Abstract
Background
Commercial sheep raised for mutton grow faster than traditional Chinese sheep breeds. Here, we aimed to evaluate genetic selection among three different types of sheep breed: two well-known commercial mutton breeds and one indigenous Chinese breed.
Results
We first combined locus-specific branch lengths and di statistical methods to detect candidate regions targeted by selection in the three different populations. The results showed that the genetic distances reached at least medium divergence for each pairwise combination. We found these two methods were highly correlated, and identified many growth-related candidate genes undergoing artificial selection. For production traits, APOBR and FTO are associated with body mass index. For meat traits, ALDOA, STK32B and FAM190A are related to marbling. For reproduction traits, CCNB2 and SLC8A3 affect oocyte development. We also found two well-known genes, GHR (which affects meat production and quality) and EDAR (associated with hair thickness) were associated with German mutton merino sheep. Furthermore, four genes (POL, RPL7, MSL1 and SHISA9) were associated with pre-weaning gain in our previous genome-wide association study.
Conclusions
Our results indicated that combine locus-specific branch lengths and di statistical approaches can reduce the searching ranges for specific selection. And we got many credible candidate genes which not only confirm the results of previous reports, but also provide a suite of novel candidate genes in defined breeds to guide hybridization breeding.
Introduction
China is the largest mutton producer in the world. According to 2012 statistics from the Food and Agriculture Organization of the United Nations, China accounts for almost one third of the world's yield of mutton (http://faostat.fao.org/). One reason for this is that there are a large number of Muslim and Mongolian residents in China and mutton is their main meat source. Meanwhile, more and more people of Han Chinese like eating mutton. As the status of mutton increases, so the deficit in the domestic supply of mutton also increases and the annual amount imported becomes ever larger. China does not have its own commercial mutton sheep breed, and the average meat production capacity of traditional Chinese breeds is lower compared with other countries. Therefore, development of a special Chinese sheep breed for meat production is needed.
Meat production traits have significant economic importance. Hybridization can quickly improve the meat quality of Chinese sheep, but cannot stabilize the inheritance of desirable traits. Identification of genomic regions that influence meat performance would enable improvement of local Chinese varieties by cross-breeding. This would have very real significance, not only to improve the weakness in Chinese mutton production, but also to improve to mutton production throughout the world.
To mine for genome selection information, the selection signal method has become popular. For the specific selection of genomic regions, pairwise FST, combined with a haplotype approach, such as REHH (Relative extended haplotype homozygosity), XPEHH (Cross population extended haplotype homozygosity)[1] or RSB (Across pairs of populations)[2] can determine the selection from a population when dealing with two groups. But it is relatively complex for multi-groups. Global FST, applied to multiple groups, cannot determine which breeds have undergone selection. At present, there are two better methods, locus-specific branch lengths (LSBL) and d i, which detect the locus specific divergence for each breed. LSBL is generally suitable for three or four groups [3], whereas d i is suitable for three or more groups [4].
In our previous study, we identified candidate genes associated with growth and meat production traits by using Illumina Ovine SNP50 BeadChip technology and genome-wide association study (GWAS) methodology to analyze three sheep populations including one indigenous Chinese sheep breed and two well-known commercial mutton sheep breeds [5]. Here we also applied these data to identify artificial selection regions using LSBL and d i statistics.
Materials and Methods
Population samples and quality control
We analyzed SNP (Single-nucleotide polymorphism) data from our previous GWAS [5]. A total of 322 sheep from three breeds, including 61 Chinese Mongolian fat-tailed (CMF), 161 German Mutton Merino (GMM) and 100 African white Dorper (AWD) sheep were analyzed. There were not any family structure and half sib family in the selected sheep. Two SNP sets were used. First, SNPs that did not pass the following three criteria were excluded: (1) SNPs with minor allele frequency > 0.01; (2) Hardy–Weinberg Equilibrium P-value > 0.000001; (3) SNPs that were located on autosomes. After quality control, there were 322 individuals and 46,752 SNPs in the genetic diversity analysis dataset. The first SNP set was then pruned using the indep-pairwise option, with a non overlapped window size of 25 SNPs, a step of 5 SNPs, and pairwise r2 threshold of 0.1, resulting in 10,260 independent SNP markers. The second SNP set was for population analysis.
Population analyses
Principal component analysis (PCA) was conducted using snpStats in R (http://cran.r-project.org). We constructed two neighbor-joining trees. One of uncorrected p-distances for individuals using SplitsTree software [6] and one of pairwise FST for populations using R package ape [7].
Statistical analyses
We first calculated pairwise FST for each locus of first SNP set between breeds using Genepop 4.2.2 software [8]. Neighbor-joining tree breed-specific population differentiation within 300 kb windows across the 26 autosomes was calculated using Locus-specific branch lengths (LSBL) [3] and d i statistics [4]. As described in Shriver et al. 2004 [3], LSBL (LGMM, LAWD, LCMF) were calculated from single locus pairwise FST distances, where LGMM = (GMM-AWD FST + GMM-CMF FST − AWD-CMF FST)/2, LAWD = (GMM-AWD FST + AWD-CMF FST − GMM-CMF FST)/2 and LCMF = (GMM-CMF FST + AWD-CMF FST − GMM-AWD FST)/2. Akey et al. [4] first described how to calculate d i statistics for each SNP; d i = , where and denote the expected value and standard deviation of pairwise FST values between breeds i and j calculated from all SNPs. Only windows with a minimum of three SNPs were considered. For each breed, windows of significance were determined as those with LSBL or d i values falling into the 99th percentile of the empirical distribution.
Gene annotation
We used the latest sheep genome release Ovis_aries_v3.1 (http://www.livestockgenomics.csiro.au/sheep/oar3.1.php) to identify relationships between significant selection windows and ovine genes. Owing to the structural imperfection and incomplete sheep genome sequence (before October, 2012), we also referenced genomic information of other species such as human, cow, mouse and rat.
Results
Population stratification
In the present study, we first performed principal component analysis on a pruned set of 10,260 genome-wide SNPs, to characterize the pattern of individual clustering in the sample set. As shown in Fig 1, PC1 (which accounts for 13.01% of the total variance) and PC2 (which accounts for 9.47% of the total variance) both separate all three population samples from each other, as the same with the former study[5].
Fig 1.
A. Animals clustered on the basis of principal component (PC) analysis using individual genotypes B. Scree-plot of proportion of variance.
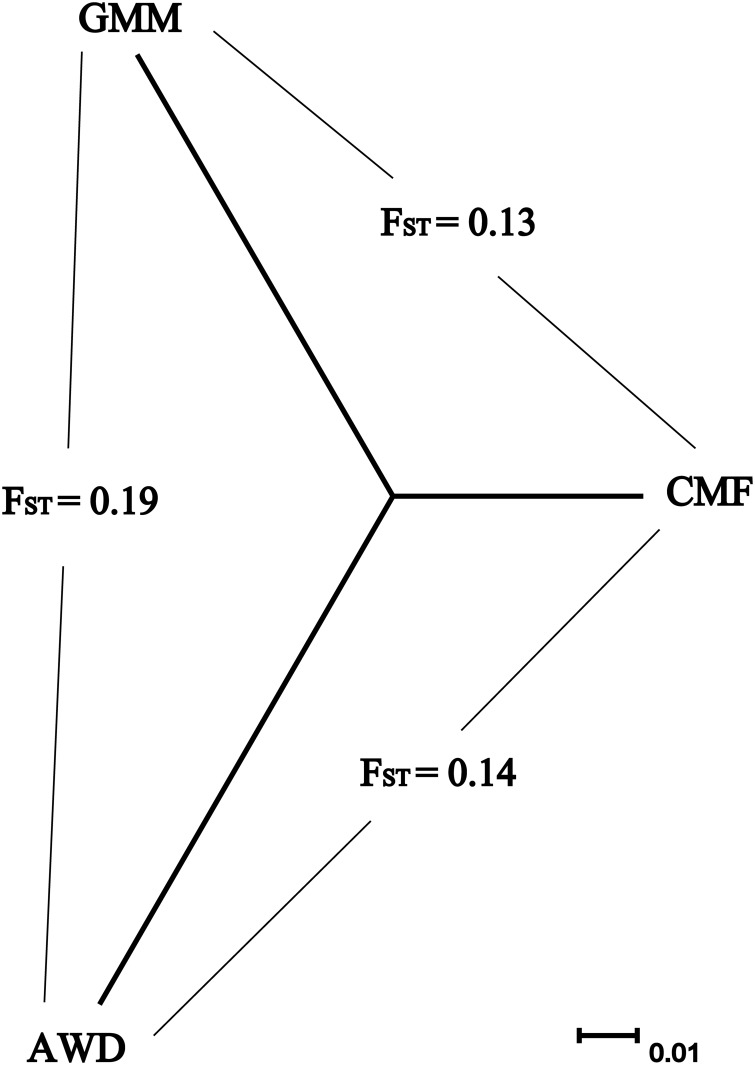
We then calculated pairwise FST [9] for the SNP data generated from the three sheep population samples (Fig 2). According to Wright’s theory [10], we found medium divergence (FST = 0.13, FST = 0.14) between CMF and GMM or AWD populations respectively, and high divergence (FST = 0.19) between AWD and GMM populations. We constructed a simple three-branch phylogeny from pairwise FST values (Fig 2) and also a neighbor-joining (NJ) tree among the individuals (S1 Fig). The results clearly showed that there were no conflicts concerning the origins of individuals assigned to each breed.
