Abstract
Introduction
Alcohols, including ethanol and isopropyl alcohol, are used in clinical practice for disinfection and infection prevention. Recent studies, however, demonstrate that alcohols may enhance biofilm production in Staphylococci.
Methods
We quantified biofilm formation in the presence of ethanol and isopropyl alcohol in six different, well-characterized strains of Staphylococcus epidermidis and Staphylococcus aureus. After 24 h of biofilm development, each strain was exposed to normal saline (NS), ethanol, or isopropyl alcohol (40%, 60%, 80% and 95%) for additional 24 h incubation. Adherent biofilms were stained and optical density was determined. Viability of strains was also determined after alcohol exposure.
Results
Ethanol increased biofilm formation in all six strains compared to normal saline (p < 0.05). There was increased biofilm formation with increasing ethanol concentration. Isopropyl alcohol also increased biofilm formation with increasing alcohol concentration in all six strains (p < 0.01 vs NS). The slime-negative, chemical mutant strain of S. epidermidis increased biofilm formation after exposure to both alcohols, likely reverting back its primary phenotype through modulation of the intercellular adhesin repressor. All strains demonstrated viability after exposure to each alcohol concentration, though viability was decreased.
Conclusion
Ethanol and isopropyl alcohol exposure increases biofilm formation of S. aureus and S. epidermidis at concentrations used in clinical settings. Ethanol and isopropyl alcohol did not eradicate viable Staphylococci from formed biofilm.
Electronic supplementary material
The online version of this article (doi:10.1007/s40121-015-0065-y) contains supplementary material, which is available to authorized users.
Keywords: Alcohol, Biofilm, Ethyl alcohol, Isopropanol, Staphylococcus aureus, Staphylococcus epidermidis
Introduction
Staphylococci, including Staphylococcus epidermidis and Staphylococcus aureus, are common biofilm-forming pathogens [1]. They frequently cause implant and catheter-associated infections, and are a significant cause of morbidity and mortality [1]. Previous studies have demonstrated increased biofilm production of S. epidermidis and S. aureus after exposure to different alcohols, including ethanol at concentrations above 40% [2, 3]. This is important since isopropyl alcohol is commonly used as a cutaneous disinfectant and ethanol is used in catheter lock solutions for the treatment and prevention of catheter-related bloodstream infections (CRBSI) [1, 4]. Although ethanol-based catheter lock solutions, including combinations with isopropyl alcohol, have been advocated for the prevention and management of CRBSI at concentrations between 25% and 100%, ethanol-based lock solutions may have unintended consequences since CRBSI are frequently caused by biofilm-forming bacteria [5, 6]. Additionally, ethanol use in lock solutions has been demonstrated to have other deleterious effects [5, 6].
We compared the effects of ethanol and isopropyl alcohol on Staphylococcal biofilms using a semi-quantitative microtiter plate assay to better understand the effect of these alcohols on biofilm formation. We also measured the viability of biofilm-embedded bacteria after exposure to ethanol or isopropyl alcohol.
Materials and Methods
Bacterial Strains
Five ATCC Staphylococcal strains were evaluated: a biofilm-producing S. epidermidis strain (ATCC 35984; RP62A [ATCC®, Manassas, Virginia]) and its isogenic, slime-negative, biofilm-deficient mutant derived from chemical mutagenesis (M7), two biofilm-forming methicillin-susceptible S. aureus strains (ATCC 35556 and ATCC 29213) and a biofilm-forming methicillin-resistant S. aureus strain (MRSA; ATCC 43300) [7–10]. ATCC 35984, ATCC 43300, and ATCC 29213 were originally isolated from clinical sources, including a catheter sepsis (ATCC 35984). Additionally, one known biofilm-forming clinical MRSA strain (L32; from blood at the Providence Veterans Affairs Medical Center) was tested [11].
Agents tested
Ethanol (Pharmco-aaper, Brookfield, CT, USA) and isopropyl alcohol (Acros, New Jersey, USA) were evaluated at concentrations of 40%, 60%, 80%, and 95% in sterile water for 24 h exposure. Normal saline (NS) was used for comparison.
