Abstract
The spectrum of neuronal migration disorders (NMD) in humans encompasses developmental brain defects with a range of clinical and pathological features. A simple classification distinguishes agyria/pachygyria, heterotopia, polymicrogyria and cortical dysplasia as distinct clinico-pathological entities. Many of these conditions are associated with intractable epilepsy. When considering the pathogenesis of NMD, a critical developmental process is the migration of neuroblasts along the processes of radial glia during the formation of the layered structure of the cerebral cortex. In addition, faulty cytodifferentiation and programmed cell death play important roles in the generation of dysplasias and heterotopias respectively. A number of genes have been identified that participate in the regulation of neuronal migration. Mouse models, in which these genes are mutated, provide insight into the developmental pathways that underlie normal and abnormal neuronal migration.
Introduction
Neuronal migration disorders (NMD) are developmental malformations of the cerebral hemispheres, frequently associated with severe epilepsy in children and adults. They can be defined as “cerebral malformations characterised by malpositioning and faulty differentiation of cortical grey matter”. Clinically and pathologically, the spectrum of NMD is complex and several classification schemes have been suggested. The purpose of this article is to review the main types of NMD in terms of their pathological and clinical characteristics, and to relate the origin of these defects to the main cellular events that comprise the development of the layered structure of the mammalian cerebral cortex. Recent studies on animal models of NMD are reviewed with the aim of defining the critical embryonic/fetal developmental events that are disturbed, leading to NMD.
Clinical/pathological subtypes of NMD
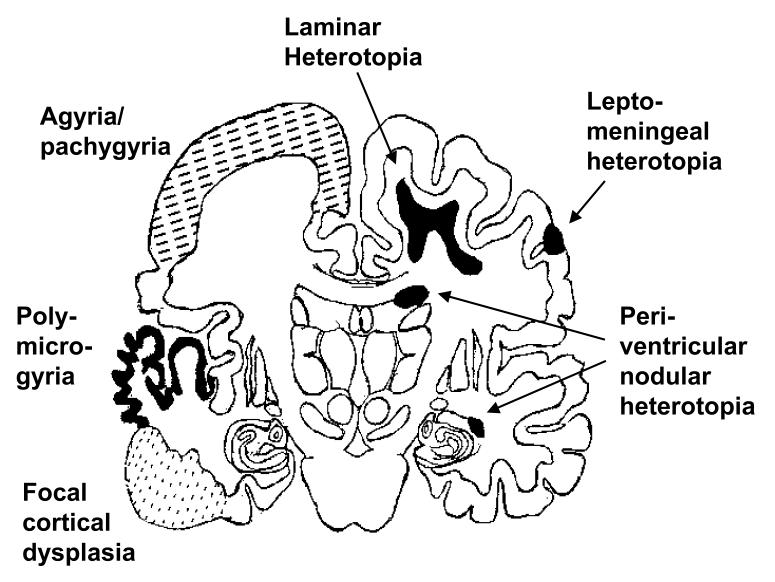
One simple pathological classification of NMD distinguishes: (1) agyria/pachygyria (including lissencephaly), (2) heterotopia, (3) polymicrogyria and (4) cortical dysplasia (Figure 1).
Figure 1.
Diagrammatic representation of the various types of ‘neuronal migration disorder’, projected onto the outline of an adult human brain section. Positioning of the different lesions types is for illustration purposes only, and does not necessarily indicate the most frequent site of occurrence.
1. Agyria/pachygyria
This refers to the condition of absent or abnormally broad gyri in the cerebral hemispheres, a condition known clinically as lissencephaly (smooth brain). Agyria/pachygyria is associated with mental retardation and severe epilepsy and comprises several subtypes. These include syndromic Miller-Dieker lissencephaly and the Isolated Lissencephaly Sequence (Dobyns et al. 1993), both of which are frequently associated with genetic deletions or mutations affecting the LIS1 gene on chromosome 17p13.3 (Hattori et al. 1994; Reiner et al. 1993). An X-linked form of lissencephaly was recently found to result from mutations in a novel gene, doublecortin, that maps to Xq22.3-Xq23 (Des Portes et al. 1998; Gleeson et al. 1998).
