Abstract
Objective
Studies investigating health effects of work and family stress usually consider these factors in isolation. The present study investigated prospective interactive effects of job strain and informal caregiving on allostatic load (AL), a multisystem indicator of physiological dysregulation.
Methods
Subjects were 7,007 British civil servants from the Whitehall II cohort study. Phase 3 (1991-1994) served as the baseline, Phases 5 (1997-1999) and 7 (2002-2004) as follow-ups. Job strain (high job demands combined with low control) and caregiving (providing care to aged or disabled relatives) were assessed at baseline. AL index (possible range 0-9) was assessed at baseline and both follow-ups based on 9 cardiovascular, metabolic and immune biomarkers. Linear mixed effect models were used to examine the association of job strain and caregiving with AL.
Results
High caregiving burden (above the sample median weekly hours of providing care) predicted higher AL levels, with the effect strongest in those also reporting job strain [b = 0.36, 95% CI: 0.01– 0.71)]; however, the interaction between job strain and caregiving was not significant (p = 0.56). Regardless of job strain, participants with low caregiving burden (below sample median) had lower subsequent AL levels than non-caregivers [b = 0.22, 95% CI: 0.06–0.37].
Conclusions
The study provides some evidence for adverse effects of stress at work combined with family demands on physiological functioning. However, providing care to others may also have health protective effects if it does not involve excessive time commitment.
Keywords: work stress, job strain, informal caregiving, allostatic load
INTRODUCTION
Stress at work has been linked to adverse health outcomes, in particular to increased risk of cardiovascular disease (CVD) (1). A recent large meta-analysis confirms an association between job strain, defined as high level of job demands and low control over job-related decisions, and coronary heart disease (CHD) (2). At the same time, the authors note inconsistencies in findings regarding job strain and CHD, and the overall effect of job strain found in the meta-analysis is small. One possible explanation for these inconsistent findings is that the extent to which work stress affects health may depend on a range factors outside work.
Even though health effects of work stress have been studied quite extensively, most of this literature considers work factors in isolation; i.e. it does not take into account other life circumstances, which affect work performance and health (3). For instance, incompatibility of family and work demands, known as work-family conflict, has been shown to affect both work outcomes, such as work satisfaction, absenteeism and work performance, and more general mental health outcomes (4,5). Previous studies have addressed the combined effect of work and family demands on sickness absence, showing that people with the highest number of demands at work and at home had the highest rates of sickness absence (6,7). However, just like findings from studies using global measures of perceived work-family conflict, these results do not address the question whether having increased demands outside workplace might amplify the effects of work stress on health.
One of the few sources of chronic stress outside the workplace that has been studied is caregiving. High levels of caregiving stress have been linked to poor mental and physical health outcomes (8), although it is less clear from the existing literature, whether caregiving per se has any adverse health effects when it is not associated with stress (9). Some researchers argue that providing care to others may in fact carry health benefits (10). For instance, Buyck et al. (11) find that caregivers with low caregiving burden report better health status than non-caregivers, while high caregiving burden is associated with poorer health status. Just like in case of work stress, it is not clear whether health effects of caregiving depend on other circumstances in the caregiver’s life.
Chronic stress is known to lead to adverse changes in multiple biological systems, including endocrine, cardiovascular, metabolic, and immune systems, which may eventually cause disease (12). A large body of literature on stress and physiological functioning has focused on single biological markers such as blood pressure or levels of the stress hormones. However emerging research on stress argues for the importance of simultaneously considering multiple indicators of stress physiology (13). Allostatic load (AL) is a multi-dimensional indicator of physiological changes resulting from stress, which is computed using biological markers of multiple biological systems simultaneously (12). Due to this multidimensionality, AL is thought to be a more comprehensive and sensitive measure of the effects of chronic stress on the body than any single biomarker (12,14). the argument being that even when the changes in each one of these systems are modest and not predictive of health outcomes, the confluence of changes across multiple physiological systems presents a health risk (15).
High levels of AL have been linked to poor health and physical and cognitive decline in later life (15,16), making it an important concept for understanding stress-related morbidity and mortality. However, there is still a lack of knowledge about life-course trajectories of AL accumulation and their predictors. The present study addresses this gap in the literature and investigates work and caregiving stress as predictors of both AL levels and longitudinal changes in AL. Thereby the study contributes to a better understanding of potential physiological mechanisms linking stress and later life morbidity and mortality.
Only few studies previously investigated psychosocial work factors as predictors of AL, with mixed results. Some studies find that increased AL is associated with higher job demands (17,18), lower decision latitude and job strain (18–20), effort-reward imbalance and exhaustion (21), burnout (22) and career instability (23). Other studies found no effect of decision latitude (17), burnout (24) or career patterns (25) on AL and a reverse association between psychological demands and AL has also been reported (20).
Previous work on the relationship between caregiving and AL is also quite scarce. Self-reported perceived caregiving stress was linked to higher AL in a cross-sectional study of middle-aged Mexican-American women (26). Roepke et al. (27) found that Alzheimer caregiving was related to higher AL in Americans who were older than 55 and free of chronic disease. Clark at al. (28) also found that caregiving was associated with an increase in endocrine AL markers during a one-year follow-up. Glover et al. report (29) mothers of pediatric cancer survivors had higher AL than mothers of healthy children.
