Abstract
Context
Conduct disorder (CD) is characterized by severe antisocial behavior that emerges in childhood (early- onset CD [EO-CD]) or adolescence (adolescence-onset CD [AO-CD]). Early-onset CD is proposed to have a neurodevelopmental basis, whereas AO-CD is thought to emerge owing to social mimicry of deviant peers. However, this developmental taxonomic theory is debated after reports of neuropsychological impairments in both CD subtypes. A critical, although unaddressed, issue is whether these subtypes present similar or distinct neurophysiological profiles. Hence, we investigated neurophysiological responses to emotional and neutral faces in regions associated with antisocial behavior (ie, the amygdala, ventromedial prefrontal cortex, insula, and orbitofrontal cortex) in individuals with EO-CD and AO-CD and in healthy control subjects.
Objective
To investigate whether EO-CD and AO-CD subjects show neurophysiological abnormalities.
Design
Case-control study.
Setting
Government research institute, university department.
Participants
Seventy-five male adolescents and young adults aged 16 to 21 years, including 27 with EO-CD, 25 with AO-CD, and 23 healthy controls.
Main Outcome Measure
Neural activations measured by functional magnetic resonance imaging while participants viewed angry, sad, and neutral faces.
Results
Comparing angry vs neutral faces, participants with both CD subtypes displayed reduced responses in regions associated with antisocial behavior compared with controls; differences between the CD subtypes were not significant. Comparing each expression with fixation baseline revealed an abnormal (increased) amygdala response to neutral but not angry faces in both groups of CD relative to controls. For sad vs neutral faces, reduced amygdala activation was observed in EO-CD relative to AO-CD and control participants. Comparing each expression with fixation revealed hypoactive amygdala responses to sadness in individuals with EO-CD relative to AO-CD participants and controls. These findings were not accounted for by attention-deficit/hyperactivity disorder symptoms.
Conclusions
Neurophysiological abnormalities are observed in both CD subtypes, contrary to the developmental taxonomic theory of CD. Additional amygdala hypofunction in relation to sad expressions might indicate why EO-CD is more severe and persistent than AO-CD.
CONDUCT DISORDER (CD) emerges in childhood or adolescence and is characterized by a pervasive pattern of aggressive and antisocial behavior.1 Individuals with CD are at increased risk of developing a range of mental and physical health problems in adulthood.2-4 Their antisocial behavior and greater use of public services means that it costs society 10 times more to raise children with CD to adulthood compared with those without conduct problems.5,6
A central issue concerns the etiology of 2 putatively distinct developmental trajectories of antisocial behavior. Following Moffitt’s influential developmental taxonomic theory,7 the DSM-IV distinguishes between the following 2 subtypes of CD: an early-onset (EO-CD) variant in which severe antisocial behavior emerges in childhood, and an adolescence-onset (AO-CD) subtype developing after 10 years of age.1 Individuals with EO-CD are more likely to display aggressive symptoms and to develop antisocial personality disorder in adulthood than those with AO-CD.1 Moffitt7 posited that only EO-CD has a neurodevelopmental basis, as evidenced by neuropsychological impairments in verbal and executive functions, together with differences in temperament and emotional reactivity. In contrast, she excluded nervous system abnormalities as contributing to adolescence-limited CD and proposed that this form of antisocial behavior reflects social mimicry of deviant peers.7 Support for Moffitt’s hypothesis would require evidence of abnormal neurophysiological function in the EO-CD variant alone, but there are reasons to suspect that this may not be found. In particular, recent behavioral studies8-10 reported equivalent impairments in the EO-CD and AO-CD subtypes on neuropsychological tasks assessing cognitive and emotional functions. This suggests possible commonalities in the neurophysiological profiles of the 2 variants, thereby providing support for a shared neurophysiological etiology. We investigated this issue for the first time, to our knowledge, in the context of a functional magnetic resonance imaging (fMRI) experiment in which participants viewed emotional and neutral facial expressions.
Previous research11,12 has shown that the neural response to facial expressions provides an effective index of abnormal brain function in individuals with conduct problems. Recent findings by our group10 in adolescents with CD demonstrated a disproportionate impairment in recognizing angry facial expressions and an additional impairment in sadness recognition in CD participants with psychopathic traits. Consequently, in the present study we characterized the neurophysiological correlates of processing these facial expressions in EO-CD and AO-CD participants relative to healthy control subjects matched for age, socioeconomic status, and performance IQ. On the basis of previous research11-16 in individuals with conduct problems showing structural abnormalities in the amygdala, ventromedial prefrontal cortex (vmPFC), insula, and orbitofrontal cortex (OFC) and reduced activation in these regions when viewing emotional stimuli, we predicted that CD participants would show a reduced neurophysiological response in these areas relative to controls. In addition, we examined the specific prediction of Moffitt’s developmental taxonomic theory that abnormal brain activations should be evident only in participants with EO-CD relative to controls.7
Given the high comorbidity between CD and attention-deficit/hyperactivity disorder (ADHD),17 some earlier studies12,18 included a comparison group of participants with ADHD to disaggregate brain abnormalities associated with CD alone from those associated with ADHD. Herein we adopted an alternative approach that involved conducting additional analyses factoring out any contribution of current and lifetime/ever ADHD symptoms. In addition, by recruiting participants from the community rather than clinics, the prevalence of comorbid illness was reduced in our sample relative to other studies.16,17,19,20
Two subsidiary hypotheses were investigated regarding the magnitude of brain responses to emotional expressions. Previous work has emphasized the importance of callous-unemotional (CU) traits in predicting reduced amygdala response to fearful facial expressions11,12 and recognition of fearful and sad expressions.10 In addition, research has demonstrated that reduced amygdala activity is associated with more aggressive symptoms.16 We therefore determined whether individual variability in CU traits, overall psychopathic traits, and/or CD symptoms (including aggressive behavior) modulated the findings.
METHODS
PARTICIPANTS
Fifty-two male adolescents and young adults with CD aged 16 to 21 years were recruited from schools, pupil referral units, and the Cambridge Youth Offending Service, Cambridge, England. Exclusion criteria included an IQ of less than 85 (estimated using the Wechsler Abbreviated Scale of Intelligence), the presence of a pervasive developmental disorder (eg, autism), or chronic physical illness. A healthy control group (no history of CD or oppositional defiant disorder and no current psychiatric illness) of 23 male adolescents were recruited from schools and colleges. To equate groups for performance IQ, controls with an estimated full-scale IQ of more than 115 were excluded. The study was approved by the local ethics committee, and all participants gave written informed consent.