Fig 2. Three-branch phylogeny constructed from pairwise FST.
Correlation of two locus specific analysis approaches
Locus-specific branch lengths (LSBL) [3] and d i statistics [4] are both summary statistical methods to measure the locus specific divergence in allele frequencies for each breed based on unbiased estimates of pairwise FST [11]. LSBL is suited to the analysis of three populations, and d i is preferred for analysis of more than three populations. When the populations number is three, both approaches can be used. In this study, we calculated genome-wide LSBL and d i values. The maximal LCMF and dCMF values were higher than those of the other two breeds (Table 1). Obviously, the mean LAWD and LGMM were higher than LCMF, and branch lengths of AWD and GMM were longer than those of CMF (Table 1, Fig 2). In other words, the CMF breed shows more loci having shorter LSBL compared with the other two breeds. Histograms of the distribution of LSBL and d i statistics for each breed are shown in Fig 3. AWD and GMM have similar LSBL distributions. But GMM and CMF are similar d i statistics distributions. Further, we used Pearson’s product-moment correlation to estimate the correlation between LSBL and d i statistics within each breed. All three breeds showed significant correlation (P-value<2.2e-16) between the two approaches. The correlations for AWD (r = 0.85) and GMM (r = 0.84) were higher than that for CMF (r = 0.68).
Table 1. The descried of LSBL and d i values for each breed.
| Value | Mean(SD) | Min | Max |
|---|---|---|---|
| LAWD | 0.079(0.13) | -0.113 | 0.838 |
| LGMM | 0.071(0.13) | -0.125 | 0.914 |
| LCMF | 0.045(0.10) | -0.083 | 0.940 |
| dAWD | -0.003(1.62) | -1.859 | 9.728 |
| dGMM | -0.012(1.61) | -1.809 | 10.1 |
| dCMF | -0.010(1.46) | -1.785 | 11.352 |
Fig 3. Histogram of the LSBL and d i statistics distribution for each breed.
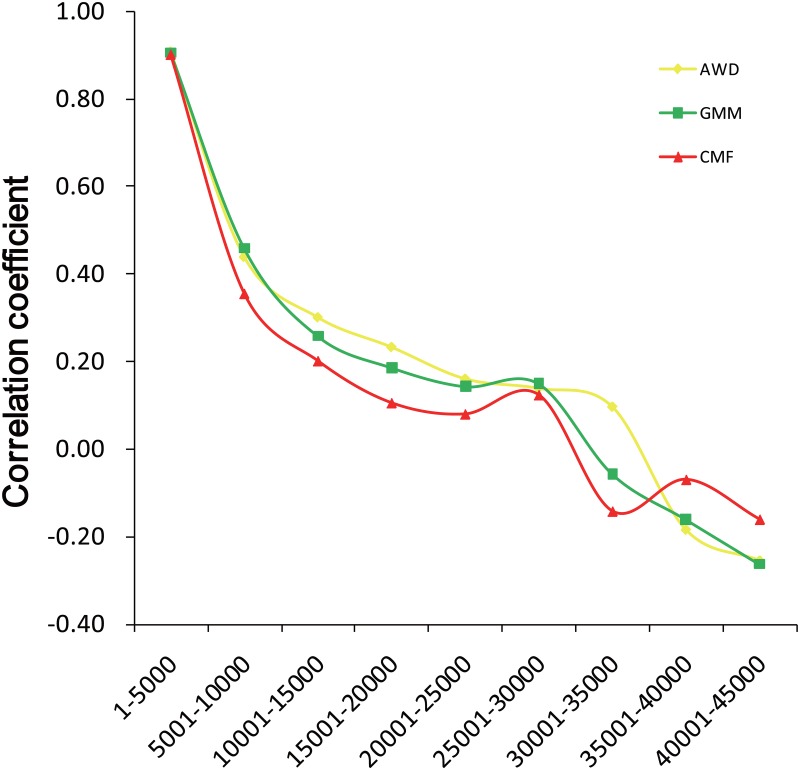
We also investigated the correlation between LSBL and d i statistics in 5000 SNPs in bin order, from high to low of LSBL value (Fig 4). The highest correlation (r>0.9) occurred in the region of the top 1–5000 SNPs in all breeds. The correlation values then sharply declined in the top 5001–10000 SNPs.
Fig 4. Correlation between LSBL and d i statistics.
Detecting breed specific selection regions
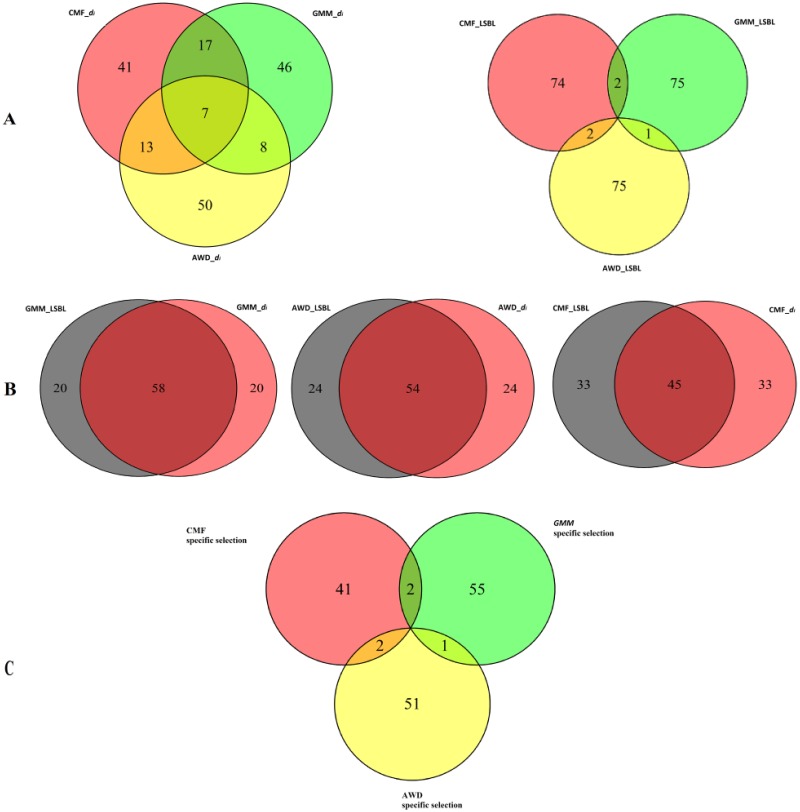
For each breed, we performed two locus-specific analyses to identify candidate regions involved in selection. These two statistical methods were calculated for autosomal SNPs in 300 kb windows, with a minimum of three SNPs per window, and defining the populations by breed. In total, 46,752 SNPs were evaluated within 7734 windows, ordered from 1 to 7734, averaging 5.97 SNPs per window (SD = 1.6). We defined candidate selection regions as those that fell into the upper 99th percentile of the empirical distribution. Within each breed, 78 windows were considered putative signatures of selection. S1 Fig shows the genome-wide distribution of the two analyses. In total, 259 of the windows met this criterion under both approaches in three breeds. Venn Diagrams were produced for the three breeds for LSBL and d i, respectively (Fig 5A). The numbers of overlapping windows for LSBL were fewer than for the d i approach. This indicates that LSBL has a greater ability to detect specific selection than d i.
Fig 5.
