Abstract
Kinetin, a cytokinin which promotes seed germination by inhibiting the action of abscisic acid, is an important molecule known to trigger various molecular mechanisms by interacting with an array of proteins shown from experimental observations in various model organisms. We report here the prediction of most probable protein targets of kinetin from spinach proteome using in silico approaches. Inverse docking and ligand-based similarity search was performed using kinetin as molecule. The former method prioritized six spinach proteins, whereas the latter method provided a list of protein targets retrieved from several model organisms. The most probable protein targets were selected by comparing the rank list of docking and ligand similarity methods. Both of these methods prioritized chitinase as the most probable protein target (ΔGpred = 5.064 kcal/mol) supported by the experimental structure of yeast chitinase 1 complex with kinetin (PDB: 2UY5) and Gliocladium roseum chitinase complex with 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione (caffeine; 3G6M) which bears a 3D similarity of 0.43 with kinetin. An in vitro study to evaluate the effect of kinetin on spinach seed germination indicated that a very low concentration of kinetin (0.5 mg/l) did not show a significant effect compared to control in inducing seed germination process. Further, higher levels of kinetin (>0.5 mg/l) constituted an antagonist effect on spinach seed germination. It is anticipated that kinetin may have a molecular interaction with prioritized protein targets synthesized during the seed germination process and reduces growth. Thus, it appears that kinetin may not be a suitable hormone for enhancing spinach seed germination in vitro.
Electronic supplementary material
The online version of this article (doi:10.1007/s12154-015-0135-3) contains supplementary material, which is available to authorized users.
Keywords: Molecular target prediction, Inverse docking, Ligand-based similarity search, Kinetin, Chitinase
Introduction
Seeds are important propagation and dispersal units for plant survival in an ecosystem. It is also crucial to maintain seed quality for the agricultural yield of crop plants [1]. Spinach (Spinacia oleracea) is a largely consumed proteinaceous food crop and necessitates large-scale production to serve the human community, which is normally hurdled by poor seed germination standards [2]. Various exogenous and endogenous factors are proven to be responsible for the inhibition of spinach seed germination and promotion of seed dormancy. Temperature of >20 °C and exposure to chemical inhibitors are the physical factors which contribute to spinach seed dormancy [2]. Several endogenous seed growth promoters and inhibitors are directly engaged in the control of seed development, dormancy and germination. Although not essential for seed germination, exogenous application of cytokinin plays a ‘permissive’ role by inhibiting the activity of abscisic acid in a number of plants [3]. It also allows gibberellins to function (directly or indirectly) in the stimulation of seed germination by inducing cellular enzymes known to weak seed coats, seed storage reserve mobilization and embryo cell elongation [4].
Among cytokinins, kinetin (6-furfurylaminopurine) is the most studied plant hormone compared with zeatin. The effect of kinetin on spinach leaf tissue and plant regeneration has been demonstrated by Al-Khayri and his coworkers [5]. Additionally, in vitro efforts on the regeneration of spinach plant from mature dry seed-derived callus culture showed that supplementation of kinetin (9.3 μM) indicated callus proliferation [6]. Earlier studies explained that the effect of exogenous application of kinetin is varied across species and exhibits slight promotion of seed germination, which can be considered as a negligible effect in comparison to gibberellic acid (GA) application [7]. Therefore, we selected kinetin to examine its effect on spinach seed germination. It is evident that cytokinins possess multifaceted biochemical roles in seed germination and promote various germination-related cellular functions by interacting with multiple proteins as shown by various experimental studies [8]. It is of prime importance to recognize the protein targets of cytokinins to better understand the molecular mechanism of the seed germination process. There are numerous proteins involved in the seed germination process. Chitinase is one of them; it performs a prime role in seed germination and seedling development and accumulates in seeds of several species as part of their developmental process, while others can be induced in response to microbial attack [9]. Gomez et al. (2002) have recently reviewed seed chitinases. Seed-specific chitinases encoded by Chia genes are classified into various classes. Several functions for plant chitinases have been proposed, such as nodulation, cleavage of Nod factors, embryogenesis, antifreeze and tissue weakening during radicle emergence [9–11]. With ongoing efforts on the development of computational strategies on medicinal plant-based drug discovery, structural bioinformatics and related methods [12–14], we present here the prediction of the most probable protein targets of kinetin from spinach proteome using approaches which are supported by experimental evidences. Inverse docking and ligand similarity methods are efficiently employed to prioritize kinetin protein targets. This prediction/hypothesis was validated by an in vitro study to understand the effect of kinetin on spinach seed germination.
Materials and methods
Spinach proteome and ligand retrieval
A non-redundant set of 22 spinach proteins (May, 2014) was retrieved from Protein Databank (PDB) [15]. The protein structures were preprocessed by removing crystallographic waters and ions and adding polar hydrogen. The protein atoms were typed with Amber03 force field [16], followed by bond orders correction and assignment of Kollman charge [17]. Subsequently, the protein structures were energy-minimized (1000 iterations) using a conjugate gradient approach in YASARA Structure software [18] and customized as protein database. The ligand structure of kinetin was downloaded from PubChem database (CID 3830) [19], and conformational analysis using conjugate gradient energy minimization protocol was carried out to retrieve a low-energy conformer to be used in further studies.
Inverse docking
Inverse docking studies were performed with 22 spinach proteins as receptors and kinetin as ligand using the idTarget docking program [20]. idTarget program employs MEDock (Maximum Entropy-based Docking) search engine [21] with integration of grid-based divide and conquer docking approach to generate dock poses which are subsequently locally minimized and rescored using robust Autodock4 scoring function [22]. A manually compiled PDB list of 22 spinach proteins was provided as receptor input, and Amber parm99SB was chosen as charge model [23]. The kinetin molecule was assigned AM1-BCC (semi-empirical Austin Model 1 (AM1) with bond charge correction (BCC)) charge [24]. The assignment of protonation state in the protein and ligand files was enabled using automated settings. The customizations in the docking run were defined using advanced settings as follows: The number of individuals and generations was set to 100 and 10000, respectively. The ligand translations inside computed ligand binding site grid were scaled to 500 search cycles, and the number of docking runs was customized to 20 for effective pose sampling. The rank list generated after target screening was utilized to study binding modes.