Participants underwent assessment for CD, oppositional defiant disorder, ADHD, major depressive disorder, generalized anxiety disorder, obsessive-compulsive disorder, posttraumatic stress disorder, and substance dependence using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version.21 Full details of the assessment can be found in the supplementary materials (supplemental text, tables, and figures; http://www.mrc-cbu.cam.ac.uk/research/emotion/san/agpsupplemental.html). In brief, participants and their caregivers underwent separate diagnostic interviews, and diagnoses were reached by combining information from both interviews. Participants were regarded as having EO-CD if they or their caregivers reported that at least 1 CD symptom and functional impairment was present before 10 years of age.1 If no symptoms were reported by the proband or caregiver during the first 10 years of life but they subsequently developed CD, a diagnosis of AO-CD was given. According to these criteria, 27 participants were classified as having EO-CD and 25 as having AO-CD. None of our participants had childhood-limited conduct problems.
Comprehensive data relating to all 18 symptoms of ADHD defined in the DSM-IV, using the ADHD supplement of the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version, were unavailable for 3 participants with EO-CD and 4 with AO-CD. Hence, the reanalysis of fMRI data factoring out the contribution of current and lifetime/ever ADHD symptoms relates to 53 participants (ie, 20 controls, 17 participants with EO-CD, and 16 with AO-CD).
Callous-unemotional and overall psychopathic traits were assessed using the CU dimension subscale and the total score on the Youth Psychopathic Traits Inventory, respectively.22 The Spielberger State-Trait Anxiety Inventory provided an additional assessment of anxiety.23
fMRI TASK
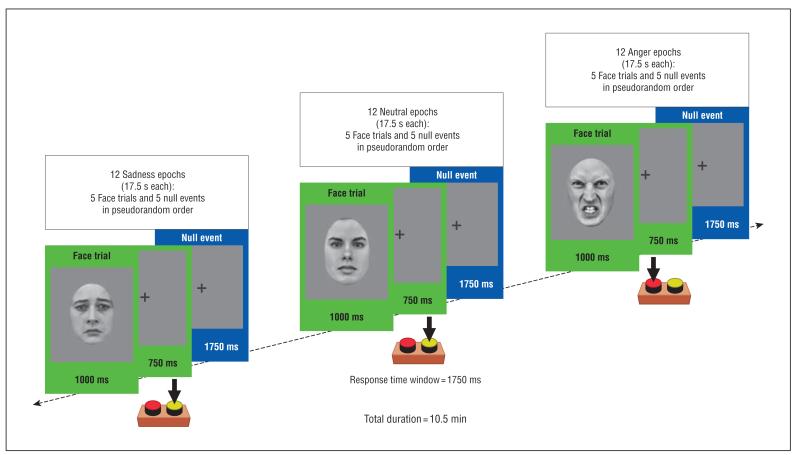
Participants categorized the sex of gray-scale photographs of angry, sad, and neutral faces (half female) posed by 30 different identities (Figure 1). The faces were selected from 2 stimulus sets24,25 on the basis of emotional ratings from an independent sample.26 Emotional ratings were also obtained from all participants in the present study after the fMRI session. Stimuli were presented in 17.5-second epochs containing 5 faces from the same category (angry, sad, or neutral) intermixed with 5 null events (fixation cross). Each face trial comprised a 1000-millisecond presentation of a face followed by a fixation cross (750 milliseconds). Null events constituted a 1750-millisecond presentation of the same fixation cross. The stimuli during each epoch were pseudorandomized with respect to trial type (face or null events) and the face’s sex and identity; no more than 3 consecutive trials were of the same trial type. The pseudorandomization enhanced design efficiency while preserving the unpredictability of stimulus onsets in naïve participants. Twelve epochs of each category were presented (60 angry, 60 sad, and 60 neutral faces; total duration, 10 minutes 30 seconds). Reaction times (RTs) and accuracy were recorded throughout.
Figure 1.
Functional magnetic resonance imaging (fMRI) paradigm and examples of stimuli used (sex discrimination). All participants were shown alternating 17.5-second epochs containing photographs of angry, sad, or neutral facial expressions (12 epochs of each). Each epoch comprised 5 face trials (green frames) interspersed with 5 null events (fixation cross) (blue frames). A full description of the paradigm is given in the “fMRI Task” subsection of the “Methods” section.
IMAGE ACQUISITION AND PREPROCESSING
Functional MRI scanning was performed on a 3-T unit (Siemens Tim Trio with a head coil gradient set; Siemens, Surrey, England) at the Cognition and Brain Sciences Unit. Whole-brain data were acquired with echo-planar T2-weighted imaging (EPI) sensitive to the blood oxygenation level–dependent signal contrast (32 axial slices, 3mm thickness; repetition time, 2000 milliseconds; echo time, 30 milliseconds; voxel size, 3×3×3 mm). Data were analyzed using statistical parametric mapping software (SPM5; http://www.fil.ion.ucl.ac.uk/spm/). The EPIs were sinc interpolated in time to correct for slice time differences and realigned to the first scan by rigid body transformations to correct for head movements. The mean EPI was computed for each subject and inspected to ensure that none showed excessive signal dropout in the medial temporal cortex and OFC. The EPIs were coregistered and normalized to the T1 standard template in the MNI space (Montreal Neurological Institute) using linear and nonlinear transformations and were smoothed with a gaussian kernel of full width at half maximum of 8 mm.
fMRI ANALYSES
For each participant, a general linear model assessed regionally specific effects of task parameters on blood oxygenation level–dependent indices of activation.27 The model included experimental factors (angry, sad, and neutral face trials and null/fixation events) and 6 realignment parameters as effects of no interest to account for residual motion-related variance. Low-frequency signal drift was removed using a high-pass filter (cutoff, 128 seconds), and an autoregressive modeling (AR[1]) of temporal autocorrelations was applied.
Contrast images for comparing angry vs neutral face trials were generated and entered into a second-level general linear model analysis of variance (ANOVA) to produce an SPM-F map that investigated the main effect of group (EO-CD, AO-CD, or control); a similar ANOVA addressed the main effect of group for the sad vs neutral comparison. Follow-up analyses decomposed the main effects of group by testing the hypothesis that CD participants, irrespective of group (ie, EO-CD and AO-CD groups combined), display reduced brain activations for angry vs neutral and sad vs neutral comparisons relative to controls. Because Moffitt’s theory predicts that neurodevelopmental abnormalities underlie EO-CD but not AO-CD,7 we also report separate comparisons between each CD group and controls for the same contrasts and examine whether a comparison of the EO-CD and AO-CD groups showed significant differences for the angry vs neutral and sad vs neutral contrasts.
