Abstract
The varicella-zoster virus (VZV) re-activation increases during ageing. Although the effects of VZV re-activation are observed in the skin (shingles) the number or functional capacity of cutaneous VZV specific T cells have not been investigated. The numbers of circulating IFN-γ secreting VZV specific CD4+ T cells are significantly decreased in old subjects however other measures of VZV-specific CD4+ T cells, including proliferative capacity to VZV antigen stimulation and identification of VZV-specific CD4+ T cells with a MHC class II tetramer (epitope of IE-63 protein), were similar in both age groups. The majority of T cells in the skin of both age groups expressed CD69, a characteristic of skin resident T cells. VZV-specific CD4+ T cells were significantly increased in the skin compared to the blood in young and old subjects and their function was similar in both age groups. In contrast the number of Foxp3+ regulatory T cells (Tregs) and expression of the inhibitory receptor PD-1 on CD4+ T cells were significantly increased in the skin of older humans. Therefore VZV-specific CD4+ T cells in the skin of older individuals are functionally competent. However their activity may be restricted by multiple inhibitory influences in situ.
Keywords: T cell, memory, skin resident, antigen-specific, ageing
INTRODUCTION
Varicella zoster virus (VZV), an alpha-herpes virus, is the causative agent of chickenpox. After resolution of the initial infection VZV enters a latent phase within dorsal root ganglia. However, later in life VZV re-activation can occur, causing herpes zoster (also known as shingles) that results from virus shedding into the skin (Arvin, 1996; Arvin, 2001; Goldblatt, 1998). Although the skin is the major site that is involved in VZV reactivation during shingles, it is not clear if this is related to changes in skin resident VZV specific T cells in this tissue. The role of a subset of memory cells in the skin, termed tissue resident memory T cells (Trm), that are poised to provide efficient and rapid immunity in this organ has been described recently (Gebhardt et al., 2009; Gebhardt et al., 2011; Jiang et al., 2012). These Trm cells have been shown to be part of the first line of defence against herpes simplex virus (HSV) or human papiloma virus (HPV) infections (Cuburu et al., 2012; Gebhardt et al., 2009; Mackay et al., 2012; Masopust et al., 2010; Tang and Rosenthal, 2010). The activation of these cells also enables the recruitment of circulating antigen-specific T cells that amplifies the response (Schenkel et al., 2013). Although most studies of Trm have focused on CD8+ populations (especially in mice), CD4+ Trm cells also reside in the skin (Clark et al., 2006; Clark et al., 2012; Mueller et al., 2013). In addition to the tissue resident Trm cells, skin also contains recirculating memory T cells which actively recirculate between blood, skin and lymphoid organs (Bromley et al., 2013; Clark et al., 2012; Jiang et al., 2012). It has been proposed that non-migrating tissue resident T cells express CD69 which was found to be necessary for S1P1 down-regulation and T cell retention (Carbone et al., 2013; Skon et al., 2013).
We have investigated quantitative and qualitative changes in VZV specific CD4+ T cells in the blood and skin in young and old subjects and showed that although the numbers of these cells were increased in the skin compared to the blood, the capacity of these cells to secrete cytokines was not altered by ageing. The vast majority of T cells in the skin of both young and old individuals expressed CD69, a marker for tissue resident memory T cell populations (Clark et al., 2012; Gebhardt et al., 2009; Jiang et al., 2012) suggesting that VZV-specific CD4+ T cells in healthy skin may be Trm cells. Although cutaneous VZV specific T cells in old individuals are functionally competent, previous studies have shown that these subjects have decreased ability to mount a recall antigen responses to cutaneous VZV antigen challenge (Agius et al., 2009; Levin et al., 2003). Our results suggest that this decrease in cutaneous VZV-specific immunity in the tissue is not directly due to a defect in CD4+ Trm cells. Instead, external influences such as the increased numbers of suppressive Tregs (Agius et al., 2009; Seneschal et al., 2012) and/or increased signalling through the inhibitory receptor PD-1 (Wherry, 2011; Zajac et al., 1998), that is highly expressed on skin resident CD4+ T cells, may create an inhibitory microenvironment that restricts effective immune responses to VZV in the skin of older humans.
RESULTS
VZV specific CD4+T cells in the blood during ageing
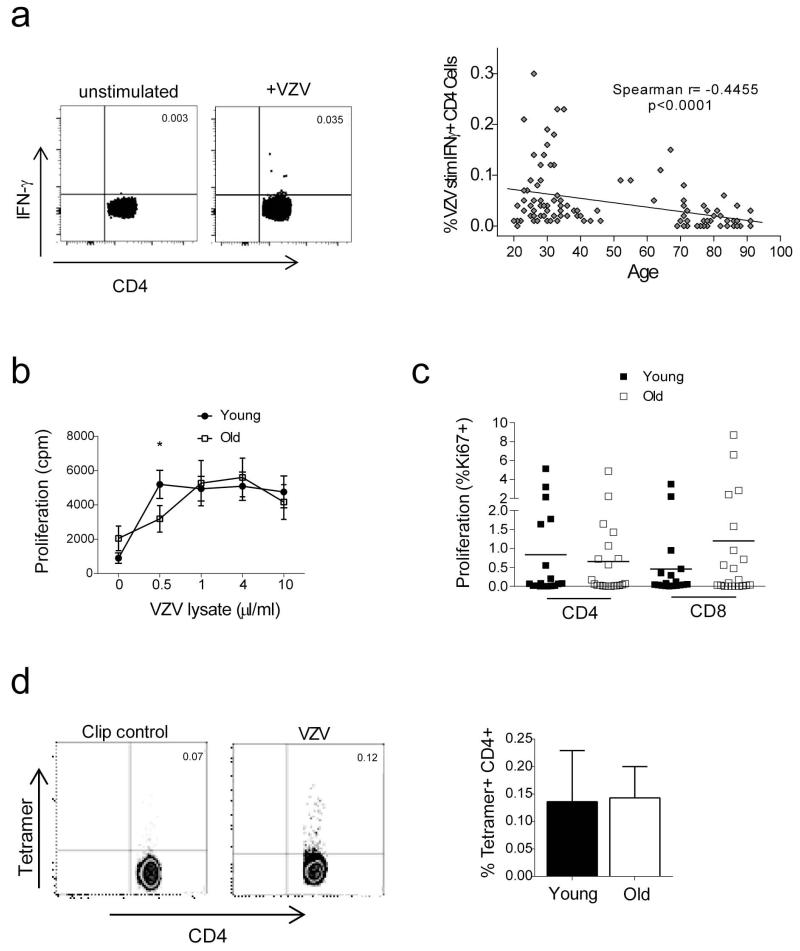
We investigated the effect of increasing age on VZV specific CD4+ T cell frequency by measuring IFN-γ and IL-2 responses following overnight stimulation of PBMC with VZV lysate. 133 donors were analysed (aged 20-91 years) and only donors serologically positive for VZV were included in the analysis. Figure 1A (left panel) shows representative dot plots of IFN-γ production for unstimulated and VZV stimulated PBMC gated on CD3+CD4+ T cells. We found a significant decrease of IFN-γ secreting cells during ageing (Figure 1A right panel, p<0.0001) confirming previous reports (Asanuma et al., 2000; Levin et al., 2003). We also examined the IL-2 responses to VZV of the donors analysed (Supplementary Figure 1) but found that host age did not significantly influence the frequency of IL-2 secreting cells.