Medium
Strains were grown overnight on Tryptic Soy Agar (TSA, Becton–Dickinson, Sparks, MD, USA). Supplemented Tryptic Soy Broth (STSB; Becton–Dickinson, Sparks, MD, USA) with 1% glucose, 2% sodium chloride, 25 mg/L calcium, and 12.5 mg/L magnesium was used to optimize biofilm production in the biofilm assay [12, 13].
Biofilm Formation Assay
Quantification of biofilm formation was conducted using the microtiter plate assay first described by Christensen et al. [14] and modified as described [8, 11–13]. Briefly, a 0.5 McFarland standard of overnight growth of test strains was diluted into STSB. Inocula (~6.5 log10 CFU/mL) were verified by plating. The inoculated medium was dispensed into wells of sterile flat-bottom 96-well polystyrene tissue culture plates (Costar no. 3596; Corning Inc., Corning, NY, USA). Plates were incubated statically at 37 °C. After 24 h of biofilm development, broth was removed and replaced with test solution and incubated at 37 °C for an additional 24 h. The solution was then removed and the plates were carefully rinsed three times with NS to remove planktonic bacteria. Adherent bacteria were dried overnight and stained with 2% crystal violet solution (Becton–Dickinson, Sparks, MD, USA). The crystal violet was then resolubilized in 95% ethanol and the optical density (OD) of stained adherent bacterial films was read at 570 nm using a SpectraMax M2 Spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
Viability
Viability of biofilm-embedded Staphylococci was evaluated using a similar 96 well plate assay [15]. After inoculation, incubation and alcohol or NS exposure as above, media was removed and wells were carefully rinsed three times with NS to remove planktonic bacteria. Wells were then filled with 200 µL of NS and plates were sonicated for 20 min in a water bath sonicator (Fisher Scientific FS20, Pittsburg, PA, USA) to disperse adherent biofilms. Viability was determined in quadruplicate on two occasions by plating aliquots from each strain and alcohol concentration. Plate counts were determined after 24 h incubation. The lower limit of detection for this method is 2.0 log10 CFU/mL.
Statistical Analysis
OD and log CFU/mL were compared between groups using analysis of variance (ANOVA) with Tukey’s post hoc test [16]. Data is presented as the mean OD with standard error of the mean using at least eight replicates for each strain and test solution combination. Statistical analysis was conducted using SPSS (release 20; SPSS, Inc. Chicago, IL). A p value of <0.05 was considered significant. Each alcohol concentration was compared to NS, and mean difference (change) in OD between alcohol and NS was determined, with a corresponding p value. Mean differences in OD are presented as a range for all the strains in the results.
Compliance with Ethics
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
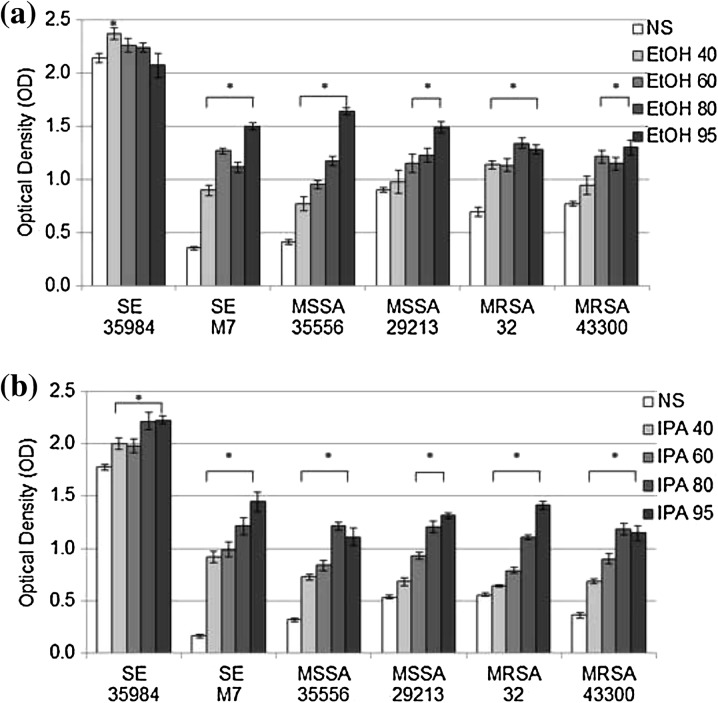
