP >30 mm Hg 3 months post-surgery, (3) any IOP <6 mm Hg, (4) any loss of ≥3 lines visual acuity from baseline or last scheduled visit, and (5) retinal tear.
Statistical Analyses
Statistical analyses were performed using SAS, version 8.2 (SAS Institute, Cary, NC, USA). The McNemar test for correlated proportions was used to analyze the primary and secondary efficacy end points. Time to uveitis recurrence was determined via Kaplan–Meier analysis. The Cochran–Mantel–Haenzsel χ 2 test was used for between-dose comparison of the frequency of uveitis recurrence post-implantation. Statistical analyses of safety variables were performed using the χ 2 test (observations were stratified according to the investigative site and the therapy used during the pre-implantation period). Distribution of time to first IOP elevation of ≥10 mm Hg was analyzed via proportional hazards regression, with observations also stratified according to investigative site and therapy used during the pre-implantation period. Adverse events were compared using the Fisher’s exact test. Descriptive statistics were calculated in all analyses and performed on the intent-to-treat population, which was defined as all enrolled patients who received implants and attended at least one post-implantation visit.
Results
Enrollment for this study began in May 2002, and the last 3-year visit occurred in April 2006. A total of 239 patients were randomized to receive the 0.59-mg (n = 117) or the 2.1-mg (n = 122) FA intravitreal implant. Although it was planned to include 250 patients, enrollment was suspended at 239 due to the severe acute respiratory syndrome epidemic in Asia and Canada. Table 2 summarizes the patient demographic characteristics. Most patients were in the fifth decade of life, and the majority were Asian. Approximately 80% (192/239) of patients had bilateral disease, and 73.6% (176/239) were using systemic immunomodulatory therapy for control of uveitis upon enrollment. There were no significant differences in baseline characteristics between treatment groups (P ≥ 0.2213). The majority of cases (178/239) had an idiopathic etiology. The most commonly known etiologies were Vogt–Koyanagi–Harada and Behçet’s disease representing 24 and 14 cases, respectively.
Table 2.
Patient baseline demographic characteristics
| Parameter | Treatment group* | All N = 239 | |
|---|---|---|---|
| 0.59-mg n = 117 | 2.1-mg n = 122 | ||
| Age (years) | |||
| Mean (standard deviation) | 42.5 (14.1) | 40.4 (12.5) | 41.4 (13.3) |
| Range (min–max) | 12.0–74.0 | 15.0–92.0 | 12.0–92.0 |
| Race, n (%) | |||
| Caucasian | 24 (20.5) | 28 (23.0) | 52 (21.8) |
| African American | 3 (2.6) | 4 (3.3) | 7 (2.9) |
| Asian | 83 (70.9) | 84 (68.9) | 167 (69.9) |
| Hispanic | 2 (1.7) | 2 (1.6) | 4 (1.7) |
| Other | 5 (4.3) | 4 (3.3) | 9 (3.8) |
| Gender, n (%) | |||
| Male | 47 (40.2) | 58 (47.5) | 105 (43.9) |
| Female | 70 (59.8) | 64 (52.5) | 134 (56.1) |
| Laterality of uveitis, n (%) | |||
| Unilateral | 23 (19.7) | 24 (19.7) | 47 (19.7) |
| Bilateral | 94 (80.3) | 98 (80.3) | 192 (80.3) |
| Previous uveitis treatment, n (%) | |||
| Systemic | 89 (76.1) | 87 (71.3) | 176 (73.6) |
| Local | 28 (23.9) | 35 (28.7) | 63 (26.4) |
* P > 0.05 for all comparisons of baseline characteristics between treatment groups (analysis of variance for continuous variables; χ 2 test for categorical variables)
A total of 211 patients (88.3%) completed the study. Twenty-eight patients (11.7%) did not complete the study: 11 patients (9.4%) from the 0.59-mg implant group and 17 patients (13.9%) from the 2.1-mg implant group. The most common reason for withdrawal (in both dose groups combined) was the occurrence of an AE. Adverse events, all resulting in explantation, were cited as the reason for withdrawal in 17 patients (n = 4, 0.59-mg implant; n = 13, 2.1-mg implant). Other reasons for withdrawal included loss to follow-up (n = 3, 0.59-mg implant; n = 1, 2.1-mg implant), death (n = 1, 0.59-mg implant; n = 2, 2.1-mg implant), subject condition no longer requiring study drug (n = 1, 2.1-mg implant), protocol violation (n = 1, 0.59-mg implant), withdrawal of consent (n = 1, 0.59-mg implant), and administrative problems (n = 1, 0.59-mg implant).
Uveitis Recurrence Rates
Uveitis recurrence rates for implanted eyes are shown in Table 3. Recurrence rates in eyes treated with the 0.59-mg implant decreased significantly from the 1-year pre-implantation period to the 1-, 2-, and 3-year post-implantation period (P < 0.0001 for all). Recurrence rates for eyes treated with the 2.1-mg implant were significantly decreased from the pre-implantation rate at the 1- and 2- year post-implantation period, but not at the 3-year post-implantation period consistent with in vitro drug release data indicating that the 2.1-mg implant was depleted of FA earlier than the 0.59-mg implant. In contrast, the uveitis recurrence rate in nonimplanted fellow eyes (both dose groups combined) increased from 19.8% (47/238 eyes) during the 1-year pre-implantation period to 49.6% (118/238), 57.6% (137/238), and 59.7% (142/238), at the 1-, 2-, and 3-year post-implantation period, respectively (P < 0.0001 versus the pre-implantation period). Fellow eye recurrence rates in the 0.59- and the 2.1-mg FA implant groups were similar. Results of the analysis of the difference between pre- and post-implantation recurrence rates for implanted study eyes and nonimplanted fellow eyes at 3 years including imputed data were consistent with those based on observed recurrence rates with the exception of the imputed 3-year post-implantation recurrence rate for all implanted study eyes (combined dose groups) which was not significantly lower than the pre-implantation recurrence rate.