Figure 1. Effect of age on circulating VZV specific CD4+ T cells.
(A) FACS analysis of intracellular IFN-γ staining following overnight stimulation with VZV viral lysate in the presence of brefeldin A. Representative dot plots of the CD4+ IFN-γ response from young and old donors are shown and unstimulated control. Cumulative data for all donors are plotted as increasing age (years) on the x axis against the percentage of antigen specific IFN-γ+ CD4+ T cells. Line of best fit was generated by linear regression and the correlation assessed by Spearman rank correlation. (B) PBMC were stimulated with a range of doses of VZV lysate and cell proliferation measured on day 7. (C) PBMCs from young and old individuals were stimulated with VZV lysate for 72 hours and then stained for the expression of Ki67, CD4 and CD8 and analysed by FACS. Data are expressed as % of CD4+ or CD8+ T cells expressing Ki67 above background (unstimulated PBMC) (n=20 young and old, horizontal line represents the mean). (D) The number of antigen specific CD4+ cells was also determined by class II tetramer staining. PBMC were stained with HLA-DRB1*1501 restricted IE63 tetramer and with CLIP control (Jones et al., 2007; Vukmanovic-Stejic et al., 2013). Each data point represents one individual (n=5 young and old individuals, mean and SEM are indicated).
We next investigated VZV specific T cell activity by assessing the ability of PBMC from young and old donors to proliferate after VZV antigen stimulation in vitro (Figure 1B, 1C). After 6 days of stimulation with a range of concentrations of VZV lysate (n=29 old and 26 young) the extent of proliferation measured by 3H-thymidine uptake was similar in young and old subjects except at the lowest dose of VZV antigen used (Figure 1B). Furthermore there were no differences in proportions of cells expressing Ki67 three days after VZV lysate stimulation (4 μl/ml) in vitro (Figure 1C). This indicates that the decrease in VZV specific cells, identified by IFN-γ secretion in the peripheral blood compartment, does not represent a global defect in the functional responses of these cells.
We also investigated the frequency of VZV specific cells in young and old subjects (Figure 1D) using a class II tetramer HLA-DRB1*1501 restricted IE63 tetramer (Jones et al., 2007; Vukmanovic-Stejic et al., 2013). No difference was observed in the number of tetramer positive VZV specific CD4+ T cells between the young and old individuals tested (Figure 1D). We confirmed that the tetramer staining was specific by showing an absence of staining when a control tetramer (CLIP) was used, and also when HLA DRB1*1501-negative individuals were tested (data not shown and (Vukmanovic-Stejic et al., 2013)).
Effect of age on leukocyte populations and global gene expression signatures in the skin
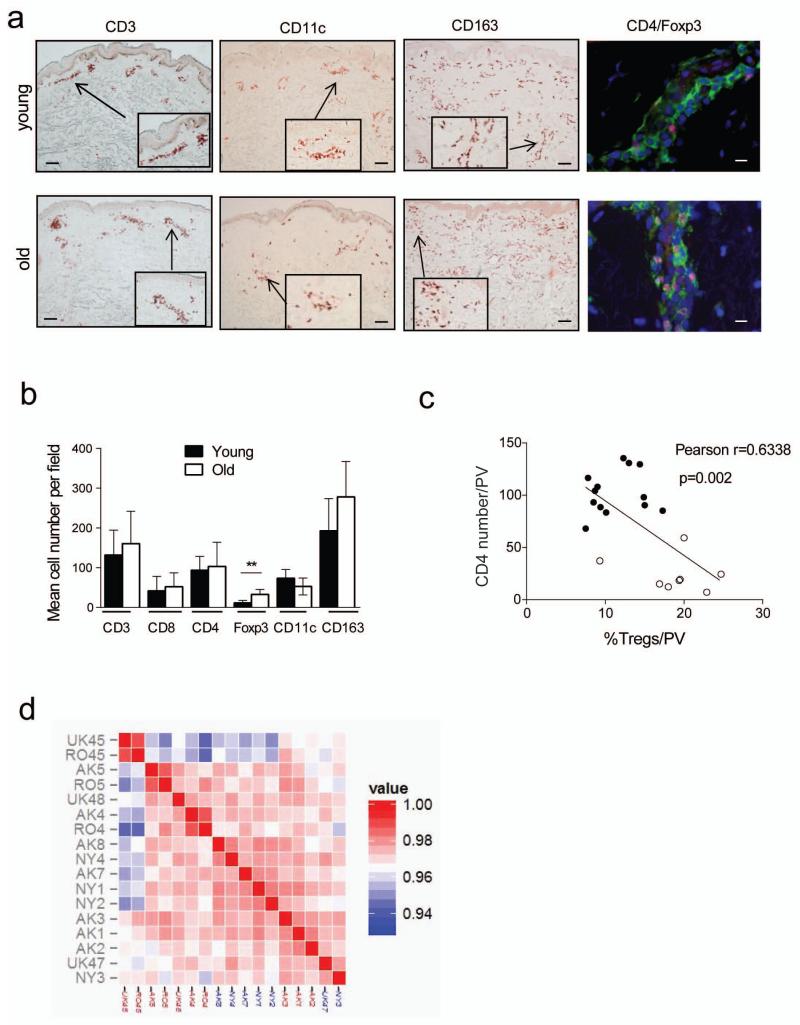
We investigated if there were numerical or functional differences in the T cells found in young and old skin. We collected 5 mm punch biopsies from individuals of both age groups and analysed them by immunohistochemistry and immunofluorescence using antibodies specific for CD3, CD4, CD11c, CD163 and Foxp3 (Figure 2A). No difference was observed in the number of CD3+, CD4+ or CD8+ T cells with age (Figure 2B, Table 1). The numbers of dermal dendritic cells (CD11c) or macrophages (CD163) (Haniffa et al., 2009; Zaba et al., 2007; Zaba et al., 2008) were also unaffected by increasing age (Figure 2B, Table 1). However there were significantly increased numbers of CD4+Foxp3+ T cells in the skin of old compared to young individuals in agreement with previous studies (Agius et al., 2009; Gregg et al., 2005; Lages et al., 2008). This difference was also observed by flow cytometric analysis of skin derived populations (Supplementary Figure 2A) The increased proportion of Foxp3+ cells in the old skin is significantly correlated with a decrease of memory CD4+ T cell infiltration and with the decrease in the clinical response after intradermal challenge with VZV antigen (Figure 2C, Supplementary Figure 2) confirming previous observations (Agius et al., 2009; Vukmanovic-Stejic et al., 2013). This is indirect evidence that these cells in older subjects have suppressive activity.
