Abstract
BACKGROUND
Hospital readmissions are expensive and they may signal poor quality of care. Whether functional status is related to hospital readmissions using a representative U.S sample remains unexplored .
OBJECTIVE
We aimed to assess the relationship between functional status and all-cause 30-day hospital readmissions using a representative sample of the US population.
DESIGN
This was a retrospective observational study (2003–2011).
PATIENTS
The study included 3,772 patients who completed the SF-12 before being hospitalized. Three hundred and eighteen (8.4 %) were readmitted within 30 days after being discharged.
MEASUREMENTS
The Medical Expenditure Panel Survey (MEPS) was employed. Functional status was measured with the Short-Form 12-Item Health Survey Version 2® (SF-12). The probability of being readmitted was estimated using a logistic model controlling for demographic characteristics, comorbid conditions, insurance coverage, physical (PCS) and mental (MCS) summaries of the SF-12, reason for hospitalization, length of hospital stay, region, and residential area.
RESULTS
A one-unit difference in PCS reduced the odds of readmission by 2 % (odds ratio 0.98 [95 % CI, 0.97 to 0.99]; p < 0.001), which implies an 18 % reduction in the odds of readmissions for a ten-unit difference (one standard deviation) in PCS. The c-statistic of the model was 0.72.
CONCLUSION
Baseline physical function is associated with hospital readmissions. The SF-12 improves the ability to identify patients at high risk of hospital readmission.
KEY WORDS: functional status, physical status, medical expenditure panel survey, hospital admission, readmission
INTRODUCTION
At least 20 % of the most frequently treated conditions in US hospitals, such as congestive heart failure, schizophrenia and renal failure, resulted in readmissions within 30 days in 2010.1 Most of these readmissions were unplanned.2 Hospital readmissions are both a clinical and financial problem, with a total annual cost of $17.4 billion for Medicare alone.3 The burden of hospital readmissions falls mostly on Medicare populations, accounting for more than half of hospital readmissions in 2011.4 Under the Accountable Care Act (ACA), the Hospital Readmissions Reduction Program aims to reduce the frequency of hospital readmissions among Medicare beneficiaries by financially penalizing hospitals with excessive readmission rates.5 The reduction of early hospital readmissions is an opportunity to control health care expenditures and improve systematic errors in clinical care and transitions in levels of care. However, the ability of hospitals to freely readmit struggling patients is a vital safety net for these individuals. The accurate prediction of readmission risk is essential to ensure that this potentially life-saving clinical option is available to minimize inequities in care delivery. Existing studies relate hospital readmissions to demographic and socioeconomic factors including age, gender,6 race,7 geographic region,8 comorbidities9 and Medicaid coverage.9–18 Other relevant factors include patient capability of self care, social support, measures of illness severity, and functional status.12,15,16 The inclusion of some of these components may help identify patients at high risk of 30-day hospital readmissions.
Existing literature suggests that lower functional status increases the probability of readmission across different settings and conditions, including the Veterans Health Administration,19 rehabilitation settings,20–22 and in acute hospitals for heart conditions,23–27 chronic obstructive pulmonary disease,15,28–30 burn injuries,31 and all-cause readmissions.19,32–34 However, acute care studies have been based on small sample sizes ranging from 45 to 938 patients. The extent to which the relationship between functional status and all-cause readmissions is generalizable to the US population remains unexplored.
The goal of this study is to assess the relationship between functional status and all-cause 30-day hospital readmissions using a representative sample of the US population.
METHODS
Data Sources
The Medical Expenditure Panel Survey (MEPS) is a nationally representative survey of the US population conducted by the Agency for Healthcare Research and Quality. MEPS began in 1996 and was designed to assess health services utilization, health care expenditure, and the scope and breadth of health insurance. Each year, a new cohort of households is selected and followed for 2 years. Within this period, there are five rounds of data collection. However, not all the components of the survey are administered each time. We employed two components of the publicly available Household Files, the Hospital Inpatient Stay Files and the Full-Year Consolidated Files. Individuals in the Hospital Inpatient Stay Files were selected and their sociodemographic information, comorbidities, Short-Form 12-Item Health Survey Version 2® (SF-12) scores, geographic region, and residential area were added from the Full-Year Consolidated Files.
Individuals with at least one hospitalization between 2003 and 2011 who completed the SF-12 before the first hospitalization were included in our sample. We used the SF-12 before the first hospital admission as a measure of functional status at baseline.35 We appended cases across 9 years of data to achieve a sufficiently large sample. For each study period, only one cohort hospitalization was selected for each patient. If more than one hospitalization per patient met the criteria, we selected the one with an earliest admission date. Only readmissions that occurred within 30 days were counted as occurrences for this study. All-cause readmissions were included; thus, readmissions may or may not be related to procedures listed on the first hospital admission.
Variables
Our outcome of interest was 30-day all-cause hospital readmission. We defined a hospital readmission as any subsequent hospitalization within 30 days of the index admission.
Functional status was measured with SF-12 Version 2. It is a self-administered questionnaire designed to cover the following eight domains of the Short-Form 36 (SF-36): physical functioning, role limitations due to physical problems, bodily pain, general health perceptions, energy and vitality, social functioning, role limitations due to emotional problems, and mental health. The SF-12 contains 12 items, which are organized into physical (PCS) and mental (MCS) summaries. PCS and MCS are standardized to the US population. Therefore, scores can be directly interpreted based on the normal US population with a mean of 50 and a standard deviation (SD) of 10. Higher scores imply better functional status. The SF-12 has shown higher precision and lower ceiling and floor effects compared to its predecessor, the SF-36 Version 1.0.36,37
We also adjusted for age (grouped into 18 to 45 years, 46 to 64 years, and 65 years and above), gender, race and ethnicity, insurance status (public, private or uninsured), diagnosed comorbidities (diabetes, high blood pressure, myocardial infarction, other heart conditions, and 18 three-digit ICD-9 categories—see Appendix), reason for hospitalization (surgical, medical or give birth), length of stay at the first hospitalization, and residential area. The medical category under reason for hospitalization includes those patients who were admitted into the hospital that received treatment or therapy, or that underwent diagnostic tests only. Finally, we also controlled for the number of days between the SF-12 completion date and the first hospitalization to account for temporal measurement differences in functional status.
