Abstract
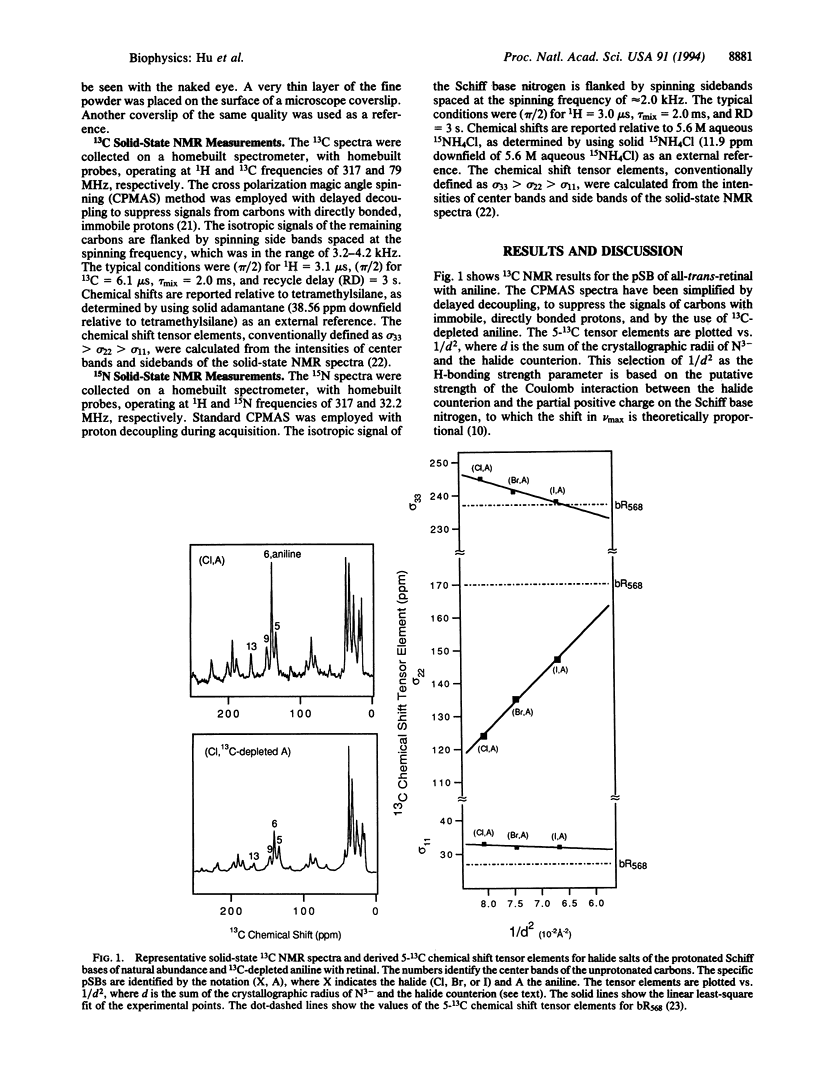
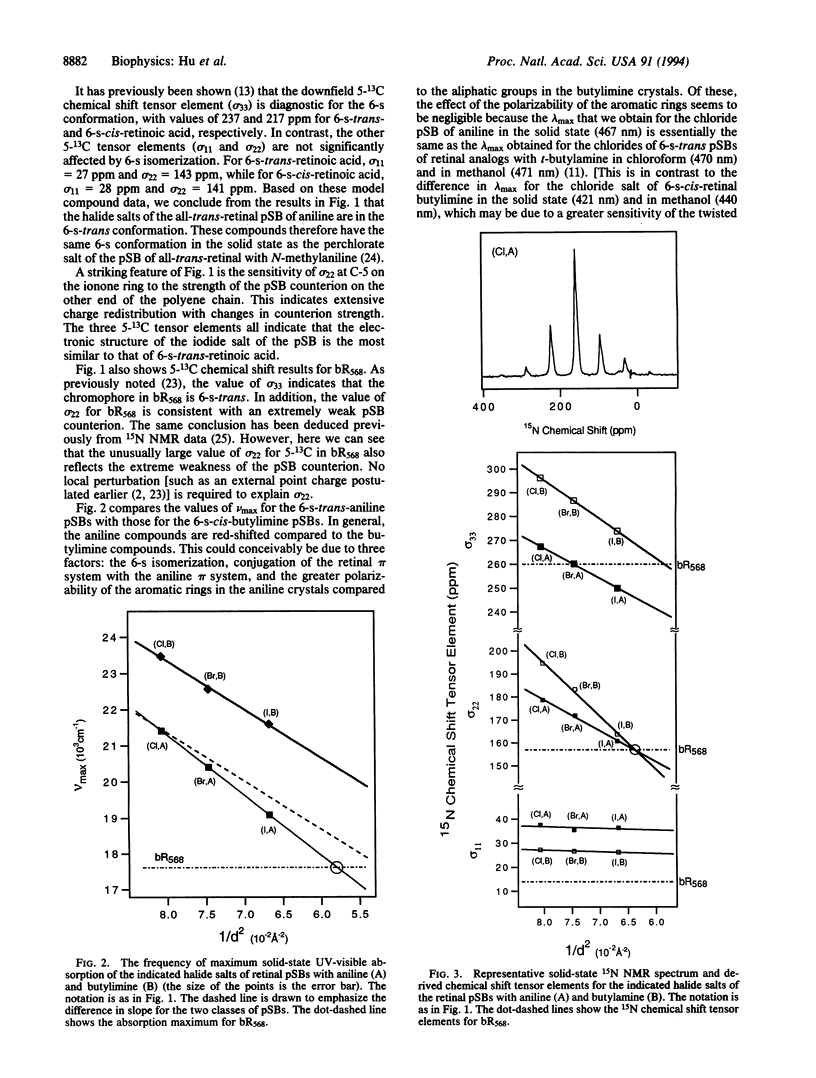
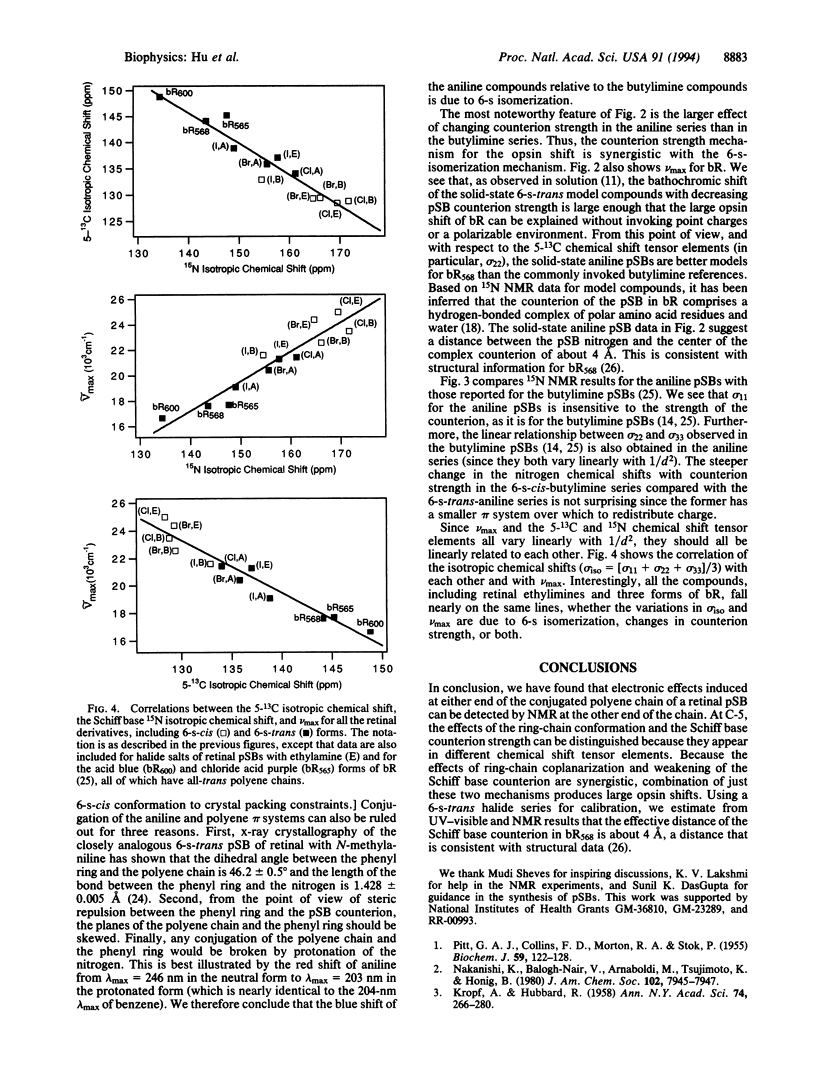
UV-visible and solid-state NMR studies of a series of 6-s-trans protonated Schiff bases of retinal with aniline show that the bathochromic shift induced by weakening the imine counterion is significantly greater in the 6-s-trans conformation than in the 6-s-cis conformation. Based on the observed magnitude of this coupling between the electronic effects of 6-s isomerization and imine counterion strength in the model compounds, the large opsin shift and unusual chemical shifts in light-adapted bacteriorhodopsin can be fully explained. These phenomena therefore do not require a negative point charge or polarizability effects in the chromophore binding pocket. The results are consistent with an effective center-to-center distance between the Schiff base and its counterion of about 4 A in light-adapted bacteriorhodopsin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatz P. E., Mohler J. H., Navangul H. V. Anion-induced wavelength regulation of absorption maxima of Schiff bases of retinal. Biochemistry. 1972 Feb 29;11(5):848–855. doi: 10.1021/bi00755a026. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Herzfeld J., Griffin R. G. Solid-state nitrogen-15 nuclear magnetic resonance study of the Schiff base in bacteriorhodopsin. Biochemistry. 1983 Jan 4;22(1):1–4. doi: 10.1021/bi00270a600. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Courtin J. M., Lugtenburg J., Herzfeld J., Mathies R. A., Griffin R. G. Solid-state 13C NMR detection of a perturbed 6-s-trans chromophore in bacteriorhodopsin. Biochemistry. 1985 Nov 19;24(24):6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Honig B., Greenberg A. D., Dinur U., Ebrey T. G. Visual-pigment spectra: implications of the protonation of the retinal Schiff base. Biochemistry. 1976 Oct 19;15(21):4593–4599. doi: 10.1021/bi00666a008. [DOI] [PubMed] [Google Scholar]
- Irving C. S., Byers G. W., Leermakers P. A. Effect of solvent polarizability on the absorption spectrum of all-trans-retinylpyrrolidiniminium perchlorate. J Am Chem Soc. 1969 Apr 9;91(8):2141–2143. doi: 10.1021/ja01036a066. [DOI] [PubMed] [Google Scholar]
- KROPF A., HUBBARD R. The mechanism of bleaching rhodopsin. Ann N Y Acad Sci. 1959 Nov 12;74(2):266–280. doi: 10.1111/j.1749-6632.1958.tb39550.x. [DOI] [PubMed] [Google Scholar]