Figure 2. Effect of age on the skin resident populations and gene expression profiles.
Punch biopsies (5 mm) were collected from normal skin of healthy young (<40 years old) and old (>70 years old) volunteers. (A) Sections were stained to detect CD4, CD8, CD3, CD163, CD11c and Foxp3 (scale bar= 100μm). Cell numbers were expressed as the mean absolute number of cells counted within the section. The frequency of Foxp3+CD4+ cells was confirmed by 3 colour IF staining (Dapi- blue, CD4-green, Foxp3- red, scale bar=10μm). (B) Graph shows numbers of different cell populations in young and old skin (n=5-7 young and old). Biopsies were collected following intradermal challenge with VZV antigen and stained for CD4 and Foxp3. Numbers of cells were counted in perivascular infiltrates (average of 5 largest perivascular infiltrates (PV) counted). Graph shows an inverse correlation between the proportion of Foxp3+ cells within CD4 population and a size of the cellular infiltrate (C) (filled circles-young, open circles-old). (D) Unsupervised clustering of the skin samples from young (labels in red) and old (labels in blue) individuals based on the expression profiles using Affymetrix HG U133 Plus 2.0 arrays.
Table 1. Numbers of different cell populations is normal skin.
| Cell type | Marker used | Range and mean (young) | Range and mean (old) | different |
|---|---|---|---|---|
| T cells | CD3 | 44-201, 131.8 | 91-291, 160.6 | no |
| CD4+ T cells | CD4 | 66-145, 93.4 | 45-159, 103 | no |
| CD8+ T cells | CD8 | 11-74, 41.6 | 24-110, 52,2 | no |
| Tregs | Foxp3 | 1-17, 11.6 | 17-50, 32.8 | 0.0095 |
| Dendritic cells | CD11c | 46-105, 73.5 | 33-83, 52.75 | no |
| Macrophages | CD163 | 95-326, 192.8 | 199-390, 278.2 | no |
| PDC | BDCA3 | 18-33, 27.4 | 13-41, 25.75 | no |
We next investigated global gene expression profiles in the skin of young and old subjects. Transcriptional analysis was performed on the skin biopsies of normal skin collected from 7 young and 7 healthy old individuals. Overall, unsupervised clustering coupled with bootstrapping did not identify stable clusters separating skin samples from young and old individuals (Figure 2D and Supplementary Figure 3A). Only 2 genes were considered differentially expressed LPPR4 (higher in young) and ADAMTSL1 (higher in old) using a typical FDR<0.05 and FCH>2. In terms of pathways, Gene Set Variation Analysis (GSVA) suggested differences in human skin pigmentation genes and cell cycle related genes (Wang et al., 2013) but not in terms of immune genes, positive or negative regulators or in any of the skin specific cytokine pathways that we have curated (Suarez-Farinas et al., 2011) (Supplementary Figure 2C).
Next, the global gene expression profiles in the young and aged skin samples were compared with that of a large collection of microarray data sets from distinct human primary cell populations (745 individual data sets) (Mabbott et al., 2013). Data were analysed using Biolayout Express3D with a Pearson correlation r=0.90 and Markov cluster (MCL) of 2.2,, and generated 140 clusters of ≥6 probe sets (genes). The network graph’s structure is derived from the clustering of genes that are expressed in a cell- or function-specific manner (Supplementary Figure 3D; Supplementary Table 1) and their contents reflect the nature of the cell populations represented in human skin. Congruent with the differential transcriptional analyses presented above (Supplementary Figure 3B, C), no significant difference was observed in the expression levels of the genes within these cell-specific (eg: keratinocytes, endothelium) and cellular activity-related clusters (eg: extracellular matrix, MHC genes) between the two age groups (Supplementary Figure 3E).
Effect of age on the extent of differentiation of CD4 T cells in blood and skin
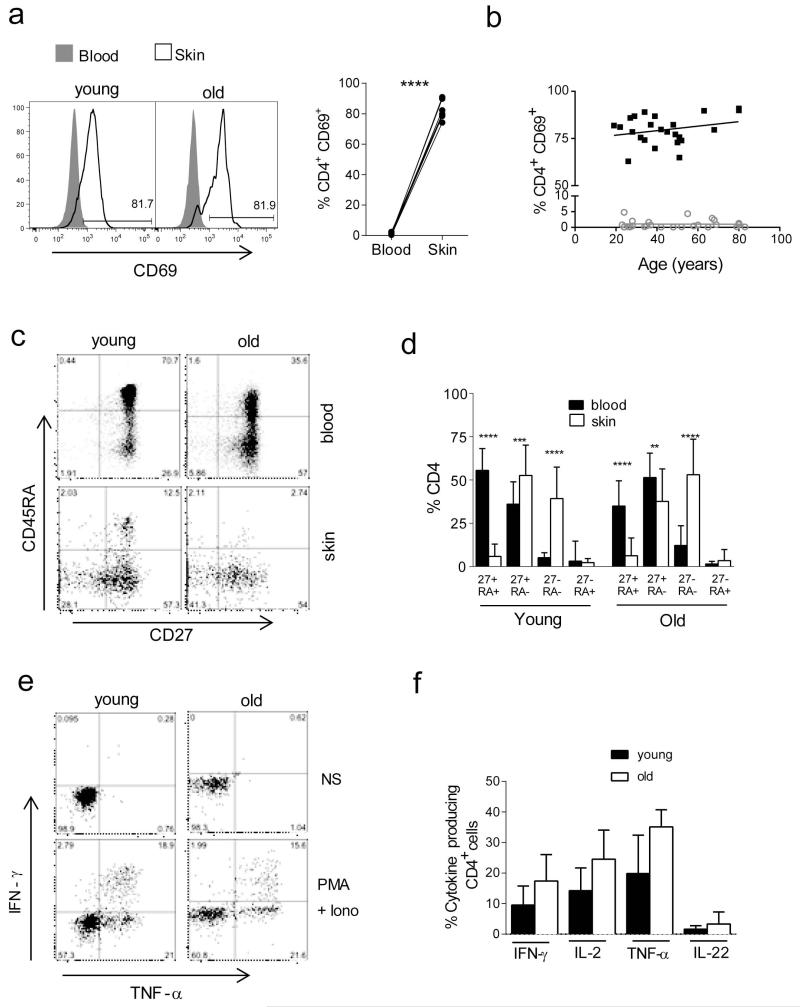
We investigated whether the T cells in skin were true skin resident T cells as defined by CD69 expression that is a marker for tissue retention(Clark et al., 2012; Gebhardt et al., 2009; Jiang et al., 2012; Skon et al., 2013), In contrast to circulating T cells 80-90% of skin derived CD4 (Figure 3A, 3B) and CD8 populations (data not shown) express CD69. In addition, only around 10% of skin derived cells express CCR7 confirming that the vast majority of cutaneous T cells are skin resident and not transient T cell populations (Clark et al., 2012; Gebhardt et al., 2009; Jiang et al., 2012). Furthermore CD69+ T cells did not express surface CD25 suggesting they were not an activated population and this was also confirmed by their small size, defined by forward and side scatter properties by flow cytometry. There was no significant change in the proportion of CD69+ cells with age (Figure 3B).