The present study investigates the interactive effect of work stress and caregiving (providing care to aged or disabled relatives) on AL. Specifically, we hypothesized that work stress and caregiving will amplify each other’s detrimental effect on allostatic load levels and the rate of its increase throughout the follow-up period.
Methods
Participants
The data were drawn from the Whitehall II cohort study. The original sample (Phase 1, recruited in 1985-1988) included 10,308 British civil service workers aged 35-55. Participants completed a questionnaire at every subsequent phase of the study, approximately two years apart, and were given medical examinations at Phases 1 (1985-1988), 3 (1991-1994), 5 (1997-1999) and 7 (2002-2004). For further details on the cohort profile, please see (30). In the present study, Phases 3, 5 and 7 were used as the baseline and two follow-ups. A supplementary analysis also used questionnaire data from Phase 4 (1995-1996). Data from Phase 1 were not used, as some of the AL biomarkers were not available at Phase 1.
Eighty six percent of Phase 1 respondents participated in Phase 3 (N = 8,815). Of them, 931 participants had incomplete or missing biomarker data for all three phases and were excluded from the study. Further 877 participants were excluded due to missing information on work stress, caregiving or one or more of the covariates (gender, age, social class, marital status, baseline longstanding illness). The resulting sample included 7,007 people (mean baseline age = 49, SD = 5.8, 30% women). Excluded participants were older, were more likely to be women, had lower social class and were more likely to report longstanding illness at baseline. The likelihood of missing AL data for one or more phases did not depend on baseline work stress or caregiving status.
Measures
Work stress was operationalized as job strain and assessed at baseline using a revised version of the Job Content Instrument (31), a widely accepted measure of psychosocial workplace characteristics. Consistent with the most common practice, job strain [yes, no] was defined as a combination of low (below sample median) decision latitude and high (above sample median) job demands. Decision latitude scale (α = 0.84) included 15 items related to decision authority (9 items, α = 0.77, e.g. “Can you choose how you do your work?”, “Do you have a good deal of say in decisions about work?”) and skill discretion (6 items, α = 0.78, e.g. “Do you have the possibility to learn new things?”, “Does your job require you to take initiative?”). Job demands scale (α = 0.67) included 4 items (e.g. “Do you have to work intensively?”, “Do others demand things that are hard to combine?”). Possible answers to ranged from 1 = “almost never” to 4 = “often”. For each scale, the overall score was calculated as the sum of the item scores.
Caregiving responsibilities were self-reported at baseline. Participants were asked whether they provided care for aged or disabled relatives and how many hours a week their caregiving responsibilities took. Because literature on caregiving and health suggests that caregiving may confer both health risks and health benefits, we wanted to make a distinction between caregivers based on the amount of their responsibilities. Thus we created a variable with three levels: no caregiving, low caregiving burden (at or below sample median, equal to 4 hours a week), and high caregiving burden (more than 4 hours a week).
Allostatic load was measured at baseline and at the 2 follow-ups. The AL index was based on 9 biological parameters: Blood pressure, body mass index (BMI), fasting insulin, fasting glucose, high density lipo-protein (HDL) cholesterol, low density lipo-protein (LDL) cholesterol, triglycerides, C-reactive protein (CRP), and interleukin-6 (IL-6). Following most common practices, AL was calculated as the number of biomarkers with values above a high-risk threshold (12,32). Clinically relevant cut-off points were used where such cut-offs have been established (see e.g. references 22 and 33 for examples of studies using clinical norms to compute AL). Otherwise, a distribution-based cut-off (75th percentile) was used following another common practice (12). The same cut-off values were used for all three Phases; the distribution-based cut-offs were established based on baseline values. Cut-off values for each biomarker are given in the Appendix. The laboratory procedure used to obtain and analyze he biomarkers have been described in detail elsewhere (34,35).
Statistical Analyses
To take advantage of the repeated measures of AL, we used Linear Mixed Effect (LME) models with a random level and slope for each subjects (36). These LME model allowed each subject to follow their own AL trajectory as a shifted and pivoted (due to the random level and slope) version of the average trajectory. These average trajectories are, just as in ordinary linear regression, allowed to depend on the covariates of interest (the fixed effects). The advantages of using the LME approach over a linear regression analyses are twofold. First, participants missing some of the AL measures need not be excluded, substantially increasing the statistical power. LME will, as an integrated part of the estimation procedure, compute likely values for the missing observations for each individual taking the observed information for that individual into consideration. This procedure will remain valid and unbiased even if missingness depends on other measured variables, e.g. age. In technical terms, the LME remains valid even when data is only missing at random compared to the more restrictive assumption of missing completely at random, which is required by a complete case analysis. Second, in addition to estimating the effect of work and caregiving on AL levels, the LME approach allowed us to investigate whether work and caregiving affected the rate of AL change (slope) over time.
In order to establish the rate of change in AL within the observed period of time and assess individual differences in the rate of AL change, we first estimated a model without any of the covariates (Model 0). Age was used as the underlying time scale. Because previous cross-sectional studies demonstrated that changes in AL over time level off as people age (37), we expected the association between age and AL to be non-linear and therefore included a quadratic age term as a fixed effect. In addition, to account for the fact that some of the effects of age might be cross-sectional cohort effects, we controlled for year of birth.