Additional analyses were conducted to determine whether the main effects of group for the angry vs neutral and sad vs neutral contrasts in the a priori regions of interest (ROIs) reflected changes in the neural response to the emotional (ie, angry or sad) and/or neutral expressions. Subject-specific contrast images were generated for each facial expression vs null/fixation events (ie, angry vs null, sad vs null, and neutral vs null) and entered into second-level analyses exploring the main effect of group for each. From the angry vs null and neutral vs null contrasts, we extracted data corresponding to the local maxima detected by the main effect of group for the angry vs neutral comparison to determine whether the latter contrast was driven by group differences to angry and/or neutral faces. Similar analyses of the sad vs null and neutral vs null contrasts were conducted from data extracted from the local maxima corresponding to the group effect of sad vs neutral.
Finally, we assessed whether individual differences in CU traits, overall psychopathic traits, and CD symptoms (ie, lifetime/ever, aggressive, or current symptoms) were correlated with the neural response for the angry vs neutral and sad vs neutral contrasts. This was examined in each group independently, in both CD groups combined, and in all participants together.
To remove any potential confounding influence of ADHD symptoms, all principal analyses were repeated including lifetime/ever and current ADHD symptoms as separate covariates of no interest.
Two approaches for thresholding second-level maps were applied. First, for a priori ROIs, the threshold was P<.05, family-wise error (FWE) correction for multiple comparisons in small volumes (ie, small volume correction [svc]).28,29 The amygdala, vmPFC, insula, and OFC were defined as ROIs given their proposed role in the pathophysiologic mechanism of CD.11,13-15,18-20,30 All ROIs were anatomical regions defined using the “aal.02” atlas for automated anatomical labeling.31 Brain regions that were not predicted a priori but met a threshold of P<.001, uncorrected, for 10 or more contiguous voxels are also reported.
RESULTS
PARTICIPANTS
Thirteen participants (6 with EO-CD, 5 with AO-CD, and 2 controls) were excluded owing to excessive movements during scanning. One additional control and another participant with EO-CD were excluded because of technical error and poor performance on the fMRI task (<60%), respectively. Table 1 summarizes the demographic and clinical characteristics of participants included in the fMRI analyses. Groups were matched for age (F2,57=1.2 [P=.21]) and performance IQ (F2,57=2.0 [P=.15]). Both CD subtypes scored higher in overall psychopathic (F2,57=10.5 [P<.001]) and CU (F2,57=6.2 [P<.005]) traits than did controls, but the participants with CD subtypes did not differ from each other on either measure (F1,38<1 [P>.70]).
Table 1. Demographic and Clinical Characteristics and fMRI Task Performances of Study Participants Included in the fMRI Analysesa.
| Participants |
|||
|---|---|---|---|
| Measure | EO-CD | AO-CD | Controls |
| Age, y | 17.7 (1.2) | 17.1 (1.0) | 17.8 (0.9) |
| Performance IQ | 101.6 (6.2) | 105.4 (6.5) | 109.0 (4.8) |
| No. of symptomsb | |||
| Current CD | 4.8 (2.5) | 4.6 (1.6) | 0.0 (0.2) |
| Lifetime/ever CD | 9.4 (1.6) | 7.0 (2.3) | 0.4 (0.6) |
| Aggressive CD | 3.8 (0.8) | 2.9 (1.2) | 0.1 (0.3) |
| Current ADHD | 6.7 (4.6) | 3.5 (3.9) | 1.3 (1.9) |
| Lifetime/ever ADHD | 8.7 (4.1) | 5.9 (4.6) | 2.4 (2.5) |
| No. of current DSM-IV comorbid diagnoses |
|||
| ADHD | 7 | 2 | 0 |
| MDD | 1 | 0 | 0 |
| Substance dependence, cannabis |
1 | 0 | 0 |
| No. of past DSM-IV comorbid diagnoses |
|||
| ADHDc | 0 | 3 | 0 |
| MDDc | 3 | 2 | 3 |
| Total YPI score | 2.4 (0.4) | 2.4 (0.3) | 2.0 (0.3) |
| CU traits | 0.7 (0.1) | 0.7 (0.1) | 0.6 (0.1) |
| STAI score | |||
| State | 27.0 (5.3) | 31.0 (8.8) | 32.0 (6.7) |
| Trait | 37.0 (7.8) | 35.0 (7.4) | 36.0 (9.0) |
| fMRI task performances Accuracy, % |
|||
| Angry | 90 (5) | 91 (6) | 91 (4) |
| Sad | 93 (6) | 94 (4) | 95 (3) |
| Neutral | 93 (5) | 92 (5) | 95 (4) |
| RTs, ms | |||
| Angry | 737 (63) | 700 (63) | 752 (96) |
| Sad | 717 (50) | 691 (59) | 720 (87) |
| Neutral | 721 (46) | 703 (66) | 723 (77) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; AO, adolescence-onset; CD, conduct disorder; CU, callous-unemotional; EO, early-onset; fMRI, functional magnetic resonance imaging; MDD, major depressive disorder; RTs, reaction times; STAI, Spielberger State-Trait Anxiety Inventory; YPI, Youth Psychopathic Traits Inventory.
Unless otherwise indicated, data are expressed as mean (SD).
For symptoms, current CD means that symptoms were present within the past 12 months; lifetime/ever CD, present at some point during the participant’s lifetime even if they were no longer present; and aggressive CD, fighting, bullying, aggressive stealing, use of a weapon in a fight, and physical cruelty.
Numbers relate to those with a past diagnosis of MDD or ADHD who are now in remission.
Participants with EO-CD displayed a trend toward more lifetime/ever ADHD symptoms (F1,38=3.39 [P=.06]), presented with more current ADHD symptoms (F1,38=4.5 [P<.05]), and endorsed more lifetime/ever (F1,38=14.4 [P<.002]) and aggressive (F1,38=8.4 [P<.01]) CD symptoms compared with participants who had AO-CD. However, the CD groups did not differ in current CD symptoms (ie, those present within the past 12 months, F1,38=0.5 [P=.82]). Finally, no significant differences between CD groups were found in state (F2,57=2.3 [P=.11]) or trait (F2,57=1.4 [P=.27]) anxiety.