Figure 3. Effect of age on the phenotype and function of skin resident T cells.
5 mm punch biopsies and peripheral blood samples were collected from n=31 young and n=23 old donors. (A) Representative FACS histograms showing ex vivo CD69 expression in CD4+ T cells in skin compared to the blood. Comparison of percentages of PD-1 expressing cells CD4+ or CD8+ T cells between skin and blood of the same donors (p<0.001 Wilcoxon paired test). (B) Cumulative data showing percentages of CD69 expressing cells amongst blood and skin derived CD4+ T cells, stratified by donor age. Spearman correlation was used to calculate significance and deviation from zero. (C) Skin cells and PBMC were stained with CD4, CD45RA and CD27 to identify 4 differentiation subsets. Representative FACS staining of PBMC (top panels) and skin derived cells (bottom panels) from young and old donors, is shown gated on the CD3+CD4+ cells. (D) Bar chart shows cumulative data for in young and old individuals. Statistical analysis of blood and skin populations was performed using a paired t-test and p-values are indicated where relevant. (E) Skin derived leukocytes were stimulated for 5 hours with PMA and ionomycin in the presence of brefeldin A and stained for CD4, IFN-γ, IL-2, TNF-α and IL-22. Representative dot plots are shown, gated on CD4+ cells (NS, non stimulated; PMA+Iono, PMA and ionomycin). (F) Graphs show % of CD4+ cells staining positive for a particular cytokine (n=5-7 young and old for each cytokine).
To determine whether excessive differentiation towards an end stage could reflect altered T cell function in the skin compared to T cells in peripheral blood we collected paired blood and skin samples from young and old individuals (n=23 old and n=31 young for CD4, n=10 old and 14 young for CD8). Based on their expression of surface CD45RA, CD27, CD4 (Figure 3C, D) and CD8 (Supplementary Figure 4A, B) T cells from both blood and skin can be subdivided into 4 subsets. The CD45RA+CD27+ population is the least differentiated and has the longest telomeres, CD45RA−CD27+ cells have intermediated telomere lengths while both CD45RA−CD27− and CD45RA+CD27− cells have relatively short telomeres and express multiple other characteristics of senescence (Di Mitri et al., 2011; Henson et al., 2014; Libri et al., 2011). In both young and old subjects, cutaneous T cells in both CD4+ (Figure 3C, D) and CD8+ populations (Supplementary Figure 4A, B) showed a significant decrease in the undifferentiated CD45RA+CD27+ population with a concomitant increase in CD27+CD45RA− and CD45RA−CD27− cells. In older subjects, the proportion of CD45RA−CD27− cells was significantly higher within skin CD4 and CD8 compartments compared to young subjects (Figure 3).
We next investigated the functional capacity of skin resident T cells isolated from punch biopsies. Following a 6 hour stimulation with PMA and ionomycin cells were stained for IL-2, IFN-γ, TNF-α and IL-22. There were no significant differences in the capacity of CD4+ (Figure 3 E, F) or CD8+ T cells from the skin of young and old individuals to secrete these inflammatory cytokines after stimulation in vitro (Supplementary Figure 4, D).
VZV-specific skin resident T cells in young and old subjects
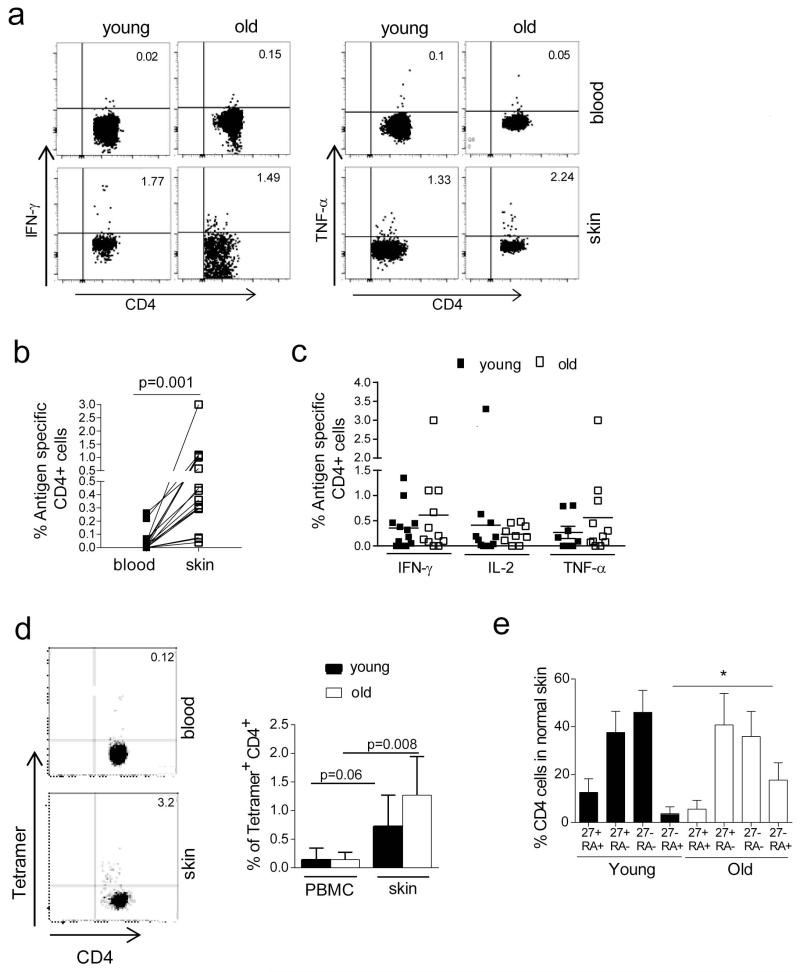
We next investigated the phenotypic and functional characteristics of VZV specific T cells in both the skin and blood of young and old subjects. Skin T cells isolated from punch biopsies, and paired blood samples, were tested for their ability to synthesize IFN-γ, TNF-α and/or IL-2 after overnight re-stimulation with VZV lysate as previously described (Vukmanovic-Stejic et al., 2013). Minimal IFN-γ or IL-2 was produced by blood or skin CD4+ T cells in the control cultures without added VZV antigens (not shown). We could identify cytokine producing CD4+ cells in skin of > 60% of young and old individuals tested (positive staining for one or more cytokines, Figure 4A). In those individuals where VZV specific cells were found in both compartments, there were significantly higher proportions of these cells in the skin (Figure 4A, 4B, p=0.001, Wilcoxon paired test, n=9 old and 15 young). The observed difference was not accounted for by the difference in the differentiation state of skin versus blood CD4 T cells as there was a significantly increased proportion of VZV-specific cells in skin compared to the circulating memory compartment. When the proportion of cytokine secreting VZV-specific CD4+ cells was compared between the skin of young and old individuals, no significant difference was observed (Figure 4C). Therefore there is no obvious age-associated reduction in the number of cytokine secreting VZV specific CD4+ T cells in the skin.