Next, we tested the main effects of job strain and caregiving on AL levels in Model 1a and on the rate of AL change with age (AL slope) in Model 1b. Job strain and caregiving were mutually controlled for in models 1a and 1b. The effects of job strain and caregiving on AL slope were assessed as a simple interaction between work and caregiving factors on the one hand and age on the other hand. Finally, we modeled the interaction between job strain and caregiving in predicting AL levels and slope (Models 2a and b respectively). Models 1 and 2 were controlled for gender, socio-economic position [administrators, professionals and executives, clerical and office support], marital status [married or cohabiting, single] and self-reported longstanding illness at baseline [yes, no]. A summary of these models is provided in the Table S1, Supplemental Digital Content 1. Analyses were conducted in R 3.0.2. using the ‘nlme’ package.
Results
Baseline study characteristics
Eighteen percent of the sample reported high job strain and eleven percent reported caregiving responsibilities. The number of caregiving hours per week ranged from 1 to 168 and was non-normally distributed. In the lower burden caregiving group, the average number of hours was 2.5 (SD = 1.1), while in the higher burden caregiving group, it was 18.0 (SD = 20.9). Table 1 shows the associations between job strain and caregiving, as well as the associations of these variables with the demographic covariates and baseline illness.
Table 1.
Baseline participants’ characteristics by job strain and caregiving status
| Job Strain | p a | Caregiving | p a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NO | YES | none | ≤ 4 hours/wk | > 4 hours/wk | |||||
| ALL participants | 5725 (82%) | 1282 (18%) | 6261 (89%) | 401 (6%) | 345 (5%) | ||||
| Caregiving | no caregiving | 5140 (82%) | 1121 (18%) | .018 | |||||
| ≤ 4 hours/wk | 322 (80%) | 79 (20%) | |||||||
| > 4 hours/wk | 263 (76%) | 82 (24%) | |||||||
| Baseline age (SD) | 49.0 (5.8) | 48.7 (5.6) | .098 | 48.7(5.8) | 50.1 (5.5) | 50.8 (5.4) | <.001 | ||
| Gender | Men | 4050(83%) | 837(17%) | <.001 | 4425 (91%) | 291 (6%) | 171 (4%) | <.001 | |
| Women | 1675 (79%) | 445(21%) | 1836 (87%) | 110 (5%) | 174 (8%) | ||||
| Marital status | Married/Cohabiting | 4472 (83%) | 906(17%) | <.001 | 4843 (90%) | 320 (6%) | 215 (4%) | <.001 | |
| Single | 1252 (77%) | 376 (23%) | 1418 (87%) | 81 (5%) | 130 (8%) | ||||
| Social class | Administrative | 2332 (86%) | 366 (14%) | <.001 | 2435 (90%) | 176 (7%) | 87 (3%) | <.001 | |
| Professional/Executive | 2484 (78%) | 706 (22%) | 2832 (89%) | 181 (6%) | 177 (6%) | ||||
| Clerical/Support | 909 (81%) | 210 (19%) | 994 (89%) | 44 (4%) | 81 (7%) | ||||
| Baseline illness | No | 3933 (83%) | 813 (17%) | <.001 | 4264 (90%) | 253 (5%) | 229 (5%) | .098 | |
| Yes | 1792 (79%) | 469 (21%) | 1997 (88%) | 148 (7%) | 116 (5%) | ||||
Two-tailed chi-square test.
Being a caregiver was associated with higher likelihood of reporting high job strain. In addition, participants who reported job strain were more likely to be women, to be single, to belong to professionals or executives and to report baseline illness. Caregivers were slightly older than noncaregivers and were more likely to be women and to be single. High caregiving burden was associated with lower socio-economic status. Caregivers were also slightly more likely to report baseline illness, however long-standing illness at baseline was not associated with the number of caregiving hours.
The AL score ranged from 0 to 9 and; 77% of all AL measurements were ≤ 3. Thirty nine percent of the sample had complete biomarker information and thus the AL index for all the three follow-up phases; 75% had at least two AL measures. Raw average AL scores for participants with all three measurements were 1.94 (SD = 1.66), 2.10 (SD = 1.67), and 2.34 (SD = 1.72) for the baseline and the two follow-ups, respectively. Table S2, Supplemental Digital Conten 1, provides average baseline AL levels and changes in AL from baseline to the end of follow-up by caregiving and job strain. Table S3, Supplemental Digital Content 1, provides correlations between caregiving, job strain and its components on one hand and individual biomarker baseline levels and changes throughout the follow-up on the other hand.