Table 3.
Uveitis recurrence rates in implanted eyes pre- and post-implantation
| Time | 0.59-mg, n (%) (n = 117) |
2.1-mg, n (%) (n = 122) |
All, n (%) (N = 239) |
|---|---|---|---|
| 1-year pre-implantation | 51 (43.6) | 50 (41.0) | 101 (42.3) |
| 1-year post-implantation |
15 (12.8) P < 0.0001 |
15 (12.3) P < 0.0001 |
30 (12.6) P < 0.0001 |
| 2 years post-implantation |
16 (13.7) P < 0.0001 |
22 (18.0) P = 0.0001 |
38 (15.9) P < 0.0001 |
| 3 years post-implantation (observed) |
20 (17.1) P < 0.0001 |
42 (34.4) P = 0.2850 |
62 (25.9) P = 0.0003 |
| 3 years post-implantation (observed + imputed) |
28 (23.9) P = 0.0023 |
53 (43.4) P = 0.6911 |
81 (33.9) P = 0.0610 |
P values were calculated via the McNemar test
Further within-subject comparison of the uveitis recurrence rate (imputed) at the 3-year follow-up period in implanted eyes vs those of nonimplanted eyes showed that the recurrence rate was significantly lower in implanted eyes compared to nonimplanted eyes for both dose groups and the combined dose group (P < 0.0001 for all).
Time to Uveitis Recurrence
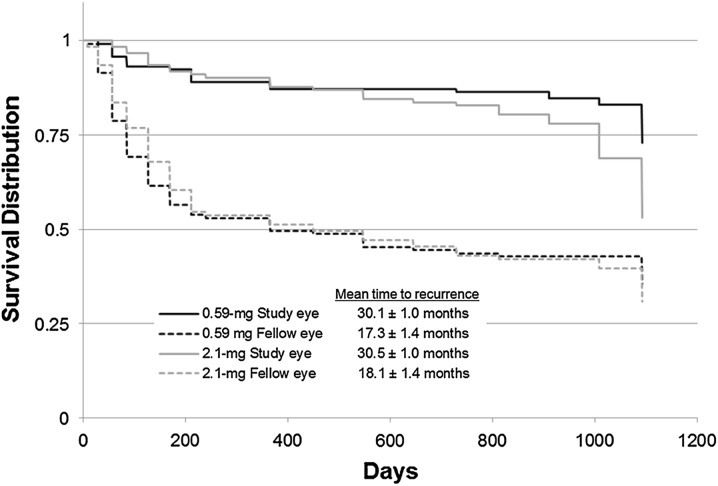
Kaplan–Meier analysis was used to evaluate time to recurrence of uveitis for implanted and nonimplanted eyes (Fig. 1). The difference in time to recurrence of uveitis in implanted versus nonimplanted eyes was statistically significant for both dose groups (P < 0.0001). Recurrences for fellow nonimplanted eyes occurred much earlier than recurrences for implanted eyes. In the 0.59-mg FA implant group, uveitis recurrence in nonimplanted fellow eyes increased rapidly during the first 150 days after implantation of the contralateral eye, whereas for implanted eyes, a significant increase in uveitis recurrence was not seen until approximately 1,000 days after implantation (P < 0.0001). Similar results were observed in the 2.1-mg implant group, although a trend toward recurrence of uveitis in the 2.1-mg group was observed beginning at approximately 24 months.
Fig. 1.
Kaplan–Meier time to uveitis recurrence for implanted study eyes versus nonimplanted fellow eyes in the 0.59- and 2.1-mg implant group. Discontinued patients were censored following their last visit. P < 0.001 for the within-treatment difference comparison of study eye versus fellow eye; P = 0.0062 for the between-treatment difference in implanted eyes
Further Kaplan–Meier analysis of the freedom from recurrence of uveitis comparing implanted eyes in the 0.59- and 2.1-mg group showed that the difference between doses was significant [hazard ratio of 1.97 (95% CI 1.21–3.21); P = 0.0062]. Recurrence began earlier for study eyes in the 2.1-mg FA implant group, at approximately 24 months, compared to the 0.59-mg FA implant dose. Again, these results were not unexpected based on known in vitro drug release data.
Kaplan–Meier analysis of implanted study eyes versus nonimplanted fellow eyes performed only for patients with bilateral disease yielded similar results: the time to recurrence of uveitis was significantly longer in implanted eyes than in fellow nonimplated eyes (P < 0.0001, data not shown).
Adjunctive Therapy
The FA intravitreal implant reduced the need for adjunctive uveitis treatment. The proportion of patients requiring adjunctive treatment to control inflammation before and after FA implantation is shown in Table 4. The proportion of patients requiring adjunctive systemic therapy decreased by an approximate 80% in the 3-year post-implantation compared to the 1-year pre-implantation period regardless of FA implant dosage. The proportion of eyes requiring adjunctive periocular injections or topical steroids was reduced by approximately 80% in eyes receiving the 0.59-mg FA implant and 60% and 50%, respectively, in eyes receiving the 2.1-mg FA implant during the 3-year implantation period compared to the 1-year pre-implantation period. In contrast, the proportion of nonimplanted fellow eyes requiring periocular injections or topical steroids in the 3-year post-implantation period increased or remained similar to the pre-implantation period.