Statistical Analysis
First, we compared the demographic characteristics (age, sex and race/ethnicity), presence of several comorbid conditions, insurance coverage, PCS, MCS, reason for hospitalization, length of hospital stay, region, and residential area between patients who were readmitted within 30 days and those not readmitted. We used the Rao-Scott chi-square test for all categorical variables, and weighted and design effect adjusted t tests for continuous variables. Potential multicollinearity was assessed using the Variance Inflation Factor (VIF).
Second, we estimated a multivariable logistic model to assess variables influencing the probability of being readmitted within 30 days, controlling for differences in patient demographics, comorbid conditions, PCS and MCS, length of stay, region, residential area, and days between the SF-12 completion date and the first hospital admission date. We analyzed selected interactions between the SF-12 and demographics, reason for hospitalization and comorbid conditions to evaluate potential improvements to the model.
Data were analyzed using SAS software (v.9.3, SAS Institute Inc., Cary, NC, USA). All analyses were conducted using PROC SURVEY procedures to account for sampling and post-stratification weights, yielding nationally representative estimates for the US. We included sampling strata information (STRATA statement), hospital information (CLUSTER statement), and individual weight information (WEIGHT statement). Odds ratios (OR), 95 % confidence interval (CI), and p values are reported based on these models. For all statistical analyses, the threshold of statistical significance was a p value below 0.05.
Model calibration was determined by the Hosmer-Lemeshow Test and inspection of observed versus predicted ratios by decile of predicted 30-day readmission risk. Model discrimination was assessed by the c statistic. The accuracy of the SF-12 parameter estimate was tested using Bonferroni and bootstrap techniques.38,39 We assessed whether the statistical significance (P value) of the study variables remained stable across 1,000 bootstrap subsamples. The Net Reclassification Improvement (NRI) was computed to evaluate whether the SF-12 improves the predictive ability of our hospital readmissions model.
Finally, missing values accounted for less than 5 % of the observations for all study variables, and were thus ignored.
The Boston University Institutional Review Board approved this study (Institutional Review Board H-33088).
RESULTS
The study sample included 3,772 individuals, and 8 % of them were readmitted within 30 days (Table 1). The mean age of hospitalized patients was 53 years, 64 % were females, 75 % Non-Hispanic White, 66 % had private insurance, 38 % were from the South region, and 21 % from rural areas. Those readmitted within 30 days were older than those who were not readmitted (P < 0.001). Of the hospitalized patients, 47 % had diagnosed high blood pressure, 17 % had diabetes, 11 % had myocardial infarction (MI) and 20 % had other heart conditions. These comorbid conditions were more prevalent among readmitted patients. For example, the prevalence of MI among readmitted patients was 24 % compared to 9 % among those who were not readmitted (p < 0.001). Mean PCS and MCS scores were 42.3 and 49.8, respectively. Yet PCS among readmitted patients was 37.5. This mean score is more than half of one SD lower, which indicates worse functional status, than those who were not readmitted (42.8) (p < 0.001). MCS scores were not significantly different between readmitted and non-readmitted patients (p = 0.329). These scores are lower (suggesting worse health status) than the norm of 50. On average, the SF-12 was completed 72 days before the first hospitalization. Approximately 40 % of total hospitalizations were surgical and 40 % were medical. However, 58 % of hospital readmissions were surgical, compared to 40 % medical (p < 0.001). Finally, mean length of stay was 3.8 days. Yet those who were readmitted had a longer length of stay at the first hospitalization compared to those who were not (4.8 vs. 3.7, p = 0.027).