A. Former: Venn diagram of selection windows from d i approach in three breeds, Latter: Venn diagram of selection windows from LSBL approach in three breeds; B. Venn diagrams of each breed’s selection windows from LSBL and d i approaches; C. Venn diagram of specific selection windows in three breeds.
To detect breed specific selection regions for each breed, we merged the window lists generated by these two approaches to identify three subsets of 54 (AWD), 58 (GMM) and 45 (CMF) windows that showed the strongest signature of selection by displaying both high LSBL and d i values (Fig 5B). Because the correlation of CMF is lower than that of AWD and GMM, the number of overlapping windows for CMF is smaller than for the other breeds. Finally, there were also five overlapping windows in the final selected windows that were selected in two breeds (Fig 5C).
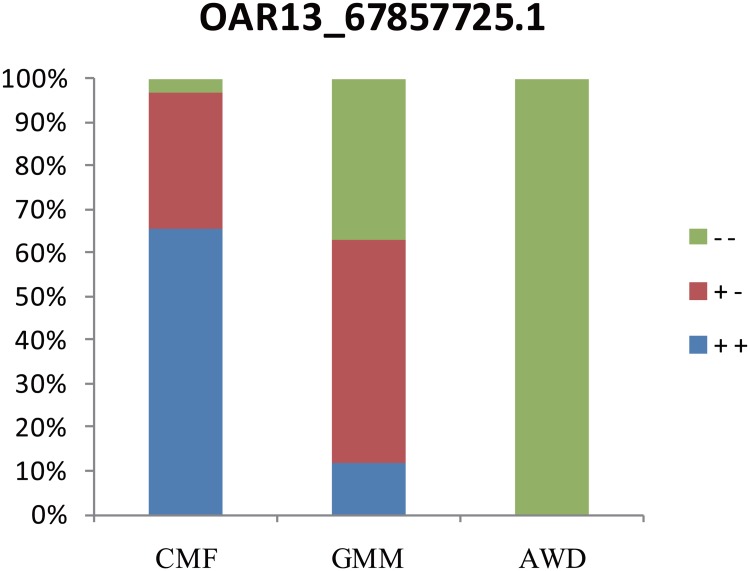
Fig 6 shows LSBL and d i values of-SNPs in five overlapping selection windows and in two nearby windows. The plot of LSBL values shows three clusters in each window. But these clusters are not clear in d i windows. All overlapping windows include 23 SNPs. Then we investigated the diversity of these SNPs. The distribution of genotypes for each SNP in the three breeds shows a stepladder, two extreme types and one middle type (S3 Fig). Fig 7 illustrates a representative SNP (OAR13_67857725.1) in window 5305. There is clearly a large difference in genotype proportion between AWD and CMF; therefore, the overlapping selection window means the two breeds, which have overlapping selection, are different in this region and maybe one or both has undergone selection.
Fig 6. The two statistic of per-SNP of three regions with three consecutive windows, the selected widows in the middle, GMM: green dot, AWD: yellow dot, CMF: red dot.
Fig 7. The diversity of OAR13_67857725.1 SNP in 3 sheep breeds.
Gene annotation
We used the latest sheep genome release, Ovis_aries_v3.1 (http://www.livestockgenomics.csiro.au/sheep/oar3.1.php), to identify relationships between significant selection windows and ovine genes. We removed uncharacterized genes and genes that overlapped among the three breeds. In total, 478 non-overlapping selected genes were annotated and 164, 201and 113 were selected in GMM, AWD and CMF breed, respectively (Table 2). Because of selective sweep or hitchiking effort, the effect of a strongly selected allele at one locus on the frequencies of neutral alleles at a linked locus, fewer genes were in fact selected[12]. We performed a further screen for each selection window. We selected genes located in or near a peak value SNP in each selection window. At last, we got 46, 51 and 32 candidate genes for GMM, AWD and CMF breed, respectively (Table 2, S1, S2 and S3 Tables). We did not screen overlapping windows.
Table 2. The annotation details in specific selected and overlapping selected region.
| Breed | No. of selected windows | No. of genes in windows | No. of genes within or near Peak SNP | |
|---|---|---|---|---|
| overlapping selected | ||||
| GMM & AWD | 2 | 9 | ||
| GMM& CMF | 2 | 3 | ||
| AWD& CMF | 1 | 5 | ||
| specific selected | ||||
| GMM | 55 | 164 | 46 | |
| AWD | 51 | 201 | 51 | |
| CMF | 41 | 113 | 32 |
Specific selection genes in each breed
Here we identified many selection genes for each breed. We focused on production, meat, reproduction and health traits because these are highly valued traits in mutton sheep production. We identified candidate genes are for enrichment of these main traits. We list below some genes previously identified to be important in each breed for various traits (Table 3).
Table 3. The information of main candidate gene of three breeds.
| Breed | Window | Chr | Region | LSBL | d i | candidate gene |
|---|---|---|---|---|---|---|
| GMM | ||||||
| 746 | 1 | 234.6–234.9 | 0.33 | 3.20 | IGSF10 | |
| 764 | 1 | 240–240.3 | 0.34 | 4.22 | PLSCR2 | |
| 1574 | 2 | 213.6–213.9 | 0.43 | 4.03 | FAM113B | |
| 1881 | 3 | 61.8–62.1 | 0.38 | 3.31 | EDAR | |
| 1984 | 3 | 93.9–94.2 | 0.42 | 4.61 | EXOC6B | |
| 2150 | 3 | 145.2–145.5 | 0.37 | 3.78 | PDZRN4 | |
| 2212 | 3 | 165–165.3 | 0.35 | 3.29 | NTN4 | |
| 2366 | 3 | 213–213.3 | 0.37 | 3.92 | MICAL3 | |
| 2999 | 5 | 66.9–67.2 | 0.36 | 4.24 | CCNB2 | |
| 3454 | 6 | 103.2–103.5 | 0.42 | 3.93 | STK32B | |
| 4579 | 10 | 55.2–55.5 | 0.34 | 3.31 | EIF3F | |
| 4794 | 11 | 39.9–40.2 | 0.35 | 3.64 | PSMD3,THRA,MSL1 | |
| 5342 | 13 | 74.7–75 | 0.32 | 3.10 | TRHR | |
| 5913 | 16 | 31.8–32.1 | 0.50 | 5.66 | GHR | |
| 6058 | 17 | 4.8–5.1 | 0.48 | 4.54 | TMEM154 | |
| 6673 | 19 | 59.1–59.4 | 0.36 | 3.34 | EEFSEC | |
| 7019 | 22 | 15–15.3 | 0.30 | 2.85 | PLCE1 | |
| 7041 | 22 | 22.2–22.5 | 0.33 | 3.28 | SUFU | |
| 7402 | 24 | 25.8–26.1 | 0.47 | 4.90 | ATP2A1, APOBR | |
| 7403 | 24 | 26.1–26.4 | 0.45 | 4.78 | ALDOA | |
| AWD | ||||||
| 756 | 1 | 237.6–237.9 | 0.35 | 3.43 | HMGB1 | |
| 1708 | 3 | 7.5–7.8 | 0.39 | 3.85 | SPTAN1 | |
| 2001 | 3 | 99.3–99.6 | 0.32 | 2.86 | IL1RL1 | |
| 2367 | 3 | 213.3–213.6 | 0.41 | 4.58 | TRIOBP | |
| 3170 | 6 | 13.8–14.1 | 0.32 | 2.97 | POL | |
| 3606 | 7 | 34.2–34.5 | 0.30 | 3.44 | SPTBN5 | |
| 3749 | 7 | 78.6–78.9 | 0.35 | 2.86 | SLC8A3 | |
| 4479 | 10 | 21.9–22.2 | 0.45 | 4.47 | TPTE2 | |
| 4504 | 10 | 29.7–30 | 0.39 | 4.59 | B3GALTL | |
| 5270 | 13 | 51.9–52.2 | 0.41 | 3.67 | TMC2 | |
| 5438 | 14 | 21.3–21.6 | 0.32 | 2.63 | RPGRIP1L, FTO | |
| 5452 | 14 | 25.5–25.8 | 0.34 | 2.95 | SETD6 | |
| 5546 | 14 | 57.9–58.2 | 0.31 | 2.76 | RPL7 | |
| 6360 | 18 | 29.4–29.7 | 0.36 | 3.35 | CIB2 | |
| 6623 | 19 | 43.5–43.8 | 0.33 | 2.76 | DNAH3 | |
| 6649 | 19 | 51.9–52.2 | 0.35 | 3.22 | SCAP | |
| 7013 | 22 | 12.3–12.6 | 0.33 | 3.75 | NUDT9 | |
| CMF | ||||||
| 286 | 1 | 89.2–89.4 | 0.32 | 4.30 | SLC16A1 | |
| 2099 | 3 | 129.6–129.9 | 0.46 | 6.02 | CRADD | |
| 2322 | 3 | 198.6–198.9 | 0.24 | 2.55 | DERA | |
| 2858 | 5 | 22.5–22.8 | 0.26 | 2.42 | SLC27A6 | |
| 3235 | 6 | 33.9–34.2 | 0.28 | 3.31 | FAM190A | |
| 3239 | 6 | 36–36.3 | 0.24 | 2.71 | HERC3 | |
| 3380 | 6 | 79.5–79.8 | 0.27 | 2.73 | TECRL | |
| 3605 | 7 | 33.9–34.2 | 0.26 | 2.85 | TYRO3 | |
| 3608 | 7 | 34.8–35.1 | 0.24 | 2.71 | CAPN3 | |
| 4834 | 11 | 52.8–53.1 | 0.28 | 2.63 | SOCS3 | |
| 5918 | 16 | 33.6–33.9 | 0.22 | 2.64 | PRKAA1 | |
| 7363 | 24 | 11.7–12 | 0.25 | 2.86 | SHISA9 | |
| 7407 | 24 | 27.6–27.9 | 0.27 | 3.08 | PHKG1 |
Underlined fonts indicate candidate gene in our former GWAS study.