Figure 4. Frequency of VZV specific CD4+ T cells is higher in the skin compared to blood and is not affected by age.
5 mm punch biopsies and peripheral blood samples were collected from healthy young and old volunteers. Skin cells and PBMC were stimulated with VZV lysate overnight and stained for intracellular cytokines-IL-2, IFN-γ and TNF-α. (A) Representative dot plots of the CD4+ IFN-γ and TNF-α response from young and old donors are shown. (B) Graph shows comparison of the frequency of cytokine secreting cells between PBMC and skin samples (n=9 old and 15 young, p=0.001 Wilcoxon paired test) each symbol represents a different individual. (C) Graph shows frequency of IFN-γ, IL-2 and TNF-α secreting VZV specific cells in young and old individuals (n≥8 young and old depending on cytokine). (D) In HLA-DR15+ donors PBMC and skin derived cells were stained with HLA-DRB1*1501 restricted IE63 tetramer. Representative FACS staining is shown on the left. Bar graph represents cumulative data (n=6 young and 5 old individuals, mean and SE and p values are indicated, Wilcoxon paired test). (E) The phenotype of skin resident VZV specific IFN-γ+ CD4 T cells was compared between young (n=12) and old individuals (n=7).
We also investigated the characteristics of VZV specific CD4+ T cells in the skin that were identified by class II tetramer binding. Paired samples of cells isolated from skin biopsies and PBMC were stained with an HLA-DRB1*1501 restricted IE63 tetramer (Jones et al., 2007). The representative tetramer staining is shown in Figure 4D (left panels). Increased proportions of VZV-specific CD4+ T cells were detected in the skin when compared to the blood in both old and young individuals (Figure 4D, Wilcoxon paired test, p=0.06 in young n=6; and p=0.008 in the old; n=5). The specificity of tetramer staining was confirmed as described above (Vukmanovic-Stejic et al., 2013). Therefore, using either intracellular cytokine staining or tetramer binding we found higher proportions of VZV specific CD4+ T cells in the skin than in blood of both young and old individuals, however there were no differences between the age groups in either compartment.
We next compared the differentiation state of skin resident VZV specific CD4+ T cells between young and old individuals. Overall, VZV specific cells identified in the skin are predominantly of the CD45RA−CD27+ and CD45RA−CD27− phenotype (Figure 4E) similar to that seen in blood (Supplementary Fig 5) in both age groups. Furthermore there was a significant increase in CD45RA+CD27− T cells, that have characteristics of end-stage differentiation or senescence (Di Mitri et al., 2011; Henson et al., 2014; Libri et al., 2011) within the VZV-specific population in the old compared to young skin. The phenotype of tetramer positive cells in skin was similar to that observed when cytokine secreting VZV specific cells were analysed (Supplementary Fig 5C). In conclusion, VZV specific CD4+ T cells in blood and in skin are functional and are not restricted by excessive differentiation.
Expression of PD-1 in skin during ageing
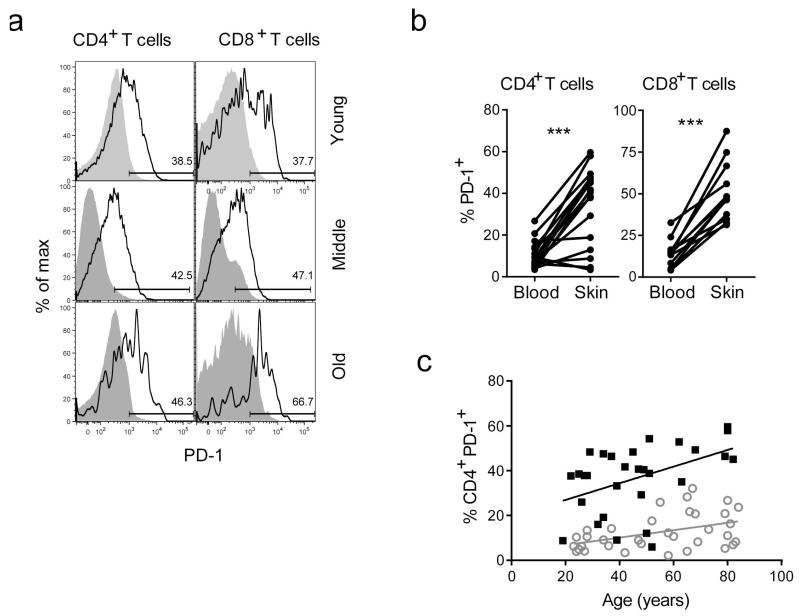
Since the numbers of skin resident VZV specific CD4+ T cells are not decreased during ageing and they are able to secrete cytokines in response to VZV antigen challenge in vitro, other factors may contribute to the impaired skin recall response to VZV antigen challenge in older subjects and to their increased susceptibility to shingles (Agius et al., 2009; Levin et al., 2003; Tang et al., 2012; Weinberg et al., 2010). The progressive loss of cytokine production and proliferative activity by CD4+ T cells as a result of chronic immune stimulation during persistent viral infections and cancer is associated with the expression of PD-1 and other inhibitory receptors (Wherry, 2011; Zajac et al., 1998). Blockade of these receptors can reverse these functional defects in these cells (Kamphorst and Ahmed, 2013). We therefore investigated whether there was increased expression of the T cell surface inhibitory receptor PD-1 in blood compared to skin cells during ageing. Paired blood and normal skin samples stained for the expression of PD-1 on CD4 (n= 17; 6 young, 6 middle aged, 5 old) and CD8+ T cells (n=−12; 4 young, 3 middle aged, 5 old). (Figure 5A, B). There was a highly significant increase in PD-1 expression on skin resident compared to circulating T cells (p < 0.001, Wilcoxon paired test). PD-1 expression was increased in both blood and skin during ageing (Figure 5C) with as many as 40% of CD4+ T cells being positive in the skin. This data suggests that T cells in the skin of old humans may be more susceptible to inhibition through PD-1 signalling.
Figure 5. Ex vivo PD-1 expression in skin and blood derived CD4+ and CD8+ T cells.
PD-1 expression was measured by FACS in PBMCs or collagenase digested skin cells derived from healthy individuals (n=34, age range 19-89). (A) Representative FACS histograms showing ex vivo PD-1 expression in CD4+ and CD8+ T cells in skin compared to the blood. (B) Comparison of percentages of PD-1 expressing cells amongst total CD4+ or CD8+ T cells between skin and blood of the same donors (p<0.001 Wilcoxon paired test). (C) Cumulative data showing percentages of PD-1 expressing cells amongst blood and skin derived CD4+ T cells, stratified by donor age. Spearman correlation was used to calculate significance and deviation from zero (* p>0.05).