Table 1.
Demographics, Comorbid Conditions, Functional Status and Characteristics of the Hospitalization for Patients Who Were and Were Not Readmitted Within 30 Daysa
| Hospitalized patients | Readmitted within 30 | Not readmitted | p value | |
|---|---|---|---|---|
| Total unweighted (n = 3772) | Unweighted (n = 318) | Unweighted (n = 3454) | ||
| Total weighted (n = 38,097,637) | Weighted (n = 3,281,319) | Weighted (n = 34,816,318) | ||
| Ageb | 52.7 (0.40) | 60.9 (0.8) | 51.9 (0.42) | <0.001 |
| Age groups | ||||
| 18–45 years | 39.9 (0.97) | 21.1 (1.77) | 40.9 (1.03) | <0.001 |
| 46–64 years | 28.3 (0.91) | 26.9 (1.57) | 28.4 (0.94) | <0.001 |
| 65 years and above | 32.5 (0.98) | 51.9 (2.53) | 30.7 (0.98) | <0.001 |
| Female | 64.5 (0.96) | 56.4 (2.48) | 65.3 (0.99) | 0.006 |
| Race/ethnicity | ||||
| Non-Hispanic white | 75.1 (0.86) | 81.1 (1.44) | 74.6 (0.89) | 0.516 |
| Hispanic | 9.7 (0.62) | 5.6 (0.65) | 10.1 (0.66) | 0.516 |
| Non-Hispanic black | 11.2 (0.55) | 9.9 (0.90) | 11.4 (0.55) | 0.516 |
| Non-Hispanic other | 3.8 (0.38) | 3.2 (0.72) | 3.9 (0.38) | 0.516 |
| Insurance | ||||
| Private | 66.1 (0.94) | 59.9 (2.01) | 66.7 (0.97) | 0.059 |
| Public only | 27.9 (0.91) | 34.2 (2.05) | 27.4 (0.93) | 0.059 |
| Uninsured | 5.9 (0.46) | 5.8 (0.92) | 5.9 (0.48) | 0.059 |
| Comorbid conditions | ||||
| High blood pressure | 46.7 (0.95) | 62.2 (2.33) | 45.2 (0.97) | <0.001 |
| Diabetes | 16.8 (0.71) | 22.3 (1.35) | 16.3 (0.75) | 0.008 |
| MI | 10.6 (0.55) | 24.2 (2.38) | 9.4 (0.53) | <0.001 |
| Other heart conditions | 19.7 (0.72) | 31.2 (2.53) | 18.6 (0.73) | <0.001 |
| SF-12b | ||||
| Physical | 42.3 (0.25) | 37.5 (0.57) | 42.8 (0.25) | <0.001 |
| Mental | 49.8 (0.22) | 49.1 (0.6) | 49.8 (0.23) | 0.329 |
| Lag SF-12 completion and hospitalization (days)b | 71.65 (0.84) | 63.7 (2.16) | 72.39 (0.87) | 0.004 |
| Reason entered the hospital | ||||
| Surgical patient | 39.6 (1.02) | 34.6 (2.06) | 40.1 (1.08) | <0.001 |
| Medical patient | 40.3 (1.01) | 58.5 (2.58) | 38.5 (1.02) | <0.001 |
| Give birth | 20.1 (0.83) | 6.84 (1.29) | 21.3 (0.87) | <0.001 |
| Length of stayb | 3.8 (0.10) | 4.78 (0.39) | 3.7 (0.10) | 0.027 |
| Region | ||||
| Northeast | 16.8 (0.87) | 17.3 (1.79) | 16.7 (0.91) | 0.223 |
| Midwest | 24.7 (0.92) | 29.3 (1.36) | 24.3 (0.96) | 0.223 |
| South | 38.3 (1.12) | 33.3 (1.41) | 38.8 (1.17) | 0.223 |
| West | 20.2 (0.91) | 20.1 (1.83) | 20.1 (0.87) | 0.223 |
| Residential area rural | 20.6 (1.21) | 24.1 (1.87) | 20.3 (1.21) | 0.135 |
MI myocardial infarction
a All values were estimated by using sampling stratification, weights and clusters, yielding nationally representative estimates for the US population with appropriate standard error (SE) estimates. Characteristics were compared for patients who were readmitted within 30 days and those who were not by using the Rao-Scott chi-square test for categorical variables, and weighted and design effect adjusted t tests for continuous variables
bValues are percentages, except for mean age, SF-12 physical and mental scales and length of hospital stay. After each value in parenthesis, we present the SE
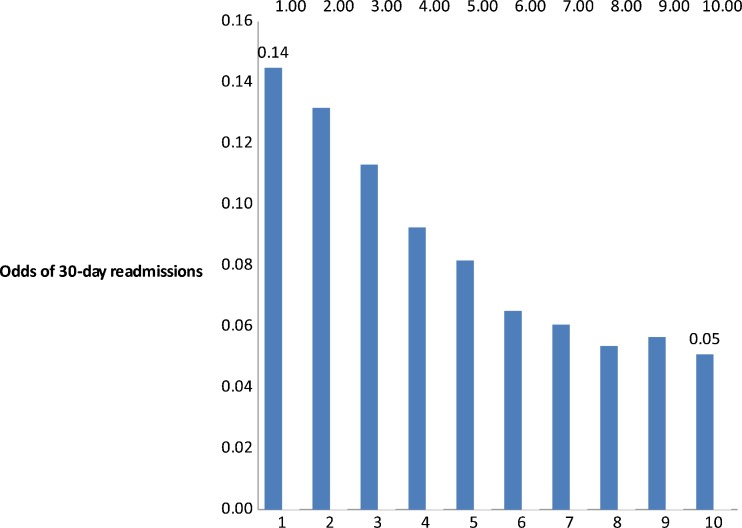
Our adjusted analyses (Table 2) suggested that PCS was a highly significant predictor of hospital readmissions. A one-unit difference in PCS reduced the odds of readmission by 2 % (odds ratio 0.98 [95 % CI, 0.97 to 0.99]; p < 0.001), which implies an 18 % reduction in the odds of readmissions for a ten-unit difference (one SD) in PCS. The association of MCS and readmission, however, was not statistically significant. Figure 1 presents mean predicted odds of 30-day readmission by PCS deciles, adjusting for all the variables in our model. Patients in the lowest PCS decile had twice the odds of 30-day readmission compared to patients in the highest PCS decile.
Table 2.