Changes of AL over Time
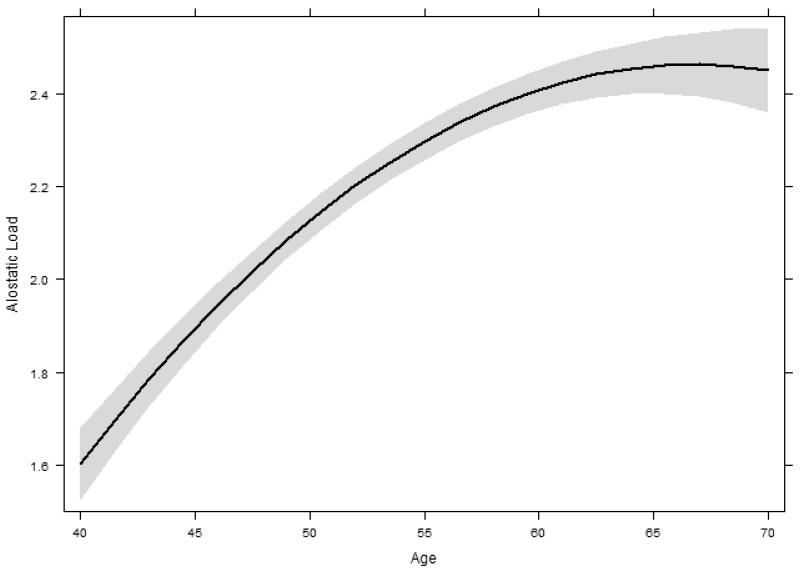
Fitting Model 0 to the data showed that AL increased with age, but as expected, the increase was non-linear (p-value for the quadratic age term < 0.001), becoming attenuated with age (Figure 1). The variance component associated with random slopes was statistically significant (p < .001) suggesting significant individual differences in AL change. There was a strong negative correlation between AL level and slope, so that individuals with lowest baseline levels had the steepest increase in AL (r = −.65).
Figure. 1.
The estimated non-linear change of AL over time based on Model 0. The trajectory is plotted for the average birth cohort (born in 1943).
Job Strain and Caregiving as Predictors of AL Levels and Slope
We investigated job strain and caregiving as predictors of AL levels and the slope of AL change over time. Because the trajectory of AL over time was found to be nonlinear, to establish the effect of job strain, caregiving and the covariates on AL trajectories, we initially included the interaction of these predictors with both first and second order age terms. However, because none of the interactions with the quadratic age term was significant, we dropped those interactions from the models (Table S1, Supplemental Digital Content 1, provides a summary of the statistical models).
Table 2 presents estimated between-group differences in AL levels associated with job strain and caregiving, averaged throughout the follow-up period. The analysis of main effects of job strain and caregiving (Model 1a, top part of Table 2) showed that job strain alone did not affect the levels of AL (p = .60). However, caregiving was a statistically significant predictor of AL levels (p = .001), with the direction of the effect depending on the caregiving burden. In those with low caregiving burden, the AL score throughout the follow-up period was on average 0.22 (95% CI: 0.06–0.37) lower than in noncaregivers, whereas in those with high caregiving burden, the AL score was 0.21 (95% CI: 0.04–0.37) higher than in non-caregivers. The effect of caregiving on the AL levels was comparable in magnitude with the effects of such well-established predictors of health as gender and social class: AL score was on average 0.52 (95% CI: 0.42–0.62) lower in women than in men and it was 0.27 (95% CI: 0.19–0.35) and 0.66 (95% CI: 0.54–0.78) lower in administrative class than, respectively, in professional/executive class and clerical/support class. Neither job strain nor caregiving affected the slope of AL (Model 1b, data not shown). In other words, the rate at which AL increased did not depend on the baseline work or caregiving stress.
Table 2.
Association of job strain and care giving with allostatic loada.
| Main effects of job strain and caregiving on allostatic load levels (Model 1a) | ||
|---|---|---|
| Job strain [NO] | Reference | |
| Job strain [YES] | −0.01 (−0.10;0.08) | |
| Caregiving [none] | Reference | |
| Caregiving [≤ 4 hours/wk] | −0.22 (−0.37;-0.06) | |
| Caregiving [> 4 hours/wk] | 0.21 (0.04;0.37) | |
| Interaction of job strain and caregivingb predicting allostatic load levels (Model 2a) | ||
| Job Strain [NO] | Caregiving [none] | Reference |
| Caregiving [≤ 4 hours/wk] | −0.20 (−0.38; −0.03) | |
| Caregiving [> 4 hours/wk] | 0.16 (−0.04; 0.35) | |
| Job strain [YES] | Caregiving [none] | −0.02 (−0.12; 0.08) |
| Caregiving [≤ 4 hours/wk] | −0.28 (−0.62; 0.06) | |
| Caregiving [> 4 hours/wk] | 0.34 (0.01; 0.68) | |
Data present differences in average AL levels throughout the follow-up period associated with job strain and caregiving and the 95% confidence intervals, adjusted for gender, age, social class, marital status and baseline longstanding illness. The table presents results from linear mixed models of both the main and the interactive effects of job strain and caregiving (Models 1a and 2a). Please see Table S1, Supplemental Digital Content 1, for details about the models.
p-value for statistical test of interaction = 0.56
The bottom of Table 2 shows the results of testing the interaction between job strain and caregiving on average AL levels (Model 2a). Regardless of whether or not participants reported job strain, caregivers with low caregiving burden had lower AL than non-caregivers. At the same time, there was a detrimental effect of high caregiving burden, as compared to no caregiving, which was approximately two times larger in those who reported job strain than in those who did not (difference in AL levels associated with high caregiving burden relative to no caregiving was 0.36 (95% CI: 0.01–0.71) vs. 0.16 (95% CI: −0.04–0.35. The interaction between job strain and caregiving was, however, not statistically significant (p = 0.56).The interaction of job strain and caregiving did not affect the slope of AL (Model 2b, data not shown).
