Abstract
Background
BMI discordant monozygotic (MZ) twins allows an examination of the causes and consequences of adiposity in a genetically controlled design. Few studies have examined longitudinal BMI discordance in MZ pairs.
Objectives
To study the development over time of BMI discordance in adolescent and adult MZ twin pairs, and to examine lifestyle, metabolic, inflammatory, and gene expression differences associated with concurrent and long-term BMI discordance in MZ pairs.
Subjects/Methods
BMI data from 2775 MZ twin pairs, collected in eight longitudinal surveys and a biobank project between 1991 and 2011, were analyzed to characterize longitudinal discordance. Lifestyle characteristics were compared within discordant pairs (ΔBMI ≥ 3 kg/m2) and biomarkers (lipids, glucose, insulin, CRP, fibrinogen, IL-6, TNF-α and sIL-6R and liver enzymes AST, ALT and GGT) and gene expression were compared in peripheral blood from discordant pairs who participated in the NTR biobank project.
Results
The prevalence of discordance ranged from 3.2% in 1991 (mean age=17, SD=2.4) to 17.4% (N=202 pairs) in 2009 (mean age=35, SD=15), and was 16.5% (N=174) among pairs participating in the biobank project (mean age=35, SD=12). Of 699 MZ with BMI data from 3-5 time points, 17 pairs (2.4%) were long-term discordant (at all available time points; mean follow-up range=6.4 years). Concurrently discordant pairs showed significant differences in self-ratings of which twin eats most (p=2.3×10−13), but not in leisure time exercise activity (p=0.28) and smoking (p>0.05). Ten out of 14 biomarkers showed significantly more unfavorable levels in the heavier of twin of the discordant pairs (p-values < 0.001); most of these biomarker differences were largest in longitudinally discordant pairs. No significant gene expression differences were identified, although high ranking genes were enriched for Gene Ontology (GO) terms highlighting metabolic gene regulation and inflammation pathways.
Conclusions
BMI discordance is uncommon in adolescent identical pairs but increases with higher pair-mean of BMI at older ages, although long-term BMI discordance is rare. In discordant pairs, the heavier twin had a more unfavorable blood biomarker profile than the genetically matched leaner twin, in support of causal effects of obesity.
Keywords: weight, obesity, diabetes, lifestyle, gene expression, lipids
Introduction
Even for highly heritable traits, there can be substantial discordance in monozygotic (MZ) twin pairs (1;2). The causes for discordance may include unequal environmental exposures (3;4), post-twinning DNA mutations (5), stochastic factors (6), and epigenetic differences between twins (7). For body-mass index (BMI), heritability estimates tend to be high (8). During foetal life, the heritability of body size increases between the second and third trimester (9). After birth, the heritability of BMI continues to increase with age during childhood, but decreases with age in adulthood (10). Similar variation has been demonstrated for the effects of genetic variants on BMI. For example, the association between FTO and BMI strengthens with age during childhood and adolescence to a maximum effect at age 20, but declines after this age (11). These observations suggest that genetic influences are not deterministic and that the impact of heritable factors at least partly depends on non-genetic factors.
Monozygotic (MZ) twins are genetically (nearly) identical (12) and therefore give insight into the potential range of variation in body weight at a given genetic background. Previous studies, however, indicated that MZ twins with large BMI discordance are rare (13). To date, at least three studies of BMI-discordant MZ twins have been described, including a well-characterized group of obesity-discordant MZ twins from Finland (13-25), a group of overweight-discordant MZ twins from the United States (26), and a BMI-discordant group from Belgium (27). In studies that reported the height of BMI-discordant twins, no significant difference in height was evident in these pairs (24;27). In the longitudinal cohort of 658 Finnish MZ twin pairs, 14 obesity-discordant MZ twin pairs with an intra-pair difference > 4 kg/m2 were identified at age 22-27 years (13;24). Retrospective data showed that the discordance had emerged around age 18 (24). Importantly, many BMI discordant pairs did not continue to be discordant when followed over time (14).
Overweight and obesity are commonly regarded as an indicator of excessive energy intake and have been linked to adverse metabolic and cardiovascular changes and to conditions including the metabolic syndrome, type 2 diabetes, coronary artery disease and depression (28-34). Growing evidence suggests that a key mechanism behind the pathogenesis of the consequences of obesity and associated conditions involves chronic over-activation of cellular stress signalling and inflammatory pathways in response to energy intake that strongly exceeds energy expenditure (35-38). Associations of overweight and obesity with blood levels of metabolic and inflammatory biomarkers are well-established based on epidemiological studies (see for example (39), but a limitation in population-based studies is that results can be (partly) confounded by genetic factors, because weight, lipid levels and glucose metabolism can be influenced by common underlying genetic influences (40). Several genes with pleiotropic effects on birth weight and type 2 diabetes have been identified (41;42), where the allele associated with lower birth weight and subsequent increased postnatal weight gain also increases the risk of Type 2 diabetes in adulthood.
Studying biomarker levels in BMI discordant MZ twins has the advantage that the relationship between differences in BMI and biomarker levels can be revealed under an identical genetic background and age. The current study had two main aims. Firstly, we aimed to examine the prevalence of BMI differences in MZ twin pairs and their development over time, analyzing BMI data from 2775 MZ twin pairs collected throughout adolescence and adulthood over a period of up to 20 years. Secondly, in subsets of these data, we examined whether differences in life style factors, metabolic and inflammatory biomarkers, and gene expression in peripheral blood are present in concurrently BMI discordant and long-term discordant MZ twin pairs.
Materials and methods
Subjects
MZ twins from the Netherlands Twin Register (NTR)(43) took part in eight longitudinal survey studies between 1991-2009 and between 2004-2011 a subgroup also participated in the NTR biobank project (44;45). BMI data were available for 2775 MZ twin pairs (including 6 pairs who were part of triplets): 1709 pairs participated in the survey studies only and 1066 pairs participated also in NTR biobank, of which 1044 pairs participated once, and 22 pairs participated twice in biobanking (interval: 3 – 7 years, mean= 5 years). Of the pairs who participated in NTR biobank, eleven pairs (including 3 pairs who were discordant for BMI) were excluded because one twin was pregnant. After quality control, data on gene expression and cell counts were available for 634 pairs. Zygosity assessment is described in the Supplemental Methods. Informed consent was obtained from participants and study protocols were approved by the Medical Ethics Committee of the VU University Medical Centre.
Anthropometric, health and lifestyle measures
Data on height and weight were obtained in eight surveys (self-report) and were measured in the NTR biobank project by a calibrated balance and a stadiometer. BMI was calculated as: weight (kg)/(height (m)2). Self-reported height data were checked for consistency over time (Supplemental Methods). Surveys also contained questions regarding demographic and lifestyle characteristics, including cigarette smoking, eating habits, leisure time exercise activities and birth weight (Supplemental Methods). Waist and hip circumference were assessed in the NTR biobank project with measurement tape. Additional measures collected at blood draw for the NTR biobank project included information regarding lipid-lowering and diabetes medication, menopause and pregnancy status. BMI difference (ΔBMI) was computed for each MZ pair as the difference between the heavier and the lighter twin, for all data points (for N pairs at each survey and the NTR biobank project, see table 1). BMI discordance was defined as ΔBMI ≥3 kg/m2, in line with the threshold applied in previous studies of BMI discordant pairs (13;26;27).
Table 1.