Adjusted Odds of Being Readmitted Within 30 Daysa
| n = 3710, Weighted n = 37,443,407 | ||
|---|---|---|
| Variable | OR (95 % CI) | p value |
| Age (ref=46–64 years) | ||
| 18–45 years | 1.10 (0.71, 1.71) | 0.665 |
| Above 65 years | 1.67 (1.19, 2.33) | 0.003 |
| Gender: Female | 1.03 (0.76, 1.39) | 0.852 |
| Race/ethnicity (ref=Non-Hispanic white) | ||
| Hispanic | 0.75 (0.48, 1.16) | 0.201 |
| Non-Hispanic black | 0.94 (0.64, 1.39) | 0.768 |
| Non-Hispanic other | 0.93 (0.45, 1.96) | 0.858 |
| Insurance (ref=uninsured) | ||
| Private | 0.87 (0.49, 1.54) | 0.637 |
| Public | 0.89 (0.47, 1.69) | 0.814 |
| High blood pressure (ref=no) | 1.03 (0.75, 1.42) | 0.851 |
| Diabetes (ref=no) | 0.89 (0.65, 1.24) | 0.501 |
| MI (ref=no) | 1.90 (1.26, 2.88) | 0.002 |
| Other heart conditions (ref=no) | 1.11 (0.76, 1.59) | 0.612 |
| ICD-9 categories (ref=Circulatory System: 390–459) b | ||
| Nervous system and sense organs (320–389) | 0.14 (0.03, 0.69) | 0.015 |
| Respiratory system (460–579) | 0.37 (0.18, 0.79) | 0.011 |
| Digestive system (520–579) | 0.47 (0.24, 0.94) | 0.033 |
| Reason for hospitalization (ref=surgical) | ||
| Give birth | 0.51 (0.21, 1.22) | 0.131 |
| Medical | 1.69 (1.16, 2.45) | 0.006 |
| SF-12 Physical | 0.98 (0.97, 0.99) | <0.001 |
| SF-12 Mental | 0.99 (0.98, 1.01) | 0.551 |
| Lag SF-12 completion and hospitalization (days) | 0.99 (0.99, 0.998) | <0.001 |
| Length of stay (days) | 1.01 (0.99, 1.04) | 0.178 |
| Region (ref=South) | ||
| Northeast | 1.15 (0.76, 1.74) | 0.498 |
| Midwest | 1.45 (1.02, 2.05) | 0.038 |
| West | 1.27 (0.91, 1.77) | 0.163 |
| Residential Area (ref=urban) | 1.12 (0.82, 1.53) | 0.464 |
OR odds ratio, MI myocardial infarction
a ORs and 95 % CIs from multivariate logistic regression using sampling stratification, weights and clusters, yielding to nationally representative estimates for the US population with appropriate SE estimates
bThe table presents the ICD-9 categories that were statistically significant. Complete model results are available from the authors upon request
Figure 1.
Adjusted odds of 30-day readmission by physical functioning (PCS) deciles. Note: Higher physical scores indicate better physical functioning. The mean and the 95 % CI of each decile were: 0.14 (0.137, 0.152), 0.13 (0.124,0.139), 0.11 (0.106, 0.121), 0.09 (0.081, 0.099), 0.08 (0.075, 0.088), 0.065 (0.060, 0.071), 0.061 (0.056, 0.065), 0.05 (0.049, 0.057), 0.056 (0.052, 0.0612), 0.051 (0.047, 0.055).
We also found that the odds of readmission were significantly associated with age, diagnosed MI, four ICD-9 groups, reason of hospitalization, the number of days between the SF-12 completion date and the first hospitalization, and residential area. Patients above 65 years of age were 67 % more likely to be readmitted within 30 days compared to those between 46 and 64 years (odds ratio 1.67 [95 % CI, 1.19 to 2.33]; p = 0.03). Being diagnosed with MI was associated with a 90 % increase in the odds of readmission (odds ratio 1.90 [95 % CI, 1.23 to 2.88]; p = 0.002). On the other hand, suffering a disease of the nervous system and sense organs, respiratory system or digestive system was associated with lower odds of readmission compared to diseases of the circulatory system. Medical patients were 69 % more likely to be readmitted compared to surgical patients (odds ratio 1.69 [95 % CI, 1.16 to 2.45]; P = 0.006). The number of days between the SF-12 completion and the first hospitalization also influenced readmissions (p < 0.001). Finally, patients in the Midwest had higher odds of 30-day readmission compared to those located in the South (odds ratio 1.45 [95 % CI, 1.02 to 2.05].
The c statistic for the logistic model was 0.72. The minimum c statistic value is 0.5; the maximum is 1.0. Values between 0.7 and 0.8 show acceptable discrimination, values 0.8 to 0.9 indicate excellent discrimination and models above 0.9 demonstrate outstanding discrimination.40 Our model is able to discriminate in the acceptable range between those who were readmitted within 30 days and those who were not. The p value of the Hosmer-Lemeshow Test equaled 0.53. Therefore, we failed to reject the null hypothesis of no difference between observed and predicted values of the response variable; thus, the model showed good calibration. We also computed the observed and expected likelihood of readmission within 30 days by decile (Appendix Table 3), and most observed over expected ratios were close to 1. The p values of Bonferroni and bootstrap inference assessing the relationship between PCS and the odds of readmissions within 30 days remained stable (p < 0.001) using either statistical method (data not shown). We also found similar values for the rest of the statistically significant variables (age, MI, ICD-9 groups and reason for hospitalization), which strengthens our results. The corresponding NRI was 0.44 (p < 0.001), indicating improved ability to predict 30-day hospital readmissions once the SF-12 was added to the model.
Table 3.
Observed and Expected Likelihood of Readmission by Decile
| Group | Total | Y30 = Readmitted within 30 | Y30 = Not readmitted | ||||
|---|---|---|---|---|---|---|---|
| Observed | Expected | O/E ratiosa | Observed | Expected | O/E ratios | ||
| 1 | 371 | 3 | 5.79 | 0.52 | 368 | 365.21 | 1.01 |
| 2 | 371 | 12 | 9.37 | 1.28 | 359 | 361.63 | 0.99 |
| 3 | 371 | 14 | 12.75 | 1.10 | 357 | 358.25 | 1.00 |
| 4 | 371 | 15 | 16.95 | 0.88 | 356 | 354.05 | 1.01 |
| 5 | 371 | 16 | 22.04 | 0.73 | 355 | 348.96 | 1.02 |
| 6 | 371 | 31 | 27.91 | 1.11 | 340 | 343.09 | 0.99 |
| 7 | 371 | 35 | 34.79 | 1.01 | 336 | 336.21 | 1.00 |
| 8 | 371 | 50 | 43.11 | 1.16 | 321 | 327.89 | 0.98 |
| 9 | 371 | 60 | 55.81 | 1.08 | 311 | 315.19 | 0.99 |
| 10 | 371 | 79 | 86.49 | 0.91 | 292 | 284.51 | 1.03 |
a Observed / Expected
The direction and significance of our reported findings were consistent across all our sensitivity analyses. We found that the relationship between PCS and the odds of being readmitted remained unchanged even after we adjusted for a potential confounder, whether the first hospitalization started with an emergency room visit, which is more prevalent among the patients who were readmitted (p < 0.001) (Appendix Table 4). Finally, we estimated our adjusted model adding hospital readmissions after 30 days to the study sample. The PCS coefficient in this analysis was comparable to the main model for readmission within 30 days (Appendix Table 5).