2. Heterotopia
These are misplaced neurons within the white matter of the cerebral hemispheres that may serve as foci for epileptogenic activity (Raymond et al. 1994). There is a wide spectrum of severity ranging from isolated grey matter nodules in the marginal zone or peri-ventricular regions, to broad ‘band’ heterotopias in the sub-cortical white matter. An X-linked gene has been identified in families exhibiting peri-ventricular heterotopias (Eksioglu et al. 1996), while the doublecortin gene has been implicated in the aetiology of band heterotopia in females (Des Portes et al. 1998; Gleeson et al. 1998).
3. Polymicrogyria
This is a condition in which there is excessive folding of an abnormally thin cortex which can be focal or generalised. In some cases, the gyri are apparently ‘fused’, giving a false impression of a smooth brain surface. The polymicrogyric appearance is evident from brain sections. Clinically, mental retardation and severe epilepsy are common findings. The aetiology is generally considered more related to environmental insults, for instance intrauterine ischaemia or infection, than to developmental abnormalities of genetic origin (Harding and Copp, 1997).
4. Focal cortical dysplasia
Nodules of atypical cortical structure are common causes of intractable epilepsy, which often require neurosurgical intervention to suppress the seizures (Taylor et al. 1971). The appearance is of abnormal neuronal and glial morphology and positioning, with associated lamination defects. Neurons are frequently enlarged, exhibit atypical Nissl staining and bizarre dendritic tree structures. ‘Balloon cells’ are characteristically found in dysplastic cortex; these cells appear to be arrested in their progression along the glial pathway of cytodifferentiation. Cells of indeterminate neuronal/glial identity are also observed (Vinters et al. 1992). More extensive lesions, with similar cytopathology, may involve large areas of one hemisphere (e.g. one form of hemimegalencephaly) or may be multi-focal, as in tuberous sclerosis.
Development of the cerebral cortex - overview of the embryonic and fetal events
The cerebral cortex arises from the telencephalic vesicle of the embryonic forebrain. Early specification of the forebrain depends on appropriate expression of a number of homeobox-containing and winged helix-containing genes, including members of the Otx, Emx, Dlx, BF, Mf, Lhx and Gsh families. Loss of function of several of these genes in mice leads to structural abnormalities in forebrain specification and development (Szucsik et al. 1997; Porter et al. 1997; Labosky et al. 1997; Yoshida et al. 1997; Matsuo et al. 1995; Qiu et al. 1995; Acampora et al. 1995). Mutations in the EMX2 gene have been found in humans with schizencephaly (Brunelli et al. 1996).
The primitive neuroepithelial cells of the embryonic neural tube are the precursors of neurons and glia within the central nervous system. These cells undergo many cycles of proliferation in the ventricular zone of the first trimester cerebral wall and then exit from the cell cycle, leave the ventricular zone and migrate centrifugally, in a series of waves, across the intermediate zone to form the layered structure of the cerebral cortex (Rakic, 1972). This process occupies the eighth to fourteenth weeks post-fertilisation in human development and occurs between embryonic days 11 and 18 in the mouse (Gillies and Price, 1993).
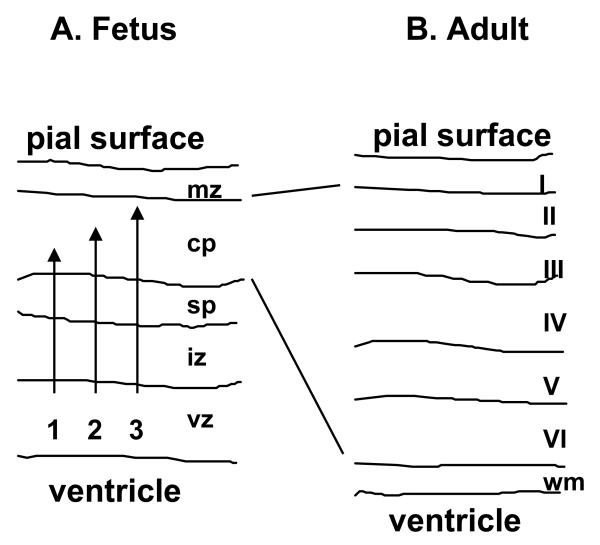
The earliest migrating neuroblasts form a transient structure, the pre-plate, between the pial surface and the intermediate zone, which subsequently becomes split into deep and superficial layers by later-migrating neuroblasts which form the cortical plate. Most pre-plate neurons are lost by programmed cell death during subsequent fetal development (Price et al. 1997; Allendoerfer and Shatz, 1994). The deep component of the pre-plate, called the sub-plate, plays a role in axonal guidance by serving as an intermediate target for the axons of thalamic neurons during their ascent to synapse with the cortical plate neurons (Allendoerfer and Shatz, 1994). The superficial layer of the pre-plate forms the cell-sparse marginal zone. The resident Cajal-Retzius neurons, which characterise the marginal zone, are the remnants of the pre-plate neurons.