Table 4.
Use of adjunctive therapy
| Eyes, n | 1-year pre-implantation, n (%) | 1-year post-implantation, n (%) | 3 years post-implantation, n (%) | P valuec | |
|---|---|---|---|---|---|
| Systemic medicationsa | |||||
| 0.59 mg | 117 | 74 (63.2) | 16 (13.9) | 14 (12.0) | <0.0001 |
| 2.1 mg | 122 | 72 (59.0) | 11 (9.2) | 16 (13.1) | <0.0001 |
| All | 239 | 146 (61.1) | 27 (11.5) | 30 (12.6) | <0.0001 |
| Periocular injections, study eyeb | |||||
| 0.59 mg | 117 | 65 (55.6) | 8 (6.8) | 11 (9.4) | <0.0001 |
| 2.1 mg | 122 | 76 (62.3) | 8 (6.6) | 30 (24.6) | <0.0001 |
| All | 239 | 141 (59.0) | 16 (6.7) | 41 (17.2) | <0.0001 |
| Periocular injections, fellow eyeb | |||||
| 0.59 mg | 117 | 26 (22.2) | 46 (39.3) | 56 (47.9) | <0.0001 |
| 2.1 mg | 121 | 31 (25.6) | 42 (34.7) | 55 (45.5) | 0.0001 |
| All | 238 | 57 (23.9) | 88 (37.0) | 111 (46.6) | <0.0001 |
| Topical corticosteroids, study eyea | |||||
| 0.59 mg | 117 | 47 (40.2) | 11 (9.6) | 9 (7.7) | <0.0001 |
| 2.1 mg | 122 | 50 (41.0) | 6 (5.0) | 25 (20.5) | 0.0079 |
| All | 239 | 97 (40.6) | 17 (7.2) | 34 (14.2) | <0.0001 |
| Topical corticosteroids, fellow eyea | |||||
| 0.59 mg | 117 | 28 (23.9) | 37 (32.2) | 25 (21.4) | 0.7055 |
| 2.1 mg | 121 | 32 (26.4) | 36 (30.3) | 35 (28.9) | 0.3035 |
| All | 238 | 60 (25.2) | 73 (31.2) | 60 (25.2) | 0.6115 |
aComparisons were made at the baseline visit and at the 1- and 3-year visits
bComparisons were made during the entire 1-year pre-implantation period and the 1- and 3-year post-implantation periods. One fellow eye (2.1-mg FA implant group) was prosthetic, and thus the sample size for fellow eyes was 238)
c P value for 1 year pre-implantation data compared with 3 year postimplantation data
Visual Acuity
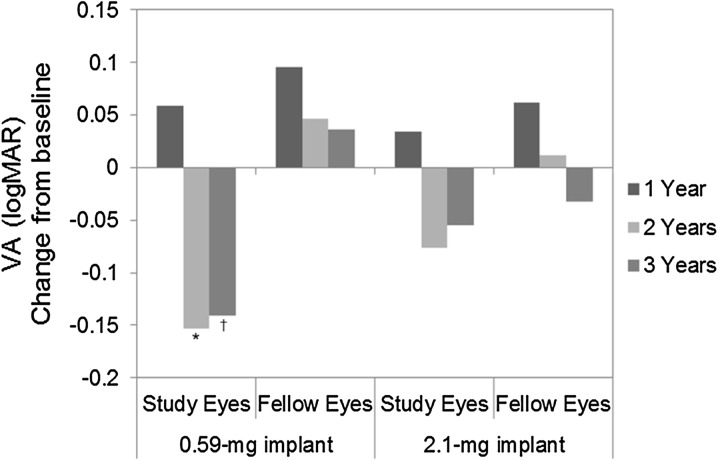
Mean changes in BCVA from baseline up to 3 years post-implantation in the 0.59- and 2.1-mg implant groups are presented in Fig. 2. The mean change from baseline in logMAR BCVA at 2 years (−0.153) and 3 years (−0.141) post-implantation in implanted eyes (0.59-mg group) was significant (P ≤ 0.0007).
Fig. 2.
Change in best-corrected visual acuity from baseline up to 3 years post-implantation in the 0.59- and 2.1-mg implant groups. A negative value represents an improvement (P values from paired t test). *P < 0.0001. † P = 0.0007. logMAR logarithm of the minimum angle resolution, VA visual acuity
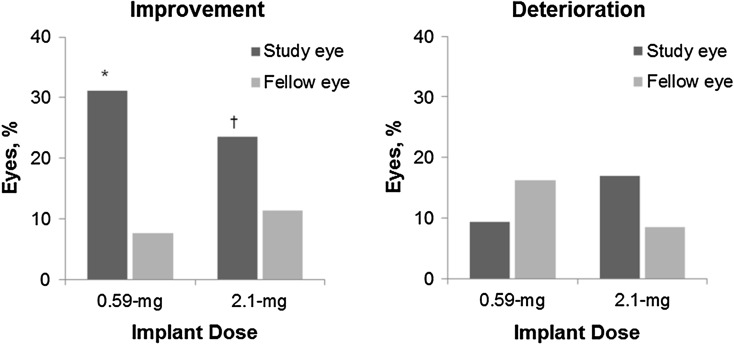
In the 0.59-mg group, 2.1-mg group, and combined dose group at the 3-year visit, 31.1% (33/106), 23.6% (25/106), and 27.4% (58/212) of implanted eyes, respectively, improved by ≥3 lines over baseline compared to 7.6% (8/105), 11.4% (12/105), and 9.5% (20/210) of nonimplanted eyes (P ≤ 0.0046 for the difference in each dose group). In contrast, there was no significant difference between implanted eyes (both doses) and fellow nonimplanted eyes in the proportion of eyes that lost ≥3 lines of BCVA from baseline at the 3-year visit (Fig. 3). Most instances of ≥3 line loss in BCVA of implanted eyes occurred during the immediate postoperative period. Loss of ≥3 lines in BCVA was also often observed between 12 and 21 months post-implantation when cataracts were most prevalent. At the 3-year follow-up visit, 9.4% (10/116) of implanted eyes in the 0.59-mg FA implant group had a loss of ≥3 lines of BCVA compared to 17.0% (18/106) of implanted eyes in the 2.1-mg implant group.