Ethanol exposure increased biofilm in all strains (Fig. 1a). In five strains, the amount of biofilm increased with increasing ethanol concentration. At 60%, 80% or 95% ethanol, more biofilm was produced than after exposure to NS (mean difference in OD vs NS 0.25–1.23, p < 0.02). One strain, the prolific biofilm-forming S. epidermidis ATCC 35984, demonstrated the inverse trend of decreased biofilm production with higher ethanol concentration, which was significantly different between 40% and 95% ethanol (−0.29, 95% CI 0.03–0.55, p < 0.02). However, differences between other concentrations were not statistically significant. Isopropyl alcohol exposure (Fig. 1b) led to increased biofilm in all strains tested, with higher biofilm production for 60%, 80%, and 95% alcohol compared to NS (mean difference in OD vs NS 0.15–1.28, p < 0.01).
Fig. 1.
Biofilm production after ethanol (a) or Isopropyl Alcohol (b) exposure for 24 h. Mean ± SEM optical density (OD) at 570 nm of stained biofilms in 96 well plates after 24 h exposure to 40%, 60%, 80%, and 95% alcohols compared to normal saline 0.9% (NS) (n = 8 each). SE 35984 S. epidermidis ATCC 35984, SE M7 S. epidermidis M7, MSSA 35556 methicillin-susceptible S. aureus ATCC 35556, MSSA 29213 methicillin-susceptible S. aureus ATCC 29213, MRSA 32 methicillin-resistant S. aureus clinical strain L32, MRSA 43300 methicillin-resistant S. aureus ATCC 43300, EtOH ethanol, SEM standard error of the mean. (Asterisk) Statistically significant compared to NS (p < 0.05). SE 35984 p = 0.04; SE M7 p < 0.01; MSSA 35556 p < 0.01; MSSA 29213 p < 0.02; MRSA 32 p < 0.01; MRSA 43300 p < 0.01. IPA isopropyl alcohol. (Asterisk) Statistically significant compared to NS (p < 0.01 for all)
Viable bacteria remained at all concentrations of both ethanol and isopropyl alcohol with a range up to 2.93 log10 CFU/mL after ethanol exposure and 3.01 log10 CFU/mL after isopropyl alcohol exposure. NS exposure yielded 2.35–4.4 log10 CFU/mL, depending on strain. For S. epidermidis ATCC 35984 and M7, the quantity of viable bacteria was reduced by all of the alcohol conditions tested (p < 0.03). Cell counts were not significantly reduced by alcohol exposure for any of the S. aureus strains tested. For all strains, viable cell count tended to decrease with increasing alcohol concentration, but these differences were not statistically significant. Some bacterial counts (CFU/mL) reached the 2.0 log10 CFU/mL lower limit of detection, but viable bacteria were present for each strain-alcohol concentration combination tested.
Discussion
Our results are similar to a previous study demonstrating increased S. aureus biofilm formation after ethanol exposure [2], however, there are conflicting reports on the viability of those biofilm bacteria [17, 18]. We found these bacteria within biofilm were viable, although viability was decreased compared to NS-exposed biofilm. In contrast to previous reports [4, 19], bacteria in biofilm were not eradicated after alcohol exposure. This may be due to different methods used to remove the biofilm from 96 well plates, as prior studies removed biofilm using cotton swabs [4, 19], whereas we sonicated the well plates.