The rationale for selecting Amber parm99SB and AM1-BCC models was made by a careful study of literature related to computational resources demanding experiments, robustness and accurateness in the calculations. Amber parm99SB is best suited for atom typing of large biomolecules such as proteins and nucleic acids, especially in the case of virtual high-throughput screening [25]. Therefore, the selection of Amber parm99SB seems appropriate for the calculation of dock poses in an online computationally expensive portal such as idTarget. On the other hand, the ligand file (here, kinetin) should be handled with an accurate atomic charge assignment scheme, and therefore we considered AM1-BCC charge model (a semiempirical scheme) rather than empirical schemes (e.g. Gasteiger). Moreover, the study on docking accuracy of AutoDock 4.2 program showed the best performance of AM1-BCC charge scheme from validation statistics of root mean square deviation (RMSD) and other parameters [26]. Hence, the choice of AM1-BCC seems reasonable for accurate charge assignment of ligand. The customizations in docking search algorithm for a comprehensive sampling of dock poses using cutoffs and thresholds together with the MEDock scoring function would possibly present a set of feasible dock poses for this study.
Ligand-based similarity search
We used ReverseScreen3D program [27] to perform a ligand-based similarity search of kinetin molecule. The advanced wizard of ReverseScreen3D computational workflow was adopted to screen available PDB proteomes from several model organisms by using 2D similarity search selection. The Tanimoto coefficients of the queried kinetin molecule against the database ligands were computed and returned as 2D score. The top 10 % of the ligand hits obtained from 2D similarity search was utilized to run 3D similarity calculations. The program employs VConf version 2.0 (VeraChem, LLC) [28] as the conformer searching engine to retrieve a low-energy conformer of the queried molecule. The conformer analysis produced 100 conformers using an energy threshold of 25 kcal/mol and tolerance of 0, respectively. A distance tolerance of 1 Å RMSD was also used to discard highly similar conformations. A geometric matching method implemented in ReverseScreen3D was used to compute atom–atom score (a modified form of Tanimoto coefficient) and returned as 3D score.
Homology model of spinach chitinase protein and docking studies
A homology model of spinach chitinase protein was developed using SwissModel program [29] due to the unavailability of experimental structure in the PDB. The protein sequence of spinach chitinase was obtained from UniProtKB database [30] using the accession number F2ZC04. The protein modeling program utilizes BlastP program [31] to select PDB templates based on sequence identities, coverage and its alignment with the queried sequence. After a run of energy minimization using conjugate gradient method (1000 iterations) in YASARA Structure software [18], the stereochemistry was assessed using Ramachandran plot [32] utility in RAMPAGE server [33].
The binding mode of modelled chitinase protein with kinetin was predicted using BSP-SLIM docking program [34]. BSP-SLIM (Binding Site Prediction with Shape-based LIgand Matching with binding pocket) identifies putative ligand binding sites by comparing the template-bound ligand information and generates poses by computing complementarities of local shape and chemical feature complementarities between ligand and the negative image of binding pockets [34]. The best dock pose was chosen based on a high docking score.
Computational site-directed mutagenesis
The chitinase–kinetin dock complex obtained from BSP-SLIM program was subjected to computational site-directed mutagenesis using ABS-Scan program [35] to understand the role of binding site residues towards kinetin recognition process. In this process, a distance cutoff of 6 Å was defined around the kinetin pose in the chitinase protein to generate a binding site. The sorted amino acids from the binding site were mutated to alanine residue in a sequential manner using a built-in Modeller [36] library. The mutants (protein structure with an alanine residue in place of a binding site residue) were subjected to a short energy minimization protocol to remove unrealistic models and subsequently evaluated using DOPE (Discrete Optimized Protein Energy) score [37]. Mutants with the lowest DOPE score were energetically evaluated using Autodock 4.1 force field [38], and a difference in the interaction energy with respect to wild (reference structure) was returned as ΔΔG value. A Jmol visualizer applet [39] was used to interpret the results graphically.
In vitro spinach seed germination
Spinach seeds were purchased from the vegetable seed market in Jamalpur, Ahmedabad, Gujarat, India. Morphologically healthy seeds were selected for experiment; these were treated with 1 % bavistin for 5 min. Subsequently, the seeds were washed with sterile distilled water (DW) for 15 min with constant shaking. Finally, seeds were surface-sterilized with 0.1 % mercuric chloride (HgCl2; 1 min) followed by extensive (three to four times) rinses with sterile DW and soaking in sterile DW as control and in various concentrations of kinetin (0.5, 1, 3, 5 and 7 mg/l) [40, 41]. After 24 h, seeds were inoculated on Murashige and Skoog (MS) medium [40] which was fortified with 3 % sucrose and solidified with 0.8 % agar-agar, supplemented with different concentrations of kinetin (0.5, 1, 3, 5 and 7 mg/l). The pH of media was adjusted to 5.8 prior to autoclaving at 121 °C temperature and 15 psi pressure for 15 min [41, 42].
We kept plain MS medium as control experiment and media supplemented with different concentrations of kinetin (0.5, 1, 3, 5 and 7 mg/l) were used for the examination of seed viability and seedling vigour of spinach. The cultures were incubated in a culture room with 25 ± 1 °C and 16-h photoperiod under white fluorescent tubes. After 45 days of inoculation, germination percentage, radical length, hypocotyle length and fresh weight of seedlings were recorded.
Results and discussion
Computational methods in predicting protein targets and its principles
We selected receptor-based (molecular docking) and ligand-based (similarity search) methods to prioritize the most probable protein targets of kinetin. Noticeably, both of these methods constitute an independent principle in its calculations, and their combination may assist in gaining confidence about the most probable protein targets in particular. Inverse docking involves docking of a ligand against a panel of protein structures, and the target hits were recognized by the best scoring protein–ligand interaction (docking) energy [43]. Ligand-based similarity search implemented in protein targets prediction is based on a reverse virtual screening concept, and the targets hit were predicted by comparing the queried molecules with a database of experimental (co-crystallized) ligands whose protein structures are available in PDB [27]. It is hypothesized that the most probable protein target may secure a significant docking energy (ΔGpred) [44]. In addition, the most probable protein targets can be retrieved if the queried ligand (here, kinetin) constitute a high similarity score with biochemically similar co-crystallized ligands [45] (e.g. adenosine) which requires that the prioritized protein targets and the selected ‘co-crystallized’ experimental protein structures should possess homology.