Fig. 3.
Proportion of eyes with an improvement (left panel) or deterioration (right panel) in visual acuity from baseline of at least 0.30 logMAR at 3 years in the 0.59- and 2.1-mg implant groups. (P values for the within-treatment comparison of study eye versus fellow eye). *P < 0.0001. † P = 0.0046. logMAR logarithm of the minimum angle resolution
Cystoid Macular Edema
The mean area of CME measured on the 300-s fluorescein angiogram decreased from 38.0 mm2 at screening to 9.3 mm2 at the 34-week post-implantation visit in eyes that received the 0.59-mg implant. For the remainder of the 3-year post-implantation follow-up period in the 0.59-mg FA implant group, mean area of CME for implanted eyes continued to decrease to a mean 3-year CME area of 6.2 or 4.6 mm2 using the last observation carried forward (LOCF). Eyes receiving the 2.1-mg implant experienced a reduction in the area of CME from 46.1 mm2 at screening to 4.7 mm2 at the 34-week post-implantation visit; however, this increased to 15.3 mm2 by the 3-year visit (LOCF mean CME was 12.8 mm2). In nonimplanted fellow eyes for both dose groups combined, the mean area of CME fluctuated within a narrow range over the 3-year post-implantation follow-up period and the area of CME at screening was very similar to that at the 3-year visit (approximately 15–20 mm2). The number of patients experiencing any reduction in the area of CME between baseline and 3-year post-implantation is presented in Table 5.
Table 5.
Number of patients experiencing reduction in the area of CME between baseline and the 3-year post-implantation visit
| Implant dose | Implanted eyes | Nonimplanted eyes | P valuea | ||
|---|---|---|---|---|---|
| N | Eyes experiencing reduction in CME, n (%) | N | Eyes experiencing reduction in CME, n (%) | ||
| 0.59 mg | 50 | 38 (76.0) | 50 | 14 (28.0) | <0.0001 |
| 2.1 mg | 48 | 30 (62.5) | 48 | 6 (12.5) | <0.0001 |
| All | 98 | 68 (69.4) | 98 | 20 (20.4) | <0.0001 |
CME cystoid macular edema
a P values were calculated via the McNemar test
Safety Outcomes
Mean (±SD) exposure to FA was 1,038.9 (188.0) days in the 0.59-mg implant group and 1,016.1 (225.1) days in the 2.1-mg implant group.
Treatment-emergent ocular AEs (including perioperative events) were reported in 99.6% (238/239) of implanted study eyes and in 81.6% (195/239) of fellow nonimplanted eyes. Table 6 presents the most frequently occurring AEs in implanted study eyes and in nonimplanted fellow eyes in each of the implant dose groups and combined. Among the most frequently observed ocular AEs reported for implanted study eyes, elevated IOP and cataract are commonly associated with ocular steroid use. Other frequently reported AEs in implanted eyes (e.g., eye pain, hypotony, conjunctival hemorrhage, and hyperemia) appear to be primarily associated with surgery. In fellow eyes, the most frequently observed ocular AEs (decreased visual acuity, cataract formation, and eye pain) were in part due to uveitic inflammation experienced when the effects of periocular corticosteroid injections were below therapeutic levels.
Table 6.