Table 4.
Multivariate Logistic Model Predicting Hospital Readmissions (Yes/No) Including Whether the First Hospitalization Started with an Emergency Room Visit (Weighted)a
| n = 3710, Weighted n = 37,443,407 | ||
|---|---|---|
| Variable | OR (95 % CI) | p value |
| Age (ref=46–64 years) | ||
| 18–45 years | 1.11 (0.71, 1,71) | 0.664 |
| Above 65 years | 1.67 (1.19, 2.34) | <0.001 |
| Gender: Female | 1.03 (0.76, 1.38) | 0.857 |
| Race/ethnicity (ref=Non-Hispanic white) | ||
| Hispanic | 0.75 (0.48, 1.16) | 0.199 |
| Non-Hispanic black | 0.94 (0.64, 1.39) | 0.765 |
| Non-Hispanic other | 0.94 (0.45, 1.54) | 0.863 |
| Insurance (ref=uninsured) | ||
| Private | 0.87 (0.49, 1.54) | 0.644 |
| Public | 0.89 (0.47, 1.69) | 0.813 |
| High blood pressure (ref=no) | 1.03 (0.75, 1.42) | 0.851 |
| Diabetes (ref=no) | 0.89 (0.64, 1.24) | 0.498 |
| MI (ref=no) | 1.91 (1.25, 2.89) | 0.003 |
| Other heart conditions (ref=no) | 1.11 (0.76, 1.61) | 0.612 |
| ICD-9 categories (ref=Circulatory System: 390–459)b | ||
| Nervous system and sense organs (320–389) | 0.14 (0.03, 0.69) | 0.016 |
| Respiratory system (460–519) | 0.37 (0.18, 0.79) | 0.009 |
| Digestive system (520–579) | 0.47 (0.24, 1.09) | 0.033 |
| Reason hospitalization (ref=surgical) | ||
| Give birth | 0.51 (0.21, 1.22) | 0.129 |
| Medical | 1.67 (1.11, 2.52) | 0.014 |
| SF-12 Physical | 0.98 (0.97, 0.99) | <0.001 |
| SF-12 Mental | 0.99 (0.98, 1.01) | 0.551 |
| Lag SF-12 completion and hospitalization (days) | 0.99 (0.991, 0.998) | <0.001 |
| Emergency room visit | 0.98 (0.68, 1.41) | 0.914 |
| Length of stay (days) | 1.01 (0.99, 1.04) | 0.178 |
| Region (ref=West) | ||
| Northeast | 0.91 (0.59, 1.38) | 0.747 |
| Midwest | 1.14 (0.81, 1.61) | 0.118 |
| South | 0.79 (0.56, 1.11) | 0.091 |
| Residential area (ref=urban) | 1.12 (0.82, 1.53) | 0.462 |
OR Odds ratio, MI myocardial infarction
a ORs and 95 % CIs from multivariate logistic regression using sampling stratification, weights and clusters, yielding to nationally representative estimates for the US population with appropriate SE estimates
b The table presents the ICD-9 categories that were statistically significant. Complete model results are available from the authors upon request
Table 5.
Multivariate Logistic Model Predicting Hospital Readmissions (Yes/No) (Weighted)a
| n = 3812, Weighted n = 38,467,643 | ||
|---|---|---|
| Variable | OR (95 % CI) | p value |
| Age (ref=46–64 years) | ||
| 18–45 years | 1.11 (0.74,1.65) | 0.614 |
| Above 65 years | 1.71 (1.26, 2.31) | <0.001 |
| Gender: Female | 0.96 (0.74, 1.25) | 0.77 |
| Race/ethnicity (ref=Non-Hispanic white) | ||
| Hispanic | 0.73 (0.49, 1.07) | 0.104 |
| Non-Hispanic black | 0.85 (0.59, 1.21) | 0.362 |
| Non-Hispanic other | 0.82 (0.41, 1.61) | 0.557 |
| Insurance (ref=uninsured) | ||
| Private | 0.95 (0.56, 1.62) | 0.672 |
| Public | 1.03 (0.58, 1.85) | 0.754 |
| High blood pressure (ref=no) | 1.01 (0.75, 1.34) | 0.953 |
| Diabetes (ref=no) | 1.11 (0.83, 1.47) | 0.475 |
| MI (ref=no) | 1.84 (1.28, 2.63) | <0.001 |
| Other heart conditions (ref=no) | 1.15 (0.83, 1.59) | 0.412 |
| ICD-9 categories (ref=Circulatory System: 390–459) b | ||
| Nervous system and sense organs (320–389) | 0.23 (0.06, 0.84) | 0.026 |
| Respiratory system (460–519) | 0.43 (0.23, 0.82) | 0.001 |
| Digestive system (520–579) | 0.55 (0.32, 0.95) | 0.034 |
| Genitourinary system (580–629) | 0.51 (0.27, 0.95) | 0.033 |
| Injury and Poisoning | 0.54 (0.31, 0.96) | 0.037 |
| Reason hospitalization (ref=surgical) | ||
| Give birth | 0.42 (0.17, 1.02) | 0.056 |
| Medical | 1.47 (1.07, 2.02) | 0.018 |
| SF-12 Physical | 0.97 (0.96, 0.98) | <0.001 |
| SF-12 Mental | 0.99 (0.98, 1.01) | 0.018 |
| Lag SF-12 completion and hospitali-zation (days) | 0.99 (0.98, 0.99) | <0.001 |
| Length of stay (days) | 1.01 (0.99, 1.03) | 0.333 |
| Region (ref=West) | ||
| Northeast | 0.96 (0.64, 1.44) | 0.893 |
| Midwest | 1.12 (0.81, 1.57) | 0.232 |
| South | 0.85 (0.62, 1.17) | 0.182 |
| Residential area (ref=urban) | 1.11 (0.82, 1.49) | 0.502 |
OR Odds ratio, MI myocardial infarction
a ORs and 95 % CIs from multivariate logistic regression using sampling stratification, weights and clusters, yielding to nationally representative estimates for the US population with appropriate SE estimates
b The table presents the ICD-9 categories that were statistically significant. Complete model results are available from the authors upon request
DISCUSSION
Using a US representative sample, we found that physical functional status (PCS) had a large and statistically significant impact on hospital readmissions within 30 days after adjusting for demographic characteristics, the most prevalent comorbid conditions, insurance coverage, mental functioning (MCS), reason of hospitalization, length of hospital stay, region, and residential area. According to our results, the physiological reserves of patients at baseline (before the first hospitalization) are associated with the probability of readmission. This is the first study to assess the association between functional status and hospital readmissions for a US representative population. The findings of this study have potentially important implications in improving the performance of existing 30-day readmission models.