To summarize, those with high caregiving burden and job strain had the highest baseline and follow-up AL scores, while those with low caregiving burden had the lowest baseline and follow-up AL scores. In non-caregivers and participants with low caregiving burden the effect of job strain was very small compared to the effect of age, while in the high caregiving burden group it was comparable in magnitude with the effect of getting a few years older. Moreover, individuals with both job strain and high caregiving burden had higher AL levels at baseline than individuals with low caregiving burden at a follow-up six years later.
Supplementary analyses
In addition to job strain, we also tested whether each of its component, job demands and decision latitude, affected AL. To this end, we reran the analyses replacing job strain with the two original continuous job demands and decision latitude variables. Neither of them had an effect on AL levels, nor did either variable modify the effect of caregiving on AL (results not shown). Furthermore, previous literature on work stress using the Job Content Instrument has included social support at work as one of the dimensions of psychosocial work environment. In Whitehall II, the social support scale (Cronbach’s α = 0.79) has six items relating to help and support from colleagues and supervisors and receiving consistent and sufficient information from supervisors (31). We also tested whether social support could buffer the effects of high caregiving burden on AL. Controlling for job demands and decision latitude, as well all other covariates, social support at work had a modest effect on AL levels. One SD increase in social support was associated with 0.09 lower AL (95% CI: 0.06 – 0.13). However, social support did not moderate the effect of caregiving on AL levels (p-value for interaction = .20).
Because the number of caregivers who also reported job strain was relatively low, we did not originally stratify our analyses by gender. Doing so would have resulted in less statistical power to test the hypothesis that job strain interacts with caregiving in predicting AL. However, considering important gender differences in caregiving roles as well as in biomarker levels, we additionally stratified the analyses of main effects of job strain and caregiving by gender. There was no effect of job strain on AL levels in either men or women. The direction of the effect of caregiving was the same in men and in women; however, the effect was much more pronounced in women than in men. In men, the effect of low caregiving burden was −0.12 (95% CI: −0.30 – 0.06) and the effect of high caregiving burden was 0.14 (95% CI: −0.09 – 0.37); in women, the effects were respectively −0.40 (95% CI: −0.69 – −0.11) and 0.26 (95% CI: 0.02 – 0.51). The interaction of caregiving with gender in predicting AL levels was not statistically significant (p = .19).
Most of the participants continued working throughout the follow-up period, and thus their exposure to job strain was likely to continue beyond baseline. To assess how stable job strain was over time, we calculated how many of participants reporting job strain at baseline also reported job strain at the two follow-ups. The subset of participants for whom job demands and decision latitude information was available for all three phases was used for this analysis (N = 2,530). To assess job strain at the two follow-ups, we used baseline cut-off values for job demands and decision latitude. Out of those who reported job strain at baseline, 28% also reported job strain at follow-up 1 and 21% reported job strain at follow-up 2, suggesting that at least a quarter of those exposed to job strain endured long-lasting work stress.
However, about a third of participants had reached retirement age (60 for women and 65 for men) before the end of the follow-up period, meaning that their exposure to job strain may have ended by the time AL was measured at one or both follow-ups. To see whether this might have affected the observed association between job strain and AL, we repeated the analyses of the main effect of job strain in the subset of participants who by the end of the follow-up period had not reached the age of retirement. The results were very similar, and we still observed no effect of job strain on AL (results not shown).
The duration of caregiving responsibilities was more difficult to ascertain using the data available. Eighty nine percent of the sample had completed questionnaires from Phase 4 of the Whitehall II study, administered approximately 3 years after the baseline questionnaire. Based on this subsample, around a half of those providing care at Phase 4 were also caregivers at baseline, while roughly a half of baseline caregivers did not report caregiving responsibilities at Phase 4. This suggests that for many participants caregiving stress was likely short in duration and its long-term effects on AL trajectories may have been limited. At the same time, baseline caregivers with low caregiving burden were less likely to also report caregiving 3 years later than those with high caregiving burden (39% vs. 55% respectively). Therefore the adverse effects of high caregiving burden on AL might have been at least in part a reflection of long-term caregiving.
Discussion
The present study investigated the interactive effect of caregiving and work stress, defined as job strain, on the levels and the trajectories of allostatic load. We found that high caregiving burden (more than 4 hours per week) predicted higher levels of AL across the follow-up period, but did not affect the rate of change of AL over time. Consistent with our hypothesis, job strain amplified the effects of high burden caregiving on AL; however, there was no statistically significant interaction between the effects of caregiving and job strain.
The analyses of main effects of job factors and caregiving showed that overall, neither job strain, nor its components job demands and decision latitude had a statistically significantly effect on levels of AL across the follow-up period. These results were replicated in a subsample of younger workers who had not reached retirement age by the end of the follow-up period. As mentioned in the introduction, the results of previous studies of work factors and AL have been mixed. These studies have also differed on the measures of work stress, and the samples are drawn from different countries, different age groups and different occupational groups, limiting comparability of the findings. Some researchers have explained the absence of the effect of work factors on AL hypothesizing that increased AL as a consequence of work conditions may not surface until later in life (24). The results of our longitudinal analyses, showing that effect of work factors did not amplify with age, do not seem to support this conjecture. Whitehall II study sample only consists of civil service workers and thus may not be representative of the range of work stress levels in the general population. On the other hand, previous studies based on the same sample have shown that the differences in job strain and its components are reflected in the risks of CVD (38,39). This discrepancy suggests that AL may not be the mechanism linking job strain to CVD in the Whitehall II cohort.