Most frequently occurring ocular adverse events
| Preferred terma | Implant dose, N = 239 | ||
|---|---|---|---|
| 0.59-mg, n (%) | 2.1-mg, n (%) | All, n (%) | |
| Study eye | |||
| Intraocular pressure increased | 82 (70.1) | 82 (67.2) | 164 (68.6) |
| Eye pain | 60 (51.3) | 63 (51.6) | 123 (51.5) |
| Visual acuity reduced | 52 (44.4) | 60 (49.2) | 112 (46.9) |
| Conjunctival hemorrhage | 42 (35.9) | 54 (44.3) | 96 (40.2) |
| Postoperative wound complication NOS | 43 (36.8) | 52 (42.6) | 95 (39.8) |
| Conjunctival hyperemia | 49 (41.9) | 41 (33.6) | 90 (37.7) |
| Cataract NOS | 42 (35.9) | 34 (27.9) | 76 (31.8) |
| Cataract NOS aggravated | 37 (31.6) | 39 (32.0) | 76 (31.8) |
| Hypotony of the eye | 29 (24.8) | 47 (38.5) | 76 (31.8) |
| Abnormal sensation in the eye | 31 (26.5) | 35 (28.7) | 66 (27.6) |
| Fellow eye | |||
| Visual acuity reduced | 27 (23.1) | 32 (26.2) | 59 (24.7) |
| Cataract NOS aggravated | 27 (23.1) | 31 (25.4) | 58 (24.3) |
| Eye pain | 24 (20.5) | 29 (23.8) | 53 (22.2) |
| Intraocular pressure increased | 28 (23.9) | 18 (14.8) | 46 (19.3) |
| Cataract NOS | 20 (17.1) | 21 (17.2) | 41 (17.2) |
| Conjunctival hyperemia | 18 (15.4) | 15 (12.3) | 33 (13.8) |
| Vitreous floaters | 11 (9.4) | 22 (18.0) | 33 (13.8) |
| Vision blurred | 14 (12.0) | 16 (13.1) | 30 (12.6) |
| Macular edema | 14 (12.0) | 15 (12.3) | 29 (12.1) |
| Posterior capsule opacification | 11 (9.4) | 14 (11.5) | 25 (10.5) |
NOS not otherwise specified
aMedical dictionary for regulatory activities (MedDRA) preferred nomenclature
The FA implant was explanted from 19 eyes (n = 5, 0.59-mg implant; n = 14, 2.1-mg implant) over the course of the 3-year post-implantation period. Seven implants were removed in the first post-implantation year, eight in the second post-implantation year, and four in the third post-implantation year. The two most common reasons attributed to these explants were elevated IOP despite maximal medical therapy (n = 2, 0.59-mg implant; n = 3, 2.1-mg implant) and hypotony associated with implant wound site leaks (n = 2, each implant dose). Other reasons for explantation, all occurring in the 2.1-mg implant group, included implant wound complication (n = 1), evisceration (n = 1), endophthalmitis (n = 1), implant protrusion (n = 3), uncontrolled glaucoma (n = 1), scleral necrosis (n = 1), scleral melt (n = 1), and retinal detachment (n = 1).
Intraocular Pressure
Table 7 presents the proportion of implant eyes and fellow eyes with IOP elevations ≥10 mm Hg at any time over the 3-year post-implantation period. The highest proportion of study eyes with IOP elevations ≥10 mm Hg in the 0.59- and the 2.1-mg FA implant groups [31.3% (35/112) and 37.8% (45/119), respectively] was seen during the 1-year post-implantation visit; thereafter, these proportions decreased throughout the remaining months of follow-up. The difference between implanted eyes and fellow eyes during the 3 years of follow-up were significant for both doses (P < 0.001); however, the differences between doses for implanted eyes were not significant (P = 0.4229).
Table 7.
Incidence of intraocular pressure increase of ≥10 mm Hg from baseline over the 3-year post-implantation period
| Time (months) | 0.59-mg Implant | 2.1-mg Implant | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study eyes | Fellow eyes | Study eyes | Fellow eyes | |||||||||
| N | Incidence | Percent | N | Incidence | Percent | N | Incidence | Percent | N | Incidence | Percent | |
| 6 | 113 | 25 | 22.1 | 111 | 2 | 1.8 | 119 | 34 | 28.6 | 115 | 7 | 6.1 |
| 12 | 112 | 35 | 31.3 | 109 | 4 | 3.7 | 119 | 45 | 37.8 | 115 | 6 | 5.2 |
| 18 | 109 | 24 | 22.0 | 108 | 3 | 2.8 | 115 | 28 | 24.3 | 111 | 3 | 2.7 |
| 24 | 107 | 17 | 15.9 | 106 | 1 | 0.9 | 110 | 23 | 20.9 | 106 | 5 | 4.7 |
| 30 | 102 | 18 | 17.6 | 100 | 8 | 8.0 | 110 | 15 | 13.6 | 108 | 5 | 4.6 |
| 36 | 103 | 12 | 11.7 | 101 | 1 | 1.0 | 106 | 9 | 8.5 | 105 | 6 | 5.7 |
| Overall | 115 | 78 | 67.8 | 114 | 24 | 21.1 | 122 | 87 | 71.3 | 118 | 22 | 18.6 |
Cases of hypotony, defined as IOP <6 mm Hg, were observed. In the 0.59-mg implant group, 40.2% (47/117) of implanted eyes experienced hypotony at sometime during the 3-year follow-up period compared to 17.9% (21/117) of the fellow nonimplanted eyes (P = 0.0002). In the 2.1-mg implant group, hypotony occurred in 61.5% (75/122) of implanted eyes during the 3-year follow-up period compared to 16.9% (20/118) of the fellow nonimplanted eyes (P < 0.0001). The difference between eyes treated with the 0.59- and the 2.1-mg implants was significant (P = 0.0007). Most instances of hypotony occurred shortly after the implantation surgery or IOP-lowering surgery.
Lens Opacification
The rate of ≥2-grade changes in lens opacities from baseline for subcapsular, nuclear, and cortical lens regions were evaluated using the LOCS II scale. Overall, ≥2-grade increases in subcapsular lens opacity were observed in 57.6% (76/132) of phakic implanted eyes (both dose groups combined) compared to 16.3% (26/160) of phakic nonimplanted fellow eyes 3 years post-implantation(P < 0.0001). Lens opacity increases in nuclear and cortical segments were observed in 23.1% (27/117) and 22.8% (26/114) of phakic implanted eyes, respectively (both dose groups combined), by 3 years post-implantation compared to 8.5% (13/153) and 9.5% (14/148), respectively, in the fellow nonimplanted eyes.