Average BMI difference between MZ twins and frequencies of various degrees of concordance and discordance at each survey and NTR biobank project.
| % of All MZ pairs | % male pairs | % female pairs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Survey no/Project, year | N pairsA | % Female | Mean Age (SD) | Mean Δ BMI (SD) | Δ BMI 0-1 | Δ BMI 1-2 | Δ BMI 2-3 | Δ BMI 3-4 | Δ BMI ≥4 | Δ BMI ≥3 | Δ BMI ≥3 |
| 1, 1991 | 590 | 58.6 | 17.2 (2.4) | 0.9 (0.9) | 67.5 | 20.2 | 9.2 | 1.5 | 1.7 | 4.1 | 2.6 |
| 2, 1993 | 771 | 60.7 | 19.5 (8.4) | 0.9 (1.4) | 68.5 | 20.5 | 7.7 | 1.6 | 1.8 | 4.0 | 3.0 |
| 3, 1995 | 626 | 61.5 | 19.2 (3.2) | 1.0 (1.0) | 65.3 | 23.6 | 6.9 | 2.4 | 1.8 | 4.1 | 4.2 |
| 4, 1997 | 563 | 67.5 | 25.4 (10.3) | 1.4 (1.4) | 53.5 | 25.2 | 9.8 | 6.6 | 5.0 | 7.1 | 13.7 |
| 5, 2000 | 827 | 73.6 | 30.1 (12.0) | 1.6 (1.6) | 45.9 | 26.4 | 15.4 | 5.2 | 7.1 | 9.6 | 13.3 |
| 6, 2002 | 804 | 73.0 | 33.1 (12.1) | 1.6 (1.7) | 47.4 | 24.5 | 13.3 | 6.7 | 8.1 | 12.4 | 15.7 |
| 7, 2004 | 1155 | 75.5 | 36.2 (13.1) | 1.7 (1.6) | 42.9 | 26.8 | 13.9 | 7.2 | 9.4 | 15.9 | 16.7 |
| 8, 2009 | 1157 | 75.4 | 34.6 (15.0) | 1.7 (1.7) | 45.4 | 25.0 | 12.2 | 8.2 | 9.2 | 14.7 | 18.3 |
| Biobank, 2004-2011 | 1055 | 69.1 | 34.9 (12.4) | 1.8 (1.9) | 42.1 | 26.3 | 15.2 | 7.1 | 9.4 | 16.6 | 16.5 |
Δ BMI = Difference between the BMIs of co-twins.
Number of complete MZ pairs.
Percentages in the table represent the percentage of MZ twin pairs with a certain BMI difference, relative to the total number of MZ twin pairs participating at each individual time point.
Blood biomarker profiles
Blood samples were collected as part of the NTR biobank project after overnight fasting (44) to assess total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol, glucose, insulin, TNF-α, IL-6, sIL-6R, fibrinogen, CRP, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma glutamyl transferase (GGT), as described in detail previously (44). Data on blood biomarkers were available for 878 (83%) – 966 (92%) complete MZ pairs who participated in NTR biobank (range is for different biomarkers). For additional information, see Supplemental Methods.
Gene expression profiles
RNA extraction (44), expression profiling, and expression quality control procedures have been described in detail previously (46). In short, gene expression in whole blood drawn for the NTR biobank project was measured with Affymetrix U219 arrays (GeneTitan), which contain 530,467 probes for 49,293 transcripts. For further information, see Supplemental Methods.
Statistical analyses BMI, lifestyle and biomarkers
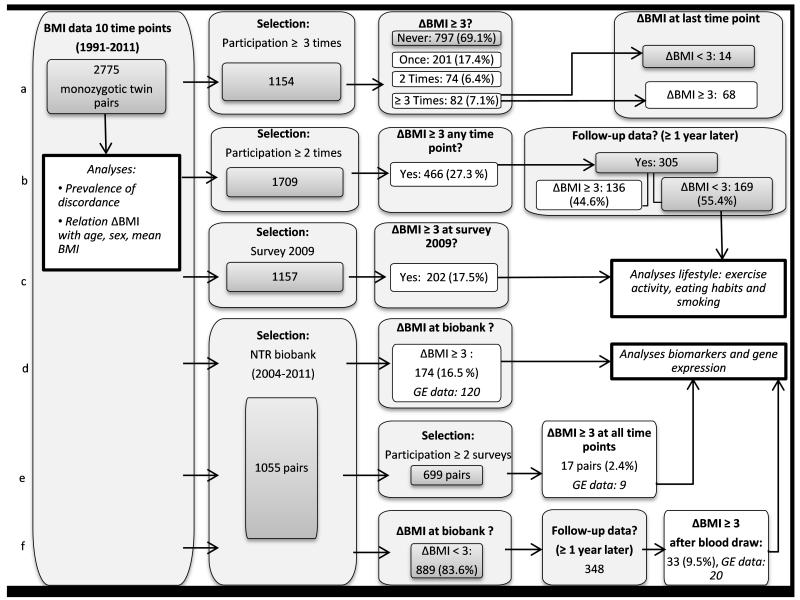
The selection of twins for each analysis is illustrated in Figure 1. Associations of ΔBMI with age, sex and BMI level were tested by linear regression analysis in SPSS version 21 with ΔBMI as outcome, and sex, age, and mean BMI of co-twins (BMI twin 1 + BMI twin 2)/2) as predictors. Here, observations corresponded to twin pairs, and one measure of BMI was included for each pair (2775 pairs in total), which was selected from the most recent time point at which both twins participated, with a preference for biobank measures of BMI. To examine the progression of BMI discordance over time, we studied data from MZ pairs with BMI data available from ≥ 3 time points (N= 1154 pairs, figure 1a) and MZ pairs with BMI data available from ≥ 2 time points (N=1709, figure 1b). Lifestyle data (exercise activity, eating habits and smoking) collected in survey 8 (2009) were studied in all pairs who were discordant for BMI at that time point (figure 1c), and blood biomarkers, gene expression, and additional measures collected as part of the NTR biobank project were examined in all MZ pairs who were discordant at blood draw (figure 1d), and in a subset of longitudinally discordant pairs, who were selected out of 699 MZ pairs who participated in the NTR biobank plus in at least two surveys (Figure 1e). Finally, to verify whether differences within MZ pairs are present before BMI discordance, biomarkers and gene expression differences were tested in a separate group of MZ pairs. These pairs were not yet discordant when blood samples were collected or at prior surveys but they became discordant ≥ 1 year after blood draw. Thus for these pairs, data on gene expression and biomarkers were not available during discordance (Figure 1f). Differences between the heavier and leaner twin from discordant pairs were tested with Wilcoxon Signed Ranks tests (ordinal data), McNemar tests (dichotomous data) and paired t-tests (continuous data) in SPSS. In total, nine lifestyle variables were compared within discordant pairs who participated in surveys and 24 variables (excluding gene expression) were compared within discordant pairs who participated in the NTR biobank. To account for multiple testing, a p-value < 0.002 (=0.05/27) was considered significant in comparisons of biomarkers and lifestyle, where 27 represents the number of independent dimensions in the data, estimated with the online software program MatSpD (http://gump.qimr.edu.au/general/daleN/matSpD/; Supplemental Methods). To rule out that small differences in age between co-twins (related to variation in the response time to questionnaires and because a subset of MZ co-twins who participated in the NTR biobank were not assessed on the same day) influenced the within-pair comparison of BMI, we tested for differences in age at assessment; there were no differences in age between discordant twins in any of the groups.
Figure 1. Flowchart of the selection procedure of MZ twin pairs included in each analysis.
All numbers in this figure represent numbers of MZ twin pairs. GE= Gene expression. Each row (a to f) illustrates the available data and selection criteria for MZ pairs included in a particular analysis. a) Frequency of BMI discordance at one, two or more longitudinal time points in MZ pairs with longitudinal BMI data. b) Number of MZ pairs who are discordant across all projects and the number of pairs who are still discordant at the first next available follow-up time point. c) Discordant pairs included in the analyses of life style data. d) MZ pairs who were discordant at blood draw and were included in the analyses of biomarkers and gene expression. e). MZ pairs who were discordant at all time points of participation and were included in the analyses of biomarkers and gene expression. f) MZ pairs who became discordant after blood draw and who were studied to examine biomarkers and gene expression difference before BMI discordance onset.
Statistical analyses gene expression
Gene expression levels corrected for a number of covariates (neutrophil, basophil, eosinophil, lymphocyte and monocyte cell counts, smoking status, age, sex, hemoglobin, hour of blood sampling, days between blood sampling and RNA extraction, plate and location on the plate, see ref (47)) were compared within discordant pairs by applying a paired t-test to all probe sets (44 241 probe sets after quality control) in R (48). All probe sets were ranked by p-value to test for enrichment of Gene Ontology (GO) terms and for enrichment of a set of candidate genes for BMI, based on loci identified by a GWAS of BMI (49). Enrichment analyses were conducted with the software packages GOrilla (50) and GSEA (51;52), as described in the Supplemental Methods. All gene expression analyses accounted for multiple testing by controlling for the false discovery rate (FDR). An FDR q-value < 0.05 was considered significant. The FDR q-value for probe sets was computed with the R function qvalue() with default settings.