We also found an increase in biofilm formation with increasing alcohol concentration. Only one strain, the prolific biofilm-forming S. epidermidis, decreased biofilm formation with increasing concentrations of ethanol. This strain was likely near maximal biofilm production possible in this assay. Small variations in biofilm formation are possible, as demonstrated by the differences in NS-exposed biofilm between the ethanol and isopropyl alcohol experiments. The differences in biofilm comparing other ethanol concentrations, such as 40% and 80% or 60% and 80% are not statistically significant for this strain.
The bactericidal effect of alcohol depends upon dehydration and denaturation of proteins [20]. Mixtures of alcohols and water (60–90% v/v) are more effective because proteins are denatured more quickly in the presence of water [20, 21]. Ethanol also causes leakage of the plasma membrane, disrupting bacterial growth and metabolism [22]. The impact of dehydration on cell death in the presence of alcohols may not be observed in catheter lock solutions since these do not dry, however denatured proteins and leaking membranes may still lead to decreased viability. The high concentrations of ethanol in catheter lock solutions increase biofilm formation in Staphylococci and also predisposes to catheter dysfunction and plasma protein precipitation [6].
Staphylococcus epidermidis M7, the isogenic slime-negative, biofilm-deficient mutant of S. epidermidis ATCC 35984 demonstrated increased OD with exposure to both alcohols; however, they were not as dense as the prolific biofilms of ATCC 35984. M7 was derived from ATCC 35984 through mitomycin C-induced mutations. M7, sometimes referred to as an accumulation-negative mutant, is distinguished from ATCC 35984 because it lacks a 140 kDa antigen called accumulation-associated protein, but it has been found to have a 200 kDa protein with similar homology [23, 24]. This strain does not accumulate on glass and polystyrene surfaces [23], but it accumulates on polyvinyl chloride disks and has been shown to produce biofilm [25–28]. The exact mechanism for the mutation is unknown but is believed to be due to alteration of the intercellular adhesin (ica) gene [10]. The ica gene regulates production of polysaccharide intercellular adhesin, the major exopolysaccharide produced in S. epidermidis and S. aureus biofilm [29]. Ethanol increases Staphylococcal biofilm formation by increasing ica expression through modulation of the repressor, icaR [2, 3, 29, 30]. It is possible that alcohol exposure and subsequent increase in ica expression allowed accumulation and biofilm formation of this strain in polystyrene plates. To our knowledge, this is the first report of any alcohol exposure to cause the M7 strain to increase biofilm formation.
Regarding limitations, we tested a small number of strains, including one clinical isolate which may have different biofilm-forming behavior. The crystal violet used in this study stains cells and does not differentiate between viable and nonviable cells or quantify extracellular matrix production. Also, we did not characterize the composition or matrix production of the biofilms. We considered that alcohol may denature bacteria in biofilm, allowing for greater penetration of the crystal violet. However, differences in biofilm formation could be observed between wells even before the crystal violet stain was added. This also would not account for the increase in ica expression noted previously [30]. Viability may be underestimated using this method, since some adherent cells were visible in the bottom of wells after 20 min of sonication, particularly the prolific biofilm-forming ATCC 35984. Sonication of well plates can fail to release cells completely [31]. There was also a tendency for the number of bacteria to be higher in the center of the well plate than along the edges where evaporation was higher, further suggesting that dehydration played a role in cell viability.
Conclusion
Staphylococci exposed to clinically relevant concentrations of ethanol and isopropyl alcohol increase biofilm formation; however, the viability of these biofilm-embedded bacteria was diminished. Future research should determine the impact of these findings on the use of various alcohol preparations in the management and prevention of infections due to biofilm-forming Staphylococci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We gratefully acknowledge Kayla Babcock and Katie Daffinee for laboratory assistance. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 2P20GM103430 through Rhode Island IDeA Network for Excellence in Biomedical Research. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The views expressed are those of the authors and do not necessarily represent the position or policy of the United States Department of Veterans Affairs.