Our results build on existing research, which reports a negative relationship between functional status and hospital readmissions. Examining inpatient rehabilitation data, Hoyer and colleagues found that the odds of readmission were three times higher for patients with the lowest functional scores compared to those with the highest scores, after adjusting for comorbidities, age, and severity of illness. However, the authors concluded that their results, based on data from a single inpatient rehabilitation hospital, needed to be replicated in a nationally representative sample to better understand their findings, scope and generalizability.22 Our study addresses this gap and corroborates their findings. The magnitude of our estimated effect is, however, lower than theirs (two times vs. three times difference in the odds of readmissions among patients with low and high scores).
Also, we found that MCS was not a significant predictor of hospital readmissions. Similarly, Hoyer and colleagues reported that Functional Independence Measures (FIM) (a standard measurement of function in inpatient rehabilitation facilities) motor domains were more highly related to unplanned readmissions than the cognitive domains.22 Pearson and colleagues found similar results among chronically ill patients using the MOS 36-Item Short-Form Health Survey (SF-36).33 However, other studies report that mental functioning also helps explain readmissions.19,41 Further research is needed to understand the interplay between physical and mental components and hospital readmissions. According to our results, medical patients were more likely to be readmitted than surgical patients across the PCS range, which has also been reported before.2,22 Future research should identify patient and system characteristics throughout the care continuum responsible for these observed differences.
Patient-reported measures of physical functioning are an important predictor of readmissions. PCS is a summary score with the greatest weights attributed to the physical function and role limitations due to physical problems. Energy and fatigue are also part of the instrument. The mere presence of comorbid conditions does not fully describe the impact of a disease on health status. A measure of functional status captures variability in impairment across individuals diagnosed with the same condition. Thus, it may be more sensitive to observed improvement in health status. Patients with multiple chronic conditions account for most hospital readmissions.42 The SF-12 could contribute to the design of effective interventions aimed at reducing hospital readmissions among complex and vulnerable patients.
Our study has several limitations unique to the data set used. First, ICD-9 codes included in our analysis were patient-reported. Specifically, they captured the first condition mentioned by the respondent as the reason for hospitalization, which may or may not correspond to the primary diagnosis on the medical record. Second, the SF-12 was completed in advance of hospital admission (median of 68 days). Thus, it acts as a measure of baseline patient functional status. However, there was notable timing variability among study subjects (standard deviation of 45 days). Our analyses suggest that this time lag provides information complementary to the SF-12 scores, and it was controlled for in the analyses. Further research should explore the optimal timing for measuring functional status and its influence on scoring. Third, we lacked clinical information characterizing patient illness severity, such as laboratory data and vital signs. Thus, our results may be confounded by the presence of a higher severity among readmitted patients. Neither provider nor system characteristics were available in the MEPS database, and they were not accounted for in our study. That said, there is no obvious reason why such variables could fully explain our findings. Given the variety of clinical problems, disease severity measurements are not routinely collected as part of administrative data sets. However, generic functional measurements may act as a surrogate marker of disease severity. Fourth, our model is not condition-specific, given data availability. Nevertheless, a generic functional measure is predictive of readmission across varied medical conditions, and therefore represents an elegant additional metric that can enhance existing readmission models. Future research should explore the impact of adding functional status as a covariate to models used by public and private insurers and how it may impact hospital profiling. Yet, given these limitations, our PCS results are highly significant (p < 0.001), large in magnitude, and they remained stable with sensitivity analyses as previously described.
Given the relative ease of administration and widespread use of the SF-12, its completion before hospitalization poses a small burden on patients that is more than compensated by the information to be gained. Programs to reduce readmissions may be more sustainable if they are focused on patients at the highest risk on the day of admission.16,43 As there are proprietary issues related to the SF-12 Version 2, we would recommend publically available short forms such as the EQ-5D,44,45 VR-12 or SF-12 version 1.0.46,47 These tools could be used to potentially identify patients at high risk of readmission, and, accordingly, address physical function as part of routine medical care and during the acute hospitalization, and tailor adequate follow-up care after discharge.
In summary, we found that lower (worse) PCS scores at baseline increase the odds of readmissions for a nationally representative sample. These results suggest that baseline physical function influences hospital readmissions, and thus, should be accounted for when predicting the risk of hospital readmissions.
Acknowledgements
Contributors
We would like to thank Bora Youn for her excellent research assistant work and Jim Rothendler for his insightful comments on the manuscript.