Results
Prevalence of BMI discordance and relation with age, sex, and mean BMI
The mean age of twins ranged from 17 years in 1991 (first survey) to 35 years in 2009 (last survey, from which lifestyle variables were analyzed). At all time points the majority of MZ twins had highly similar BMIs (Table 1), with 87.7-89.0% of pairs in surveys 1-3 showing a BMI difference < 2 kg/m2. The percentage of discordant MZ pairs (ΔBMI ≥ 3) ranged from 3.2% in survey 1 to 17.4% in survey 8, when relatively more older pairs were included. To illustrate: if both twins have a height of 175 cm, a ΔBMI of 1 between co-twins corresponds to a weight difference of 3.1 kg, ΔBMI of 2 corresponds to a weight difference of 6.1 kg and ΔBMI of 3 to 9.2 kg.
Within-pair differences increased at each successive survey, together with the age and BMI of twins. In a linear regression analysis with ΔBMI as outcome and sex and age as predictors, ΔBMI was larger in female pairs and increased significantly with the age of twins (Psex= 7.2 × 10−3, Page= 4.6 × 10−21). However, when mean BMI of the twins was added as predictor, ΔBMI was only significantly associated with sex and mean BMI (Psex= 4.9 × 10−5, PmeanBMI=8.7 × 10−84, Page=0.22). On average, ΔBMI was 0.8 (SD=0.7) in pairs with a mean BMI in the underweight range (BMI<18), 1.3 (SD=1.3) in pairs with a ‘normal’ mean BMI (18-25), 2.5 (SD=2.4) in pairs with a mean BMI in the overweight range (25-30) and 3.9 (SD=3.9) in obese pairs (BMI >30), suggesting that ΔBMI increases with the mean BMI of a pair. We next ranked the twin pairs on the basis of the BMI-class (underweight, normal weight, overweight, or obese) of the leaner twin. A BMI difference ≥ 3 between co-twins was evident in 10.1% of pairs where the leaner twin had a BMI in the underweight range, 12.3% of pairs where the leaner twin had a normal BMI, 21.7% of pairs where the leaner twin was overweight, and 45.5% of pairs where the leaner twin was obese.
Progression of BMI discordance over time
To examine the progression of BMI discordance over time, we studied data from pairs who participated in at least three NTR projects (Supplemental Table 1), and found that 30.9% was discordant at least once, but only 7.1 % of all pairs had a Δ BMI ≥3 at three or more time points; still, some of these pairs did not cross the threshold for discordance at the most recent project in which they participated (N=14, Figure 1a). These findings suggest that it is not uncommon for MZ twins to show episodes of discordance and converge later, while long-term BMI discordance is rare. Across all time points, we identified 305 BMI-discordant MZ twin pairs (Δ BMI ≥3, at any time point) with follow-up data after being identified as discordant (on average 3 years later, Figure 1b). At follow-up, the average BMI difference between co-twins had decreased, and 169 pairs (55.4%) were no longer discordant (“converging pairs”), due to weight gain of the leaner twin (mean 5.2 kg, SD=6.1) and weight loss of the heavier twin (−2.9 kg, SD=6.8; Supplemental Table 2). The following combinations were observed among converging pairs: leaner twin gained weight and heavier twin lost weight (45.6%), both twins gained weight (27.2%), both twins lost weight (11.8%), the leaner twins’ weight was stable while the heavier twin lost weight (7.7%), or the heavier twin’s weight was stable while the leaner twin gained weight (7.7%). Overall, 80.4% of initially leaner twins from converging pairs gained weight, and 65.1% of initially heavier twins lost weight. For 98 converging pairs, we also had BMI data before they became discordant (on average 3 years earlier). These data showed that 79.6% of the heavier twins and 76.5% of the leaner twins had ended up heavier after discordance in comparison to their BMI before discordance (average weight change in all converging pairs over the entire period of on average 6.5 years: heavier twin; mean=+4.8kg, SD=6.5, leaner twin; mean=+4.9kg, SD=6.9), and suggest that discordance mainly reflects one twin starting out earlier on a trajectory of weight gain.
Lifestyle
To assess whether BMI discordance in MZ twins is related to lifestyle differences, survey data collected in 2009 from 202 discordant pairs were studied (Figure 1c; Table 2). Heavier and leaner twins equally often reported to participate in leisure time exercise regularly (62.9% of leaner twins and 57.1% of heavier twins participated in exercise on a regular basis, P=0.28). Discordant twins also did not differ in the number of reported hours of exercise per week (P=0.58) but a difference was noticed in response to a question that asked twins about their relative food intake (P=2.3 × 10−13). To the question “Who of you eats most?” 50.3% of the heavier twins responded with “I eat most” versus 6.3% of the leaner twins. 43.2% of the leaner twins reported that their co-twin eats most, while 3.1% of the heavier twins reported that their co-twin eats most. Heavier twins also reported to go on a diet more often compared to their leaner co-twins (P=2.1 × 10−5). Discordant twins did not differ significantly in smoking status (Pcurrent smoking=0.054, Pever smoked=0.50).
Table 2.
Leisure time exercise activity and eating habits of BMI discordant pairs (ΔBMI ≥ 3) who participated in NTR survey 8 (2009).
| Heavier twins | Leaner twins | P-valueA | |
|---|---|---|---|
| N pairs | 202 | 202 | |
| N male/female pairs | 42/160 | 42/160 | |
| Age (years) | 40.2(16.0) | ||
| BMI (kg/m2) | 28.0 (4.2) | 23.4 (3.6) | 1.6×10−89 |
| Do you participate in leisure time exercise activity regularly? | 0.28 | ||
| Yes | 57.1% | 62.9% | |
| No | 42.9% | 37.1% | |
| Frequency leisure time exercise activity | 0.58 | ||
| Almost never | 30.7% | 31.6% | |
| 1-5 hours per week | 56.1% | 51.8% | |
| 5-10 hours per week | 11.10% | 13% | |
| > 10 hours per week | 2.10% | 3.6% | |
| Ever been on a diet | 2.1 × 10−5 | ||
| Never | 40.1% | 54.8% | |
| A few times | 29.2% | 25.6% | |
| Multiple times | 16.8% | 11.6% | |
| Often | 10.9% | 5.5% | |
| Always on a diet | 3.0% | 2.5% | |
| Fear to gain weight | 0.01 | ||
| Not scared | 29.2% | 37.2% | |
| A little bit scared | 42.6% | 38.2% | |
| Quite scared | 17.3% | 18.6% | |
| Very scared | 8.4% | 6.0% | |
| Extremely scared | 2.5% | 0.0% | |
| How fast do you normally eat? | 0.64 | ||
| Very slow | 0.5% | 1.0% | |
| Slowly | 6.0% | 8.0% | |
| Medium | 60.0% | 56.2% | |
| Fast | 30.5% | 33.8% | |
| Very fast | 3.0% | 1.0% | |
| Do you normally eat until you feel full? | 0.07 | ||
| I stop with eating before I feel full | 34.5% | 40.1% | |
| I stop with eating when I feel full | 57.5% | 55.4% | |
| I continue eating, even when I feel full | 8.0% | 4.5% | |
| Who of you eats most? | 2.3 ×10−13 | ||
| I do | 50.3% | 6.5% | |
| We eat just as much | 29.2% | 27.6% | |
| My co-twin | 3.1% | 43.2% | |
| I do not know | 17.4% | 22.6% | |
| Smoking | |||
| Current smoker | 27.4% | 34.8% | 0.05 |
| Ever smoked | 69.9% | 72.3% | 0.50 |
P-value from a paired t-test (BMI), McNemar test (Regular sports and smoking), or Wilcoxon signed rank test (all others). Mean (SD) or percentages are displayed.
We hypothesized that changes in smoking status may potentially contribute to shifts in BMI discordance and therefore compared smoking status in pairs who were initially discordant but concordant after a period of on average 3 years (“converging pairs” described in the previous section). We observed a larger percentage of individuals who quit smoking (14.1%) among the initially leaner twins compared to the initially heavier twins (4.3%). Of the initially leaner twins who quit smoking, all except for one twin gained weight (mean change=+9.8 kg, range=−2 kg – +27 kg). Of the (initially) heavier twins, 14.5% had started smoking (of which 70 % lost weight: mean change=−2.56 kg, range= −10 kg – +8kg) versus 2.8% of the initially leaner twins. Although this pattern is in line with changes in BMI discordance being related to changes in smoking status of twins, the difference in smoking status over time in converging pairs was nominally significant only (p=0.012), and a similar trend of quitting smoking was noticed among the leaner twins from pairs of who were still BMI discordant at follow-up (Supplemental Table 2).