Over the 3-year post-implantation follow-up period, 94.9% (129/136) of phakic implanted eyes (both doses combined) had cataract surgery compared to 30.4% (51/168) of phakic nonimplanted fellow eyes. Most of the cataract surgeries in implanted eyes occurred between 1 year and 18 months post-implantation.
Visual Fields
Visual field sensitivity was quantified as the mean deficit (MD), measured in decibels (dB). At 3 years post-implantation, MD decreased by 0.47 dB in the 2.1-mg implant group and improved by 0.35 dB in the 0.59-mg implant group (P = 0.3967). There were no statistically significant within-group changes in MD for study or fellow eyes for each dose group and for both dose groups combined. In the 0.59-mg implant group, 9.8% (10/102) of implanted eyes had a ≥10-dB reduction in MD at any time during the 3-year post-implantation follow-up period compared to 3.9% (4/102) in the fellow nonimplanted eyes (P = 0.001). In the 2.1-mg implant group, 16.0% (17/106) of implanted eyes had a ≥10-dB reduction in MD at any time during the 3-year post-implantation follow-up period compared to 2.1% (2/96) in the fellow nonimplanted eyes (P = 0.001). There were no statistically significant differences between treatments.
Nonocular Adverse Events
Nonocular AEs were reported in 86.6% (207/239) of patients in both dose groups combined. The most frequently observed nonocular AEs were headache [29.3% (70/239)], nasopharyngitis [17.2% (41/239)], arthralgia [16.3% (39/239)], and pyrexia [15.1% (36/239)].
Over the course of the study, three patients died: one in the 0.59-mg implant group (abdominal aortic aneurysm) and two in the 2.1-mg implant group (sudden cardiac arrest, pneumonia). None of the deaths was related to the study drug.
Discussion
The results of this study demonstrate that the FA intravitreal implant is effective in the treatment of NIPU in a predominantly Asian patient population. The FA intravitreal implant effectively reduced rates of uveitis recurrence and improved visual outcomes compared to nonimplanted fellow eyes. The degree of improvement relative to the nonimplanted fellow eyes was especially notable given that most patients had bilateral NIPU and had the more severely affected eye implanted. Uveitis recurrence rates in eyes treated with the FA intravitreal implant (combined dose groups) decreased from 42.3% during the 1-year pre-implantation period to 25.9% during the 3-year post-implantation period, and control was maintained through 3 years. Visual acuity in implanted eyes was stabilized or improved in 80% of eyes with a significant number of eyes demonstrating a ≥3 line improvement at 3 years post-implantation. Furthermore, eyes treated with the FA intravitreal implant had a reduced need for adjunctive systemic therapy or periocular or topical corticosteroid injections to control uveitis. These data are consistent with two previous studies evaluating the FA intravitreal implant in the treatment of NIPU [18, 20]. Both the marketed 0.59-mg FA intravitreal implant (Retisert) and the 2.1-mg FA intravitreal implant demonstrated similar efficacy, although the higher-dose implant tended to have a shorter time to uveitis recurrence. This was attributed to a shorter lifespan of the 2.1-mg implant and consistent with known in vitro release rate data, suggesting that the drug reservoir is depleted more rapidly in the higher-dose implant.
The main cause of visual loss in patients with posterior uveitis is CME [21, 22]. The ability of the FA implant to reduce CME through control of inflammation led to good visual acuity outcomes. These improved visual acuity outcomes were observed despite the formation of cataracts in the majority of implanted patients. Cataract formation and progress are common in eyes with uveitis and attributable both to the inflammatory progress and to the chronic use of corticosteroids to control the disease. [16] In this study, nearly all (94.9%) implanted phakic eyes underwent cataract surgery during the 3-year follow-up with a high incidence of reduction in BCVA of ≥3 lines observed between 12 and 18 months post-implantation—the period during follow-up when most cataract surgeries were performed in implanted eyes. However, following cataract removal, implant eye visual acuity improved, with a significant improvement in mean BCVA compared with baseline in the 0.59-mg implant group at 2 and 3 years post-implantation and with 27.4% of implanted eyes (both dose groups combined) improved by ≥3 lines over baseline at 3 years post-implantation, a significantly greater proportion than that observed in fellow nonimplanted eyes (P < 0.0001). Notably, in a follow-up post hoc analysis of post-surgical outcomes in eyes requiring cataract extraction from an earlier Phase II/III study of the FA implant in the treatment of NIPU, Sheppard et al. [23] reported better vision and less intraocular inflammation following cataract surgery in implanted eyes compared with nonimplanted eyes. These results were more remarkable, in that in the study, as well as in the current study, the FA-implanted eye represented each patient’s worse eye. At 1 and 3 months post-cataract extraction, mean improvement in visual acuity was significantly greater in implanted than nonimplanted eyes (P ≤ 0.0047) and significantly fewer AC cells were seen in implanted than nonimplanted eyes (P ≤ 0.0084) [23].
Elevated IOP is also common in uveitic eyes due to the occlusion of aqueous outflow by inflammatory debris and/or formation of peripheral anterior synechia [24, 25]. Herbert et al. [26] reported the prevalence of elevated IOP in uveitis patients to be as high as 41.8%; with 29.8% of cases requiring treatment to manage the elevated IOP. The proportion of implanted eyes experiencing elevations in IOP in this study was higher (67.8% and 71.3% of eyes in the 0.59- and the 2.1-mg implant groups, respectively), due to the fact that corticosteroid treatment itself may also lead to reduced aqueous outflow through a variety of mechanisms [27, 28]. While the majority of implanted eyes with IOP elevations were successfully treated with IOP-lowering medications, 33% of implanted eyes with IOP elevations required glaucoma-filtering procedures to control IOP. Details of the topical IOP-lowering medications and filtering procedures utilized in this study and the two previous FA implant studies have been described by Goldstein et al. [29]. Trabeculectomy was the most common surgical procedure in this and previous studies, and surgical procedures were deemed successful in 85.1% of eyes at 1 year (postoperative IOP of 6–21 mm Hg with or without additional IOP-lowering medication).