Specific selection genes in GMM breed Production traits
Two important genes TRHR and APOBR as candidate association with body mass [13, 14]. PDS5B showed negative covariance between average daily weight gain and backfat thickness [15]. IGSF10 is differentially expressed in cattle with high and low residual feed intake [16]. Meat traits: GHR is a well-known gene that not only effects meat production and quality but also reproduction traits in many species [17, 18]. STK32B is a QTL(quantitative trait loci) for marbling score in Hanwoo [19]. ALDOA, which encodes a glycolytic metabolic enzyme, was expressed at around 2-fold lower levels in the longissimus muscle of Wagyu-sired fetuses at day 195 compared with Piedmontese-sired fetuses [20]. FAM113B is expressed in dairy cattle at least twice the level of that in beef cattle [21]. NTN4 was down-regulated in differentiated adiposities compared with intramuscular fibroblast-like cells [22]. Reproduction traits: PLSCR2 is a candidate endometrial gene in the regulation of conceptus growth and elongation [23]. EIF3F gene transcripts were more highly enriched in brilliant cresyl blue (BCB)+ oocytes compared with BCB− oocytes [24]. CCNB2 was identified as significantly associated with developmental competence of bovine oocytes [25]. PDZRN4 is associated with sperm motility of Holstein-Friesian cattle and EEFSEC is related to buffalo bull fertility [26, 27]. Health traits: TMEM154 can reduce lentivirus susceptibility in sheep [28] and GWAS indicate this gene to be associated with susceptibility to and control of ovine lentivirus [29]. MICAL3 is associated with immune response traits in Canadian Holstein cattle [30]. ATP2A1 is associated with pseudomyotonia, a muscle function disorder, in cattle [31]. PSMD3 shows significant association with the mean corpuscular volume [32]. Other traits: GMM is merino fine wool sheep, so wool trait was also selected when in process of breeding. Unsurprisingly, we found three important genes involved in wool trait. EDAR is associated with hair thickness in human [33]. Mutation in Mpzl3, a gene encoding a predicted adhesion protein, is responsible for rough coat mice with severe skin and hair abnormalities [34]. THRA is located at quantitative. Meanwhile, we found three genes looks association with milk traits. Such as, EXOC6B is a candidate gene for teat morphology and function [35]. PLCE1 is associated with total protein weight in milk and SUFU is associated with the mammary system, somatic cell count and survival [36].
Specific selection genes in AWD breed Production traits
FTO is associated with BMI in human and growth rate and fat mass in pig [37–40]. SCAP, part of the INSIG-SCAP-SREBP pathway, is involved in obesity risk in Chinese children [41]. Mutations in B3GALTL can cause disproportionate short stature in human, and developmental delay [42]. Reproduction traits: SLC8A3 is a transporter that can potentially increase the availability of L-alanine and L-histidine for gap junctional transfer in oocytes [43]. SETD6 is involved in the transcriptional regulation of gonadotropin-releasing hormone [44]. Health traits: SPTAN1 is a candidate gene for parasite resistance in livestock [45]. CIB2 is associated with influencing interleukin levels in African Americans [46]. HMGB1 is involved in mastitis in dairy cattle [47]. TRIOBP and TMC2 can cause recessive hearing loss in human [48, 49]. NUDT9 is a candidate gene for an inherited cataract in sheep [50]. Mutations in SPTBN5 and RPGRIP1L cause retinitis pigmentosa [51, 52]. A SNP mutation in DNAH3 is involved in recurrent airway obstruction in European horses [53]. A functional SNP in IL1RL1 is associated with asthma in human [54]. Other traits: TPTE2 may be directly or indirectly related to epithelial cells or skin development [44] and is a candidate gene associated with wool traits in Chinese Merino Sheep [55].
Specific selection genes in CMF breed Production traits
TECRL is associated with withers height in racing quarter horse [56]. SLC27A6 is part of the peroxisome proliferator-activated receptor (PPAR) signaling pathway, which is associated with carcass conformation in cattle [57]. Meat trait: FAM190A is a QTL associated with weight after slaughter in Hanwoo cattle [58]. CRADD is associated with muscle compactness [59]. PHKG1 causes high glycogen content and low meat quality in pig skeletal muscle [60]. CAPN3 is related to meat quality traits in chickens [61]. Reproduction traits: TYRO3 modulates female reproduction by influencing gonadotropin-releasing hormone [62]. SLC16A1 plays an important role in the transport of mevalonate and ketone bodies [63] and may be involved in differences in efficiency of reproduction in cattle[64]. Health traits: SOCS3 is associated with somatic cell score trait in cattle and is expressed in goat milk fat globules in response to experimental intramammary infection with Staphylococcus aureus [65]. Other traits: In milk traits, PRKAA1 is associated with fat percentage and may have effects on fat metabolism affecting milk production traits in cattle [66]. DERA is a positional candidate gene for milk fat percentage in the German Holstein-Friesian population [67]. HERC3 is associated with milk production performance in Chinese Holstein cattle [68].
Overlapping selection regions
According to the above analysis, overlapping windows means there are differences between the two selected breeds. In Table 4, 17 selected genes in these overlapping regions are annotated.
Table 4. The genes in overlapping selection windows.
| LSBL | di | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chr | window | SNP No. | Region | GMM | AWD | CMF | GMM | AWD | CMF | Selected genes |
| 1 | 288 | 4 | 89.7–90.0 | -0.07 | 0.30 | 0.24 | 0.75 | 3.29 | 3.17 | LRIG2, RPS6 |
| 2 | 1246 | 5 | 114.3–114.6 | 0.33 | -0.09 | 0.26 | 3.84 | 0.85 | 3.60 | - |
| 2 | 1588 | 4 | 219.6–219.9 | 0.29 | 0.30 | -0.04 | 3.54 | 3.52 | 1.80 | BCS1L, CYP27A1, PRKAG3, RNF25, STK36, TTLL4, WNT10A, WNT6, ZNF142 |
| 6 | 3241 | 6 | 37.2–37.5 | 0.37 | 0.00 | 0.31 | 5.20 | 2.55 | 5.14 | FAM184B, NCAPG, LCORL |
| 13 | 5305 | 4 | 62.7–63.0 | -0.02 | 0.49 | 0.24 | 2.58 | 6.08 | 4.91 | RALY, EIF2S2, CHMP4B |
Blot fonts as candidate gene. Underlined fonts indicate values in the top first percentiles.
Firstly, two overlapping windows were detected between GMM and CMF breeds. There is no gene involved in the 1246 window. We then identified two well-known genes, NCAPG and its near neighbor LCORL within 37.2–37.5Mb on OAR6, which are reported to be involved in fetal growth, stillbirth, and carcass size in sheep and other livestock (Table 4). GWAS revealed that these two genes are associated with body weight in Australian Merino sheep[69]. Kijas et al. suggest that variation in the NCAPG/LCORL region also influences production traits in sheep [70]. In horses, GWAS indicates LCORL/NCAPG as a candidate region for withers height [71]. In cattle, LCORL and NCAPG genes are associated with feed intake and weight gain [72] and body frame size [73]. Xu et al. detected that LCORL/NCAPG have undergone positive selection in five distinct cattle breeds [74].