BEHAVIORAL FINDINGS
Accuracy or correct RT on the fMRI sex discrimination task were submitted to a 3×3 ANOVA examining group and expression. Neither measure showed an effect of group (accuracy, F2,57=0.6 [P=.54]; RT, F2,57=1.4 [P=.24]) or group×expression interaction (accuracy, F2,57=1.0 [P=.37]; RT, F2,57=1.6 [P=.16]) (Table 1). Emotional ratings of facial expressions obtained after scanning were submitted to a 3×3 ANOVA examining group and expression that showed no main effect of group (F2,57=0.8 [P=.44]) or group×expression interaction (F2,57=0.2 [P=.89]) (supplemental Figure 1).
fMRI RESULTS
Main Effect of Group for Angry Compared With Neutral Faces
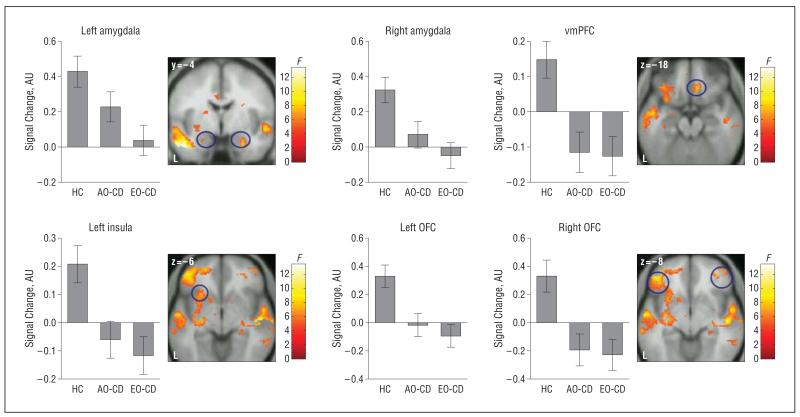
An ANOVA comparing the main effect of group for the angry vs neutral contrast identified several regions, including our ROIs (Figure 2 and Table 2). Follow-up analyses demonstrated that the combined CD group displayed reduced responses in ROIs and other regions relative to healthy controls (supplemental Table 1). In addition, separate comparisons between each CD group and the control group revealed that AO-CD and EO-CD participants displayed reduced brain responses relative to controls when viewing angry vs neutral faces (supplemental Table 2). Neither the ROIs nor any other region showed significant differences between the AO-CD and EO-CD groups for the angry vs neutral contrast (P>.20, FWE, svc in all ROIs). The inverse comparisons between groups (ie, combined CD group>controls; EO-CD group>controls; and AO-CD group>controls) revealed no suprathreshold voxels.
Figure 2.
Statistical parametric map (SPM-F) displaying the main effect of group for the contrast of angry vs neutral faces. Statistics and coordinates are given in Table 2. Bar graphs display mean (SE) signal change. Color bars ranging from red to yellow represent F statistics. For display purposes, maps are thresholded at P<.005, uncorrected. AO-CD indicates adolescence-onset conduct disorder; AU, arbitrary units; EO-CD, early-onset CD; HC, healthy controls; L, left; OFC, orbitofrontal cortex; and vmPFC, ventromedial prefrontal cortex.
Table 2. Main Effect of Group for the Contrast of Angry vs Neutral Faces.
| Cerebral Region | Side | Local Maxima, F | Cluster Size, No. of Voxels |
MNI Coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| OFC | Left | 9.42a | 167 | −46 | 44 | −8 |
| Right | 8.98a | 90 | 42 | 46 | −12 | |
| vmPFC | Right | 8.89a | 14 | 10 | 32 | −18 |
| Insula | Left | 7.12a | 103 | −26 | 20 | −6 |
| Amygdala | Left | 6.13a | 12 | −24 | −4 | −22 |
| Right | 7.27a | 15 | 26 | −4 | −26 | |
| dmPFC | Right | 10.45 | 214 | 8 | 26 | 52 |
| Left | 9.29 | 198 | −8 | 28 | 42 | |
| DLPFC | Right | 12.26 | 440 | 50 | 28 | 34 |
| Left | 9.42 | 167 | −46 | 44 | −10 | |
| Inferior parietal cortex | Right | 8.73 | 104 | 48 | −46 | 38 |
| Inferior temporal gyrus | Right | 8.23 | 59 | 60 | −36 | −16 |
| Left | 13.43 | 115 | −48 | −6 | −26 | |
| Fusiform gyrus | Left | 12.41 | 101 | −56 | −2 | −28 |
| Middle temporal gyrus | Right | 8.25 | 59 | 62 | −28 | −16 |
| Superior temporal sulcus/gyrus | Right | 10.63 | 39 | 48 | −18 | −16 |
| Putamen | Left | 8.17 | 11 | −32 | −16 | −6 |
| Thalamus | Right | 11.72 | 33 | 4 | −14 | 2 |
| Left | 10.59 | 23 | −4 | −16 | 2 | |
| Cerebellum | Left | 9.79 | 13 | −32 | −78 | −46 |
Abbreviations: DLPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; MNI, Montreal Neurological Institute; OFC, orbitofrontal cortex; vmPFC, ventromedial prefrontal cortex.
P < .05, familywise error (small volume correction), for a priori regions of interest. Activations in all other regions met the criteria P < .001, uncorrected, for 10 or more contiguous voxels.