Inverse docking
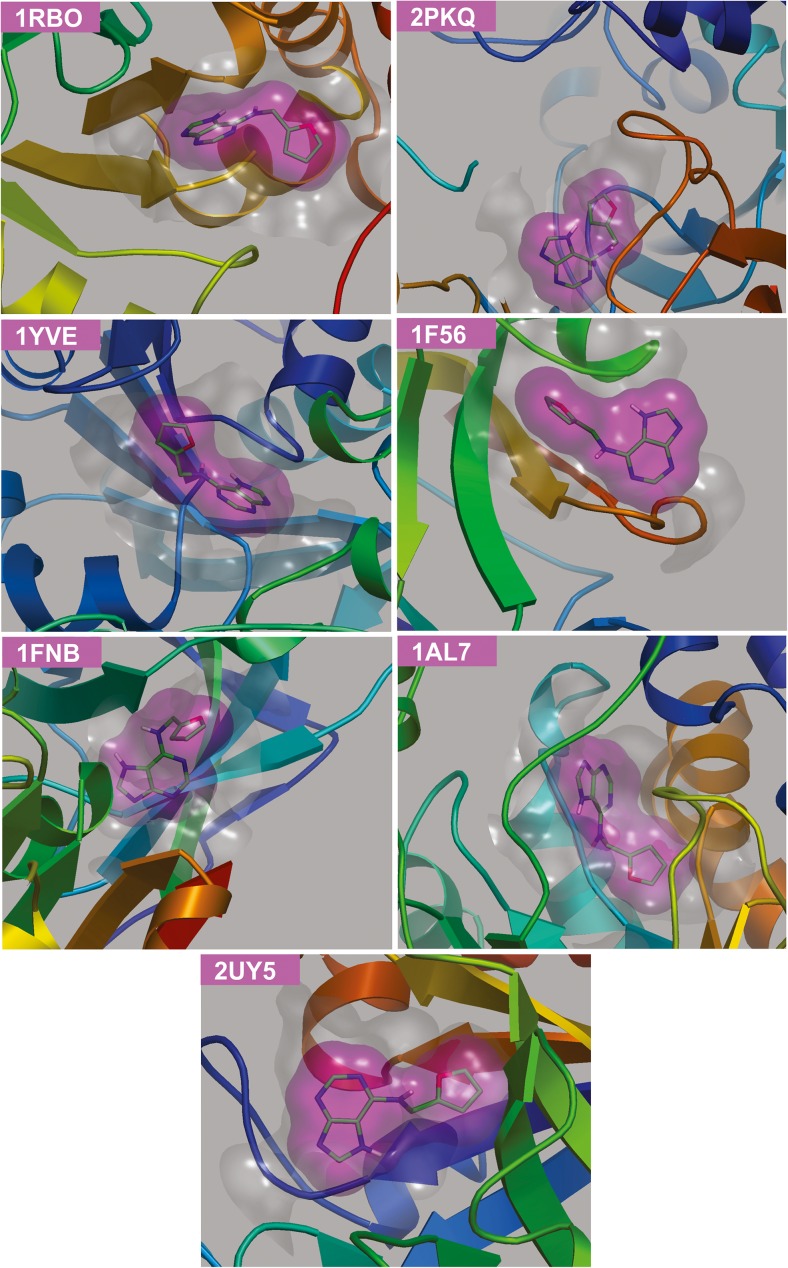
We considered a non-redundant set of 22 protein structures from spinach PDB proteome (May, 2014) and preprocessed them using standard molecular mechanics protocol (Table 1). idTarget inverse docking program was effectively applied to prioritize protein targets, which employ divide and conquer docking principle [20]. These calculations selected six spinach proteins (PDB entries: 1YVE, 1FNB, 2PKQ, 1AL7, 1 F56 and 1RBO) with a docking energy (ΔGpred) in the range of −7.58 and −8.26 kcal/mol (Fig. 1). The selected protein hits also secured significant predicted inhibitory constants (Kipred) in micromolar and nanomolar concentrations (Table 2). Of note, ketol-acid reductoisomerase (1YVE) and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco; 1RBO) possessed better docking energies (ΔGpred = −8.24 and −8.26 kcal/mol) and Kipred (910.8 nM and 880.6 nM), respectively. Intriguingly, the idTarget program also utilizes ligand similarity protocol to facilitate the user in the identification of similar experimental structures deposited in the PDB, whose co-crystallized ligand constitutes a high similarity with the queried ligand [20]. We recognized chitinase 1 complex with kinetin as the experimental protein structure from Saccharomyces cerevisiae (yeast) [46], which secured a ligand Tanimoto coefficient of 1 (the value 1 denotes identical ligands). The yeast chitinase 1 protein also possessed significant ΔGpred (−7.51 kcal/mol) and Kipred (3.1 μM) values (Table 2), respectively. We expect that kinetin may interact effectively with spinach chitinase protein (Fig. 1) to affect seed germination-related functions.
Table 1.
List of non-redundant protein structures from spinach proteome in PDB (May, 2014)
| S. no | Protein | PDB entry |
|---|---|---|
| 1 | Ketol-acid reductoisomerasea/acetohydroxy acid isomeroreductase | 1YVE |
| 2 | Acyl carrier protein 1a | 2FVA |
| 3 | Aquaporin | 1Z98 |
| 4 | ATP synthase subunit ca | 2W5J |
| 5 | ATP synthase subunit alphaa | 1KMH |
| 6 | Betaine aldehyde dehydrogenasea | 4A0M |
| 7 | Beta-trypsin | 4AOR |
| 8 | Chlorophyll a–b binding proteina | 1RWT |
| 9 | Superoxide dismutase [Cu–Zn]a | 1SRD |
| 10 | Ferredoxin-NADP+ reductase | 1FNB |
| 11 | Fructose 1,6-bisphosphatase | 1SPI |
| 12 | Glyceraldehyde-3-phosphate dehydrogenase Aa | 2PKQ |
| 13 | Peroxisomal (S)-2-hydroxy-acid oxidase/glycolate oxidase | 1AL7 |
| 14 | Oxygen-evolving enhancer 2/PsbP | 2VU4 |
| 15 | Oxygen-evolving enhancer 3/PsbQ | 1NZE |
| 16 | Photosystem I reaction centera | 2O01 |
| 17 | Plantacyanin | 1 F56 |
| 18 | Plastocyanina | 2PCF |
| 19 | Lumazine synthase | 1C2Y |
| 20 | Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) | 1RBO |
| 21 | Cytochrome b6-f complex iron-sulfur subunit (Rieske iron–sulfur protein)a | 1RFS |
| 22 | Thioredoxin F | 1F9M |
aChloroplast proteins
Fig. 1.
Docked poses of kinetin with the prioritized protein targets of spinach and yeast chitinase 1 (2UY5). The kinetin and the co-crystallized molecules are represented in green and yellow stick models, respectively
Table 2.