Funders
This study had no formal funding source.
Conflict of Interests
The authors declare that they have no conflicts of interest.
APPENDIX
ICD-9 Codes Categories (Based on MEPS)
- 1=001–139
Infectious and parasitic diseases
- 2=140–239
Neoplasms
- 3=240–279
Endocrine, nutritional and metabolic diseases, and immunity disorders
- 4=280–289
Diseases of the blood and blood-forming organs
- 5=290–319
Mental disorders
- 6=320–389
Diseases of the Nervous System and Sense Organs
- 7=390–459
Diseases of the circulatory system
- 8=460–519
Diseases of the respiratory system
- 9=520–579
Diseases of the digestive system
- 10=580–629
Diseases of the genitourinary system
- 11=630–677
Complications of Pregnancy, Childbirth, and the Puerperium
- 12=680–709
Diseases of the skin and subcutaneous tissue
- 13=710–739
Diseases of the musculoskeletal system and connective tissue
- 14=740–759
Congenital anomalies
- 15=760–779
Certain conditions originating in the perinatal period
- 16=780–799
symptoms, signs, and ill-defined conditions
- 17=800–999
injury and poisoning
- 18=v01–v83
Supplementary classification of factors influencing health status and contact with health services.
REFERENCES
- 1.Elixhauser A, Steiner C. Readmissions to U.S. hospitals by diagnosis, 2010: statistical brief #153. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Health Care Policy and Research (US); 2006 [cited 2014 May 23]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK154385/
- 2.Robert Wood Johnson Foundation. The Revolving Door: A Report on U.S. Hospital Readmissions [Internet]. RWJF. [cited 2014 May 23]. Available from: http://www.rwjf.org/en/research-publications/find-rwjf-research/2013/02/the-revolving-door--a-report-on-u-s--hospital-readmissions.html
- 3.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 4.Hines AL, Barrett ML, Jiang HJ, Steiner CA. Conditions with the largest number of adult hospital readmissions by Payer, 2011 [Internet]. Rockville, MD: Agency for Healthcare Research and Quality; 2014 Apr. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb172-Conditions-Readmissions-Payer.pdf [PubMed]
- 5.The Affordable Care Act is the combination of the Patient Protection and Affordable Care Act (PPACA), P.L. 111–148. Sect. 3025.
- 6.Hannan EL, Racz MJ, Walford G, Ryan TJ, Isom OW, Bennett E, et al. Predictors of readmission for complications of coronary artery bypass graft surgery. JAMA. 2003;290(6):773–80. doi: 10.1001/jama.290.6.773. [DOI] [PubMed] [Google Scholar]
- 7.Allaudeen N, Vidyarthi A, Maselli J, Auerbach A. Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54–60. doi: 10.1002/jhm.805. [DOI] [PubMed] [Google Scholar]
- 8.Aranda JM, Johnson JW, Conti JB. Current trends in heart failure readmission rates: analysis of medicare data. Clin Cardiol. 2009;32(1):47–52. doi: 10.1002/clc.20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donze J, Lipsitz S, Bates DW, Schnipper JL. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ. 2013;347(dec16 4):f7171. doi: 10.1136/bmj.f7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang HG, Wier LM. All cause hospital readmission among non-elderly Medicaid patients in 2007 [Internet]. Rockville, MD: HCUP, Agency for Healthcare Research and Quality; 2010 Apr. Report No.: 89. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb89.jsp [PubMed]
- 11.Medicare Hospital Readmissions Reduction Program [Internet]. Health Affairs - Health Policy Briefs. [cited 2014 May 23]. Available from: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=102
- 12.American Hospital Association. Examining the drivers of readmissions and reducing unnecessary readmissions for better patient care [Internet]. 2011 Sep. Available from: http://www.aha.org/research/policy/2011.shtml
- 13.Joynt KE, Jha AK. A path forward on medicare readmissions. N Engl J Med. 2013;368(13):1175–7. doi: 10.1056/NEJMp1300122. [DOI] [PubMed] [Google Scholar]
- 14.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–98. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osman IM, Godden DJ, Friend JA, Legge JS, Douglas JG. Quality of life and hospital re-admission in patients with chronic obstructive pulmonary disease. Thorax. 1997;52(1):67–71. doi: 10.1136/thx.52.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, Boult C. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504. doi: 10.1093/geront/48.4.495. [DOI] [PubMed] [Google Scholar]
- 17.Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–107. doi: 10.1001/jamainternmed.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, Gonsahn MD, Nerenz DR. Socioeconomic status and readmissions: evidence from an urban teaching hospital. Health Aff. 2014;33(5):778–85. doi: 10.1377/hlthaff.2013.0816. [DOI] [PubMed] [Google Scholar]
- 19.Smith DM, Giobbie-Hurder A, Weinberger M, Oddone EZ, Henderson WG, Asch DA, et al. Predicting non-elective hospital readmissions: a multi-site study. Department of Veterans Affairs Cooperative Study Group on Primary Care and Readmissions. J Clin Epidemiol. 2000;53(11):1113–8. doi: 10.1016/S0895-4356(00)00236-5. [DOI] [PubMed] [Google Scholar]
- 20.Ottenbacher KJ, Karmarkar A, Graham JE, et al. Thirty-day hospital readmission following discharge from postacute rehabilitation in fee-for-service medicare patients. JAMA. 2014;311(6):604–14. doi: 10.1001/jama.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9(5):277–82. doi: 10.1002/jhm.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyer EH, Needham DM, Miller J, Deutschendorf A, Friedman M, Brotman DJ. Functional status impairment is associated with unplanned readmissions. Arch Phys Med Rehabil. 2013;94(10):1951–8. doi: 10.1016/j.apmr.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raymond T, Arrigain S, Landers S, Gorodeski E. Patient-reported health related quality of life (Eq-5d) predicts readmission risk. J Am Coll Cardiol. 2012;59(13s1):E1890. doi: 10.1016/S0735-1097(12)61891-9. [DOI] [Google Scholar]
