Abstract
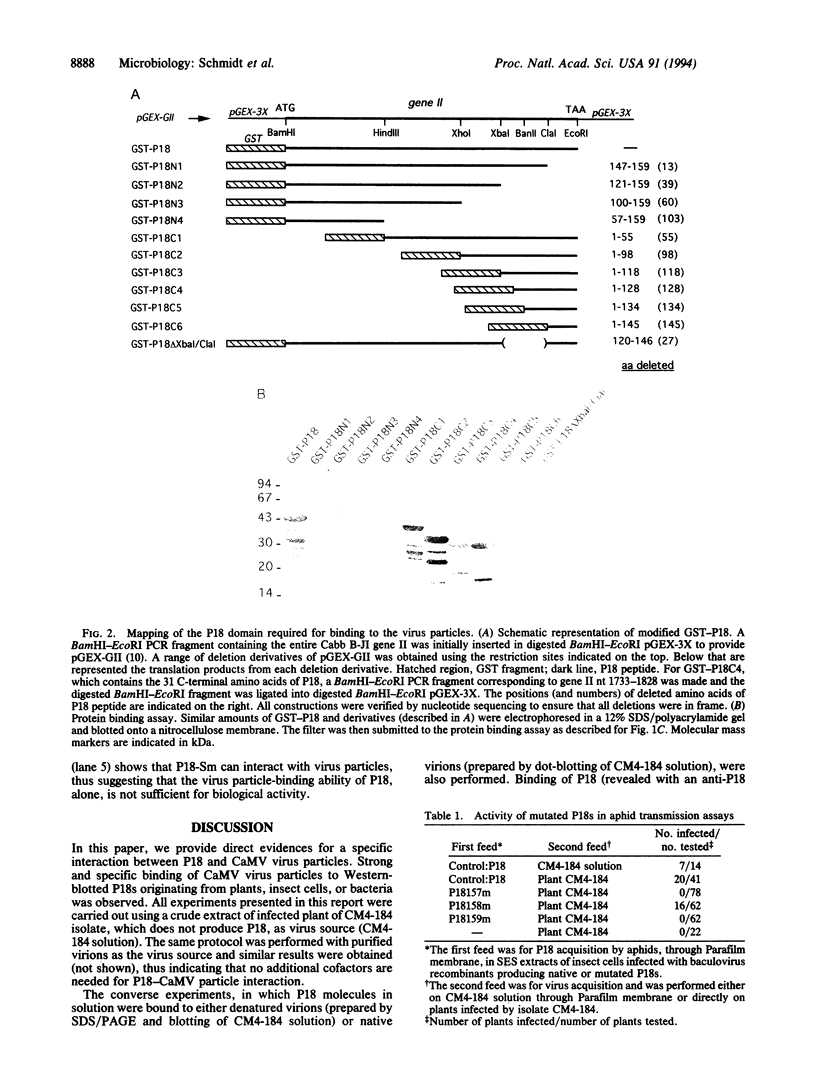
Cauliflower mosaic virus (CaMV) aphid transmission factor (ATF or P18) is presumed to interact with both virus particles and vector mouthparts, thereby mediating virus aphid transmission. We developed a protein-protein binding assay and our results clearly show that virus particles bind strongly and specifically to P18 whether P18 was obtained from plants, a baculovirus expression system, or the pGEX-3X Escherichia coli expression system. We overproduced, using the pGEX-3X expression system, various fragments of P18 and thereby demonstrated that the C-terminal 31 amino acid residues are responsible for the interaction. Using PCR-based mutagenesis, 2 amino acid residues essential for interaction were identified. Point substitutions (amino acids 157 from Ile to Asn or 159 from Gly to Ser) were sufficient to abolish the interaction, whereas another mutation (amino acid 158 from Ile to Ser) had no effect on P18 virus binding. We evaluated whether there was a correlation between the ability of P18 to interact with CaMV particles and its biological activity. Aphid transmission assays were carried out and we demonstrated that the loss of the virus binding capacity had a dramatic effect on the ability of P18 to mediate aphid transmission. Thus, our results suggest that binding between P18 and virus particles is likely to be one of the molecular mechanisms involved in CaMV aphid transmission.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atreya C. D., Raccah B., Pirone T. P. A point mutation in the coat protein abolishes aphid transmissibility of a potyvirus. Virology. 1990 Sep;178(1):161–165. doi: 10.1016/0042-6822(90)90389-9. [DOI] [PubMed] [Google Scholar]
- Atreya P. L., Atreya C. D., Pirone T. P. Amino acid substitutions in the coat protein result in loss of insect transmissibility of a plant virus. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7887–7891. doi: 10.1073/pnas.88.17.7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balàzs E., Guilley H., Jonard G., Richards K. Nucleotide sequence of DNA from an altered-virulence isolate D/H of the cauliflower mosaic virus. Gene. 1982 Oct;19(3):239–249. doi: 10.1016/0378-1119(82)90013-0. [DOI] [PubMed] [Google Scholar]
- Blanc S., Cerutti M., Chaabihi H., Louis C., Devauchelle G., Hull R. Gene II product of an aphid-nontransmissible isolate of cauliflower mosaic virus expressed in a baculovirus system possesses aphid transmission factor activity. Virology. 1993 Feb;192(2):651–654. doi: 10.1006/viro.1993.1081. [DOI] [PubMed] [Google Scholar]
- Blanc S., Cerutti M., Usmany M., Vlak J. M., Hull R. Biological activity of cauliflower mosaic virus aphid transmission factor expressed in a heterologous system. Virology. 1993 Feb;192(2):643–650. doi: 10.1006/viro.1993.1080. [DOI] [PubMed] [Google Scholar]
- Blanc S., Schmidt I., Kuhl G., Esperandieu P., Lebeurier G., Hull R., Cerutti M., Louis C. Paracrystalline structure of cauliflower mosaic virus aphid transmission factor produced both in plants and in a heterologous system and relationship with a solubilized active form. Virology. 1993 Nov;197(1):283–292. doi: 10.1006/viro.1993.1589. [DOI] [PubMed] [Google Scholar]