Secondly, there are two windows that are different between AWD and CMF breeds. One region, 89.7 to 90.0 Mb on OAR10, coincides with LRIG2 and RPS6 genes. RPS6 is a candidate gene in a QTL region affecting growth and reproduction traits in swine [75]. The other region, from 62.7 to 63.0 Mb, on OAR13 included three genes, RALY, EIF2S2 and CHMP4B (Table 4). Another nearby gene, ASIP, regulates pigmentation in mice, while duplication of ASIP in sheep controls a series of alleles for black and white coat color [76]. The ASIP region is one of four known melanoma-susceptibility regions and includes the four genes (RALY, EIF2S2, CHMP4B and ASIP) [77]. In Kijas et al. research, a SNP s51670.1 has peak value of global FST in similar region, ASIP as candidate gene, on OAR13 [78]; here this SNP also has peak value of LSBL and d i in 5305 windows (Fig 7). In a recent GWAS analysis ASIP was associated with white versus non-white coat-color variation in sheep [79].
Thirdly, only one region was different between AWD and GMM, at 219.6–219.9 Mb on OAR4. There are nine genes involved (Table 4), three of which have been are reported. The most important gene is PRKAG3 (protein kinase, AMP-activated, gamma 3 noncatalytic subunit), which increases fatty acid oxidation and glucose uptake to satisfy muscle energy demands [80] and is a candidate gene associated with meat quality and production traits in pig [81] and cattle [82]. A mutation in PRKAG3 is associated with excess glycogen content in pig skeletal muscle [83]. Recently GWAS analysis indicated that PRKAG3 affected meat pH and color in crossbred commercial pig lines [84]. The other two genes are WNT10A and WNT6, which are strongly co-expressed in human SW480 cells [85]. Wnt6 is an early negative regulator of limb chondrogenesis and ectoderm development in the chicken embryo [86]. Interestingly, Christodoulides et al. identified a proband with early onset obesity that is heterozygous for a WNT10 C256Y mutation, which blocks adipogenesis [87].
Discussion
Three breeds of sheep were investigated in this study; CMF comes from China, GMM originates from Germany and AWD was originally developed in South Africa. The FST results showed significant genetic divergence between GMM and AWD (FST = 0.19) and medium divergence between CMF and GMM (FST = 0.13) or AWD (FST = 0.14). This is consistent with domestic sheep being first domesticated in Asia, the Fertile Crescent, and then dispersing to Europe and Africa [88]. The PCA and neighbor-joining tree clearly separate these three population samples from each other.
In the present study, we used two locus specific analysis approaches to detect candidate regions targeted by selection. Both of them calculated for each breed based on pairwise FST. From the previously describe, the d i approach measures the standardized locus-specific deviation in levels of population structure [4]. However, the LSBL approach geometrically isolates allele frequency change [3].
First we compared the values of these two statistical approaches. The two methods had a high correlation, especially in the selected regions. For example, the highest correlation (r>0.9) occurred in the region of the top 1–5000 SNPs by LSBLs in all three breeds. Furthermore, the result shows the high correlation of LSBL and d i in AWD and GMM, while lower in CMF. It might be relevant with the evolution process of these three breeds. AWD and GMM are notable commercial breeds in the world, which developed through strict selection pressure. However, CMF is local breed which mainly selected for body weight and conformation in recent years [5].
We then calculated the mean value respectively of the two approaches for autosomal SNPs in 300 kb windows for each breed. Interestingly, LSBL had a greater ability to detect specific selection than d i. We merged the window lists generated by these two approaches to identify breed specific selection regions. In total, 142 windows showed the strongest signature of selection, five of which overlapped. This means that the two breeds are different in these regions and one or both may have undergone selection.
We have defined candidate genes in selection windows located at or near a peak value SNPs. Some genes were identified in earlier sheep selection studies, such as NF1 and ASIP [78], RNF180 and GHR [89]. GHR, identified in the GMM breed, is an important growth-related gene that, not only affects meat production and quality, but also reproduction traits [17, 18]. Two genes were detected in sheep by GWAS, such as TPTE2 [55], TMEM154 [29]. In our previous study four genes, POL, RPL7, MSL1 and SHISA9, are associated with growth and meat production traits [5]. We notice that there are only a littler common results in these two studies, although using the same data. Because the sample sizes were too small, we combined three population data as a whole object in our GWA study. But herein, we respectively detected the specific selection for each breed.
Therefore, our study provides additional information for interpreting selection in different domestic sheep breeds. Production, meat, reproduction and health traits of sheep were investigated because these are highly valued traits in mutton sheep production. So the candidate genes enrich for these main traits. For production traits, there are two genes, APOBR and FTO, are associated with BMI [14, 37]. For reproduction traits, we found no major genes controlling reproduction prolificacy, such as GDF9 and BMPR1B; however, we found some genes which can influence development of the oocyte and sperm. For example, EIF3F, CCNB2 and SLC8A3 affect oocyte development [24, 25, 43] and PDZRN4 and EEFSEC affect sperm [26, 27]. For meat traits, ALDOA, STK32B and FAM190A are related to marbling in cattle[19, 20, 58]. For wool traits, EDAR was selected in the GMM breed and is associated with hair thickness [33]. AWD has a characteristic of molting, and TPTE2 is related to epithelial cells or skin development [44]. For health traits, we noticed that association of candidate genes related to disease resistance traits is more common in Chinese compared with Mongolian commercial mutton sheep. This shows that the artificial selection of Mongolian sheep has not received sufficient attention. An important gene was found, TMEM154, which can control and reduce lentivirus susceptibility in sheep [28, 29]. Currently, there is no vaccine to prevent ovine lentivirus infection and no cost-effective treatment for infected animals. This gene should therefore be used in breeding projects. In the AWD breed, we found a lot of genes associated with disease (except for immune related genes). These included sensory disorders and respiratory system diseases. Interestingly, some genes related to milk traits were selected in GMM and CMF breeds, both of which are from the Northern hemisphere, but not in AWD.
It is worth mentioning that the early growth speed of Chinese Mongolian sheep is too slow compared with commercial breeds. This is because the Chinese Mongolian sheep is a fat-tailed sheep and deposition of tail fat reduces early growth speed. We therefore focused on the pathways and genes associated with fat formation. Interestingly, five such genes (SOCS2, SOCS3, PPP1CC, PHKG1 and PRKAA1) are in the insulin signaling pathway. SOCS2 and SOCS3 (suppressor of cytokine signaling 2 and 3), regulate insulin signaling in different tissues by impacting on the insulin receptor and insulin receptor substrates [90]. PPP1CC, also known as PPP1G, is a subunit of protein phosphatase 1. It is a glycogen-associated phosphatase responsible for dephosphorylation and subsequent inactivation of glycogen synthase and is universal in skeletal muscle [91]. PHKG1, causes high glycogen content and low meat quality in pig skeletal muscle [60]. PRKAA1/2 acts as an energy sensor, sensing an increased AMP/ATP ratio, and is known to regulate substrates that mediate metabolic activity, such as phosphorylation of acetyl coA carboxylase (ACACA, also known as ACC) [92]. Furthermore, studies have shown that PDGF promotes proliferation and inhibits differentiation of preadipocytes [93, 94]. Real-time quantitative PCR indicates that PDGFD is expressed at a higher level in adipose tissue than in normal human tissues, except the thyroid [95]. Insulin also stimulates cell growth and differentiation, and promotes the storage of substrates in fat, liver and muscle by stimulating lipogenesis, glycogen and protein synthesis, and inhibiting lipolysis, glycogenolysis and protein breakdown [96]. We therefore suggest that these genes affect fat-tail formation but this requires further study.
In this study, we also found some different selection regions between breeds; however, we were unable to determine in which breed the candidate gene was selected. For instance, CMF has a black head and legs, while the AWD are white. It appears as though ASIP, a key gene of pigmentation, may provide evidence for selection in CMF. According to the same principle, the LCORL/NCAPG region was selected in GMM, which grows faster and has a bigger carcass than CMF. Of course, not all genes can be judged, such like PRKAG3 affecting meat pH and color, because the relevant data was lacking. These genes, in addition to RPS6, WNT10A and WNT6, require further study.