Unlike job strain, caregiving predicted AL levels. Participants who reported the largest number of caregiving hours had the highest levels of physiological dysregulation. However, participants with low caregiving burden had lower levels of AL than people without any caregiving responsibility. Several previous studies finding that caregivers are less likely to report limiting long-term illness have explained these results by ill people self-selecting out of caregiving roles (9). Yet, in our study, we found that at baseline, caregivers were slightly more likely to report longstanding illness, while caregiving burden was not related to baseline self-reported longstanding illness, suggesting that the positive effects of small amount of caregiving on AL is unlikely to be explained by a self-selection bias. Instead, these positive effects might be mediated through enhanced psychological well-being, for instance, added sense of reward and fulfillment or improved relationship with care recipients (10,40). This explanation is also consistent with the hypothesis that a combination of multiple social roles may be beneficial for health and well-being (41). To summarize, our findings are consistent with studies finding both benefits and risks in caregiving and suggest, that in small amount caregiving may have protective effects on the body, while as demands associated with caregiving increase, its toll on the body increases as well.
The majority of previous studies of AL have been cross-sectional, and the existing prospective studies only assess AL at follow-up, limiting our understanding of how stress affects the rate of AL accumulation over time. In the present study, the differences in AL attributed to caregiving did not increase over time, which may partly be due to the fact that caregiving was likely limited in time. Indeed half of those reporting caregiving at baseline did not have any caregiving role three years later. Future research is needed to assess the effects of more persistent types of stress on the rates of AL accumulation.
While the generalizability of the findings to occupations other than civil service is limited, a large sample representative of a wide age range and a repeated measures design are among the strengths of the present study. The use of the multisystemic index of physiological dysregulation has both advantages and trade-offs. On the one hand, as stress affects multiple biological systems, the AL index is a more comprehensive measure of physiological changes resulting from stress than any biological parameter considered in isolation. On the other hand, because there is no standardized ways to measure AL, the comparability of our results to those of other studies is limited (32). Furthermore, compared to some of more recent studies (see reference 32), our AL index was based on a relatively small number of available biomarkers and did not include, for instance, any of the endocrine measures commonly considered in the AL literature. Finally, just like in any longitudinal study, attrition and non-random missingness of the data in Whitehall II create a potential bias in results. Using linear mixed model mostly solves this issue, but only if missingness status is dependent on nothing but measured variables (i.e. missing at random).
In conclusion, the study provides some evidence for the synergetic adverse effects of stress at work and family demands on physiological functioning. These results underscore the need for future studies to consider the effects of occupational factors within a broader non-work context.
Supplementary Material
Acknowledgments
We thank all participating women and men in the Whitehall II Study, as well as all Whitehall II research scientists, study and data managers and clinical and administrative staff who make the study possible.
Source of Funding: This research was supported by the Danish National Work Environment Foundation (Grant No. 12-2013-03). The UK Medical Research Council, British Heart Foundation, and the US National Institutes of Health (R01HL36310, R01AG013196) have supported collection of data in the Whitehall II Study. Jenny Head is partially supported by the Economic and Social Research Council (ES/K01336X/1 and ES/L002892/1).
Abbreviations
- AL
Allostatic Load
- BMI
body mass index
- CVD
Cardiovascular Disease
- CRP
C-reactive protein
- HDL
high density lipo-protein
- IL-6
interleukin-6 (IL-6)
- LME
Linear Mixed Effect
- LDL
low density lipo-protein
APPENDIX.
High-risk cut-off values used in calculating the allostatic load index
| Biological risk | Cut-off point | Reference |
|---|---|---|
| High blood pressure | 140 / 90 mmHg | (42) |
| High BMI | 25 kg/m2 | (43) |
| High fasting insulin | 8.6 uiu/ml | distribution-based |
| High fasting glucose | 5.5 mmol/L | (44) |
| Low HDL cholesterol | 1.03 mmol/L | (45) |
| High LDL cholesterol | 4.9 mmol/La | (45) |
| High Triglycerides | 1,7 mmol/L | (46) |
| High CRP | 3 mg/L | (47) |
| IL6 | 2.06 pg/ml | distribution-based |
National Cholesterol Education Program (NCEP) lists several cut-off values for LDL. The value of 4.9 mmol/L is the cut-off for very high LDL cholesterol corresponding to the highest risk of morbidity. Values lower than that also present health risks. However, because in out sample HDL cholesterol values were on average high (4.4 mmol/L at Phase 3), we chose such a high threshold to reflect the most vulnerable group among the participants.
Footnotes
Conflicts of Interest
The authors have no conflict of interest to report.