- Kliger D. S., Milder S. J., Dratz E. A. Solvent effects on the spectra of retinal Schiff bases--I. models for the bathochromic shift of the chromophore spectrum in visual pigments. Photochem Photobiol. 1977 Mar;25(3):277–286. doi: 10.1111/j.1751-1097.1977.tb06911.x. [DOI] [PubMed] [Google Scholar]
- Mathies R., Stryer L. Retinal has a highly dipolar vertically excited singlet state: implications for vision. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2169–2173. doi: 10.1073/pnas.73.7.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITT G. A., COLLINS F. D., MORTON R. A., STOK P. Studies on rhodopsin. VIII. Retinylidenemethylamine, an indicator yellow analogue. Biochem J. 1955 Jan;59(1):122–128. doi: 10.1042/bj0590122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot H. J., Harbison G. S., Herzfeld J., Griffin R. G. Nuclear magnetic resonance study of the Schiff base in bacteriorhodopsin: counterion effects on the 15N shift anisotropy. Biochemistry. 1989 Apr 18;28(8):3346–3353. doi: 10.1021/bi00434a033. [DOI] [PubMed] [Google Scholar]
- de Groot H. J., Smith S. O., Courtin J., van den Berg E., Winkel C., Lugtenburg J., Griffin R. G., Herzfeld J. Solid-state 13C and 15N NMR study of the low pH forms of bacteriorhodopsin. Biochemistry. 1990 Jul 24;29(29):6873–6883. doi: 10.1021/bi00481a017. [DOI] [PubMed] [Google Scholar]