DISCUSSION
Recent studies have shown that skin resident memory T (Trm) cells play an important role in providing protection against re-exposure to, or re-activation of, local persisting pathogens (Clark et al., 2012; Cuburu et al., 2012; Gebhardt et al., 2009; Mackay et al., 2012; Masopust et al., 2010; Mueller et al., 2013; Tang and Rosenthal, 2010). Furthermore, these Trm cell populations may be regulated independently of the circulating memory T cell pools of cells (Carbone et al., 2013; Clark et al., 2012; Gebhardt et al., 2011; Mueller et al., 2013; Schenkel et al., 2013). Considering the skin manifestation of both primary and secondary varicella disease, it is unusual that VZV specific T cells have only previously been studied in the peripheral blood (Asanuma et al., 2000; Hayward and Herberger, 1987; Patterson-Bartlett et al., 2007; van Besouw et al., 2012; Weinberg and Levin, 2010). We first investigated whether there was a general decrease in the number or functional capacity of skin resident T cells in older subjects. Based on the expression of CD69, that was expressed by almost all skin derived CD4 and CD8 T cells, together with the lack of CCR7 expression and the fact that skin biopsies were collected from normal, unchallenged skin we concluded that our skin derived cells represent a skin resident population (Clark et al., 2012; Gebhardt et al., 2009; Jiang et al., 2012; Skon et al., 2013). However contribution of recirculating T cells transiently patrolling the skin cannot be completely discounted (Zhu et al., 2013). A key observation was that there were significantly more VZV specific CD4+ T cells in the skin compared to the blood in both young and old individuals. Since the skin contains more T cells than the blood (Clark, 2010; Clark et al., 2006), the decrease in VZV-specific T cells in the circulation during ageing may not represent a global decrease of these cells in vivo as they may simply have re-located to the skin. This observation coupled to the fact that other methods of evaluating VZV-specific T cells do not indicate a reduction of these cells in the blood suggests that there may not be a general defect of VZV-specific T cell numbers or function during ageing.
We found no differences in numbers of dendritic cells and macrophages between both age groups, and transcriptional profiling of young and old normal skin did not show any significant differences in the genes involved in mononuclear phagocyte function or immune responses. Therefore the general skin microenvironment at a steady state appears very similar in young and old individuals suggesting that the reduced recall response to antigen in the skin during ageing (Agius et al., 2009) probably occurs downstream of antigen challenge.
We investigated whether Trm cells in the skin of older humans were inhibited in situ, as this could explain why there is a reduced recall response to VZV skin test antigen challenge and also the predisposition to VZV reactivation in vivo. We showed previously that Foxp3+ Tregs that are identified in the skin are suppressive and there is a significant inverse correlation between the number of these cells present and the size of the delayed type hypersensitivity response after VZV challenge ((Vukmanovic-Stejic et al., 2013) and Figure 2C, Supplementary Figure 2). The increase in Tregs during ageing has also been observed in the blood of normal subjects (Gregg et al., 2005; Vukmanovic-Stejic et al., 2006) and has also been reported in the tissues of old mice (Lages et al., 2008; Raynor et al., 2012). This supports the possibility that the increase in Treg cell numbers and their suppressive activity may contribute to decreased VZV specific responses in older subjects.
Signalling through PD-1 and other inhibitory receptors can inhibit T cell responses both in vitro and in vivo (Wherry, 2011; Zajac et al., 1998). The blockade of these receptors can reverse these functional defects in these cells and this has led to the use of PD-1 blocking strategies in the treatment of melanoma and other skin cancers (Flemming, 2012; Kamphorst and Ahmed, 2013; Lu et al., 2014). In addition, it has been shown recently that interplay between Tregs and PD-1 signalling regulates immune responsiveness and the control of viral infection in vivo (Penaloza-MacMaster et al., 2014). We observed very high PD-1 expression on skin compared to blood T cells that increased with age. This suggests that inhibitory signalling by different mechanisms may actively regulate immune responsiveness in the skin, especially during ageing. The reason for the increase in Treg numbers or PD-1 expression in the skin during ageing is unclear. It has been shown previously that infection with persistent viruses such as CMV in humans can induce PD-1 expression and it is possible that more of the old subjects we have studied are CMV+ than the young cohort (Henson et al., 2014). Alternatively, these changes may be linked to the increased inflammation (inflammageing) that is observed in elderly subjects (Franceschi et al., 2000).
Our studies highlight the importance of studying human immunity in additional compartments to peripheral blood. The availability of a vaccine (Zostavax) to prevent herpes zoster in older subjects has reduced the incidence of shingles in the elderly (Gershon, 2007; Levin et al., 2008; Weinberg et al., 2009) although efficacy of the vaccine is only 38% in the very old (>80yrs). This indicates that it is essential to study the mechanisms that are responsible for the declining immunity during ageing to rationalize ways by which immunity in general and vaccine responses in particular could be improved in the elderly.
MATERIALS AND METHODS
Subjects
Healthy individuals who had a history of previous chickenpox infection (n=94, median age = 32.5 years, age range 20-92 years, 38 male, 56 female) were recruited for the study. Subjects were grouped as young <40, middle aged 40-65 and old >60 years old. This work was approved by the Ethics Committee of the Royal Free Hospital, London. All volunteers provided written informed consent and study procedures were performed in accordance with the principles of the declaration of Helsinki. Individuals with history of neoplasia, immunosuppressive disorders or inflammatory skin disorders and individuals on immunosuppressive medication were excluded. In some cases skin from healthy individuals was obtained from donors undergoing routine plastic surgery at Guy’s and St. Thomas’ hospitals (approved by the Institutional Review Board of Guy’s Hospital). To examine the clinical response to VZV, healthy individuals were injected intradermally with Varicella Zoster Virus (VZV) skin test antigen from The Research Foundation for Microbial Diseases of Osaka University (BIKEN) as described previously(Agius et al., 2009; Vukmanovic-Stejic et al., 2011).
Skin biopsies
Punch biopsies (5 mm diameter) from the upper volar region of the forearm were obtained from 30 young and 25 old volunteers. Biopsies were frozen in optimal cutting temperature compound (OCT, Bright Instrument Company Ltd, Huntingdon, Uk). 6 μm sections were cut and then fixed in ethanol and acetone and stored at −80°C. For functional analysis of skin cells 5 mm punch biopsies were digested overnight with 0.8mg/ml of collagenase IV (Sigma Aldrich, Gillingham, UK) as described (Vukmanovic-Stejic et al., 2013). For the analysis of the DTH response to VZV challenge skin biopsies were collected at different times post injection, frozen and used for histological analysis (Agius et al., 2009).