References
- 1.Steptoe A, Kivimäki M. Stress and cardiovascular disease: an update on current knowledge. Annu. Rev. Public Health. 2013;34:337–54. doi: 10.1146/annurev-publhealth-031912-114452. [DOI] [PubMed] [Google Scholar]
- 2.Kivimäki M, Nyberg ST, Batty GD, Fransson EI, Heikkilä K, Alfredsson L, Bjorner J, Borritz M, Burr H, Casini A, Clays E, De Bacquer D, Dragano N, Ferrie JE, Geuskens GA, Goldberg M, Hamer M, Hooftman WE, Houtman IL, Joensuu MJ, Markus KF, Knutsson A, Koskenvuo M, Koskinen A, Kouvonen A, Kumari M, Madsen IEH, Marmot MG, Nielsen ML, Nordin M, Oksanen T, Pentti J, Rugulies R, Salo P, Siegrist J, Singh-Manoux A, Suominen SB, Väänänen A, Vahtera J, Virtanen M, Westerholm PJM, Westerlund H, Zins M, Steptoe A, Theorell T. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012;380(9852):1491–7. doi: 10.1016/S0140-6736(12)60994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kivimaki M, Vahtera J, Elovainio M, Lillrank B, Kevin MV. Death or Illness of a Family Member, Violence, Interpersonal Conflict, and Financial Difficulties as Predictors of Sickness Absence: Longitudinal Cohort Study on Psychological and Behavioral Links. Psychosom. Med. 2002;64(5):817–825. [PubMed] [Google Scholar]
- 4.Amstad FT, Meier LL, Fasel U, Elfering A, Semmer NK. A meta-analysis of work-family conflict and various outcomes with a special emphasis on cross-domain versus matching-domain relations. J. Occup. Health Psychol. 2011;16(2):151–69. doi: 10.1037/a0022170. [DOI] [PubMed] [Google Scholar]
- 5.Leineweber C, Baltzer M, Magnusson Hanson LL, Westerlund H. Work-family conflict and health in Swedish working women and men: a 2-year prospective analysis (the SLOSH study) Eur. J. Public Health. 2012 doi: 10.1093/eurpub/cks064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabbath EL, Melchior M, Goldberg M, Zins M, Berkman LF. Work and family demands: predictors of all-cause sickness absence in the GAZEL cohort. Eur. J. Public Health. 2012;22(1):101–6. doi: 10.1093/eurpub/ckr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melchior M, Berkman LF, Niedhammer I, Zins M, Goldberg M. The mental health effects of multiple work and family demands. A prospective study of psychiatric sickness absence in the French GAZEL study. Soc. Psychiatry Psychiatr. Epidemiol. 2007;42(7):573–82. doi: 10.1007/s00127-007-0203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: a review of the literature. J. Am. Acad. Nurse Pract. 2008;20(8):423–8. doi: 10.1111/j.1745-7599.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly D, Connolly S, Rosato M, Patterson C. Is caring associated with an increased risk of mortality? A longitudinal study. Soc. Sci. Med. 2008;67(8):1282–90. doi: 10.1016/j.socscimed.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Cohen CA, Colantonio A, Vernich L. Positive aspects of caregiving: rounding out the caregiver experience. Int. J. Geriatr. Psychiatry. 2002;17(2):184–8. doi: 10.1002/gps.561. [DOI] [PubMed] [Google Scholar]
- 11.Buyck J-F, Bonnaud S, Boumendil A, Andrieu S, Bonenfant S, Goldberg M, Zins M, Ankri J. Informal caregiving and self-reported mental and physical health: results from the Gazel Cohort Study. Am. J. Public Health. 2011;101(10):1971–9. doi: 10.2105/AJPH.2010.300044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 13.McEwen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism. 2003;52:10–16. doi: 10.1016/s0026-0495(03)00295-6. [DOI] [PubMed] [Google Scholar]
- 14.Gallo LC, Fortmann AL, Mattei J. Allostatic load and the assessment of cumulative biological risk in biobehavioral medicine: challenges and opportunities. Psychosom. Med. 2014;76(7):478–80. doi: 10.1097/PSY.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc. Natl. Acad. Sci. U. S. A. 2001;98(8):4770–5. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Read S, Grundy E. Allostatic load and health in the older population of England: a crossed-lagged analysis. Psychosom. Med. 2014;76(7):490–6. doi: 10.1097/PSY.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnorpfeil P, Noll A, Schulze R, Ehlert U, Frey K, Fischer JE. Allostatic load and work conditions. Soc. Sci. Med. 2003;57(4):647–656. doi: 10.1016/s0277-9536(02)00407-0. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Wang S, Zhang J-Q, Li W. Assessing the cumulative effects of stress: The association between job stress and allostatic load in a large sample of Chinese employees. Work Stress. 2007;21(4):333–347. [Google Scholar]
- 19.Li W, Zhang J-Q, Sun J, Ke JH, Dong ZY, Wang S. Job stress related to glyco-lipid allostatic load, adiponectin and visfatin. Stress Heal. 2007;23(4):257–266. [Google Scholar]
- 20.Juster R-P, Moskowitz DS, Lavoie J, D’Antono B. Sex-specific interaction effects of age, occupational status, and workplace stress on psychiatric symptoms and allostatic load among healthy Montreal workers. Stress. 2013;16(6):616–29. doi: 10.3109/10253890.2013.835395. [DOI] [PubMed] [Google Scholar]
- 21.Bellingrath S, Weigl T, Kudielka BM. Chronic work stress and exhaustion is associated with higher allostastic load in female school teachers. Stress. 2009;12(1):37–48. doi: 10.1080/10253890802042041. [DOI] [PubMed] [Google Scholar]