Prioritized protein list of spinach PDB proteome obtained from idTarget screening (receptor-based approach)
| S. no | Proteins | PDB entry | idTarget results (kinetin as ligand) | |
|---|---|---|---|---|
| ΔG pred (kcal/mol) | K i pred | |||
| Prioritized proteins from spinach PDB proteome | ||||
| 1 | Ketol-acid reductoisomerase/acetohydroxy acid isomeroreductase | 1YVE | −8.24 | 910.8 nM |
| 2 | Ferredoxin-NADP+ reductase | 1FNB | −8.15 | 1.1 μM |
| 3 | Glyceraldehyde-3-phosphate dehydrogenase A | 2PKQ | −7.97 | 1.4 μM |
| 4 | Peroxisomal (S)-2-hydroxy-acid oxidase/glycolate oxidase | 1AL7 | −7.58 | 2.8 μM |
| 5 | Plantacyanin | 1 F56 | −7.66 | 2.4 μM |
| 6 | Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) | 1RBO | −8.26 | 880.6 nM |
| Proteins from other PDB proteomes ranked by ligand similarity search | ||||
| 7 | Chitinase 1 (Saccharomyces cerevisiae) | 2UY5 | −7.51 | 3.1 μM |
Ligand-based similarity search
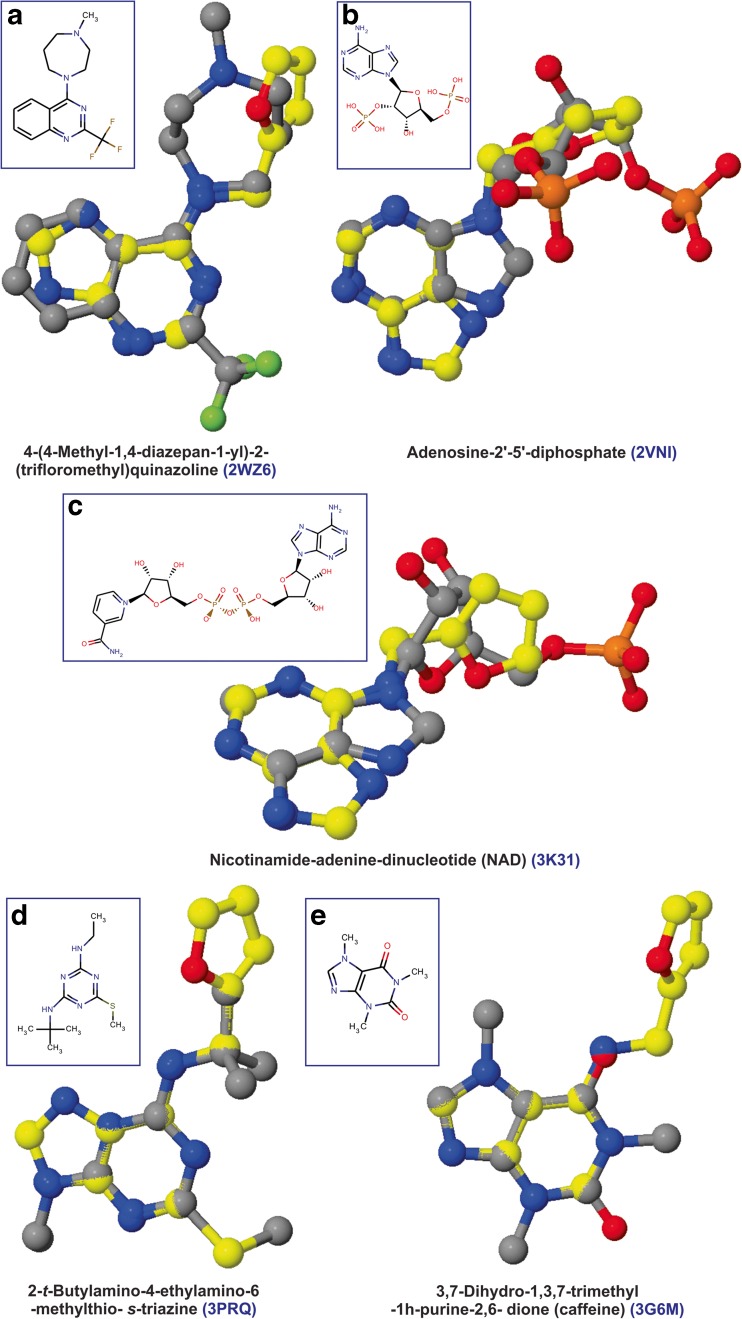
ReverseScreen3D is a reverse virtual screening program which attempts to predict probable protein targets based on ‘queried ligand–co-crystallized ligand’ similarity metric [27]. We had given no emphasis on selecting spinach PDB proteome preferences and considered the available PDB proteome from various model organisms prepared by the ReverseScreen3D program for its calculations. These computations helped us in (a) studying kinetin (or similar ligands) binding chemical space available in PDB structures, (b) studying protein targets sharing homology with the spinach proteins which do not secure significant docking energies due to limitations in docking scoring schemes and regarded as ‘non-hits’ and (3) recognizing crucial spinach proteins whose experimental structure is not available in PDB. These considerations had a positive impact on prioritizing spinach protein targets. We used kinetin as the query molecule to calculate the hit list of protein targets based on 2D score, and subsequently, the top 10 % of the 2D score-based hit list was utilized to compute 3D ligand similarities. We had given no prominence to choose targets based on 2D and 3D scores but considered the 3D score-based hit list as the final rank list. We relied on selecting protein targets having homology with the spinach PDB proteome because all the top-scoring protein targets were not available in the PDB proteome of spinach and due to the consideration of complete ReverseScreen3D proteomes. The manually curated 3D score-based rank list (Table 3) ensured the selection of homologous spinach proteins which secured insignificant ΔGpred values. These include superoxide dismutase [Cu-Zn] (2WZ6; spinach homologous protein: 1SRD) and photosystem Q(B) (3PRQ; 1NZE) proteins from Homo sapiens and Thermosynechococcus elongatus (strain BP-1), respectively. The selection of these targets can be attributed to the similarities of aminopurine moiety of kinetin with 4-(4-methyl-1,4-diazepan-1-yl)-2-trifloromethyl) quinazoline (2D score = 0.28; 3D score = 0.46) and 2-t-butylamino-4-ethylamino-6-methylthio-s-triazine (0.25; 0.39) (Fig. 2). We recognized NADPH-ferredoxin reductase (1FNB) as the most probable target which secured ΔGpred and Kipred of −8.15 kcal/mol and 1.1 μM, respectively, and possessed better ligand similarity with adenosine-2′–5′-diphosphate (2VNI; 3D score = 0.43). Interestingly, ReverseScreen3D rank list also prioritized chitinase from Gliocladium roseum (3G6M) as one among the targets owing to the ligand similarity of kinetin with caffeine (3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione) [46]. Thus, it is advantageous to select protein targets based on homology than 2D and 3D scores.