Conclusions
In the present study, we used the two approaches, LSBL and d i statistics, to detect selection regions in three different sheep breeds (populations). These approaches clearly identified selected regions in each breed, and provided many candidate genes, including some well-known genes. Overall, growth, meat and health traits are undergoing different levels of selection in these three breeds, but the choice of focus differs for each breed according to origin, local preferences and environment.
Supporting Information
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to Inner Mongolia Sunit Purebred Sheep Stud, Inner Mongolia German Mutton Purebred Sheep Stud, and Tianjin Aoqun Animal Husbandry Propriety Limited, especially to Lady Hua Chen, for providing the experimental animals and helping with the sampling. We also thank Hangxing Ren, Jiasen Liu, Guobin Lu, Lingyang Xu, Jian Lu, Shifang Zhang, Xiaoning Zhang, Yanfei Lv, and Dan Sun (CAAS) for their generous assistance. This research was supported by the National Key Technology R&D Program of China (2011BAD28B05-2).
Data Availability
All data underlying the findings in the study are freely available in the manuscript and supplemental files.
Funding Statement
This research was supported by the National Key Technology R&D Program of China (2011BAD28B05-2)(http://www.most.gov.cn/eng/index.htm). LZ received funding from the Chinese Academy of Agricultural Sciences(2014ywf-yb-7) (http://en.gscaas.net.cn/). The funder had collected and analyzed data, and decided to publish.
References
- 1. Sabeti PC, Varilly P, Fry B, Lohmueller J, Hostetter E, Cotsapas C, et al. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449(7164):913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang K, Thornton KR, Stoneking M. A new approach for using genome scans to detect recent positive selection in the human genome. PLoS biology. 2007;5(7):e171 10.1371/journal.pbio.0050171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shriver MD, Kennedy GC, Parra EJ, Lawson HA, Sonpar V, Huang J, et al. The genomic distribution of population substructure in four populations using 8,525 autosomal SNPs. Human genomics. 2004;1(4):274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akey JM, Ruhe AL, Akey DT, Wong AK, Connelly CF, Madeoy J, et al. Tracking footprints of artificial selection in the dog genome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(3):1160–5. 10.1073/pnas.0909918107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang L, Liu J, Zhao F, Ren H, Xu L, Lu J, et al. Genome-wide association studies for growth and meat production traits in sheep. PloS one. 2013;8(6):e66569 10.1371/journal.pone.0066569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huson DH. SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics. 1998;14(1):68–73. . [DOI] [PubMed] [Google Scholar]
- 7. Paradis E, Claude J, Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20(2):289–90. . [DOI] [PubMed] [Google Scholar]
- 8. Rousset F. genepop'007: a complete re-implementation of the genepop software for Windows and Linux. Molecular ecology resources. 2008;8(1):103–6. 10.1111/j.1471-8286.2007.01931.x . [DOI] [PubMed] [Google Scholar]
- 9. Weir BS. Genetic data analysis Methods for discrete population genetic data: Sinauer Associates, Inc. Publishers; 1990. [Google Scholar]
- 10. Wright S. Evolution and the genetics of populations, volume 4: variability within and among natural populations: University of Chicago press; 1984. [Google Scholar]
- 11. Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. evolution. 1984:1358–70. [DOI] [PubMed] [Google Scholar]
- 12. Stephan W. Genetic hitchhiking versus background selection: the controversy and its implications. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1544):1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu XG, Tan LJ, Lei SF, Liu YJ, Shen H, Wang L, et al. Genome-wide association and replication studies identified TRHR as an important gene for lean body mass. American journal of human genetics. 2009;84(3):418–23. 10.1016/j.ajhg.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nature genetics. 2010;42(11):937–48. 10.1038/ng.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rothschild M, Kim E, Plastow G, editors. Novel Tools Provide New Opportunities for Genetic Improvement of Swine 10th World Congress on Genetics Applied to Livestock Production; 2014: Asas. [Google Scholar]
- 16. Chen Y, Gondro C, Quinn K, Herd RM, Parnell PF, Vanselow B. Global gene expression profiling reveals genes expressed differentially in cattle with high and low residual feed intake. Animal genetics. 2011;42(5):475–90. 10.1111/j.1365-2052.2011.02182.x . [DOI] [PubMed] [Google Scholar]
- 17. Crisà A, Marchitelli C, Pariset L, Contarini G, Signorelli F, Napolitano F, et al. Exploring polymorphisms and effects of candidate genes on milk fat quality in dairy sheep. Journal of dairy science. 2010;93(8):3834–45. 10.3168/jds.2009-3014 [DOI] [PubMed] [Google Scholar]
- 18. Komisarek J, Michalak A, Walendowska A. The effects of polymorphisms in DGAT 1, GH and GHR genes on reproduction and production traits in Jersey cows. Animal Science Papers and Reports. 2011;29(1):29–36. [Google Scholar]
- 19. Lee J, Lia Y, Kim J. Detection of QTL for Carcass Quality on Chromosome 6 by Exploiting Linkage and Linkage Disequilibrium in Hanwoo. Asian-Australasian Journal of Animal Sciences. 2012;25(1):17–21. 10.5713/ajas.2011.11337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lehnert SA, Reverter A, Byrne KA, Wang Y, Nattrass GS, Hudson NJ, et al. Gene expression studies of developing bovine longissimus muscle from two different beef cattle breeds. BMC developmental biology. 2007;7(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sadkowski T, Jank M, Zwierzchowski L, Oprzadek J, Motyl T. Transcriptomic index of skeletal muscle of beef breeds bulls. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2009;60 Suppl 1:15–28. . [PubMed] [Google Scholar]
- 22. SeongLan Y, SangMi L, ManJong K, HangJin J, ByungChan S, JinTae J, et al. Identification of differentially expressed genes between preadipocytes and adipocytes using affymetrix bovine genome array. Journal of Animal Science and Technology. 2009;51(6):443–52. [Google Scholar]
- 23. Spencer T. Insights into Conceptus Elongation in Ruminants: Roles of Ovarian Progesterone and the Uterus. J Biol Chem. 2000;275:32106 10851241 [Google Scholar]
- 24. Janowski D, Salilew-Wondim D, Torner H, Tesfaye D, Ghanem N, Tomek W, et al. Incidence of apoptosis and transcript abundance in bovine follicular cells is associated with the quality of the enclosed oocyte. Theriogenology. 2012;78(3):656–69. e5 10.1016/j.theriogenology.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 25. Mourot M, Dufort I, Gravel C, Algriany O, Dieleman S, Sirard MA. The influence of follicle size, FSH-enriched maturation medium, and early cleavage on bovine oocyte maternal mRNA levels. Molecular reproduction and development. 2006;73(11):1367–79. [DOI] [PubMed] [Google Scholar]
- 26. Hering D, Olenski K, Kaminski S. Genome-wide association study for poor sperm motility in Holstein-Friesian bulls. Animal reproduction science. 2014;146(3):89–97. [DOI] [PubMed] [Google Scholar]
- 27. Verma A, Rajput S, De S, Kumar R, Chakravarty AK, Datta TK. Genome-wide profiling of sperm DNA methylation in relation to buffalo (Bubalus bubalis) bull fertility. Theriogenology. 2014;82(5):750–9. e1 10.1016/j.theriogenology.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 28. Heaton MP, Clawson ML, Chitko-Mckown CG, Leymaster KA, Smith TP, Harhay GP, et al. Reduced lentivirus susceptibility in sheep with TMEM154 mutations. PLoS genetics. 2012;8(1):e1002467 10.1371/journal.pgen.1002467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. White SN, Mousel MR, Herrmann-Hoesing LM, Reynolds JO, Leymaster KA, Neibergs HL, et al. Genome-wide association identifies multiple genomic regions associated with susceptibility to and control of ovine lentivirus. PloS one. 2012;7(10):e47829 10.1371/journal.pone.0047829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thompson-Crispi KA, Sargolzaei M, Ventura R, Abo-Ismail M, Miglior F, Schenkel F, et al. A genome-wide association study of immune response traits in Canadian Holstein cattle. BMC genomics. 2014;15(1):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grünberg W, Sacchetto R, Wijnberg I, Neijenhuis K, Mascarello F, Damiani E, et al. Pseudomyotonia, a muscle function disorder associated with an inherited ATP2A1 (SERCA1) defect in a Dutch Improved Red and White cross-breed calf. Neuromuscular Disorders. 2010;20(7):467–70. 10.1016/j.nmd.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Yang J, Yu M, Liu B, Fan B, Zhu M, et al. Polymorphism detection of porcine PSMC3, PSMC6 and PSMD3 genes and their association with partial growth, carcass traits, meat quality and immune traits. Canadian journal of animal science. 2005;85(4):475–80. [Google Scholar]