Conflict of interest
Megan Luther declares research funding from Pfizer and Cubist. Sarah Bilida declares no conflict of interest. Leonard Mermel declares Theravance, Astellas, CareFusion, Fresenius Medical, Marvao Medical research funding and/or consultancy. Kerry LaPlante declares Cubist, Astellas, Theravance, Forest, Davol, Marvao Medical, and Pfizer research funding, advisor, and/or consultancy.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. Erratum in: Clin Infect Dis. 2010 Apr 1;50(7):1079. Dosage error in article text. Clin Infect Dis. 2010 Feb 1;50(3):457. [DOI] [PMC free article] [PubMed]
- 2.Redelman CV, Maduakolam C, Anderson GG. Alcohol treatment enhances Staphylococcus aureus biofilm development. FEMS Immunol Med Microbiol. 2012;66(3):411–418. doi: 10.1111/1574-695X.12005. [DOI] [PubMed] [Google Scholar]
- 3.Milisavljevic V, Tran LP, Batmalle C, Bootsma HJ. Benzyl alcohol and ethanol can enhance the pathogenic potential of clinical Staphylococcus epidermidis strains. Am J Infect Control. 2008;36(8):552–558. doi: 10.1016/j.ajic.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Qu Y, Istivan TS, Daley AJ, Rouch DA, Deighton MA. Comparison of various antimicrobial agents as catheter lock solutions: preference for ethanol in eradication of coagulase-negative staphylococcal biofilms. J Med Microbiol. 2009;58(Pt 4):442–450. doi: 10.1099/jmm.0.006387-0. [DOI] [PubMed] [Google Scholar]
- 5.Restrepo D, Laconi NS, Alcantar NA, et al. Inhibition of heparin precipitation, bacterial growth, and fungal growth with a combined isopropanol-ethanol locking solution for vascular access devices. J Pediatr Surg. 2015;50(3):472–477. doi: 10.1016/j.jpedsurg.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Mermel LA, Alang N. Adverse effects associated with ethanol catheter lock solutions: a systematic review. J Antimicrob Chemother. 2014;69(10):2611–9. [DOI] [PubMed]
- 7.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37(6):1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luther MK, Mermel LA, LaPlante KL. Comparison of ML8-X10 (a prototype oil-in-water micro-emulsion based on a novel free fatty acid), taurolidine/citrate/heparin and vancomycin/heparin antimicrobial lock solutions in the eradication of biofilm-producing staphylococci from central venous catheters. J Antimicrob Chemother. 2014;69(12):3263–3267. doi: 10.1093/jac/dku281. [DOI] [PubMed] [Google Scholar]
- 9.Polonio RE, Mermel LA, Paquette GE, Sperry JF. Eradication of biofilm-forming Staphylococcus epidermidis (RP62A) by a combination of sodium salicylate and vancomycin. Antimicrob Agents Chemother. 2001;45(11):3262–3266. doi: 10.1128/AAC.45.11.3262-3266.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumacher-Perdreau F, Heilmann C, Peters G, Gotz F, Pulverer G. Comparative analysis of a biofilm-forming Staphylococcus epidermidis strain and its adhesion-positive, accumulation-negative mutant M7. FEMS Microbiol Lett. 1994;117(1):71–78. doi: 10.1111/j.1574-6968.1994.tb06744.x. [DOI] [PubMed] [Google Scholar]
- 11.LaPlante KL, Mermel LA. In vitro activities of telavancin and vancomycin against biofilm-producing Staphylococcus aureus, S. epidermidis, and Enterococcus faecalis strains. Antimicrob Agents Chemother. 2009;53(7):3166–3169. doi: 10.1128/AAC.01642-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaPlante KL, Mermel LA. In vitro activity of daptomycin and vancomycin lock solutions on staphylococcal biofilms in a central venous catheter model. Nephrol Dial Transplant. 2007;22(8):2239–2246. doi: 10.1093/ndt/gfm141. [DOI] [PubMed] [Google Scholar]