- 22.Juster R-P, Sindi S, Marin M-F, Perna A, Hashemi A, Pruessner JC, Lupien SJ. A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology. 2011;36(6):797–805. doi: 10.1016/j.psyneuen.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Kinnunen M-L, Kaprio J, Pulkkinen L. Allostatic Load of Men and Women in Early Middle Age. J. Individ. Differ. 2005;26(1):20–28. [Google Scholar]
- 24.Langelaan S, Schaufeli WB, Doornen LJP, Bakker AB, Rhenen W. Is burnout related to allostatic load? Int. J. Behav. Med. 2007;14(4):213–221. doi: 10.1007/BF03002995. [DOI] [PubMed] [Google Scholar]
- 25.Johansson G, Huang Q, Lindfors P. A life-span perspective on women’s careers, health, and well-being. Soc. Sci. Med. 2007;65(4):685–97. doi: 10.1016/j.socscimed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Gallo LC, Jiménez JA, Shivpuri S, Espinosa de los Monteros K, Mills PJ. Domains of chronic stress, lifestyle factors, and allostatic load in middle-aged Mexican-American women. Ann. Behav. Med. 2011;41(1):21–31. doi: 10.1007/s12160-010-9233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roepke SK, Mausbach BT, Patterson TL, Von Känel R, Ancoli-Israel S, Harmell AL, Dimsdale JE, Aschbacher K, Mills PJ, Ziegler MG, Allison M, Grant I. Effects of Alzheimer caregiving on allostatic load. J. Health Psychol. 2011;16(1):58–69. doi: 10.1177/1359105310369188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark MS, Bond MJ, Hecker JR. Environmental stress, psychological stress and allostatic load. Psychol. Health Med. 2007;12(1):18–30. doi: 10.1080/13548500500429338. [DOI] [PubMed] [Google Scholar]
- 29.Glover DA, Stuber M, Poland RE. Allostatic load in women with and without PTSD symptoms. Psychiatry. 2006;69(3):191–203. doi: 10.1521/psyc.2006.69.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marmot M, Brunner E. Cohort Profile: the Whitehall II study. Int. J. Epidemiol. 2005;34(2):251–6. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 31.Bosma H, Marmot MG, Hemingway H, Nicholson AC, Brunner E, Stansfeld SA. Low job control and risk of coronary heart disease in Whitehall II (prospective cohort) study. BMJ. 1997;314(7080):558–558. doi: 10.1136/bmj.314.7080.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol. Res. Nurs. 2012;14(4):311–46. doi: 10.1177/1099800412455688. [DOI] [PubMed] [Google Scholar]
- 33.Glei DA, Goldman N, Wu C-H, Weinstein M. Does Exposure to Stressors Predict Changes in Physiological Dysregulation? Ann. Behav. Med. 2013;46(1):121–126. doi: 10.1007/s12160-013-9485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215–21. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamer M, Sabia S, Batty GD, Shipley MJ, Tabák AG, Singh-Manoux A, Kivimaki M. Physical activity and inflammatory markers over 10 years: follow-up in men and women from the Whitehall II cohort study. Circulation. 2012;126(8):928–33. doi: 10.1161/CIRCULATIONAHA.112.103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS (Statistics and Computing) Springer; New York, NY: 2000. [Google Scholar]
- 37.Crimmins EM, Johnston M, Hayward M, Seeman TE. Age differences in allostatic load: an index of physiological dysregulation. Exp. Gerontol. 2003;38(7):731–734. doi: 10.1016/s0531-5565(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 38.Kivimäki M, Head J, Ferrie JE, Brunner E, Marmot MG, Vahtera J, Shipley MJ. Why is evidence on job strain and coronary heart disease mixed? An illustration of measurement challenges in the Whitehall II study. Psychosom. Med. 2006;68(3):398–401. doi: 10.1097/01.psy.0000221252.84351.e2. [DOI] [PubMed] [Google Scholar]
- 39.Kuper H, Marmot M. Job strain, job demands, decision latitude, and risk of coronary heart disease within the Whitehall II study. J. Epidemiol. Community Health. 2003;57(2):147–53. doi: 10.1136/jech.57.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolff JL, Dy SM, Frick KD, Kasper JD. End-of-life care: findings from a national survey of informal caregivers. Arch. Intern. Med. 2007;167(1):40–6. doi: 10.1001/archinte.167.1.40. [DOI] [PubMed] [Google Scholar]
- 41.Lahelma E, Arber S, Kivelä K, Roos E. Multiple roles and health among British and Finnish women: the influence of socioeconomic circumstances. Soc. Sci. Med. 2002;54(5):727–740. doi: 10.1016/s0277-9536(01)00105-8. [DOI] [PubMed] [Google Scholar]
- 42.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 43.WHO [Accessed January 24, 2014];Global Database on Body Mass Index. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 44.American Diabetes Association [Accessed March 17, 2014];Diagnosing Diabetes and Learning About Prediabetes. http://www.diabetes.org/are-you-at-risk/prediabetes/
- 45.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 46.American Heart Association [Accessed January 24, 2014];Triglycerides. http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/Triglycerides_UCM_306029_Article.jsp.
- 47.Ridker PM. Cardiology Patient Page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation. 2003;108(12):e81–5. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.