Individual Contribution of Angry and Neutral Faces to the Main Effect of Group
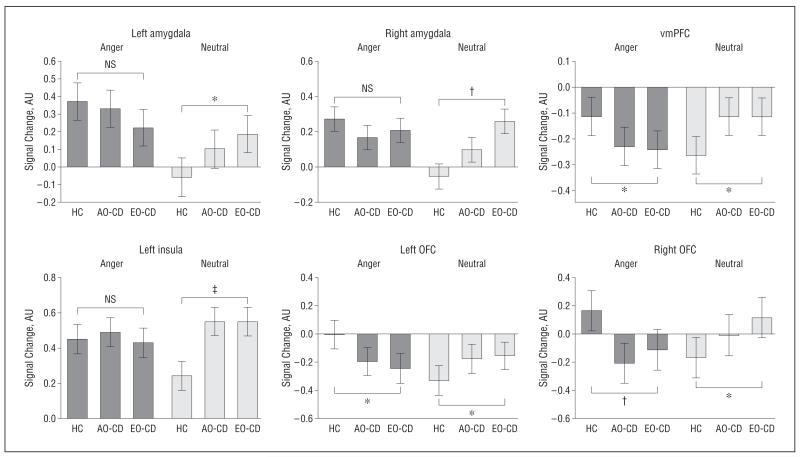
Additional analyses comparing angry and neutral expressions relative to fixation baseline (null events) revealed that the main effect of group for the angry vs neutral contrast in the amygdala was driven by a differential group response to neutral rather than angry facial expressions in the left amygdala (anger, F2,57=1.1 [P>.3]; neutral, F2,57=3.9 [P<.03]) and right amygdala (anger, F2,57= 1.4 [P > .2]; neutral, F2,57= 7.5 [P < .002]) (Figure 3). A similar pattern was found in the left insula (anger, F2,57=1.1 [P=.31]; neutral, F2,57=5.1 [P<.01]) (Figure 3). In contrast, the main effects of group found in vmPFC and bilateral OFC reflected significant group differences for both angry and neutral expressions (vmPFC: anger, F2,57=4.6 [P<.02] and neutral, F2,57=4.8 [P<.02]; left OFC: anger, F2,57=4.5 [P<.02] and neutral, F2,57=4.0 [P<.03]; and right OFC: anger, F2,57=6.0 [P<.005] and neutral, F2,57=3.9 [P<.03]) (Figure 3).
Figure 3.
Relative contribution of angry and neutral faces alone (each vs null/fixation events) to the brain activations shown in Figure 2. Bar graphs display mean (SE) signal change. NS indicates not statistically significant. For other abbreviations, see the legend to Figure 2. *P<.05; †P<.005; ‡P<.01. Detailed statistics are given in the “Results” section.
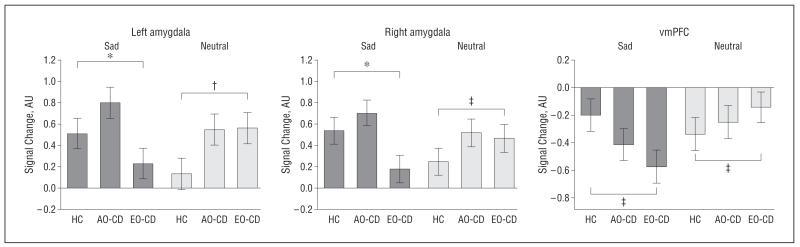
Main Effect of Group for Sad Compared With Neutral Faces
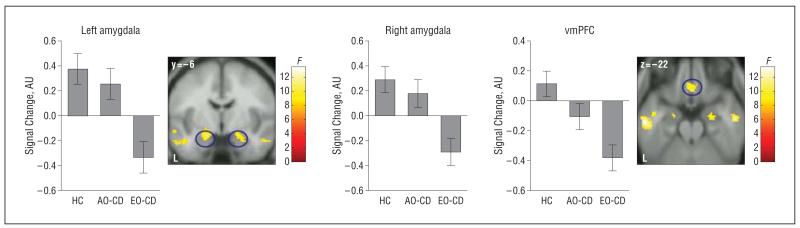
An ANOVA comparing the main effect of group for the sad vs neutral contrast identified significant differences in the amygdala, vmPFC, and other regions (Figure 4 and Table 3). Follow-up analyses showed that the combined CD group displayed reduced activation in the same ROIs relative to controls (supplemental Table 3). In addition, participants with EO-CD displayed reduced activations in all 4 ROIs relative to controls, whereas no significant differences were found between controls and participants with AO-CD (supplemental Table 4). Again, the inverse comparisons between groups (ie, combined CD group>controls; EO-CD group>controls; AO-CD group>controls) did not reveal any suprathreshold voxels.
Figure 4.
Statistical parametric map (SPM-F) displaying the main effect of group for the contrast of sad vs neutral faces. Statistics and coordinates are given in Table 3. Bar graphs display mean (SE) signal change. Color bars ranging from red to yellow represent F statistics. For display purposes, maps are thresholded at P<.005, uncorrected. For other abbreviations, see the legend to Figure 2.
Table 3. Main Effect of the Group for Contrast of Sad vs Neutral Faces.
| Cerebral Region | Side | Local Maxima, F | Cluster Size, No. of Voxels |
MNI Coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| vmPFC | 8.91a | 50 | 0 | 28 | −22 | |
| Amygdala | Left | 9.76a | 33 | −20 | −6 | −16 |
| Right | 8.21a | 11 | 22 | −6 | −14 | |
| DLPFC | Left | 9.74 | 58 | −10 | 56 | 34 |
| Superior temporal sulcus/gyrus | Left | 12.09 | 303 | −56 | −16 | −22 |
| Right | 11.78 | 411 | 56 | −10 | −24 | |
| Putamen | Left | 10.00 | 12 | −26 | −16 | −8 |
| Right | 7.69 | 18 | 14 | 6 | −12 | |
| Cerebellum | Right | 10.04 | 278 | 4 | −58 | −48 |
Abbreviations: See Table 2.
P < .01, familywise error (small volume correction) for a priori regions of interest. Activations in all other regions met the criteria P < .001, uncorrected, for 10 or more contiguous voxels.
A comparison of the AO-CD vs EO-CD groups (AO-CD>EO-CD) showed reduced responses in EO-CD in only the following 2 regions: bilateral amygdala (left: x, y, z coordinates, −20, −6, −20 [t=3.7; P=.003, FWE svc]; right: x, y, z coordinates, 18, −6, −14 [t=3.5; P=.006, FWE svc]) (supplemental Figure 2) and right anterior superior temporal sulcus/gyrus (x, y, z coordinates, 54, −10, −20 [t=3.64; P<.001, uncorrected]). The reverse contrast (EO-CD>AO-CD) did not reveal any suprathreshold voxels.
Individual Contribution of Sad and Neutral Faces to the Main Effect of Group
Additional analyses comparing sad and neutral expressions relative to fixation baseline revealed that neutral and sad faces contributed to group differences for the sad vs neutral contrast in the left amygdala (sad, F2,57=7.07 [P<.003]; neutral, F2,57=5.1 [P<.01]), right amygdala (sad, F2,57=6.6 [P<.003]; neutral, F2,57=4.2 [P<.03]), and the vmPFC (sad, F2,57=4.3 [P<.02]; neutral, F2,57=3.9 [P<.05]) (Figure 5).
Figure 5.
Relative contribution of sad and neutral faces alone (each vs null/fixation events) to the brain activations shown in Figure 4. Bar graphs display mean (SE) signal change. For the abbreviations, see the legend to Figure 2. *P<.005; †P<.01; ‡P<.05. Detailed statistics are given in the “Results” section.