- 33. Fujimoto A, Kimura R, Ohashi J, Omi K, Yuliwulandari R, Batubara L, et al. A scan for genetic determinants of human hair morphology: EDAR is associated with Asian hair thickness. Human molecular genetics. 2008;17(6):835–43. 10.1093/hmg/ddm355 . [DOI] [PubMed] [Google Scholar]
- 34. Cao T, Racz P, Szauter KM, Groma G, Nakamatsu GY, Fogelgren B, et al. Mutation in Mpzl3, a novel [corrected] gene encoding a predicted [corrected] adhesion protein, in the rough coat (rc) mice with severe skin and hair abnormalities. The Journal of investigative dermatology. 2007;127(6):1375–86. 10.1038/sj.jid.5700706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pausch H, Jung S, Edel C, Emmerling R, Krogmeier D, Götz KU, et al. Genome-wide association study uncovers four QTL predisposing to supernumerary teats in cattle. Animal genetics. 2012;43(6):689–95. 10.1111/j.1365-2052.2012.02340.x [DOI] [PubMed] [Google Scholar]
- 36. Cecchinato A, Ribeca C, Chessa S, Cipolat-Gotet C, Maretto F, Casellas J, et al. Candidate gene association analysis for milk yield, composition, urea nitrogen and somatic cell scores in Brown Swiss cows. Animal. 2014:1–9. 10.1017/S1751731114001098 . [DOI] [PubMed] [Google Scholar]
- 37. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan B, Du Z-Q, Rothschild MF. The fat mass and obesity-associated (FTO) gene is associated with intramuscular fat content and growth rate in the pig. Animal biotechnology. 2009;20(2):58–70. 10.1080/10495390902800792 [DOI] [PubMed] [Google Scholar]
- 39. Scuteri A, Sanna S, Chen W-M, Uda M, Albai G, Strait J, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS genetics. 2007;3(7):e115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fontanesi L, Scotti E, Buttazzoni L, Davoli R, Russo V. The porcine fat mass and obesity associated (FTO) gene is associated with fat deposition in Italian Duroc pigs. Animal genetics. 2009;40(1):90–3. 10.1111/j.1365-2052.2008.01777.x [DOI] [PubMed] [Google Scholar]
- 41. Liu F-H, Song J-Y, Shang X-R, Meng X-R, Ma J, Wang H-J. The Gene-Gene Interaction of INSIG-SCAP-SREBP Pathway on the Risk of Obesity in Chinese Children. BioMed research international. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lesnik Oberstein SA, Kriek M, White SJ, Kalf ME, Szuhai K, den Dunnen JT, et al. Peters Plus Syndrome Is Caused by Mutations in B3GALTL, a Putative Glycosyltransferase. The American Journal of Human Genetics. 2006;79(3):562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biology of reproduction. 2005;73(2):351–7. [DOI] [PubMed] [Google Scholar]
- 44. Levy D, Kuo AJ, Chang Y, Schaefer U, Kitson C, Cheung P, et al. SETD6 lysine methylation of RelA couples GLP activity at chromatin to tonic repression of NF-κB signaling. Nature immunology. 2011;12(1):29 10.1038/ni.1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sayre B, Harris G. Systems genetics approach reveals candidate genes for parasite resistance from quantitative trait loci studies in agricultural species. Animal genetics. 2012;43(2):190–8. 10.1111/j.1365-2052.2011.02231.x [DOI] [PubMed] [Google Scholar]
- 46. Ayele FT, Doumatey A, Huang H, Zhou J, Charles B, Erdos M, et al. Genome-wide associated loci influencing interleukin (IL)-10, IL-1Ra, and IL-6 levels in African Americans. Immunogenetics. 2012;64(5):351–9. 10.1007/s00251-011-0596-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li L, Huang J, Zhang X, Ju Z, Qi C, Zhang Y, et al. One SNP in the 3′-UTR of HMGB1 gene affects the binding of target bta-miR-223 and is involved in mastitis in dairy cattle. Immunogenetics. 2012;64(11):817–24. 10.1007/s00251-012-0641-1 [DOI] [PubMed] [Google Scholar]
- 48. Shahin H, Walsh T, Sobe T, Abu Sa’ed J, Abu Rayan A, Lynch ED, et al. Mutations in a novel isoform of TRIOBP that encodes a filamentous-actin binding protein are responsible for DFNB28 recessive nonsyndromic hearing loss. The American Journal of Human Genetics. 2006;78(1):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nature genetics. 2002;30(3):277–84. [DOI] [PubMed] [Google Scholar]
- 50. Wilson GR, Morton JD, Palmer DN, McEwan JC, Gately K, Anderson RM, et al. The locus for an inherited cataract in sheep maps to ovine chromosome 6. Molecular vision. 2012;18:1384 [PMC free article] [PubMed] [Google Scholar]
- 51. Borràs E, de Sousa Dias M, Hernan I, Pascual B, Mañé B, Gamundi MJ, et al. Detection of novel genetic variation in autosomal dominant retinitis pigmentosa. Clinical genetics. 2013;84(5):441–52. 10.1111/cge.12151 [DOI] [PubMed] [Google Scholar]
- 52. Fahim AT, Bowne SJ, Sullivan LS, Webb KD, Williams JT, Wheaton DK, et al. Polymorphic variation of RPGRIP1L and IQCB1 as modifiers of X-linked retinitis pigmentosa caused by mutations in RPGR Retinal Degenerative Diseases: Springer; 2012. p. 313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shakhsi-Niaei M, Klukowska-Roetzler J, Droegemueller C, Gerber V, Leeb T. The equine DNAH3 gene: SNP discovery and exclusion of an involvement in recurrent airway obstruction (RAO) in European Warmblood horses. ARCHIV FUR TIERZUCHT-ARCHIVES OF ANIMAL BREEDING. 2013;56:1–10. [Google Scholar]
- 54. Akhabir L, Sandford A. Identification of a functional SNP in an asthma gene: IL1RL1. Allergy, asthma, and clinical immunology: official journal of the Canadian Society of Allergy and Clinical Immunology. 2010;6(Suppl 3):P1. [Google Scholar]
- 55. Wang Z, Zhang H, Yang H, Wang S, Rong E, Pei W, et al. Genome-wide association study for wool production traits in a Chinese Merino sheep population. PloS one. 2014;9(9):e107101 10.1371/journal.pone.0107101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Meira C, Fortes M, Farah M, Porto-Neto L, Curi R, Moore S, et al. , editors. A genome-wide association study for height at withers in racing quarter horse. Proc Assoc Advmt Anim Breed Genet; 2013. [Google Scholar]
- 57. Doran AG, Berry DP, Creevey CJ. Whole genome association study identifies regions of the bovine genome and biological pathways involved in carcass trait performance in Holstein-Friesian cattle. BMC genomics. 2014;15(1):837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim J, Li Y, Lee J, Lee Y. Application of linkage disequilibrium mapping methods to detect QTL for carcass quality on chromosome 6 using a high density SNP map in Hanwoo. Asian-Aust J Anim Sci. 2011;24:457–62. [Google Scholar]
- 59. Raschetti M, Castiglioni B, Caroli A, Guiatti D, Pagnacco G, Chessa S. SNP identification in swine candidate genes for meat quality. Livestock Science. 2013;155(2):165–71. [Google Scholar]
- 60. Ma J, Yang J, Zhou L, Ren J, Liu X, Zhang H, et al. A Splice Mutation in the PHKG1 Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle. PLoS genetics. 2014;10(10):e1004710 10.1371/journal.pgen.1004710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Felício A, Boschiero C, Balieiro J, Ledur M, Ferraz J, Michelan Filho T, et al. Identification and association of polymorphisms in CAPN1 and CAPN3 candidate genes related to performance and meat quality traits in chickens. Genetics and Molecular Re-search. 2013;12(1):472 10.4238/2013.February.8.12 [DOI] [PubMed] [Google Scholar]