- Chaabihi H., Ogliastro M. H., Martin M., Giraud C., Devauchelle G., Cerutti M. Competition between baculovirus polyhedrin and p10 gene expression during infection of insect cells. J Virol. 1993 May;67(5):2664–2671. doi: 10.1128/jvi.67.5.2664-2671.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenault K. D., Steffens D. L., Melcher U. Nucleotide Sequence of Cauliflower Mosaic Virus Isolate NY8153. Plant Physiol. 1992 Sep;100(1):542–545. doi: 10.1104/pp.100.1.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert S., Shepherd R. J., Gardner R. C. Insertional mutagenesis of the cauliflower mosaic virus genome. Gene. 1983 Nov;25(2-3):201–208. doi: 10.1016/0378-1119(83)90224-x. [DOI] [PubMed] [Google Scholar]
- Delseny M., Hull R. Isolation and characterization of faithful and altered clones of the genomes of cauliflower mosaic virus isolates Cabb B-JI, CM4-184, and Bari I. Plasmid. 1983 Jan;9(1):31–41. doi: 10.1016/0147-619x(83)90029-x. [DOI] [PubMed] [Google Scholar]
- Espinoza A. M., Usmany M., Pirone T. P., Harvey M., Woolston C. J., Medina V., Vlak J. M., Hull R. Expression of cauliflower mosaic virus ORF II in a baculovirus system. Intervirology. 1992;34(1):1–12. doi: 10.1159/000150257. [DOI] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Givord L., Xiong C., Giband M., Koenig I., Hohn T., Lebeurier G., Hirth L. A second cauliflower mosaic virus gene product influences the structure of the viral inclusion body. EMBO J. 1984 Jun;3(6):1423–1427. doi: 10.1002/j.1460-2075.1984.tb01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govier D. A., Kassanis B. A virus-induced component of plant sap needed when aphids acquire potato virus Y from purified preparations. Virology. 1974 Oct;61(2):420–426. doi: 10.1016/0042-6822(74)90278-5. [DOI] [PubMed] [Google Scholar]
- Govier D. A., Kassanis B. Evidence that a component other than the virus particle is needed for aphid transmission of potato virus Y. Virology. 1974 Jan;57(1):285–286. doi: 10.1016/0042-6822(74)90129-9. [DOI] [PubMed] [Google Scholar]
- Harrison B. D., Robinson D. J. Molecular variation in vector-borne plant viruses: epidemiological significance. Philos Trans R Soc Lond B Biol Sci. 1988 Oct 31;321(1207):447–462. doi: 10.1098/rstb.1988.0102. [DOI] [PubMed] [Google Scholar]
- Homann H. E., Willenbrink W., Buchholz C. J., Neubert W. J. Sendai virus protein-protein interactions studied by a protein-blotting protein-overlay technique: mapping of domains on NP protein required for binding to P protein. J Virol. 1991 Mar;65(3):1304–1309. doi: 10.1128/jvi.65.3.1304-1309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R., Sadler J., Longstaff M. The sequence of carnation etched ring virus DNA: comparison with cauliflower mosaic virus and retroviruses. EMBO J. 1986 Dec 1;5(12):3083–3090. doi: 10.1002/j.1460-2075.1986.tb04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lung M. C., Pirone T. P. Acquisition factor required for aphid transmission of purified cauliflower mosaic virus. Virology. 1974 Jul;60(1):260–264. doi: 10.1016/0042-6822(74)90383-3. [DOI] [PubMed] [Google Scholar]
- Richins R. D., Scholthof H. B., Shepherd R. J. Sequence of figwort mosaic virus DNA (caulimovirus group). Nucleic Acids Res. 1987 Oct 26;15(20):8451–8466. doi: 10.1093/nar/15.20.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd R. J., Wakeman R. J., Romanko R. R. DNA in cauliflower mosaic virus. Virology. 1968 Sep;36(1):150–152. doi: 10.1016/0042-6822(68)90127-x. [DOI] [PubMed] [Google Scholar]
- Woolston C. J., Covey S. N., Penswick J. R., Davies J. W. Aphid transmission and a polypeptide are specified by a defined region of the cauliflower mosaic virus genome. Gene. 1983 Jul;23(1):15–23. doi: 10.1016/0378-1119(83)90212-3. [DOI] [PubMed] [Google Scholar]