Correlations Between Brain Responses and Psychopathic Traits
Multiple regression analyses in SPM did not reveal any brain regions that showed a correlation with individual scores on the CU subscale or the total Youth Psychopathic Traits Inventory score for the comparisons of angry vs neutral and sad vs neutral faces in each group considered independently, in CD groups combined, or across all participants together (P>.15 for all comparisons, FWE svc in the ROIs).
Correlations Between Brain Responses and CD Symptoms
In each group (EO-CD, AO-CD, and control) considered independently or in a combined CD group, no brain regions showed a correlation with individual scores on CD symptom severity (lifetime/ever, aggressive, or current symptoms) for the comparisons of angry vs neutral or sad vs neutral faces (P>.15 for all comparisons, FWE svc in the ROIs). Across all subjects, however, significant negative correlations were observed between CD symptoms (lifetime/ever, aggressive, or current symptoms) and neural responses in the ROIs for the comparisons of angry vs neutral and sad vs neutral faces (supplemental Figure 3 and Figure 4).
Overall, these results indicate that more lifetime symptoms, increased aggressive behavior, and more severe current CD symptoms were each associated with abnormally reduced responses in brain areas implicated in antisocial or aggressive behavior when processing emotional relative to neutral faces.
Effects of ADHD Comorbidity
For the 53 individuals (20 controls, 17 participants with EO-CD, and 16 with AO-CD) who had complete data of ADHD symptoms available, we repeated all the principal analyses (ie, main effect of group for angry vs neutral and sad vs neutral faces, correlations between brain responses and CD symptoms), including current and lifetime/ever ADHD symptoms as covariates of no interest. The main effect of group showed the same pattern as that reported previously for all a priori ROIs (supplemental Tables 5, 6, and 7). The same was true for the correlation analyses (supplemental Figure 5 and Figure 6), apart from the negative correlation between current CD symptoms and the vmPFC response to sad vs neutral faces, which was no longer significant.
Separate regression analyses exploring the effect of current and lifetime/ever ADHD symptoms alone revealed no significant effect in the ROIs (even at the reduced threshold of P<.05, uncorrected) for the contrast of angry vs neutral faces. However, similar analyses for the contrast of sad vs neutral faces revealed a positive correlation between measures of ADHD symptoms and right insula activation (supplemental Figure 7).
COMMENT
Moffitt7 proposed that EO-CD, but not AO-CD, has a neurodevelopmental basis; hence, only the EO-CD variant should be characterized by neurophysiological abnormalities. Our study is, to our knowledge, the first to investigate this neurodevelopmental hypothesis using fMRI. Although our findings are consistent with Moffitt’s hypothesis that the EO-CD variant has a neural basis, contrary to the developmental taxonomic theory, we also provide evidence of abnormal neurophysiological function in the AO-CD subtype. In summary, we showed that a combined group of participants with EO-CD and AO-CD, or each CD group independently, displayed abnormally reduced brain responses when viewing angry vs neutral faces relative to controls. This was true for each of our a priori ROIs implicated in antisocial behavior (ie, the amygdala, vmPFC, OFC, and insula). No significant differences were found between CD subtypes (P>.20), further corroborating the finding that both CD variants showed similar patterns of abnormal neural activation when processing angry vs neutral faces. These findings fit with previous evidence demonstrating that both CD subtypes show marked and equivalent impairments in neuropsychological tasks, such as affective decision making and facial expression recognition and measures of peripheral physiological function (ie, blunted cortisol and heart rate responses to psychosocial stress).8,9
For the sad vs neutral contrast, bilateral amygdala and anterior superior temporal sulcus/gyrus activations were abnormally reduced in the EO-CD compared with the AO-CD groups. These additional dysfunctions may reflect the pathophysiological distinction between the 2 variants such that the EO-CD subtype is associated with more widespread or severe neural abnormalities.4 Given that angry and sad expressions convey different types of social information (relating to social threat/punishment vs distress/submission, respectively), it is possible that the reduced amygdala response to sad expressions in EO-CD reflects an insensitivity to social cues of distress or submission, although this remains to be established in future research. Nevertheless, our current findings demonstrate a putative neural contribution to the etiology of both subtypes and suggest that the social mimicry hypothesis accounting for the emergence of AO-CD is at least insufficient.
Our results require validating in prospective studies that use a repeated fMRI design from childhood into adolescence. This would establish the developmental emergence of neural markers of CD subtypes and ensure an AO group with no history of CD symptoms in childhood. For example, the present cross-sectional findings cannot determine whether the abnormal neural responses precede the emergence of the syndrome in both subtypes or whether the slightly more restricted neural abnormalities in the AO-CD group arise at a different and later point in the life course compared with the EO-CD subtype. In addition, assessing the age at onset of CD symptoms using retrospective information is not optimal and may have led to some participants with AO-CD being misclassified as having EO-CD or vice versa. However, we attempted to circumvent this problem by obtaining detailed information from the volunteers and their parents and asking them to consider salient life landmarks (such as the transition from primary to secondary school) to assist accurate recall when providing age-at-onset information.
Current neurobiological models of psychopathic and antisocial behavior emphasize the critical role of the amygdala.30,32,33 Reduced function of this region in psychopathic individuals is thought to impair the processing of distress cues (eg, fearful or sad faces), which, in turn, would increase the likelihood that such individuals engage in antisocial behavior to achieve their goals (eg, instrumental aggression).30 This model is supported by several studies showing impaired recognition of fearful and sad faces in youths and adults with psychopathy10,34-37 (but not others, such as Glass and Newman38 and Kosson et al39) and reduced amygdala response to fearful vs neutral faces in youths with conduct problems and CU traits.11,12 However, it is unclear whether the abnormal amygdala activity in these studies reflects reduced activation to the emotional expression, increased activation to the neutral expression, or a combination of both factors. By comparing individual expressions (angry, sad, and neutral) with a low-level baseline (null/fixation), we showed that reduced amygdala activation for angry vs neutral faces in participants with CD (relative to controls) reflected an altered (ie, increased) amygdala response to neutral but not angry faces. In contrast, the reduced amygdala response for sad vs neutral, found specifically in participants with EO-CD, also reflected a reduced response to sad faces.
A hyperactive amygdala response to neutral faces has also been observed in other conditions, such as schizophrenia or pediatric bipolar disorder.40,41 Collectively, these findings emphasize the importance of a low-level baseline to disaggregate the contributions of emotional vs neutral stimuli in functional activations derived from comparing the two.