Blood biomarkers
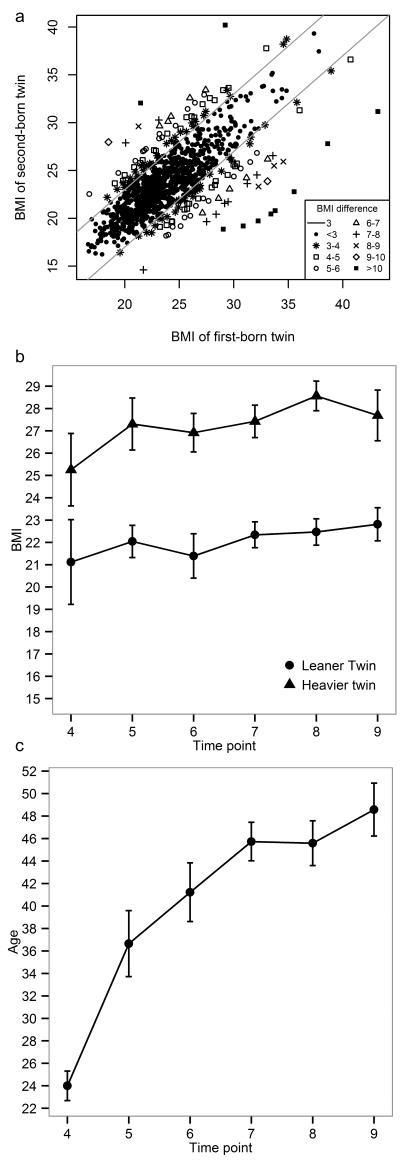
There were 174 MZ twin pairs with a BMI difference ≥ 3 BMI kg/m2 at blood draw (16.5%). Their average age was 38.5 years and 69 % were female. The BMI of the heavier twins was on average 5.1 kg/m2 (22%) larger compared to the leaner co-twins (range= 3-13, Figure 2a), and the weight, waist circumference and hip circumference of heavier twins were on average 14.7 kg, 11.0 cm and 8.4 cm larger respectively, compared to their leaner co-twins (Table 3, first four columns). Heavier twins had significantly (p < 0.002) higher levels of glucose, insulin, total cholesterol, LDL, triglycerides, CRP, IL-6, sIL-6R, and GGT, a lower level of HDL cholesterol (p-values: 6.0×10−13-0.001), and a nominally significant trend of higher fibrinogen (p=2.2×10−3), compared to their leaner co-twins. Discordant twins did not differ significantly (p ≥ 0.002) in height, birth weight, plasma levels of TNF-α, AST and ALT, menopause status, and use of lipid-lowering medication or diabetes medication (p-values: 0.04-0.88). These findings illustrate that the heavier twins from genetically identical BMI-discordant pairs show less favourable biomarker profiles, a pattern that is in line with reports from population-based studies on the relationships between BMI and biomarkers.
Figure 2. BMI of MZ twin pairs who participated in the NTR biobank project (N=1055 pairs) and longitudinal BMI of 17 longitudinally discordant MZ pairs.
a) Concordant pairs (Δ BMI < 3) are denoted by filled circles (N=881) and discordant pairs (Δ BMI ≥ 3 kg/m2) are indicated by other symbols (N=174). The grey lines indicate the threshold for discordance. B) Mean BMI of longitudinally discordant twins across NTR projects, with data from the heavier twins denoted by triangles and data from the leaner twins denoted by circles. c) Mean age of longitudinally discordant twins across NTR projects. Error bars represent standard errors. Time points 4-7 and 9 are surveys and time point 8 represents the time point of blood draw (NTR biobank). Note: None of these pairs participated in surveys 1-3. The following number of twins participated at each time point (N=leaner twin/heavier twin): 4: N=3/3, 5: N=10/10, 6: N=7/10, 7: N=15/15, 8: N=17/17, 9: N=14/11.
Table 3.
Characteristics at the moment of blood draw of all BMI discordant MZ twin pairs (Δ BMI ≥ 3 kg/m2) who participated in the NTR biobank project, and for the longitudinally discordant subset.
| All discordant pairs | Longitudinally discordant subset | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Heavier Twin | Leaner Twin | Mean difference (heavier - leaner twin) | P-value | Heavier Twin | Leaner Twin | Mean difference (heavier - leaner twin) | P-value | |
| N | 174 | 174 | 17 | 17 | ||||
| N male/female pairs | 54/120 | 54/120 | 1/16 | 1/16 | ||||
| Age (years) | 38.5 (12.6) | 45.6 (11.6) | ||||||
| Birth weight (g) | 2394 (600) | 2378 (614) | 16 | 0.16 | 2366 (742) | 2538 (752) | −172 | 0.27 |
| BMI (kg/m2) | 28.4 (4.2) | 23.3 (3.7) | 5.1 | 8.7×10−71 | 28.6 (2.7) | 22.5 (2.4) | 6.1 | 5.1×10−7 |
| Weight (kg) | 84.3 (14.8) | 69.6 (13.9) | 14.7 | 3.7×10−68 | 80.1 (9.6) | 63.1 (9.2) | 17.0 | 1.9×10−7 |
| Height (cm) | 172.1 (8.2) | 172.4 (8.4) | 0.3 | 0.32 | 167.4(5.6) | 167.3 (5.3) | 0.1 | 0.84 |
| Waist (cm) | 91.2 (12.0) | 80.1 (11.3) | 11.1 | 1.5×10-47 | 92.2 (10.3) | 76.6 (8.5) | 15.6 | 3.9×10-8 |
| Hip (cm) | 109. 2 (8.3) | 100.8 (8.0) | 8.4 | 7.2×10-47 | 109.7 (5.8) | 98.6 (6.9) | 11.1 | 8.1×10-10 |
| WHR (cm/cm) | 0.84 (0.08) | 0.79 (0.08) | 0.05 | 1.1×10-15 | 0.84 (0.08) | 0.78 (0.07) | 0.06 | 6.6×10−5 |
| Glucose (mmol/L) | 5.4 (0.8) | 5.1 (0.6) | 0.3 | 3.4×10-8 | 5.4 (0.6) | 5.1 (0.4) | 0.3 | 0.06 |
| Insulin (μIU/ml) | 12.3 (12.4) | 7.4 (4.5) | 4.9 | 5.6×10-13 | 10.1 (6.0) | 5.3 (2.6) | 4.8 | 0.03 |
| Total Chol (mmol/L) | 5.2 (1.2) | 4.9 (1.1) | 0.3 | 2.0×10-4 | 5.9 (1.5) | 5.2 (1.4) | 0.7 | 0.03 |
| LDL (mmol/L) | 3.2 (1.1) | 2.9 1.0) | 0.3 | 5.4×10-4 | 3.6 (1.4 | 3.2 (1.5) | 0.4 | 0.15 |
| HDL (mmol/L) | 1.3 (0.3) | 1.5 (0.3) | −0.2 | 2.2×10-7 | 1.5 (0.3) | 1.6 (0.3) | −0.1 | 0.20 |
| Triglycerides (mmol/L) | 1.5 (0.9) | 1.2 (0.5) | 0.3 | 8.1×10-11 | 1.5(0.9) | 0.9 (0.4) | 0.6 | 9.2×10-4 |
| CRP (mg/L) | 4.5 (5.6) | 2.7 (4.2) | 1.8 | 4.6×10-11 | 4.4(4.6) | 1.2 (1.4) | 3.2 | 4.9×10-4 |
| TNFα (pg/ml) | 1.1 (1.3) | 1.1(1.4) | 0.0 | 0.41 | 1.95 (3.7) | 1.05 (1.3) | 0.9 | 0.86 |
| IL-6 (pg/ml) | 1.7 (1.3) | 1.5 (1.4) | 0.2 | 1.1×10-3 | 2.3 (1.9) | 1.4 (0.8) | 0.9 | 0.12 |
| sIL-6R (pg/mL) | 42506.5 (11168.4) | 40093.9 (11051.6) | 2412.6 | 4.6×10-4 | 42645 (13375) | 38672 (13340) | 3973 | 0.14 |
| Fibrinogen (g/L) | 3.0 (0.7) | 2.8 (0.8) | 0.2 | 2.2 × 10-3 | 3.3 (0.7) | 2.6 (0.6) | 0.7 | 1.3×10-5 |
| AST (U/L) | 22.4 (6.3) | 21.5 (6.5) | 0.9 | 0.04 | 22.9 (6.9) | 22.4 (10.0) | 0.5 | 0.32 |
| ALT (U/L) | 12.3 (7.7) | 11.4 (5.2) | 0.9 | 0.68 | 12.6 (6.6) | 12.0 (5.4) | 0.6 | 0.90 |
| GGT (U/L) | 32.7 (23.4) | 27.6 (19.6) | 5.1 | 5.4 × 10-4 | 29.4 (18.0) | 20.6 (10.6) | 8.8 | 1.9 × 10-3 |
| N (%) using lipid-lowering medication | 5 (2.9%) | 7 (4%) | −2 | 0.73 | 1 (5.9%) | 1 (5.9%) | 0 | 0.99 |
| N (%) using diabetes medication | 1 (0.6%) | 1 (0.6%) | 0 | 0.99 | 0(0%) | 0 (0%) | 0 | 0.99 |
| N (% of female twins) menopause | 20 (16.7%) | 16 (13.3%) | 4 | 0.22 | 6(37.5) | 6 (37.5%) | 0.99 | |
Numbers in the table represent Mean (SD) or N (%)
Waist= Waist circumference, Hip= Hip circumference, WHR=Waist-to-hip ratio, Total Chol=Total cholesterol, AST= aspartate aminotransferase, ALT= alanine aminotransferase, GGT= gamma glutamyl transferase.