PBMC preparation
Heparinized blood samples were collected from healthy volunteers and patients with acute disease. PBMCs were prepared by density centrifugation on Ficoll-Paque (Amersham Biosciences, Little Chalfont, UK) and re-suspended in complete medium.
Flow Cytometric Analysis
Multi-parameter analysis of skin and blood T cell phenotype was performed on LSR II or BD Fortessa using FACS Diva software (both BD Biosciences, Oxford, UK) and further analyzed using FlowJo software (TreeStar, Inc) as previously described (Agius et al., 2009; Vukmanovic-Stejic et al., 2013). PBMCs or skin cells were stained with different combinations of antibodies including CD3, CD4, CD8, CD45RA, CD28, CD27 (BD Biosciences) Ki67, CLA, CCR7 and PD-1 (clone EH12.2H7; Biolegend, London, UK). All surface staining was performed for 30 minutes on ice. Isotype control staining and FMO controls were used to set the quadrants. Ki67 staining (clone B56, BD Bioscience) was performed by intracellular staining using the Foxp3 Staining Buffer Set (Miltenyi Biotec, Bisley, UK). For intracellular cytokine staining cells were stimulated with VZV lysate (Virusys corporation, Taneytown, MD) or SEB as positive control and incubated for 15 hours at 37°C, 5% CO2 in the presence of 5 μg/ml Brefeldin A (Sigma-Aldrich, Gillingham, UK). Unstimulated controls were always included. Following stimulations cells were stained for surface markers for 30 minutes at 4°C, washed, fixed and permeabilised (Fix & Perm Cell Permeabilisation Kit, Invitrogen, Paisley, UK) before staining for IL-2, IFN-γ and TNF-α (all from BD Biosciences, Oxford, UK).
Tetramer staining
Tetramer staining was performed as previously described using DRB1*1501 iTAg MHCII tetramer complexed to VZV IE63 peptide 24 (QRAIERYAGAETAEY) (Beckman Coulter, High Wycombe, UK); CLIP peptide (PVSKMRMATPLLMQA) was used as a control (Jones et al., 2007; Vukmanovic-Stejic et al., 2013). Briefly, cells were first incubated with 2 μg/ml HLA Class II tetramers for 1-2 hours at 37°C in the dark, washed in PBS and then stained for different surface markers as needed. We analysed the tetramer expression within the CD4+ T cell subset by gating on the lymphocytes and excluding B cells, monocytes and dead cells (Via-Probe positive population).
Immunofluorescence
6 μm skin sections collected from normal or VZV injected skin were blocked with Dako non-serum protein block for 20 minutes, followed by incubation with primary antibodies (biotin anti-human Foxp3 and mouse anti-human CD4) overnight at 4°C, followed by incubation with strepCy3 and anti-mouse IgG1 Alexa Fluor 488 for 1hr at room temperature as described (Vukmanovic-Stejic et al., 2008). Cell numbers were expressed as the mean absolute cell number per perivascular infiltrate where 5 largest perivascular infiltrates present in the upper and mid dermis of each section were counted (Vukmanovic-Stejic et al., 2008).
Immunohistochemistry
Skin sections from normal skin (n=6 young, n=6 old) were stained with purified mouse anti-human CD3 antibody, purified mouse anti-human CD8 antibody (both Dakocytomation, Ely, UK), purified mouse anti-human CD4 and CD11c antibodies (BD Biosciences, Oxford, UK) or purified mouse anti-human CD163 antibody (Acris, Herford, Germany). Sections were counterstained using rabbit anti-mouse horseradish-peroxidase conjugated antibody (Dakocytomation, Ely, UK) and immunostaining revealed using chromagen 3′-diaminobenzidine tetrahydrochloride. The number of positive cells/mm2 was counted manually using computer-assisted image analysis (NIH Image 6.1; http://rsb.info.nih.gov/nih-image).
Transcriptional analysis
3 mm punch biopsies were collected from young and old individuals and immediately frozen in RNAlater and stored at −20°C until use. Tissues were homogenized and total RNA extracted using RNeasy Mini Kit (Qiagen, Manchester, UK). Target amplification and labelling was performed according to standard protocols using Nugen Ovation WB Kit. RNA was hybridized to Affymetrix Human Genome U133 2.0 plus arrays. Affymetrix gene chips were scanned for spatial artefacts using the Hirshlight package (Suarez-Farinas et al., 2005). Gene expression measures were obtained using the GCRMA algorithm (Wu and Irizarry, 2004). Group comparisons were made using the moderated t-test and P-values were adjusted for multiple hypotheses using the Benjamini-Hochberg procedure.
Network analysis of the genes expressed within skin samples and in various human cell populations was performed as described previously (Mabbott et al., 2013). Briefly, human skin-punch microarray data were combined with a large collection of other primary cell gene-expression data sets (745 individual microarray data sets), available from the GEO database on the Affymetrix Human Genome U133 Plus 2.0 expression array platform (GSE49910) and analysed using Biolayout Express3D software (Freeman et al., 2007). The graph of these data was then explored to understand the significance of the gene clusters, identify those expressed by the young and old skin samples and their functional relationships to the other cell populations represented.
Statistics
Statistical analysis was performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego, California, USA). Non-parametric tests were predominantly utilised as data sets were not normally distributed. The Wilcoxon matched pairs test or a paired t-test was used when comparing two groups of matched data and a 2-tailed Mann-Whitney test was used when comparing two unpaired groups.
Supplementary Material
Acknowledgments
This work was funded by grants from the Medical Research Council, the Biotechnology and Biological Sciences Research Council, The British Skin Foundation and Dermatrust.
Abbreviations
- VZV
varicella zoster virus
- Treg
regulatory T cells
- CMV
cytomegalovirus
- HSV
herpes simplex virus
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication. NPG are providing this early version of the manuscript as a service to our customers. The manuscript will undergo copyediting, typesetting and a proof review before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers apply.