Table 3.
Homologous proteins of spinach PDB proteome obtained from ReverseScreen3D screening (ligand-based approach)
| S. no | Protein | Organism | PDB entry | 2D score | 3D score |
|---|---|---|---|---|---|
| 1 | Superoxide dismutase [Cu–Zn] | Homo sapiens | 2WZ6 | 0.28 | 0.46 |
| 2 | NADPH-ferredoxin reductase | Rhodobacter capsulatus | 2VNI | 0.25 | 0.43 |
| 3 | Enoyl-(acyl-carrier protein) reductase |
Anaplasma phagocytophilum
(strain HZ) |
3K31 | 0.25 | 0.39 |
| 4 | Photosystem Q(B) protein | Thermosynechococcus elongatus (strain BP-1) | 3PRQ | 0.25 | 0.39 |
| 5 | Chitinase | Gliocladium roseum | 3G6M | 0.24 | 0.43 |
Fig. 2.
a–e 3D ligand superposition of co-crystallized ligands (grey ball and stick model) from selected homologous proteins of spinach targetome (ranked by ReverseScreen3D ligand similarity search) with kinetin (yellow ball and stick model). The 2D structures of the co-crystallized ligands are shown in the inset
Comparing rank list of receptor- and ligand-based methods
The comparison of protein target rank lists from inverse docking and ligand similarity studies may provide confidence in prioritizing protein targets based on common target criterion. This task recognized NADPH-ferredoxin reductase as the common target due to significant docking energy and kinetin similarity with adenosine-2′–5′-diphosphate, respectively. The unavailability of the precise mechanism of NADPH-ferredoxin reductase in the seed germination process precludes the priority task and requires further studies. Spinach chitinase can also be regarded as the most probable target of kinetin due to its inhibition by kinetin in yeast studies [46] and chemical similarities of kinetin with caffeine in the experimental structure of G. roseum chitinase [47]. These observations were judged purely by protein homology and ligand similarity criteria.
Experimental evidences of prioritized protein targets from model organisms
The experimental evidences of proteins from various model organisms can be effectively used to hypothesize the prioritize protein target molecular functions. Literature mining provided experimental evidences for four (out of six) inverse docking-based prioritized targets. These include superoxide dismutase [Cu-Zn] (1SRD), ferredoxin- NADP+ reductase (1FNB), glycolate oxidase (1AL7) and rubisco (1RBO) proteins. The literature survey recognized only photosystem Q(B) protein (spinach: 1NZE; homology: 3PRQ) amongst the curated hit list of ReverseScreen3D calculations.
A direct effect of kinetin on superoxide dismutase activity (SOD) has been noticed in plants [8]. Tobacco and sal (Shorea robusta Gaertn. f.) studies showed a significantly high level of SOD in kinetin-treated seeds that functions in the inhibition or reduction of membrane damage caused by superoxide anions [48, 49]. Substantial increase in the peroxisomal enzyme activities, viz. catalase, isocitrate lyase, hydroxypyruvate reductase and glycolate oxidase, was observed after supplementation of kinetin (50 μg/ml) in sunflower (Helianthus annuus L.) seedlings [50]. An indirect evidence from the proteomic study of adenine isopentenyl transferase gene-based cytokinin synthesis suggests that cytokinin regulation correlates with the expression of six leaf and nine root proteins in perennial grass species including putative oxygen-evolving complex (photosystem Q(B)), rubisco small subunit, glycolate oxidase, ferredoxin-NADP reductase precursor proteins, etc. [51]. These evidences only account for the role of kinetin and recognized proteins studied in various plant systems. The exact interaction mechanism of kinetin with spinach protein targets needs further investigations.
Chitinase as the most probable protein target of kinetin
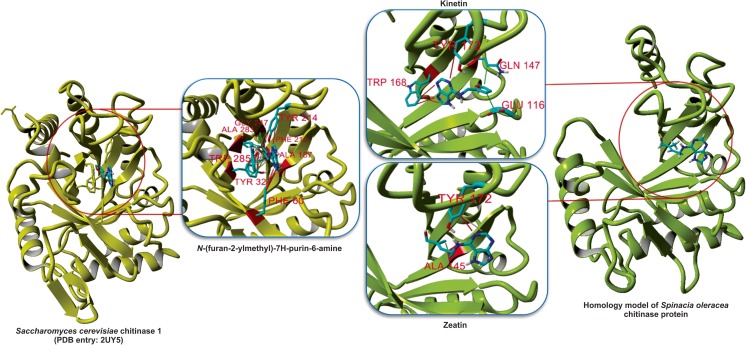
The experimental yeast chitinase 1 protein complex with kinetin and kinetin similarity with the caffeine molecule represented spinach chitinase as the most probable protein target of kinetin. A homology model of spinach chitinase was developed based on chitinase from Parkia platycephala (2GSJ) experimental structure [52] (refer to Supplementary Text), and its interaction with kinetin was studied using the conventional molecular docking method. Kinetin inhibits yeast chitinase 1 competitively with Ki of 3.2 μM [46] and exhibited a unique binding pattern enriched with π contacts, H bonds and van der Waals interactions (Fig. 3). The docked pose of kinetin in modelled spinach chitinase protein was generated using BSP-SLIM docking program [34], which secured a better docking score of 5.064 kcal/mol.
Fig. 3.
The interaction of kinetin with yeast chitinase 1 was deduced from the experimental structure (2UY5), and its contact with spinach chitinase proteins was predicted from docking approach
The majority of contacts in the kinetin docked pose were weak contacts and electrostatic interactions conferred by binding site residues viz. Asp112, Asp114, Glu116, Ala145 and Trp168, respectively. It can be discerned that active site residues (Asp114—electrostatic, Glu116—van der Waals and Tyr 172—H bond interactions) are engaged in ligand contacts. H bonds were developed between the N and O atoms of kinetin with Tyr172 and Gln170 residues. In silico site-directed mutagenesis experiment on the developed chitinase–kinetin complex using ABS-Scan [35] revealed the major contribution of Trp168 amino acid towards kinetin recognition (Fig. 4). However, the active site residues, Asp114 and Tyr172, constituted a moderate effect for kinetin binding. The residue Glu116 possessed a very weak contact as suggested by the dock pose which, as a result, did not participate in binding site due to a distance of 9.15 Å from the terminal atom of the purine moiety from kinetin. Trp168 established π contact with furan group of kinetin, which can be a considered as anchoring region for furan group orientation and signifies the binding pose of kinetin. Additionally, residues including Ile19, Trp168, Gln170 and Phe202 were found to play a major role in ligand recognition despite the lower contribution in terms of Autodock score in comparison to wild (native) kinetin–chitinase complex. The ΔΔG mutants developed from Ile19, Trp168, Trp168, Gln170 and Phe202 secured more than 0.2 kcal/mol variations, which indicate the importance of these residues in kinetin binding (Table S1).