Gene expression
Of the 174 pairs who were discordant at blood draw, whole blood gene expression data were available for 120 pairs. None of the probe sets identified a difference in expression between discordant twins reaching genome-wide significance (FDR q-values > 0.05, for the top 100 probes see Supplemental Table 3), and discordant twins also showed no difference in the expression of BMI candidate genes from GWAS (Supplemental Table 3). GO enrichment analysis based on p-value rank from the gene expression comparison of discordant twins highlighted significant enrichment of a number of GO terms (FDR q-value < 0.05) related to broad metabolic categories (e.g. regulation of metabolic process, cellular macromolecule metabolic process), suggesting that BMI discordance is associated with small but widespread differences in the expression in blood of genes related to metabolism (Supplemental Table 3). Other GO processes significantly enriched among high ranking genes included hepatocyte differentiation and negative regulation of type I interferon production, and enriched GO components included Golgi apparatus, NLRP3 inflammasome complex, and mitochondrial outer membrane, amongst others.
Biomarkers and gene expression related to prolonged BMI discordance
To examine biomarker and gene expression differences related to long-term BMI discordance, we studied a sub-group of 17 pairs who had a BMI difference ≥ 3 at all NTR projects in which they participated (3-5 time points, stretching on average 6.4 years, range 3-12 years; Figure 2b and c). These pairs showed similar differences in blood biomarkers (Table 3, last three columns), although not all effects were statistically significant in this smaller sample. For ten of the fourteen biomarkers, the effect size was larger in longitudinally discordant pairs than in the total group of pairs who were BMI discordant at blood draw (Supplemental Figure 1). This pattern was strongest for fibrinogen (Mean Difference in all discordant pairs=0.2 g/L, P=2.2 ×10−3; Mean Difference in longitudinally discordant pairs = 0.7 g/L, P=1.3 × 10−5). Comparison of gene expression profiles (Supplemental Table 4), which were available for a subset of 9 longitudinally discordant pairs, revealed no genome-wide significant differences for individual probe sets, and no significant GO terms were found. Longitudinally discordant pairs also showed no difference in the expression of candidate genes for BMI. Effect sizes for genome-wide probe sets (mean difference in expression of the heavier twin – leaner twin) were moderately correlated with the effect sizes observed in all discordant pairs (r=0.31, p< 0.001, Supplemental Figure 2), suggesting partial correspondence of gene expression effects in all discordant pairs versus longitudinally discordant pairs.
Blood biomarkers and gene expression before onset of BMI discordance
Finally, we examined whether differences in molecular profiles precede BMI discordance, by studying 33 MZ pairs who were not yet discordant at blood draw, but who became discordant afterwards (mean=3.1 years after blood draw, range 1-6, figure 1f). When first identified as discordant, the heavier and leaner twins had mean BMIs of 27.1 and 23.0, respectively, while their BMIs were on average 24.9 and 23.8, respectively, when blood samples were collected. Prior to BMI discordance, none of the blood biomarkers differed significantly (Table 4). A comparison of the effect sizes in the three groups illustrates that within-pair differences were largest for most biomarkers in the longitudinally discordant pairs while they were smallest in MZ pairs before BMI discordance (Supplemental Figure 1), suggesting that the adverse blood profile observed in heavier twins from BMI discordant pairs (Table 3) represents a consequence of the higher BMI. No significant differences in gene expression (data available for 20 pairs) were evident in MZ pairs before BMI discordance (Supplemental Table 5). No significant differences in gene expression (data available for 20 pairs) were evident in MZ pairs before BMI discordance (Supplemental Table 5). Even though not statistically significant, we explored whether genome-wide expression differences between twins before discordance (n twins pairs=20) were of comparable magnitude as the expression differences observed between twins during discordance (n twin pairs =120). We computed the correlation between effect sizes observed before and during discordance (where effect sizes refer to the mean expression differences at 44 241 probe sets between the heavier and leaner twin). This correlation (r=−0.04, p< 0.001, Supplemental Figure 3) indicated that MZ twins do not exhibit comparable differences in gene expression prior to BMI discordance as observed during BMI discordance.
Table 4.
Characteristics of MZ twin pairs who participated in the NTR biobank project and who became discordant (Δ BMI ≥3 kg/m2) after blood draw.
| MZ pairs who became discordant after blood draw |
||||
|---|---|---|---|---|
| Heavier Twin | Leaner Twin | Mean difference (Heavier-leaner twin) | P-value | |
| N | 33 | 33 | ||
| N male/female pairs | 7/26 | 7/26 | ||
| Age post-blood draw | 34.7 (11.0) | |||
| BMI post-blood draw(kg/m2) | 27.1 (3.2) | 23.0 (3.1) | 4.1 | 2.2 × 10−25 |
| Weight post-blood draw(kg)A | 80.9 (11.2) | 69.0 (9.3) | 11.9 | 2.9 × 10−18 |
| Age at blood draw (years) | 31.5 (11.2) | |||
| BMI at blood draw (kg/m2) | 24.9 (3.9) | 23.8 (3.8) | 1.1 | 1.5 × 10−6 |
| Weight at blood draw (kg) | 74.5 (13.7) | 71.3 (12.4) | 3.2 | 4.2 × 10−5 |
| Height at blood draw (cm) | 172.8 (8.0) | 173.2 (8.0) | −0.4 | 0.37 |
| Waist at blood draw (cm) | 84.0 (11.2) | 80.8 (10.6) | 3.2 | 7.1 × 10−4 |
| Hip at blood draw (cm) | 104.2 (7.1) | 101.6 (7.7) | 2.6 | 1.4 × 10−3 |
| WHR at blood draw (cm/cm) | 0.8 (0.1) | 0.8 (0.1) | 0 | 0.26 |
| Birth weight (g) | 2638.6 | 2516.7 | 121.9 | 0.72 |
| Glucose (mmol/L) | 5.3 (0.5) | 5.0 (0.6) | 0.3 | 0.11 |
| Insulin (μIU/ml) | 9.1 (5.5) | 8.4 (5.4) | 0.7 | 0.16 |
| Total Chol (mmol/L) | 4.9 (1.0) | 4.8 (0.9) | 0.1 | 0.55 |
| LDL (mmol/L) | 2.9 (0.9) | 2.9 (0.8) | 0.0 | 0.78 |
| HDL (mmol/L) | 1.4 (0.4) | 1.4 (0.3) | 0.0 | 0.37 |
| Triglycerides (mmol/L) | 1.1 (0.5) | 1.2 (0.6) | −0.1 | 0.71 |
| CRP (mg/L) | 5.7 (17.7) | 2.8 (3.4) | 2.9 | 0.72 |
| TNFα (pg/ml) | 0.9 (0.3) | 1.1(1.0) | −0.2 | 0.32 |
| IL-6 (pg/ml) | 2.0 (3.1) | 1.4 (0.9) | 0.6 | 0.55 |
| sIL-6R (pg/mL) | 40664.0 | 42012.5 | −1348.5 | 0.34 |
| Fibrinogen (g/L) | 2.6 (0.8) | 2.5 (0.7) | 0.1 | 0.28 |
| AST (U/L) | 20.0 (6.7) | 19.1 (4.8) | 0.9 | 0.66 |
| ALT (U/L) | 11.0 (6.7) | 9.8 (3.8) | 1.2 | 0.51 |
| GGT (U/L) | 24.2 (10.3) | 22.8 (8.1) | 1.4 | 0.39 |
| N (%) using lipid-lowering medication at blood draw | 1 (3%) | 0 (0%) | 1 | 0.99 |
| N (%) using diabetes medication at blood draw | 0(0%) | 0 (0%) | 0 | n.a. |
| N (% of female twins) | 2 (7.7%) | 1 (3.8%) | 1 | 0.99 |
Characteristics of MZ twin pairs who were concordant for BMI (Δ BMI < 3) at blood draw and at all time points of participation prior to blood draw, but who became discordant (Δ BMI ≥ 3) ≥ 1 year after blood draw. The selection procedure of these twins is illustrated in figure 1f. All characteristics in this table refer to data collected at the moment of blood draw (when the twins were concordant for BMI), except for birth weight (based on data from multiple longitudinal surveys), the classification of “heavier” and “leaner” twins (which is based on the moment when twins were discordant), and variables marked withA.