References
- Agius E, Lacy KE, Vukmanovic-Stejic M, et al. Decreased TNF-{alpha} synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. The Journal of Experimental Medicine. 2009;206:1929–40. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin AM. Varicella-zoster virus. ClinMicrobiolRev. 1996;9:361–81. doi: 10.1128/cmr.9.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin AM. Varicella-zoster virus: molecular virology and virus-host interactions. Current opinion in microbiology. 2001;4:442–9. doi: 10.1016/s1369-5274(00)00233-2. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Sharp M, Maecker HT, et al. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. JInfectDis. 2000;181:859–66. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Yan S, Tomura M, et al. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. 2013;190:970–6. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone FR, Mackay LK, Heath WR, et al. Distinct resident and recirculating memory T cell subsets in non-lymphoid tissues. Curr Opin Immunol. 2013;25:329–33. doi: 10.1016/j.coi.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–70. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Chong B, Mirchandani N, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–9. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- Clark RA, Watanabe R, Teague JE, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. SciTranslMed. 2012;4:117ra7. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuburu N, Graham BS, Buck CB, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. The Journal of clinical investigation. 2012;122:4606–20. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mitri D, Azevedo RI, Henson SM, et al. Reversible senescence in human CD4+CD45RA+ JImmunol. 2011;187:2093–100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- Flemming A. Cancer: PD1 makes waves in anticancer immunotherapy. Nature reviews Drug discovery. 2012;11:601. doi: 10.1038/nrd3806. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Freeman TC, Goldovsky L, Brosch M, et al. Construction, visualisation, and clustering of transcription networks from microarray expression data. PLoS computational biology. 2007;3:2032–42. doi: 10.1371/journal.pcbi.0030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. NatImmunol. 2009;10:524–30. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Whitney PG, Zaid A, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–9. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- Gershon AA. Varicella-zoster vaccine. 2007 [PubMed] [Google Scholar]
- Goldblatt D. The immunology of chickenpox. A review prepared for the UK Advisory Group on Chickenpox on behalf of the British Society for the Study of Infection. The Journal of infection. 1998;36(Suppl 1):11–6. doi: 10.1016/s0163-4453(98)80150-3. [DOI] [PubMed] [Google Scholar]
- Gregg R, Smith CM, Clark FJ, et al. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. ClinExpImmunol. 2005;140:540–6. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniffa M, Ginhoux F, Wang X-N, et al. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. The Journal of Experimental Medicine. 2009;206:371–85. doi: 10.1084/jem.20081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward AR, Herberger M. Lymphocyte responses to varicella zoster virus in the elderly. Journal of clinical immunology. 1987;7:174–8. doi: 10.1007/BF00916011. [DOI] [PubMed] [Google Scholar]
- Henson SM, Lanna A, Riddell NE, et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8+ T cells. The Journal of clinical investigation. 2014;124:4004–16. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–31. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Black AP, Malavige GN, et al. Phenotypic analysis of human CD4+ T cells specific for immediate-early 63 protein of varicella-zoster virus. EurJ Immunol. 2007;37:3393–403. doi: 10.1002/eji.200737648. [DOI] [PubMed] [Google Scholar]
- Kamphorst AO, Ahmed R. Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol. 2013;25:381–8. doi: 10.1016/j.coi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lages CS, Suffia I, Velilla PA, et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–48. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MJ, Oxman MN, Zhang JH, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. The Journal of infectious diseases. 2008;197:825–35. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. JInfectDis. 2003;188:1336–44. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- Libri V, Azevedo RI, Jackson SE, et al. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27+ T cells: the potential involvement of interleukin-7 in this process. Immunology. 2011;132:326–39. doi: 10.1111/j.1365-2567.2010.03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Lee-Gabel L, Nadeau MC, et al. Clinical evaluation of compounds targeting PD-1/PD-L1 pathway for cancer immunotherapy. Journal of oncology pharmacy practice: official publication of the International Society of Oncology Pharmacy Practitioners. 2014 doi: 10.1177/1078155214538087. [DOI] [PubMed] [Google Scholar]
- Mabbott NA, Baillie JK, Brown H, et al. An expression atlas of human primary cells: inference of gene function from coexpression networks. BMC genomics. 2013;14:632. doi: 10.1186/1471-2164-14-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Stock AT, Ma JZ, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proceedings of the National Academy of Sciences. 2012;109:7037–42. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–64. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Gebhardt T, Carbone FR, et al. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–61. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- Patterson-Bartlett J, Levin MJ, Lang N, et al. Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine. 2007;25:7087–93. doi: 10.1016/j.vaccine.2007.07.051. [DOI] [PubMed] [Google Scholar]
- Penaloza-MacMaster P, Kamphorst AO, Wieland A, et al. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med. 2014;211:1905–18. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor J, Lages CS, Shehata H, et al. Homeostasis and function of regulatory T cells in aging. Current Opinion in Immunology. 2012;24:482–7. doi: 10.1016/j.coi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, et al. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–13. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneschal J, Clark RA, Gehad A, et al. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–84. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–93. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Haider A, Wittkowski KM. “Harshlighting” small blemishes on microarrays. BMC bioinformatics. 2005;6:65. doi: 10.1186/1471-2105-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Tintle SJ, Shemer A, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. JAllergy ClinImmunol. 2011;127:954–64. doi: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Moriishi E, Okamoto S, et al. A community-based survey of varicella-zoster virus-specific immune responses in the elderly. Journal of Clinical Virology. 2012;55:46–50. doi: 10.1016/j.jcv.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Tang VA, Rosenthal KL. Intravaginal infection with herpes simplex virus type-2 (HSV-2) generates a functional effector memory T cell population that persists in the murine genital tract. Journal of reproductive immunology. 2010;87:39–44. doi: 10.1016/j.jri.2010.06.155. [DOI] [PubMed] [Google Scholar]
- van Besouw NM, Verjans GM, Zuijderwijk JM, et al. Systemic varicella zoster virus reactive effector memory T-cells impaired in the elderly and in kidney transplant recipients. Journal of medical virology. 2012;84:2018–25. doi: 10.1002/jmv.23427. [DOI] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Agius E, Booth N, et al. The kinetics of CD4Foxp3 T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J ClinInvest. 2008;118:3639–50. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Rustin MH, Nikolich-Zugich J, et al. Immune responses in the skin in old age. CurrOpinImmunol. 2011;23:525–31. doi: 10.1016/j.coi.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Sandhu D, Sobande TO, et al. Varicella Zoster-Specific CD4+Foxp3+ T Cells Accumulate after Cutaneous Antigen Challenge in Humans. J Immunol. 2013;190:977–86. doi: 10.4049/jimmunol.1201331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Zhang Y, Cook JE, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. JClinInvest. 2006;116:2423–33. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF Synergistically Modulate Cytokine Expression while Suppressing Melanogenesis: Potential Relevance to Psoriasis. The Journal of investigative dermatology. 2013;133:2741–52. doi: 10.1038/jid.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Lazar AA, Zerbe GO, et al. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. The Journal of infectious diseases. 2010;201:1024–30. doi: 10.1086/651199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Levin MJ. VZV T cell-mediated immunity. CurrTopMicrobiolImmunol. 2010;342:341–57. doi: 10.1007/82_2010_31. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Zhang JH, Oxman MN, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. The Journal of infectious diseases. 2009;200:1068–77. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wu Z, Irizarry RA. Preprocessing of oligonucleotide array data. Nature biotechnology. 2004;22:656–8. doi: 10.1038/nbt0604-656b. author reply 8. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, et al. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J ClinInvest. 2007;117:2517–25. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Krueger JG, Lowes MA. Resident and “Inflammatory” Dendritic Cells in Human Skin. J Invest Dermatol. 2008;129:79–88. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral Immune Evasion Due to Persistence of Activated T Cells Without Effector Function. The Journal of Experimental Medicine. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Peng T, Johnston C, et al. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature. 2013;497:494–7. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.