Fig. 4.
The effect of computational mutagenesis for binding site amino acids deduced from the kinetin–chitinase dock pose. a The level of contribution (low to high) of amino acids essential for kinetin recognition is graphically shown and b its evaluations using Autodock 4.1 scoring function for chitinase mutants and resulting ΔΔG
Chitinase, the cell wall-modifying enzyme belonging to β-1,3-glucanases protein family, is transcriptionally induced during seed induction and germination processes, and its expression is strongly associated with the activity of phytohormones and environmental factors [1]. Noticeably, the inhibition of chitinases is an important molecular function in the defense against seed pathogens wherein a pathogen attacks the seed protective layers as the first line of infection, and the inhibition of cell wall-modifying enzymes including plant chitinases is crucial for pathogens to establish infection [53]. The chitinase activity is also well understood in tomato [54] and muskmelon seeds [55]. Moreover, studies on the role of chitinases in plant growth processes showed that ethylene (a stress hormone) induces some chitinase isoforms [56]. Induction studies of chitinases in Nicotiana tabacum in in vitro conditions revealed that the presence of cytokinin repressed the expression of chitinases genes [57]. Such down-regulation mechanism requires the interaction of kinetin, in which we anticipate that chitinase–kinetin complex plays an active role.
In vitro study reveals the effect of kinetin in spinach seed germination
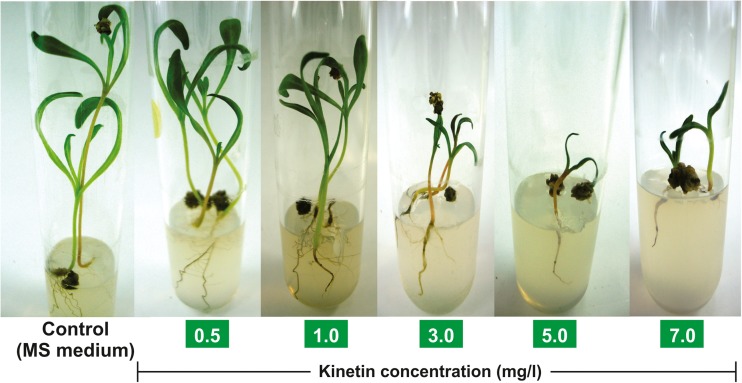
An in vitro study was performed to study the effects of kinetin in the germination of spinach seeds. This study provided evidence about the negative effect of kinetin in seed germination and the hypothesized interactaction with prioritized protein targets. The seed viability and seedling vigourness of spinach was studied (Fig. 5) in MS medium supplemented with various concentrations of kinetin (Table 4), which showed that a very low concentration of kinetin (0.5 mg/l) does not show a significant effect compared to control in inducing seed germination process. Kinetin exhibited a marked elongation in the seedling and an increase in the number of leaves at 0.5 mg/l concentration comparable to control, demonstrating an insignificant kinetin effect. This lower level of kinetin activity can be linked to the predicted Ki values in nanomolar and micromolar concentrations shown in the inverse docking experiment. The higher levels of kinetin (>0.5 mg/l) exhibited strong growth antagonist activities. It shows that kinetin interferes with the seed growth mechanism by interacting with germination-related proteins. Thus, it appears that kinetin may not be a suitable hormone for enhancing spinach seed germination in vitro, and further experiments are needed to unravel the associated molecular mechanism.
Fig. 5.
Effect of kinetin in various concentrations on spinach seed germination. Murashige–Skoog (MS) medium without supplementation of kinetin was used as control
Table 4.
Study of seed viability and seedling vigour of spinach plant on MS media supplemented with different types of kinetin
| Medium number | Kinetin concentration (mg/l) | Seed viability | Seed vigourness a | ||||
|---|---|---|---|---|---|---|---|
| Germination (%) | Hypocotyle length (cm) | Radical length (cm) | Seedling length (cm) | Seedling fresh weight (g) | Number of leaves | ||
| 1 | – | 72.73 | 4.34 ± 0.3 | 5.16 ± 0.51 | 9.5 ± 0.70 | 0.11 ± 0.02 | 3.25 ± 0.31 |
| 2 | 0.5 | 75 | 3.25 ± 0.15 | 4.19 ± 0.46 | 7.44 ± 0.54 | 0.10 ± 0.01 | 3.2 ± 0.41 |
| 3 | 1 | 65 | 2.66 ± 0.34 | 3.45 ± 0.38 | 6.11 ± 0.55 | 0.09 ± 0.01 | 2.62 ± 0.35 |
| 4 | 3 | 58.33 | 2.16 ± 0.24 | 1.39 ± 0.17 | 3.55 ± 0.33 | 0.03 ± 0.02 | 1.14 ± 0.32 |
| 5 | 5 | 45.84 | 1.33 ± 0.16 | 0.65 ± 0.15 | 1.97 ± 0.23 | 0.06 ± 0.03 | 1.09 ± 0.31 |
| 6 | 7 | 68.18 | 0.92 ± 0.14 | 0.71 ± 0.15 | 1.63 ± 0.27 | 0.03 ± 0.01 | 2.00 ± 0.19 |
aThe values are expressed as average ± standard error from five replicates in each hormone concentration
Conclusions
The protein targets of kinetin were predicted using in silico approaches. Inverse docking and ligand similarity methods prioritized probable protein targets available from spinach PDB proteome. We studied chitinase as the most probable protein target of kinetin owing to its significant docking score, ligand similarity with caffeine, availability of yeast experimental structure complex with kinetin and experimental evidences on seed defense mechanisms. The binding mode of the spinach chitinase–kinetin complex was explored, which revealed active site amino acids involved in kinetin binding and an equal contribution of van der Waals and electrostatic interactions with two H bonds. An in vitro study focused on the effect of kinetin in spinach seed germination correlates with the experimental evidences that kinetin antagonistically affects seed viability and vigourness.
Electronic supplementary material
(DOCX 3070 kb)
Acknowledgments
Sivakumar Prasanth Kumar acknowledges support from the Department of Science and Technology, New Delhi, India, as INSPIRE Senior Research Fellowship.
Compliance with ethical standards
The authors declare that no competing interests exist.
Footnotes
Sivakumar Prasanth Kumar and Vilas R. Parmar contributed equally to this work.