As might be expected, there were more ocular AEs in implanted eyes compared with nonimplanted fellow eyes. Adverse events in nonimplanted eyes appeared to be reflective, in large part, of the underlying uveitic process (e.g., reduced visual acuity, cataract, eye pain, increased IOP, conjunctival hyperemia, vitreous floaters, blurred vision, macular edema), while AEs in implanted eyes were consistent with the surgical procedure and corticosteroid delivered (e.g., increased IOP, eye pain, conjunctival hermorrhage, postoperative wound complications, cataract formation). Notably, there were no nonocular AEs considered treatment related in either implant group. This finding is likely due to the negligible systemic exposure to FA following implantation of the FA implant. In a previous study of patients who received the intravitreal implant, and had blood samples taken at various times after implantation, plasma levels of FA were below the limit of detection [30].
The dose-controlled design of this study, along with the lack of randomization regarding the treatment eye in bilateral cases, precludes definitive distinction from regression to the mean as the explanation for the apparent treatment effects on many of the clinical findings, including visual acuity and recurrent inflammation. The biologic plausibility and magnitude of the results, however, suggest that treatment effects were the primary contributor to the results.
Conclusion
The results of this study demonstrate the efficacy of the FA intravitreal implant in the treatment of NIPU in a population of predominantly Asian patients. The FA intravitreal implant led to both significant reductions in uveitis recurrence rates and improvements in visual acuity. Elevated IOP and cataract formation were the most common AEs, consistent with the natural history of the disease and the treatment used. Unlike previous studies, there were more complications related to implant site wound leaks. It is therefore especially important that the physician pays careful attention to wound closure in the uveitic patient treated with an FA implant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Bausch + Lomb sponsored this study and participated in its design and conduct, as well as data collection, management, and analysis and interpretation of the data. Bausch + Lomb funded the article processing charges associated with this publication. The authors thank and acknowledge all the investigators in the Fluocinolone Acetonide Uveitis Study Group and the members of the Data and Safety Monitoring Committee (see below) as well as the Retinal Diseases and Image Analysis Center (University Hospitals Case Medical Center, Cleveland, Ohio). All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Fluocinolone Acetonide Uveitis Study Group Principal Investigators
Thomas M. Aaberg Jr, MD, Grand Rapids, Michigan; J. Biswas, MD, Chennai, India; Diana Conrad, MD, Brisbane, Queensland, Australia; Jean Deschenes, MD, Montreal, Quebec, Canada; Robert Devenyi, MD, Toronto, Ontario, Canada; S. P. Garg, MD, New Delhi, India; William Hodge, MD, MPH, PhD, Ottawa, Ontario, Canada; Phil Hooper, MD, London, Ontario, Canada; Juan S. Lopez, MD, Quezon City, Philippines; Rajiv Maturi, MD, Indianapolis, Indiana; Peter McCluskey, MD, Darlinghurst, New South Wales, Australia; Don Perez-Ortiz, MD, Tampa, Florida; Theodore Rabinovitch, MD, Toronto, Ontario, Canada; William Rodden, MD, Ashland, Oregon; Virender S. Sangwan, MD, Hyderabad, India; Dennis Lam Shun-Chiu, MD, Kowloon, Hong Kong; Richard Stawell, MD, East Melbourne, Victoria, Australia; Mei-Ling Tay-Kearney, MD, Nedlands, Western Australia, Australia; Harvey Siy Uy, MD, Makati City, Philippines.
Data and Safety Monitoring Board Members
Alexander Brucker, MD, Scheie Eye Institute, Philadelphia, Pennsylvania (Chair); Karen Gehrs, MD, University of Iowa, Iowa City, Iowa; Lee Jampol, MD, Northwestern University, Chicago, Illinois; Mark Johnson, MD, University of Michigan, Ann Arbor, Michigan; Theodore Colton, ScD, Boston University, Boston, Massachusetts; David Musch, PhD, University of Michigan, Ann Arbor, Michigan; Robert Levine, MD, Yale University, New Haven, Connecticut.
Conflict of Interest
Virender S. Sangwan and P. Andrew Pearson declare no conflict of interest. Hemanth Paul was an employee of Bausch + Lomb at the time of the study. Timothy Comstock was an employee of Bausch + Lomb at the time of the study.
Compliance with Ethics Guidelines
The study received Institutional Review Board approval at each center. Before study entry and providing written informed consent, each patient received a full explanation of study procedures. An independent Data Safety Monitoring Board assessed the safety and efficacy data as the study progressed and alerted the sponsor if any issues arose. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Funding
Bausch + Lomb, Rochester, NY, USA.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
Trial Registration
Clinical Trials. gov #NCT0456482.