- 62. Pierce A, Bliesner B, Xu M, Nielsen-Preiss S, Lemke G, Tobet S, et al. Axl and Tyro3 modulate female reproduction by influencing gonadotropin-releasing hormone neuron survival and migration. Molecular Endocrinology. 2008;22(11):2481–95. 10.1210/me.2008-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Adams HA, Southey BR, Everts RE, Marjani SL, Tian CX, Lewin HA, et al. Transferase activity function and system development process are critical in cattle embryo development. Functional & integrative genomics. 2011;11(1):139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Strillacci MG, Frigo E, Schiavini F, Samoré AB, Canavesi F, Vevey M, et al. Genome-wide association study for somatic cell score in Valdostana Red Pied cattle breed using pooled DNA. BMC genetics. 2014;15(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li H, Wang Z, Moore S, Schenkel F, Stothard P. Genome-wide scan for positional and functional candidate genes affecting milk production traits in Canadian Holstein Cattle Proc 9th WCGALP, Leipzig, Germany: 2010;26. [Google Scholar]
- 67. Wang X. Identification and Characterization of Candidate Genes for Complex Traits in Cattle 2012. [Google Scholar]
- 68. Pan D, Zhang S, Jiang J, Jiang L, Zhang Q, Liu J. Genome-wide detection of selective signature in Chinese Holstein. PloS one. 2013;8(3):e60440 10.1371/journal.pone.0060440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Al-Mamun H, Clark S, Kwan P, Gondro C, editors. Genome-Wide Association Study on Body Weight Reveals Major Loci on OAR6 in Australian Merino Sheep 10th World Congress on Genetics Applied to Livestock Production; 2014: Asas. [Google Scholar]
- 70. Kijas JW, Naumova A. Haplotype-based analysis of selective sweeps in sheep. Genome. 2014;57(999):1–5. [DOI] [PubMed] [Google Scholar]
- 71. Tetens J, Widmann P, Kühn C, Thaller G. A genome-wide association study indicates LCORL/NCAPG as a candidate locus for withers height in German Warmblood horses. Animal genetics. 2013;44(4):467–71. 10.1111/age.12031 [DOI] [PubMed] [Google Scholar]
- 72. Lindholm-Perry AK, Kuehn LA, Oliver WT, Sexten AK, Miles JR, Rempel LA, et al. Adipose and muscle tissue gene expression of two genes (NCAPG and LCORL) located in a chromosomal region associated with cattle feed intake and gain. PloS one. 2013;8(11):e80882 10.1371/journal.pone.0080882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Setoguchi K, Watanabe T, Weikard R, Albrecht E, Kühn C, Kinoshita A, et al. The SNP c. 1326T> G in the non-SMC condensin I complex, subunit G (NCAPG) gene encoding a p. Ile442Met variant is associated with an increase in body frame size at puberty in cattle. Animal genetics. 2011;42(6):650–5. 10.1111/j.1365-2052.2011.02196.x [DOI] [PubMed] [Google Scholar]
- 74. Xu L, Bickhart DM, Cole JB, Schroeder SG, Song J, Van Tassell CP, et al. Genomic signatures reveal new evidences for selection of important traits in domestic cattle. Molecular biology and evolution. 2014. 10.1093/molbev/msu333 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Smith TP, Showalter AD, Sloop KW, Rohrer GA, Fahrenkrug SC, Meier BC, et al. Identification of porcine Lhx3 and SF1 as candidate genes for QTL affecting growth and reproduction traits in swine. Animal genetics. 2001;32(6):344–50. . [DOI] [PubMed] [Google Scholar]
- 76. Norris BJ, Whan VA. A gene duplication affecting expression of the ovine ASIP gene is responsible for white and black sheep. Genome research. 2008;18(8):1282–93. 10.1101/gr.072090.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barrett JH, Iles MM, Harland M, Taylor JC, Aitken JF, Andresen PA, et al. Genome-wide association study identifies three new melanoma susceptibility loci. Nat Genet. 2011;43(11):1108–13. 10.1038/ng.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kijas JW, Lenstra JA, Hayes B, Boitard S, Porto Neto LR, San Cristobal M, et al. Genome-wide analysis of the world's sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS biology. 2012;10(2):e1001258 10.1371/journal.pbio.1001258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li M, Tiirikka T, Kantanen J. A genome-wide scan study identifies a single nucleotide substitution in ASIP associated with white versus non-white coat-colour variation in sheep (Ovis aries). Heredity. 2013;112(2):122–31. 10.1038/hdy.2013.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Winder W, Holmes B, Rubink D, Jensen E, Chen M, Holloszy J. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. Journal of applied physiology. 2000;88(6):2219–26. [DOI] [PubMed] [Google Scholar]
- 81. Fontanesi L, Davoli R, Nanni Costa L, Beretti F, Scotti E, Tazzoli M, et al. Investigation of candidate genes for glycolytic potential of porcine skeletal muscle: Association with meat quality and production traits in Italian Large White pigs. Meat science. 2008;80(3):780–7. 10.1016/j.meatsci.2008.03.022 . [DOI] [PubMed] [Google Scholar]
- 82. Li W-F, Li J-Y, Gao X, Xu S-Z, Yue W-B. Association analysis of PRKAG3 gene variants with carcass and meat quality traits in beef cattle. African Journal of Biotechnology. 2014;11(8):1855–61. [Google Scholar]
- 83. Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000;288(5469):1248–51. . [DOI] [PubMed] [Google Scholar]
- 84. Zhang C, Wang Z, Bruce H, Kemp R, Charagu P, Miar Y, et al. , editors. Genome-wide association studies (GWAS) identifies a QTL close to PRKAG3 affecting meat pH and colour in crossbred commercial pig lines Proceedings, 10th World Congress of Genetics Applied to Livestock Production; 2014; BC, Canada. [Google Scholar]
- 85. Kirikoshi H, Sekihara H, Katoh M. WNT10A and WNT6, clustered in human chromosome 2q35 region with head-to-tail manner, are strongly coexpressed in SW480 cells. Biochemical and biophysical research communications. 2001;283(4):798–805. 10.1006/bbrc.2001.4855 . [DOI] [PubMed] [Google Scholar]
- 86. Geetha-Loganathan P, Nimmagadda S, Christ B, Huang R, Scaal M. Ectodermal Wnt6 is an early negative regulator of limb chondrogenesis in the chicken embryo. BMC Dev Biol. 2010;10:32 10.1186/1471-213X-10-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Christodoulides C, Scarda A, Granzotto M, Milan G, Dalla Nora E, Keogh J, et al. WNT10B mutations in human obesity. Diabetologia. 2006;49(4):678–84. 10.1007/s00125-006-0144-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lawson Handley LJ, Byrne K, Santucci F, Townsend S, Taylor M, Bruford MW, et al. Genetic structure of European sheep breeds. Heredity. 2007;99(6):620–31. 10.1038/sj.hdy.6801039 . [DOI] [PubMed] [Google Scholar]
- 89. Zhang L, Mousel MR, Wu X, Michal JJ, Zhou X, Ding B, et al. Genome-wide genetic diversity and differentially selected regions among Suffolk, Rambouillet, Columbia, Polypay, and Targhee sheep. PloS one. 2013;8(6):e65942 10.1371/journal.pone.0065942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Galic S, Sachithanandan N, Kay TW, Steinberg GR. Suppressor of cytokine signalling (SOCS) proteins as guardians of inflammatory responses critical for regulating insulin sensitivity. The Biochemical journal. 2014;461(2):177–88. 10.1042/BJ20140143 . [DOI] [PubMed] [Google Scholar]
- 91. Newgard CB, Brady MJ, O'Doherty RM, Saltiel AR. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes. 2000;49(12):1967–77. . [DOI] [PubMed] [Google Scholar]
- 92. Zhong W, Xie Y, Abdallah M, Awonuga AO, Slater JA, Sipahi L, et al. Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction. 2010;140(6):921–30. 10.1530/REP-10-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Artemenko Y, Gagnon A, Aubin D, Sorisky A. Anti-adipogenic effect of PDGF is reversed by PKC inhibition. Journal of cellular physiology. 2005;204(2):646–53. [DOI] [PubMed] [Google Scholar]
- 94. Holmstrom TE, Mattsson CL, Falting JM, Nedergaard J. Differential signalling pathways for EGF versus PDGF activation of Erk1/2 MAP kinase and cell proliferation in brown pre-adipocytes. Experimental cell research. 2008;314(19):3581–92. 10.1016/j.yexcr.2008.09.007 . [DOI] [PubMed] [Google Scholar]
- 95. LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, et al. PDGF-D, a new protease-activated growth factor. Nature cell biology. 2001;3(5):517–21. 10.1038/35074593 . [DOI] [PubMed] [Google Scholar]
- 96. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All data underlying the findings in the study are freely available in the manuscript and supplemental files.