References
- 1.Leubner-Metzger G. Functions and regulation of β-1,3-glucanases during seed germination, dormancy release and after-ripening. Seed Sci Res. 2003;13:17–34. doi: 10.1079/SSR2002121. [DOI] [Google Scholar]
- 2.Katzman LS, Taylor AG, Langhans RW. Seed enhancements to improve spinach germination. Hortscience. 2001;36:979–981. [Google Scholar]
- 3.Hartmann HT, Kester DE, Geneve RL, Davies FT. In: Hartmann and Kester’s plant propagation: principles and practices. Hartmann HT, Kester DE, editors. Prentice Hall: New Jersey; 2001. [Google Scholar]
- 4.Khan AA. Cytokinins: permissive role in seed germination. Science. 1971;171:853–859. doi: 10.1126/science.171.3974.853. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khayri JM, Huang FH, Morelock TE. Regeneration of spinach from leaf callus. HortSci. 1991;26:913–914. [Google Scholar]
- 6.Al-Khayri JM, Huang FH, Morelock TE, Busharar TA. In vitro plant regeneration of spinach from mature seed-derived callus. In Vitro Cell Dev Biol. 1992;28:64–66. doi: 10.1007/BF02823020. [DOI] [Google Scholar]
- 7.Robert CE, Douglas GF. Interactions of applied hormones in the germination of Lepidium virginicum seeds. Ohio J Sci. 1977;77:236–239. [Google Scholar]
- 8.Barciszewski J, Rattan SIS, Siboska G, Clark BFC. Kinetin—45 years on. Plant Sci. 1999;148:37–45. doi: 10.1016/S0168-9452(99)00116-8. [DOI] [Google Scholar]
- 9.Gomez L, Allona I, Casado R, Aragoncillo C. Seed chitinases. Seed Sci Res. 2002;12:217–230. doi: 10.1079/SSR2002113. [DOI] [Google Scholar]
- 10.Domon JM, Neutelings G, Roger D, David A, David H. A basic chitinase-like protein secreted by embryogenic tissues of Pinus caribaea acts on arabinogalactan proteins extracted from the same cell lines. J Plant Physiol. 2000;156:33–39. doi: 10.1016/S0176-1617(00)80269-2. [DOI] [Google Scholar]
- 11.Krishnaveni S, Liang GH, Muthukrishnan S, Manickam A. Purification and partial characterization of chitinases from sorghum seeds. Plant Sci. 1999;144:01–07. doi: 10.1016/S0168-9452(99)00050-3. [DOI] [Google Scholar]
- 12.Kumar SP, Pandya HA, Jasrai YT. A computational model for non-conserved mature miRNAs from the rice genome. SAR QSAR Environ Res. 2014;25:205–220. doi: 10.1080/1062936X.2013.875941. [DOI] [PubMed] [Google Scholar]
- 13.Kumar SP, Jha PC, Pandya HA, Jasrai YT. Implementation of pseudoreceptor-based pharmacophore queries in the prediction of probable protein targets: explorations in the protein structural profile of Zea mays. Mol BioSyst. 2014;10:1833–1844. doi: 10.1039/C4MB00058G. [DOI] [PubMed] [Google Scholar]
- 14.Kumar SP, Pandya HA, Desai VH, Jasrai YT. Compound prioritization from inverse docking experiment using receptor-centric and ligand-centric methods: a case study on Plasmodium falciparum Fab enzyme. J Mol Recognit. 2014;27:215–229. doi: 10.1002/jmr.2353. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein FC, Koetzle TF, Williams GJB. The protein data bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977;112:535–542. doi: 10.1016/S0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- 16.Duan Y, Wu C, Chowdhury S, Lee MC, Xiong G, Zhang W, Yang R, Cieplak P, Luo R, Lee T, Caldwell J, Wang J, Kollman P. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem. 2003;24:1999–2012. doi: 10.1002/jcc.10349. [DOI] [PubMed] [Google Scholar]
- 17.Singh UC, Kollman PA. An approach to computing electrostatic charges for molecules. J Comput Chem. 1984;5:129–145. doi: 10.1002/jcc.540050204. [DOI] [Google Scholar]
- 18.Krieger E, Darden T, Nabuurs SB, Finkelstein A, Vriend G. Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins. 2004;57:678–683. doi: 10.1002/prot.20251. [DOI] [PubMed] [Google Scholar]
- 19.Bolton E, Wang Y, Thiessen PA, Bryant SH. In: The annual reports in computational chemistry. Cornell W, editor. Washington: American Chemical Society; 2008. [Google Scholar]
- 20.Wang JC, Chu PY, Chen CM, Lin JH. idTarget: a web server for identifying protein targets of small chemical molecules with robust scoring functions and a divide-and-conquer docking approach. Nucleic Acids Res. 2012;40:W393–W399. doi: 10.1093/nar/gks496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang DT, Oyang YJ, Lin JH. MEDock: a web server for efficient prediction of ligand binding sites based on a novel optimization algorithm. Nucleic Acids Res. 2005;33:W233–W238. doi: 10.1093/nar/gki586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JC, Lin JH, Chen CM, Perryman AL, Olson AJ. Robust scoring functions for protein–ligand interactions with quantum chemical charge models. J Chem Inf Model. 2011;51:2528–2537. doi: 10.1021/ci200220v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. A second generation force-field for the simulation of proteins, nucleic-acids, and organic-molecules. J Am Chem Soc. 1995;117:5179–5197. doi: 10.1021/ja00124a002. [DOI] [Google Scholar]
- 24.Jakalian A, Jack DB, Bayly CI. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J Comput Chem. 2002;23:1623–1641. doi: 10.1002/jcc.10128. [DOI] [PubMed] [Google Scholar]
- 25.Hospital A, Andrio P, Fenollosa C, Cicin-Sain D, Orozco M, Gelpí JL. MDWeb and MDMoby: an integrated web-based platform for molecular dynamics simulations. Bioinformatics. 2012;28:1278–1279. doi: 10.1093/bioinformatics/bts139. [DOI] [PubMed] [Google Scholar]
- 26.Hou X, Du J, Zhang J, Du L, Fang H, Li M. How to improve docking accuracy of AutoDock4.2: a case study using different electrostatic potentials. J Chem Inf Model. 2013;53:188–200. doi: 10.1021/ci300417y. [DOI] [PubMed] [Google Scholar]
- 27.Kinnings SL, Jackson RM. ReverseScreen3D: a structure-based ligand matching method to identify protein targets. J Chem Inf Model. 2011;51:624–634. doi: 10.1021/ci1003174. [DOI] [PubMed] [Google Scholar]
- 28.Chang CE, Gilson MK. Tork: conformational analysis method for molecules and complexes. J Comput Chem. 2003;24:1987–1998. doi: 10.1002/jcc.10325. [DOI] [PubMed] [Google Scholar]