Characteristics based on information collected ≥ 1year after blood draw (range 1-6, mean=3.1 years); this is the time point at which the BMI difference of these twins first passed the threshold of discordance (Δ BMI ≥ 3).
Waist= Waist circumference, Hip= Hip circumference, WHR=Waist-to-hip ratio, Total Chol=Total cholesterol, AST= aspartate aminotransferase, ALT= alanine aminotransferase, GGT= gamma glutamyl transferase. Numbers in the table represent Mean (SD) or N (%)
Discussion
We described longitudinal BMI data collected between 1991 and 2011 in adolescent and adult MZ twin pairs to examine the prevalence of BMI discordance (defined as ΔBMI ≥3 kg/m2) in genetically identical individuals and its development over time, and examined possible associations of BMI discordance with discordance in lifestyle factors, biomarkers, and gene expression profiles. The majority of MZ twin pairs was highly concordant for BMI, but temporary differences in BMI were not uncommon, particularly when mean BMI increased at higher ages. However, when followed over time, only a minority of MZ twins stayed discordant for a prolonged time period. Of the pairs who were identified as discordant at any NTR project, 55.4% were no longer discordant after a period of on average 3 years, and in a group of 699 MZ twin pairs who participated ≥ 3 times in longitudinal NTR projects (including the NTR biobank), only 17 pairs (2.4%) were discordant at all time points (over a period of on average 6.5 years). These findings illustrate the difficulty to find long-term BMI discordant MZ twins and suggest that BMI discordance is generally not a stable characteristic of MZ twins. This observation carries an important message regarding the etiology of BMI: the fact that large differences in BMI in most MZ twin pairs do not last long emphasizes the important impact of genetic influences on weight regulation. Based on our findings and previous reports of convergence among initially BMI-discordant pairs (14) we conclude that genetically identical individuals who exhibit stable lifetime BMI discordance will be very rare.
The fact that (temporary) BMI discordance in MZ twins nonetheless occurs highlights the role of non-genetic influences on BMI. We found that food intake, assessed by asking each twin which of them eats most, showed the strongest difference between BMI-discordant twins of the lifestyle variables, and found no difference in self-reported frequency of leisure time exercise. This finding suggests that large BMI differences between genetically identical subjects are more strongly related to food intake than to current voluntary exercise participation. A limitation of our study is that the assessment of relative food intake, as well as other lifestyle measures and a subset of our BMI data, were based on self-report. The comparison of relative food intake among co-twins in particular might be biased by the twin’s perception of their weight difference. Nonetheless, it has also been reported that self-report of twins about their relative food intake may provide more reliable information with regard to which twin eats most in comparison to self-report of absolute food intake. Thus, in a previous study of obesity-discordant twins that assessed three measures; self-reported absolute food intake, self-reported relative food intake of twins, and measured energy turn-over (using double-labeled water technology), it was found that discordant twins showed no difference in self-reported absolute food intake (because obese twins tended to under-report their own energy intake, as suggested by data on measured energy intake). By contrast, the data on self-reported relative intake for several types of food (which twin eats most) corresponded well with predicted relative food intake of twins based on their difference in measured energy intake (53).
We found that convergence of the BMIs of initially discordant twins was related to both weight gain of the leaner twins and weight loss of the heavier twins, but an interesting question that remains to be examined by future studies is to which extent becoming BMI-discordant and converging after discordance in MZ twins is due to intentional efforts to lose or gain weight in one of the twins. Previous studies have shown that after weight loss following caloric restriction or an increase in physical exercise, most individuals tend to regain the lost weight (54;55). In addition to the difficulty to lose weight, “overeating” experiments have shown that most lean subjects eventually return to their original weight following diet-induced weight gain (56). This tendency of individuals to return to a certain “set-point” of body weight has been attributed to homeostatic regulation of body fat mass, which triggers for example compensatory responses (e.g. increased appetite and energy efficiency) when the brain senses a reduction of energy stores through changes in circulating levels of adipocyte-secreted signals such as the hormone leptin (57). Genetic regulation of this homeostatic system may explain our observation that most MZ twin pairs eventually converge to a similar BMI, after a period of discordance.
We observed that BMI discordance occurred more frequently among heavier twins. A significant relationship between the mean trait value of MZ co-twins and the difference of that trait between co-twins may reflect genotype-by-environment interaction (58). When the impact of environmental influences on BMI depends on the genetic vulnerability of the twins, MZ twins who are highly vulnerable to the impact of environmental influences that promote weight gain (as reflected by a high mean BMI of co-twins) are expected to show the largest divergence in response to unequal environmental exposures. A second possible explanation is that the BMI of heavier persons may fluctuate more, because people often respond to weight gain by efforts to lose weight (e.g. by going on a diet). Unless such fluctuations in BMI occur at exactly the same time in MZ co-twins, they will lead to the observation of a greater percentage of discordant pairs in the higher BMI range. Finally, the larger percentage of BMI discordance among heavier twins might be related to the pathogenesis of obesity. Obesity is associated with a deterioration of homeostatic weight regulation (59). It could thus be hypothesized that increasing variation of BMI between MZ co-twins in the higher BMI range is related to the decreasing capacity of the twins’ bodies to regulate body weight after repeated weight gain.
It is well-established that obesity is associated with adverse changes in blood levels of biomarkers that are reflective of an increased risk of developing cardiovascular disease and type 2 diabetes. These markers include dysregulated blood lipid levels and glucose homeostasis, and a pro-inflammatory state. Studying variation in these markers in BMI-discordant twins has the advantage that genetic pleiotropy is ruled out as a potential explanation for the association by design. If the association between a higher BMI and adverse blood biomarkers in the population would solely exist because genetic variants that predispose to a high BMI also cause the adverse changes in biomarkers, MZ twins who are discordant for BMI should show equal levels of these biomarkers because MZ twins have the same genetic vulnerability. However, we found that MZ twin pairs who were discordant at the moment of blood draw exhibited significant differences in all metabolic biomarkers (with the heavier twin having an unfavorable metabolic profile) and heavier twins had significantly higher blood levels of IL-6, sIL-6R, CRP and GGT. Effect sizes ranged from a 0.14 standard deviation increase in IL-6 levels in the heavier twins to a 1.1 SD increase in insulin. Differences in biomarkers were more pronounced in twins with longitudinal evidence for discordance with effect sizes ranging up to a 2.3 SD increase in CRP in heavier twins of longitudinally discordant pairs. To illustrate the meaning of these differences, we assessed whether individuals had elevated fasting levels of triglycerides or glucose, or reduced levels of HDL cholesterol – according to the revised criteria from the National Cholesterol Education Program’s Adult Treatment Panel III (NCEP ATP III) (60). Of all discordant pairs, 35.7% of heavier twins versus 22.4% of leaner twins had elevated fasting glucose levels (or used glucose-lowering medication), 31.4% of heavier twins versus 16.0% of leaner twins had high triglyceride levels (or used lipid-lowering medication) and 40.8% of heavier twins versus 30.8% of leaner twins had low HDL levels (or used lipid-lowering medication). Of the longitudinally discordant pairs, the percentages were as follows: elevated glucose; 41.2% of heavier twins and 12.5% of leaner twins, elevated triglycerides; 37.5% of heavier twins and 6.7% of leaner twins, low HDL levels; 43.8% of heavier twins and 26.7% of leaner twins. Importantly, MZ pairs who became discordant after blood draw showed no significant differences in biomarker levels prior to BMI discordance. This pattern suggests that adverse changes in these biomarkers are caused by a change in weight and worsen over time as a consequence of larger adiposity in the heavier twins from discordant MZ pairs. The fact that no differences in biological markers were present in genetically identical subjects before their weight difference emerged highlights that these biomarkers are not predictive of BMI but that a high BMI is driving unfavorable changes in these biomarkers.