The increased amygdala response to neutral expressions in CD accords with previous findings showing that aggressive subjects tend to interpret neutral expressions as aversive, which in turn might explain “why aggressive individuals are easily provoked into negative interactions and conflicts with others.”42(p8452) In the present study, the CD groups did not rate neutral expressions as more angry or sad than controls; however, this may reflect the different nature of our current and previous tasks,42 that is, rating neutral faces for anger and sadness in the present study vs categorizing exemplars of 6 facial expressions.42 We also note that a previous study showed no differences in amygdala response to angry vs neutral faces in children with disruptive behavior disorders and CU traits relative to healthy controls.12 However, this may reflect the relative sensitivity of the paradigm used in the 2 experiments because, unlike our study, the control group in the previous investigation12 did not show an amygdala response to angry vs neutral expressions.
Conduct disorder has also been associated with dysfunction in other brain regions, including the vmPFC, insula, and OFC.13,15,18,20 It is therefore of note that CD subtypes combined showed abnormal activations to angry vs neutral and sad vs neutral contrasts in the vmPFC, whereas the insula and the OFC showed abnormal responses for angry vs neutral. Converging evidence from human and comparative research suggests that the OFC may be more specialized for simple emotional responses, whereas the vmPFC might play a distinct role in more complex aspects of emotional behavior, such as social interactions.43 Hence, abnormal function of both prefrontal regions in individuals with CD might explain their highly dysregulated emotional behavior and marked social deficits. The abnormal insular response we detected in both CD subtypes might be related to decreased gray matter in this region and to the reduced empathy observed in individuals with CD.20
In addition, we observed reduced anterior superior temporal sulcus/gyrus responses in participants with CD relative to controls for angry vs neutral and sad vs neutral contrasts and in participants with EO-CD relative to those with AO-CD or to controls for the sad vs neutral contrast. A previous investigation of children with conduct problems and CU traits also found an abnormal response in this region,12 which has been implicated in a range of social cognitive functions including perception of facial and vocal expressions,44 eye gaze,45 and theory of mind.46 Hence, its dysfunction might be related to abnormal social development in CD.
As found previously,8,9 individuals with CD scored significantly higher than healthy controls in CU traits or overall psychopathic traits. Thus, our findings of neurophysiological abnormalities in CD are largely consistent with previous research demonstrating reduced amygdala activations in youths with CU traits.11,12 However, variation in these dimensions was not significantly related to the neural response to angry vs neutral or sad vs neutral faces. This was true when considering each group independently, combining the CD subgroups, or including all participants. Moreover, the CD subtypes did not differ in psychopathic or CU traits, so any differences between these groups do not appear to reflect differences on these measures. However, we cannot exclude that different clinical measures of psychopathy and/or different emotional stimuli than those used herein might identify relationships between brain abnormalities and psychopathic traits, as found previously.12 By contrast, the number of lifetime/ever, aggressive, and current CD symptoms each correlated negatively with neural responses in some or all of the a priori ROIs across groups, demonstrating that more severe clinical phenotypes are associated with increased brain abnormalities. Critically, all of these findings were unaffected when controlling for lifetime/ever and current ADHD symptoms, demonstrating that our effects cannot be attributed to comorbid ADHD.
In conclusion, our observation of neurophysiological abnormalities in the EO-CD and AO-CD subtypes is difficult to reconcile with the developmental taxonomic theory of CD.7 The findings are broadly consistent with previous work from our group showing that both CD subtypes are equally impaired on behavioral and psychophysiological measures of emotional function, including facial expression recognition10 and fear conditioning.47 Our results for the sad vs neutral contrast demonstrate that it is also possible to reveal differences in neural activations between the CD subtypes, which may reflect more marked neurophysiological abnormalities in EO-CD. Furthermore, we demonstrated that more severe CD symptoms are associated with an increased abnormal neural response in brain areas implicated in antisocial behavior and that dysfunctional brain responses may depend on differential contributions of emotional and neutral facial expressions. Although EO-CD is more likely than AO-CD to develop into a life course–persistent pattern of antisocial behavior, clinical outcomes are variable in both subtypes.48,49 Further neuroimaging strategies embedded within longitudinal studies might therefore offer an opportunity to develop neural markers for predicting onset and prognosis in this highly heterogeneous condition.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by project grant 083140 (Drs Goodyer and Fairchild) and grant 077029 (Dr Rowe) from the Wellcome Trust; Medical Research Council project code U.1055.02.001.00001.01 (Dr Calder); and the Betty Behrens Research Fellowship at Clare Hall in Cambridge University (Dr Passamonti). This research was completed within the National Institute of Health Research Collaboration for Leadership in Applied Health Research and Care for Cambridgeshire and Peterborough.
Additional Contributions: We thank our participants, their parents, and their teachers for taking part in the study. Giuseppina Morganti, PGCE, provided help with the reference section. The Cambridge Youth Offending Service, the schools, and pupil referral units helped with participant recruitment.
Footnotes
Financial Disclosure: None reported.
Online-Only Material: Supplemental text, tables, and figures are available on the authors’ Web site at http://www.mrc-cbu.cam.ac.uk/research/emotion/san/agpsupplemental.html.