- 29.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 30.The UniProt Consortium Activities at the Universal Protein Resource (UniProt) Nucleic Acids Res. 2014;42:D191–D198. doi: 10.1093/nar/gkt1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran GN, Ramakrishnan C, Sasisekharan V. Stereochemistry of polypeptide chain configurations. J Mol Biol. 1963;7:95–99. doi: 10.1016/S0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- 33.RAMPAGE Server. Available at: http://ravenbioccam.ac.uk/rampage.php, Accessed May 2014
- 34.Lee HS, Zhang Y. BSP-SLIM: a blind low-resolution ligand–protein docking approach using predicted protein structures. Proteins. 2012;80:93–110. doi: 10.1002/prot.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anand P, Nagarajan D, Mukherjee S, Chandra N. ABS-Scan: in silico alanine scanning mutagenesis for binding site residues in protein–ligand complex. F1000Research. 2014;3:214. doi: 10.12688/f1000research.5165.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eswar N, Eramian D, Webb B, Shen MY, Sali A. Protein structure modeling with MODELLER. Methods Mol Biol. 2008;426:145–159. doi: 10.1007/978-1-60327-058-8_8. [DOI] [PubMed] [Google Scholar]
- 37.Shen MY, Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006;15:2507–2524. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huey R, Morris GM, Olson AJ, Goodsell DS. A semiempirical free energy force field with charge-based desolvation. J Comput Chem. 2007;28:1145–1152. doi: 10.1002/jcc.20634. [DOI] [PubMed] [Google Scholar]
- 39.Jmol: an open-source Java viewer for chemical structures in 3D. Available at http://www.jmol.org/, Accessed March 2015
- 40.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 41.Rai S, Sharma M, Jain M, Awasthi A, Purshottam D, Nair N, Sharma A. Rapid in vitro production of cloned plants of Uraria picta (Jacq.) DC—a rare medicinal herb in long-term culture. Appl Biochem Biotechnol. 2010;162:1929–1937. doi: 10.1007/s12010-010-8970-8. [DOI] [PubMed] [Google Scholar]
- 42.Parmar VR, Jasrai YT. Micropropagation of an important medicinal plant Aloe barbadensis Mill (Aloe vera L.) for field plantation. Res J BioTechnol. 2009;4:07–10. doi: 10.1002/biot.200990011. [DOI] [Google Scholar]
- 43.Chen YZ, Zhi DG. Ligand–protein inverse docking and its potential use in the computer search of protein targets of a small molecule. Proteins. 2001;43:217–226. doi: 10.1002/1097-0134(20010501)43:2<217::AID-PROT1032>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 44.Kitchen DB, Decornez H, Furr JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 2004;3:935–949. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- 45.Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, Shoichet BK. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25:197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- 46.Hurtado-Guerrero R, van Aalten DM. Structure of Saccharomyces cerevisiae chitinase 1 and screening-based discovery of potent inhibitors. Chem Biol. 2007;14:589–599. doi: 10.1016/j.chembiol.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, Gan Z, Lou Z, Tao N, Mi Q, Liang L, Sun Y, Guo Y, Huang X, Zou C, Rao Z, Meng Z, Zhang KQ. Crystal structure and mutagenesis analysis of chitinase CrChi1 from the nematophagous fungus Clonostachys rosea in complex with the inhibitor caffeine. Microbiology. 2010;156:3566–3574. doi: 10.1099/mic.0.043653-0. [DOI] [PubMed] [Google Scholar]
- 48.Kurepa J, Herouart D, Van Montagu M, Inze D. Differential expression of Cu-, Zn- and Fe-superoxide dismutase genes of tobacco during development, oxidative stress and hormonal treatment. Plant Cell Physiol. 1997;38:463–470. doi: 10.1093/oxfordjournals.pcp.a029190. [DOI] [PubMed] [Google Scholar]
- 49.Chaitanya KSK, Naithani SC. Kinetin-mediated prolongation of viability in recalcitrant sal (Shorea robusta Gaertn. f.) seeds at low temperature: role of kinetin in delaying membrane deterioration during desiccation-induced injury. J Plant Growth Regul. 1998;17:63–69. doi: 10.1007/PL00007018. [DOI] [Google Scholar]
- 50.Theimer RR, Anding G, Matzner P. Kinetin action on the development of microbody enzymes in sunflower cotyledons in the dark. Planta. 1976;128:41–47. doi: 10.1007/BF00397177. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Gianfagna T, Huang B. Proteomic changes associated with expression of a gene (ipt) controlling cytokinin synthesis for improving heat tolerance in a perennial grass species. J Exp Bot. 2010;61:3273–3289. doi: 10.1093/jxb/erq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavada BS, Moreno FB, da Rocha BA, de Azevedo Jr WF, Castellón RE, Goersch GV, Nagano CS, de Souza EP, Nascimento KS, Radis-Baptista G, Delatorre P, Leroy Y, Toyama MH, Pinto VP, Sampaio AH, Barettino D, Debray H, Calvete JJ, Sanz L. cDNA cloning and 1.75 A crystal structure determination of PPL2, an endochitinase and N-acetylglucosamine-binding hemagglutinin from Parkia platycephala seeds. FEBS J. 2006;273:3962–3974. doi: 10.1111/j.1742-4658.2006.05400.x. [DOI] [PubMed] [Google Scholar]
- 53.Freeman BC, Beattie GA. An overview of plant defenses against pathogens and herbivores. Plant Health Instr. 2008 [Google Scholar]
- 54.Wu CT, Bradford KJ. Class I chitinase and β-1,3-glucanase are differentially regulated by wounding, methyl jasmonate, ethylene, and gibberellin in tomato seeds and leaves. Plant Physiol. 2003;133:263–273. doi: 10.1104/pp.103.024687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witmer X, Nonogaki H, Beers EP, Bradford KJ, Welbaum GE. Characterization of chitinase activity and gene expression in muskmelon seeds. Seed Sci Res. 2003;13:167–178. doi: 10.1079/SSR2003134. [DOI] [Google Scholar]
- 56.Kasprzewska A. Plant chitinases—regulation and function. Cell Mol Biol Lett. 2003;8:809–824. [PubMed] [Google Scholar]
- 57.van Buuren M, Neuhaus JM, Shinshi H, Ryals J, Meins FJ. The structure and regulation of homeologous tobacco endochitinase genes of Nicotiana sylvestris and N. tomentosiformis origin. Mol Gen Genet. 1992;232:460–469. doi: 10.1007/BF00266251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 3070 kb)