While MZ twins share the same genome, it is possible that, in addition to differences in lifestyle, differences in the regulation of genes between twins may contribute to differences in their BMIs. Although we noticed significant enrichment of various Gene Ontology terms among genes with a stronger expression difference in 120 discordant pairs, highlighting metabolic regulation and processes that have been previously linked to the pathogenesis of obesity (e.g. type I interferon signaling (61) and NLRP3 inflammasome complex (62)), we found no statistically significant associations for the expression level of individual probe sets with BMI discordance. We next zoomed in to a set of candidate genes for BMI that were identified through genome-wide association analysis of BMI, which implies that genetic variants (single nucleotide polymorphisms) in or nearby these genes are associated with variation in BMI in the population. Since genetic variation at these loci is shared within MZ pairs, differences in expression between BMI-discordant twins would indicate a role of environmental influences or epigenetic mechanisms in the regulation of these genes. Yet, we did not find differences in the expression of these well-established BMI loci, thus finding no evidence for BMI discordance being the result of differential gene regulation, however, we only studied gene expression in peripheral blood, and it is possible that causal regulatory pathways underlying BMI discordance are confined to other tissues. Of note, previous studies have reported differences in the expression of candidate genes related to e.g. lipid metabolism in peripheral blood between overweight versus normal weight children (63) and between at-risk obese versus metabolically healthy obese adult individuals (64).
In conclusion, the prevalence of BMI discordance in MZ pairs is low and ranged from 3.2% in 1991 (mean age=17, SD=2.4) to 17.4% in 2009 (mean age=35, SD=15). Only 2.4% of MZ pairs showed a stable long-term difference in BMI (3-5 time points, average range 6.5 years). Comparing the heavier and leaner twins, we found significant differences in self-reported food intake relative to the co-twin, and found clinically meaningful differences in metabolic and inflammatory biomarkers that arise as a consequence of the difference in adiposity and that are more pronounced in long-term discordant pairs. Other lifestyle variables such as smoking, voluntary exercise and gene expression did not differ between the heavier and leaner twins.
Supplementary Material
Acknowledgements
This work was supported by: Genetics of Mental Illness: A lifespan approach to the genetics of childhood and adult neuropsychiatric disorders and comorbid conditions [ERC-230374]. We like to thank the twins who participated in this study.
Footnotes
Conflict of interest
All authors declare no conflict of interest.
Supplementary information is available at International Journal of Obesity’s website.
Text summary of Supplementary Files: Supplementary files include 1 word document (Supplemental material.doc) and three excel workbooks (Supplemental Table 3.xls; Supplemental Table 4.xls; Supplemental Table 5.xls). Supplemental material.doc contains Supplemental Methods, Supplemental Figure 1, 2, 3, and Supplemental Tables 1 and 2.
References
- 1.van Dongen J, Slagboom PE, Draisma HH, Martin NG, Boomsma DI. The continuing value of twin studies in the omics era. Nat Rev Genet. 2012;13(9):640–653. doi: 10.1038/nrg3243. [DOI] [PubMed] [Google Scholar]
- 2.Zwijnenburg PJG, Meijers Heijboer H, Boomsma DI. Identical but not the same: The value of discordant monozygotic twins in genetic research. Am J Med Genet B Neuropsychiatr Genet. 2010;153(6):1134–1149. doi: 10.1002/ajmg.b.31091. [DOI] [PubMed] [Google Scholar]
- 3.Halfvarson J, Jess T, Magnuson A, Montgomery SM, Orholm M, Tysk C, et al. Environmental factors in inflammatory bowel disease: a co-twin control study of a Swedish-Danish twin population. Inflamm Bowel Dis. 2006;12(10):925–933. doi: 10.1097/01.mib.0000228998.29466.ac. [DOI] [PubMed] [Google Scholar]
- 4.Lehn H, Derks EM, Hudziak JJ, Heutink P, van Beijsterveldt TC, Boomsma DI. Attention problems and attention-deficit/hyperactivity disorder in discordant and concordant monozygotic twins: evidence of environmental mediators. J Am Acad Child Adolesc Psychiatry. 2007;46(1):83–91. doi: 10.1097/01.chi.0000242244.00174.d9. [DOI] [PubMed] [Google Scholar]
- 5.Vadlamudi L, Dibbens LM, Lawrence KM, Iona X, McMahon JM, Murrell W, et al. Timing of de novo mutagenesis--a twin study of sodium-channel mutations. N Engl J Med. 2010;363(14):1335–1340. doi: 10.1056/NEJMoa0910752. [DOI] [PubMed] [Google Scholar]
- 6.Wong CC, Caspi A, Williams B, Houts R, Craig IW, Mill J. A longitudinal twin study of skewed X chromosome-inactivation. PLoS One. 2011;6(3):e17873. doi: 10.1371/journal.pone.0017873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weksberg R, Shuman C, Caluseriu O, Smith AC, Fei YL, Nishikawa J, et al. Discordant KCNQ1OT1 imprinting in sets of monozygotic twins discordant for Beckwith-Wiedemann syndrome. Hum Mol Genet. 2002;11(11):1317–1325. doi: 10.1093/hmg/11.11.1317. [DOI] [PubMed] [Google Scholar]
- 8.Schousboe K, Willemsen G, Kyvik KO, Mortensen J, Boomsma DI, Cornes BK, et al. Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin Research. 2003;6(5):409–421. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- 9.Mook-Kanamori DO, van Beijsterveldt CE, Steegers EA, Aulchenko YS, Raat H, Hofman A, et al. Heritability estimates of body size in fetal life and early childhood. PLoS One. 2012;7(7):e39901. doi: 10.1371/journal.pone.0039901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elks CE, den HM, Zhao JH, Sharp SJ, Wareham NJ, Loos RJ, et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol (Lausanne) 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy R, Wills AK, Wong A, Elks CE, Wareham NJ, Loos RJ, et al. Life course variations in the associations between FTO and MC4R gene variants and body size. Hum Mol Genet. 2010;19(3):545–552. doi: 10.1093/hmg/ddp504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye K, Beekman M, Lameijer EW, Zhang Y, Moed MH, van den Akker EB, et al. Aging as accelerated accumulation of somatic variants: whole-genome sequencing of centenarian and middle-aged monozygotic twin pairs. Twin Res Hum Genet. 2013;16(6):1026–1032. doi: 10.1017/thg.2013.73. [DOI] [PubMed] [Google Scholar]
- 13.Naukkarinen J, Rissanen A, Kaprio J, Pietilainen KH. Causes and consequences of obesity: the contribution of recent twin studies. Int J Obes (Lond) 2012;36(8):1017–1024. doi: 10.1038/ijo.2011.192. [DOI] [PubMed] [Google Scholar]
- 14.Graner M, Seppala-Lindroos A, Rissanen A, Hakkarainen A, Lundbom N, Kaprio J, et al. Epicardial fat, cardiac dimensions, and low-grade inflammation in young adult monozygotic twins discordant for obesity. Am J Cardiol. 2012;109(9):1295–1302. doi: 10.1016/j.amjcard.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Hakala P, Rissanen A, Koskenvuo M, Kaprio J, Ronnemaa T. Environmental factors in the development of obesity in identical twins. Int J Obes Relat Metab Disord. 1999;23(7):746–753. doi: 10.1038/sj.ijo.0800923. [DOI] [PubMed] [Google Scholar]
- 16.Kannisto K, Pietilainen KH, Ehrenborg E, Rissanen A, Kaprio J, Hamsten A, et al. Overexpression of 11beta-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: studies in young adult monozygotic twins. J Clin Endocrinol Metab. 2004;89(9):4414–4421. doi: 10.1210/jc.2004-0153. [DOI] [PubMed] [Google Scholar]
- 17.Kaye SM, Pietilainen KH, Kotronen A, Joutsi-Korhonen L, Kaprio J, Yki-Jarvinen H, et al. Obesity-related derangements of coagulation and fibrinolysis: a study of obesity-discordant monozygotic twin pairs. Obesity (Silver Spring) 2012;20(1):88–94. doi: 10.1038/oby.2011.287. [DOI] [PubMed] [Google Scholar]