- 24.Hansen TB, Thygesen LC, Zwisler AD, Helmark L, Hoogwegt M, Versteeg H, et al. Self-reported health-related quality of life predicts 5-year mortality and hospital readmissions in patients with ischaemic heart disease. Eur J Prev Cardiol. 2014. [DOI] [PubMed]
- 25.Rodríguez-Artalejo F, Guallar-Castillón P, Pascual CR, Otero CM, Montes AO, García AN, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165(11):1274–9. doi: 10.1001/archinte.165.11.1274. [DOI] [PubMed] [Google Scholar]
- 26.Mejhert M, Kahan T, Persson H, Edner M. Predicting readmissions and cardiovascular events in heart failure patients. Int J Cardiol. 2006;109(1):108–13. doi: 10.1016/j.ijcard.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Mommersteeg PMC, Denollet J, Spertus JA, Pedersen SS. Health status as a risk factor in cardiovascular disease: a systematic review of current evidence. Am Heart J. 2009;157(2):208–18. doi: 10.1016/j.ahj.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Gudmundsson G, Gislason T, Janson C, Lindberg E, Hallin R, Ulrik CS, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J. 2005;26(3):414–9. doi: 10.1183/09031936.05.00078504. [DOI] [PubMed] [Google Scholar]
- 29.Almagro P, Barreiro B, Ochoa de Echaguen A, Quintana S, Rodriguez Carballeira M, Heredia JL, et al. Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease. Respiration. 2006;73(3):311–7. doi: 10.1159/000088092. [DOI] [PubMed] [Google Scholar]
- 30.Carneiro R, Sousa C, Pinto A, Almeida F, Oliveira JR, Rocha N. Risk factors for readmission after hospital discharge in chronic obstructive pulmonary disease. The role of quality of life indicators. Rev Port Pneumol. 2010;16(5):759–77. doi: 10.1016/S0873-2159(15)30070-2. [DOI] [PubMed] [Google Scholar]
- 31.Schneider JC, Gerrard P, Goldstein R, Divita MA, Niewczyk P, Ryan CM, et al. Predictors of transfer from rehabilitation to acute care in burn injuries. J Trauma Acute Care Surg. 2012;73(6):1596–601. doi: 10.1097/TA.0b013e318270d73d. [DOI] [PubMed] [Google Scholar]
- 32.Holloway JJ, Thomas JW. Factors influencing readmission risk: implications for quality monitoring. Health Care Finance Rev. 1989;11(2):19–32. [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson S, Stewart S, Rubenach S. Is health-related quality of life among older, chronically ill patients associated with unplanned readmission to hospital? Aust NZ J Med. 1999;29(5):701–6. doi: 10.1111/j.1445-5994.1999.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson A, Rasekaba TM, Graco M, Berlowitz DJ, Hawthorne G, Lim WK. Relationship between health-related quality of life, and acute care re-admissions and survival in older adults with chronic illness. Health Qual Life Outcomes. 2013;11(1):136. doi: 10.1186/1477-7525-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to SCORE VERSION 2 of the SF-12v2® Health Survey (With a Supplement Documenting SF-12® Health Survey) Lincoln: QualityMetric Incorporated; 2002. [Google Scholar]
- 36.Kazis LE. The veterans SF-36 health status questionnaire: development and application in the veterans health administration. Medical Outcomes Trust Monitor. 2000;5(1).
- 37.Kazis LE, Miller DR, Clark JA, Skinner KM, Lee A, Ren XS, Spiro IIIA, Rogers WH, Ware JE., Jr Improving the response choices on the veterans SF-36 health survey role functioning scales: results from the veterans health study. J Ambul Care Manag. 2004;27(3):263–80. doi: 10.1097/00004479-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 39.Hallahan C. Extending the SAS system statistical capabilities [Internet]. Available from: http://www.sascommunity.org/sugi/SUGI92/Sugi-92-229%20Hallahan.pdf
- 40.Jr DWH, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3. Hoboken: Wiley; 2013. p. 528. [Google Scholar]
- 41.Mistry R, Rosansky J, McGuire J, McDermott C, Jarvik L, UPBEAT Collaborative Group Social isolation predicts re-hospitalization in a group of older American veterans enrolled in the UPBEAT Program. Unified Psychogeriatric Biopsychosocial Evaluation and Treatment. Int J Geriatr Psychiatr. 2001;16(10):950–9. doi: 10.1002/gps.447. [DOI] [PubMed] [Google Scholar]
- 42.Sommers A, Cunningham P. Physician visits after hospital discharge: implications for reducing readmissions. National institute for health care reform. 2011;(6).
- 43.Amarasingham R, Moore BJ, Tabak YP, Drazner MH, Clark CA, Zhang S, et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Med Care. 2010;48(11):981–8. doi: 10.1097/MLR.0b013e3181ef60d9. [DOI] [PubMed] [Google Scholar]
- 44.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 45.EuroQol Group EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 46.Kazis LE, Selim AJ, Rogers W, Qian SX, Brazier J. Monitoring outcomes for the medicare advantage program: methods and application of the VR-12 for evaluation of plans. J Ambul Care Manag. 2012;35(4):263–76. doi: 10.1097/JAC.0b013e318267468f. [DOI] [PubMed] [Google Scholar]
- 47.Kazis LE, Nethercot VA, Ren XS, Lee A, Selim A, Miller DR. Medication effectiveness studies in the United States Veterans Administration health care system: a model for large integrated delivery systems. Drug Dev Res. 2006;67(3):217–26. doi: 10.1002/ddr.20080. [DOI] [Google Scholar]