- 13.Luther MK, Arvanitis M, Mylonakis E, LaPlante KL. Activity of daptomycin or linezolid in combination with rifampin or gentamicin against biofilm-forming Enterococcus faecalis or E. faecium in an in vitro pharmacodynamic model using simulated endocardial vegetations and an in vivo survival assay using Galleria mellonella larvae. Antimicrob Agents Chemother. 2014;58(8):4612–4620. doi: 10.1128/AAC.02790-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amorena B, Gracia E, Monzon M, et al. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J Antimicrob Chemother. 1999;44(1):43–55. doi: 10.1093/jac/44.1.43. [DOI] [PubMed] [Google Scholar]
- 16.Koseki H, Yonekura A, Shida T, et al. Early staphylococcal biofilm formation on solid orthopaedic implant materials: in vitro study. PLoS One. 2014;9(10):e107588. doi: 10.1371/journal.pone.0107588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenep LE, Shenep MA, Cheatham W, et al. Efficacy of intravascular catheter lock solutions containing preservatives in the prevention of microbial colonization. J Hosp Infect. 2011;79(4):317–322. doi: 10.1016/j.jhin.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers ST, Pithie A, Gallagher K, Liu T, Charles CJ, Seaward L. Treatment of Staphylococcus epidermidis central vascular catheter infection with 70% ethanol locks: efficacy in a sheep model. J Antimicrob Chemother. 2007;59(4):779–782. doi: 10.1093/jac/dkl542. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhury A, Rangineni J, Venkatramana B. Catheter lock technique: in vitro efficacy of ethanol for eradication of methicillin-resistant staphylococcal biofilm compared with other agents. FEMS Immunol Med Microbiol. 2012;65(2):305–308. doi: 10.1111/j.1574-695X.2012.00950.x. [DOI] [PubMed] [Google Scholar]
- 20.Ali Y, Dolan MJ, Fendler EJ, Larson EL. Alcohols. In: Block SS, editor. Disinfection, sterilization, and preservation. Philadelphia: Lippincott Williams & Wilkin; 2001. pp. 229–254. [Google Scholar]
- 21.Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory Committee (HICPAC) Guideline for Disinfection and Sterilization in Healthcare Facilities: CDC; 2008 [cited 2015 4 Apr]. Available from: http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf.
- 22.Ingram LO. Ethanol tolerance in bacteria. Crit Rev Biotechnol. 1990;9(4):305–319. doi: 10.3109/07388558909036741. [DOI] [PubMed] [Google Scholar]
- 23.Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65(2):519–524. doi: 10.1128/iai.65.2.519-524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun D, Accavitti MA, Bryers JD. Inhibition of biofilm formation by monoclonal antibodies against Staphylococcus epidermidis RP62A accumulation-associated protein. Clin Diagn Lab Immunol. 2005;12(1):93–100. doi: 10.1128/CDLI.12.1.93-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam B, Baillie GS, Douglas LJ. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J Med Microbiol. 2002;51(4):344–349. doi: 10.1099/0022-1317-51-4-344. [DOI] [PubMed] [Google Scholar]
- 26.Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55(Pt 8):999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 27.Al-Fattani MA, Douglas LJ. Penetration of Candida biofilms by antifungal agents. Antimicrob Agents Chemother. 2004;48(9):3291–3297. doi: 10.1128/AAC.48.9.3291-3297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwank S, Rajacic Z, Zimmerli W, Blaser J. Impact of bacterial biofilm formation on in vitro and in vivo activities of antibiotics. Antimicrob Agents Chemother. 1998;42(4):895–898. doi: 10.1128/aac.42.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cue D, Lei MG, Lee CY. Genetic regulation of the intercellular adhesion locus in staphylococci. Front Cell Infect Microbiol. 2012;2:38. doi: 10.3389/fcimb.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korem M, Gov Y, Rosenberg M. Global gene expression in Staphylococcus aureus following exposure to alcohol. Microb Pathog. 2010;48(2):74–84. doi: 10.1016/j.micpath.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R. Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob Agents Chemother. 2007;51(1):78–83. doi: 10.1128/AAC.00154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.