- 18.Mustelin L, Pietilainen KH, Rissanen A, Sovijarvi AR, Piirila P, Naukkarinen J, et al. Acquired obesity and poor physical fitness impair expression of genes of mitochondrial oxidative phosphorylation in monozygotic twins discordant for obesity. Am J Physiol Endocrinol Metab. 2008;295(1):E148–E154. doi: 10.1152/ajpendo.00580.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietilainen KH, Rissanen A, Kaprio J, Makimattila S, Hakkinen AM, Westerbacka J, et al. Acquired obesity is associated with increased liver fat, intra-abdominal fat, and insulin resistance in young adult monozygotic twins. Am J Physiol Endocrinol Metab. 2005;288(4):E768–E774. doi: 10.1152/ajpendo.00381.2004. [DOI] [PubMed] [Google Scholar]
- 20.Pietilainen KH, Kannisto K, Korsheninnikova E, Rissanen A, Kaprio J, Ehrenborg E, et al. Acquired obesity increases CD68 and tumor necrosis factor-alpha and decreases adiponectin gene expression in adipose tissue: a study in monozygotic twins. J Clin Endocrinol Metab. 2006;91(7):2776–2781. doi: 10.1210/jc.2005-2848. [DOI] [PubMed] [Google Scholar]
- 21.Pietilainen KH, Bergholm R, Rissanen A, Kaprio J, Hakkinen AM, Sattar N, et al. Effects of acquired obesity on endothelial function in monozygotic twins. Obesity (Silver Spring) 2006;14(5):826–837. doi: 10.1038/oby.2006.96. [DOI] [PubMed] [Google Scholar]
- 22.Pietilainen KH, Sysi-Aho M, Rissanen A, Seppanen-Laakso T, Yki-Jarvinen H, Kaprio J, et al. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects--a monozygotic twin study. PLoS One. 2007;2(2):e218. doi: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietilainen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keranen H, et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 2008;5(3):e51. doi: 10.1371/journal.pmed.0050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietiläinen KH, Rissanen A, Laamanen M, Lindholm AK, Markkula H, Yki-Jarvinen H, et al. Growth patterns in young adult monozygotic twin pairs discordant and concordant for obesity. Twin Res. 2004;7(5):421–429. doi: 10.1375/1369052042335368. [DOI] [PubMed] [Google Scholar]
- 25.Rissanen A, Hakala P, Lissner L, Mattlar CE, Koskenvuo M, Ronnemaa T. Acquired preference especially for dietary fat and obesity: a study of weight-discordant monozygotic twin pairs. Int J Obes Relat Metab Disord. 2002;26(7):973–977. doi: 10.1038/sj.ijo.0802014. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell KS, Mazzeo SE, Aggen SH, Maes HH, Kendler KS, Neale MC, et al. Characteristics of monozygotic male and female twins discordant for overweight: a descriptive study. Eat Behav. 2008;9(3):366–369. doi: 10.1016/j.eatbeh.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souren NY, Tierling S, Fryns JP, Derom C, Walter J, Zeegers MP. DNA methylation variability at growth-related imprints does not contribute to overweight in monozygotic twins discordant for BMI. Obesity (Silver Spring) 2011;19(7):1519–1522. doi: 10.1038/oby.2010.353. [DOI] [PubMed] [Google Scholar]
- 28.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111(11):1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 29.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Festa A, D’Agostino R, Jr., Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51(4):1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 31.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 32.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 33.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117(10):2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 37.Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab. 2012;15(1):10–18. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metabolism. 1996;45(2):235–240. doi: 10.1016/s0026-0495(96)90060-8. [DOI] [PubMed] [Google Scholar]
- 40.Elder SJ, Lichtenstein AH, Pittas AG, Roberts SB, Fuss PJ, Greenberg AS, et al. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J Lipid Res. 2009;50(9):1917–1926. doi: 10.1194/jlr.P900033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freathy RM, Bennett AJ, Ring SM, Shields B, Groves CJ, Timpson NJ, et al. Type 2 diabetes risk alleles are associated with reduced size at birth. Diabetes. 2009;58(6):1428–1433. doi: 10.2337/db08-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horikoshi M, Yaghootkar H, Mook-Kanamori DO, Sovio U, Taal HR, Hennig BJ, et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat Genet. 2013;45(1):76–82. doi: 10.1038/ng.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boomsma DI, de Geus EJ, Vink JM, Stubbe JH, Distel MA, Hottenga JJ, et al. Netherlands Twin Register: from twins to twin families. Twin Res Hum Genet. 2006;9(6):849–857. doi: 10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- 44.Willemsen G, de Geus EJ, Bartels M, van Beijsterveldt CE, Brooks AI, Estourgie-van Burk GF, et al. The Netherlands Twin Register biobank: a resource for genetic epidemiological studies. Twin Res Hum Genet. 2010;13(3):231–245. doi: 10.1375/twin.13.3.231. [DOI] [PubMed] [Google Scholar]
- 45.Willemsen G, Vink JM, Abdellaoui A, den BA, van Beek JH, Draisma HH, et al. The adult Netherlands twin register: twenty-five years of survey and biological data collection. Twin Res Hum Genet. 2013;16(1):271–281. doi: 10.1017/thg.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen R, Batista S, Brooks AI, Tischfield JA, Willemsen G, van GG, et al. Sex differences in the human peripheral blood transcriptome. BMC Genomics. 2014;15(1):33. doi: 10.1186/1471-2164-15-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright FA, Sullivan PF, Brooks AI, Zou F, Sun W, Xia K, et al. Heritability and genomics of gene expression in peripheral blood. Nat Genet. 2014;46(5):430–437. doi: 10.1038/ng.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. URL http://www.R-project.org/ [Google Scholar]
- 49.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 52.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pietilainen KH, Korkeila M, Bogl LH, Westerterp KR, Yki-Jarvinen H, Kaprio J, et al. Inaccuracies in food and physical activity diaries of obese subjects: complementary evidence from doubly labeled water and co-twin assessments. Int J Obes (Lond) 2010;34(3):437–445. doi: 10.1038/ijo.2009.251. [DOI] [PubMed] [Google Scholar]
- 54.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safer DJ. Diet, behavior modification, and exercise: a review of obesity treatments from a long-term perspective. South Med J. 1991;84(12):1470–1474. [PubMed] [Google Scholar]
- 56.Sims EA, Horton ES. Endocrine and metabolic adaptation to obesity and starvation. Am J Clin Nutr. 1968;21(12):1455–1470. doi: 10.1093/ajcn/21.12.1455. [DOI] [PubMed] [Google Scholar]
- 57.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jinks JL, Fulker DW. Comparison of the biometrical genetical, MAVA, and classical approaches to the analysis of the human behavior. Psychol Bull. 1970;73(5):311–349. doi: 10.1037/h0029135. [DOI] [PubMed] [Google Scholar]
- 59.Hussain SS, Bloom SR. The regulation of food intake by the gut-brain axis: implications for obesity. Int J Obes (Lond) 2013;37(5):625–633. doi: 10.1038/ijo.2012.93. [DOI] [PubMed] [Google Scholar]
- 60.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 61.Wang XA, Zhang R, Zhang S, Deng S, Jiang D, Zhong J, et al. Interferon regulatory factor 7 deficiency prevents diet-induced obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2013;305(4):E485–E495. doi: 10.1152/ajpendo.00505.2012. [DOI] [PubMed] [Google Scholar]
- 62.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez J, Priego T, Pico C, Ahrens W, De HS, Fraterman A, et al. Blood cells as a source of transcriptional biomarkers of childhood obesity and its related metabolic alterations: results of the IDEFICS study. J Clin Endocrinol Metab. 2012;97(4):E648–E652. doi: 10.1210/jc.2011-2209. [DOI] [PubMed] [Google Scholar]
- 64.Telle-Hansen VH, Halvorsen B, Dalen KT, Narverud I, Wesseltoft-Rao N, Granlund L, et al. Altered expression of genes involved in lipid metabolism in obese subjects with unfavourable phenotype. Genes Nutr. 2013;8(4):425–434. doi: 10.1007/s12263-012-0329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.