References
- 1.Durrani OM, Tehrani NN, Marr JE, et al. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88:1159–1162. doi: 10.1136/bjo.2003.037226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forster DJ. General approach to the uveitis patient and treatment strategies. In: Yanoff M, Duker JS, editors. Ophthalmology. 2. St. Louis: Mosby; 2004. pp. 1115–1120. [Google Scholar]
- 3.Larson T, Nussenblatt RB, Sen HN. Emerging drugs for uveitis. Expert Opin Emerg Drugs. 2011;16:309–322. doi: 10.1517/14728214.2011.537824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218:223–236. doi: 10.1159/000078612. [DOI] [PubMed] [Google Scholar]
- 5.Anglade E, Whitcup SM. The diagnosis and management of uveitis. Drugs. 1995;49:213–223. doi: 10.2165/00003495-199549020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Foster SC. The Ocular Immunology and Uveitis Foundation. Available from http://www.uveitis.org/patients/education/ glossary/t-z#UVEITIS. Accessed March 18, 2014.
- 7.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–336. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakefield D, Chang JH. Epidemiology of uveitis. Int Ophthalmol Clin. 2005;45:1–13. doi: 10.1097/01.iio.0000155938.83083.94. [DOI] [PubMed] [Google Scholar]
- 9.Becker MD, Smith JR, Max R, Fiehn C. Management of sight-threatening uveitis: new therapeutic options. Drugs. 2005;65:497–519. doi: 10.2165/00003495-200565040-00005. [DOI] [PubMed] [Google Scholar]
- 10.Acharya N, Young L. Sustained-release drug implants for the treatment of intraocular disease. Int Ophthalmol Clin. 2004;44:33–39. doi: 10.1097/00004397-200404430-00006. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe GJ, Ben-Nun J, Guo H, et al. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology. 2000;107:2024–2033. doi: 10.1016/S0161-6420(00)00466-8. [DOI] [PubMed] [Google Scholar]
- 12.Hsu J. Drug delivery methods for posterior segment disease. Curr Opin Ophthalmol. 2007;18:235–239. doi: 10.1097/ICU.0b013e3281108000. [DOI] [PubMed] [Google Scholar]
- 13.Sarao V, Veritti D, Boscia F, Lanzetta P. Intravitreal steroids for the treatment of retinal disease. Sci World J. 2014;2014:989501. doi: 10.1155/2014/989501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group, Kempen JH, Altawheel MM et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior and panuveitis. The multicenter uveitis steroid treatment trials. Ophthalmology. 2011;118:1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen QD, Callanan D, Dugel P, et al. Treating chronic noninfectious posterior segment uveitis: the impact of cumulative damage. Proceedings of an expert panel roundtable discussion. Retina. 2006;26(suppl):1–16. doi: 10.1097/01.iae.0000250601.15893.5f. [DOI] [PubMed] [Google Scholar]
- 16.Mohammad DA, Sweet BV, Elner SG. Retisert: is the new advance in treatment of uveitis a good one? Ann Pharmacother. 2007;41:449–454. doi: 10.1345/aph.1H540. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe GJ, McCallum RM, Branchaud B, et al. Long-term follow-up results of a pilot trail of a fluocinolone acetonide implant to treated posterior uveitis. Ophthalmology. 2005;112:1192–1198. doi: 10.1016/j.ophtha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Callanan DG, Jaffe GJ, Martin DF, et al. Treatment of posterior uveitis with a fluocinolone acetonide implant: 3-year clinical trial results. Arch Ophthalmol. 2008;126:1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe GJ, Martin D, Callanan D, et al. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113:1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Pavesio C, Sierhut M, Bairi K. Evaluation of an intravitreal fluocinolone acetonide implant versus standard systemic therapy in noninfectious posterior uveitis. Ophthalmology. 2010;117:567–575. doi: 10.1016/j.ophtha.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Tomkins-Netzer O, Talat L, Bar A, et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. 2014 doi: 10.1016/j.ophtha.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Lardenoye CWTA, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113(8):1446–1449. doi: 10.1016/j.ophtha.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Sheppard JD, Nguyen QD, Usner DW, et al. Post-cataract outcomes in patients with noninfectious posterior uveitis treated with the fluocinolone acetonide intravitreal implant. Clin Ophthalmol. 2012;6:79–85. doi: 10.2147/OPTH.S24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moorthy RS, Mermoud A, Baerveldt G, et al. Glaucoma associated with uveitis. Surv Ophthalmol. 1997;41:361–394. doi: 10.1016/S0039-6257(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 25.Ritch R. Pathophysiology of glaucoma in uveitis. Trans Ophthalmol Soc UK. 1981;101(Pt 3):321–324. [PubMed] [Google Scholar]
- 26.Herbert HM, Viswanathan A, Jackson H, Lightman SL. Risk factors for elevated intraocular pressure in uveitis. J Glaucoma. 2004;13:96–99. doi: 10.1097/00061198-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Tripathi RC, Parapuram SK, Tripathi BJ, et al. Corticosteroids and glaucoma risk. Drugs Aging. 1999;15:439–450. doi: 10.2165/00002512-199915060-00004. [DOI] [PubMed] [Google Scholar]
- 28.Conti SM, Kertes PJ. The use of intravitreal corticosteroids, evidence-based and otherwise. Curr Opin Ophthalmol. 2006;17:235–244. doi: 10.1097/01.icu.0000193107.00089.ee. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein DA, Godfrey DG, Hall A, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol. 2007;125:1478–1485. doi: 10.1001/archopht.125.11.ecs70063. [DOI] [PubMed] [Google Scholar]
- 30.Retisert® (fluocinolone acetonide intravitreal implant) 0.59 mg prescribing information. Rochester: Bausch & Lomb, Inc.; 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021737s019.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.