REFERENCES
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 2.Colman I, Murray J, Abbott RA, Maughan B, Kuh D, Croudace TJ, Jones PB. Outcomes of conduct problems in adolescence: 40 year follow-up of national cohort. BMJ. 2009;338:a2981. doi: 10.1136/bmj.a2981. doi:10.1136/bmj.a2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odgers CL, Caspi A, Broadbent JM, Dickson N, Hancox RJ, Harrington H, Poulton R, Sears MR, Thomson WM, Moffitt TE. Prediction of differential adult health burden by conduct problem subtypes in males. Arch Gen Psychiatry. 2007;64(4):476–484. doi: 10.1001/archpsyc.64.4.476. [DOI] [PubMed] [Google Scholar]
- 4.Moffitt TE, Arseneault L, Jaffee SR, Kim-Cohen J, Koenen KC, Odgers CL, Slutske WS, Viding E. Research review: DSM-V conduct disorder: research needs for an evidence base. J Child Psychol Psychiatry. 2008;49(1):3–33. doi: 10.1111/j.1469-7610.2007.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott S, Knapp M, Henderson J, Maughan B. Financial cost of social exclusion: follow up study of antisocial children into adulthood. BMJ. 2001;323(7306):191. doi: 10.1136/bmj.323.7306.191. doi:10.1136/bmj.323.7306.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moffitt T. Sex Differences in Antisocial Behaviour. Cambridge University Press; Cambridge, England: 2001. [Google Scholar]
- 7.Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychol Rev. 1993;100(4):674–701. [PubMed] [Google Scholar]
- 8.Fairchild G, van Goozen SHM, Stollery SJ, Aitken MRF, Savage J, Moore SC, Goodyer IM. Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biol Psychiatry. 2009;66(2):162–168. doi: 10.1016/j.biopsych.2009.02.024. doi:10.1016/j.biopsych.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer IM. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biol Psychiatry. 2008;64(7):599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairchild G, Van Goozen SH, Calder AJ, Stollery SJ, Goodyer IM. Deficits in facial expression recognition in male adolescents with early-onset or adolescenceonset conduct disorder. J Child Psychol Psychiatry. 2009;50(5):627–636. doi: 10.1111/j.1469-7610.2008.02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry. 2009;166(1):95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- 12.Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 13.De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, Hodgins S, Viding E. Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132(pt 4):843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- 14.Huebner T, Vloet TD, Marx I, Konrad K, Fink GR, Herpertz SC, Herpertz-Dahlmann B. Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2008;47(5):540–547. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- 15.Stadler C, Sterzer P, Schmeck K, Krebs A, Kleinschmidt A, Poustka F. Reduced anterior cingulate activation in aggressive children and adolescents during affective stimulation: association with temperament traits. J Psychiatr Res. 2007;41(5):410–417. doi: 10.1016/j.jpsychires.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57(1):7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Klein RG, Abikoff H, Klass E, Ganeles D, Seese LM, Pollack S. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073–1080. doi: 10.1001/archpsyc.1997.01830240023003. [DOI] [PubMed] [Google Scholar]
- 18.Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS, Chen G, Towbin KE, Leibenluft E, Pine DS, Blair JR. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65(5):586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, Shah NJ, Konrad K, Herpertz-Dahlmann B. Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry. 2008;49(7):781–791. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 20.Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37(1):335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Andershed H, Kerr M, Stattin H. Understanding the abnormal by studying the normal. Acta Psychiatr Scand Suppl. 2002;(412):75–80. doi: 10.1034/j.1600-0447.106.s412.17.x. [DOI] [PubMed] [Google Scholar]
- 23.Spielberger C, Gorsuch R, Lushene P, Vagg P, Jacobs A. Manual for the State-Trait Anxiety Inventory (Form Y) Inc. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- 24.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundqvist D, Flykt A, Ohman A. The Karolinska Directed Emotional Faces (KDEF) Dept of Neurosciences, Karolinska Hospital; Stockholm, Sweden: 1998. [Google Scholar]
- 26.Ewbank MP, Lawrence AD, Passamonti L, Keane J, Peers PV, Calder AJ. Anxiety predicts a differential neural response to attended and unattended facial signals of anger and fear. Neuroimage. 2009;44(3):1144–1151. doi: 10.1016/j.neuroimage.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 27.Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2(4):189–210. doi:10.1002/hbm.460020402. [Google Scholar]
- 28.Friston KJ. Testing for anatomically specified regional effects. Hum Brain Mapp. 1997;5(2):133–136. doi: 10.1002/(sici)1097-0193(1997)5:2<133::aid-hbm7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4(1):58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. doi:10.1002/(SICI)1097-0193(1996)4:1<58: AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 30.Blair RJ. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Dev Psychopathol. 2005;17(3):865–891. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- 31.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 32.Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142(2-3):107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychol Bull. 2007;133(1):149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- 34.Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J Abnorm Child Psychol. 2001;29(6):491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- 35.Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, Solak E, Abeygunawardane AI. Attention to the eyes and fear-recognition deficits in child psychopathy. Br J Psychiatry. 2006;189:280–281. doi: 10.1192/bjp.bp.105.018150. [DOI] [PubMed] [Google Scholar]
- 36.Marsh AA, Blair RJ. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci Biobehav Rev. 2008;32(3):454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolan M, Fullam R. Face affect recognition deficits in personality-disordered offenders: association with psychopathy. Psychol Med. 2006;36(11):1563–1569. doi: 10.1017/S0033291706008634. [DOI] [PubMed] [Google Scholar]
- 38.Glass SJ, Newman JP. Recognition of facial affect in psychopathic offenders. J Abnorm Psychol. 2006;115(4):815–820. doi: 10.1037/0021-843X.115.4.815. [DOI] [PubMed] [Google Scholar]
- 39.Kosson DS, Suchy Y, Mayer AR, Libby J. Facial affect recognition in criminal psychopaths. Emotion. 2002;2(4):398–411. doi: 10.1037/1528-3542.2.4.398. [DOI] [PubMed] [Google Scholar]
- 40.Hall J, Whalley HC, McKirdy JW, Romaniuk L, McGonigle D, McIntosh AM, Baig BJ, Gountouna VE, Job DE, Donaldson DI, Sprengelmeyer R, Young AW, Johnstone EC, Lawrie SM. Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry. 2008;64(1):70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103(23):8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Best M, Williams JM, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci U S A. 2002;99(12):8448–8453. doi: 10.1073/pnas.112604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudebeck PH, Bannerman DM, Rushworth MF. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci. 2008;8(4):485–497. doi: 10.3758/CABN.8.4.485. [DOI] [PubMed] [Google Scholar]
- 44.Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA. Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci. 1998;265(1408):1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calder AJ, Jenkins R, Cassel A, Clifford CW. Visual representation of eye gaze is coded by a nonopponent multichannel system. J Exp Psychol Gen. 2008;137(2):244–261. doi: 10.1037/0096-3445.137.2.244. [DOI] [PubMed] [Google Scholar]
- 46.Hein G, Knight RT. Superior temporal sulcus: it’s my area, or is it? J Cogn Neurosci. 2008;20(12):2125–2136. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- 47.Fairchild G, Van Goozen SH, Stollery SJ, Goodyer IM. Fear conditioning and affective modulation of the startle reflex in male adolescents with early-onset or adolescence-onset conduct disorder and healthy control subjects. Biol Psychiatry. 2008;63(3):279–285. doi: 10.1016/j.biopsych.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: follow-up at age 26 years. Dev Psychopathol. 2002;14(1):179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- 49.Robins LN. Sturdy childhood predictors of adult antisocial behaviour: replications from longitudinal studies. Psychol Med. 1978;8(4):611–622. doi: 10.1017/s0033291700018821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.