The cortical plate is constructed from inside to outside, with later-arriving cells migrating past early-arriving cells, to populate progressively more superficial layers (Sidman and Rakic, 1973). The cortical plate thus expands as development proceeds, giving rise to the bulk (layers II to VI) of the definitive cerebral cortex (Figure 2). Studies with the developing ferret brain have shown that the final layer occupied by the migrating neuroblasts is determined while the cells are still resident in the ventricular zone, prior to the onset of migration (McConnell and Kaznowski, 1991). It appears that a ‘clock’ is running in the ventricular zone, closely related to the cell cycle, so that progressively later-emerging post-mitotic neuroblasts are determined to migrate to more superficial layers of the cortex than earlier-emerging neuroblasts. The precise nature of this cellular clock is unknown.
Figure 2.
Diagram to illustrate the formation of the cerebral cortex. This process involves the centrifugal migration of waves of post-mitotic neuroblasts along the processes of radial glia (A), to establish the ‘inside-out’ structure of the cortical plate (shown schematically in B). Late migrating neurons pass earlier migrating neurons, and take up the most superficial positions in the cortex.
Possible cellular disturbances underlying human “neuronal migration disorders”
Although many types of developmental brain disorder are conventionally grouped within the NMD spectrum, close examination of each pathology suggests that disordered neuronal migration, although critical in the generation of many defects, cannot explain the entire range of disorders. Other cellular processes, for instance aberrant neuronal cytodifferentiation and disturbance of programmed cell death, may also be important pathogenetic factors.
1. Abnormalities of cytodifferentiation
The lesions of focal cortical dysplasia show clear morphological signs of disturbed cytodifferentiation, with aberrant expression of markers of neuronal and glial differentiation in dysplastic lesions. It is possible that dysplastic lesions are generated when the development of neuroepithelial cells becomes arrested at various stages in the progression towards increased cell specialisation. Clonal analysis in experimental animals indicates that the decision to follow a pathway of neuronal or glial differentiation may occur early in neuroepithelial differentiation, even prior to the cessation of cell proliferation and exit from the ventricular layer of the neural tube (Grove et al. 1993). This would suggest that dysplastic lesions containing cells with intermediate neuronal/glial characteristics must have a very early origin during prenatal development. On the other hand, recent studies have identified putative stem cells in the postnatal nervous system that retain the ability to differentiate along either neuronal or glial lineages (McKay, 1997). This finding would suggest, therefore, that dysplastic lesions may arise at almost any stage of development, as a result of the aberrant differentiation of groups of previously multipotential stem cells.
A third possibility is that normally differentiated cortical cells undergo a genetic change, triggering the development of a dysplastic lesion, and that some of the cells within the lesion begin to ‘re-express’ genes normally only expressed by cells at an earlier stage of differentiation, or genes that characterise a different cell lineage. Somatic mutation is commonly observed in the generation of tumours of many types (knudson, 1986), and has also been inferred from the finding of ‘loss of heterozygosity’ for polymorphic DNA markers in the lesions of tuberous sclerosis (Green et al. 1994). It remains to be determined whether somatic mutation or chromosomal damage, followed by clonal expansion, plays a significant role in the development of dysplastic cortical lesions.
2. Disturbance of programmed cell death
Anomalies of programmed cell death (apoptosis) have been implicated in the pathogenesis of certain types of NMD, particularly heterotopias (Volpe, 1996; Rorke, 1994). These suggestions have followed the demonstration that certain neuronal populations, particularly those derived from the early migrating pre-plate neuroblasts, die by apoptosis during brain development (Allendoerfer and Shatz, 1994). In recent years, a great deal of information has emerged on the cellular process of apoptosis, in which cells die as part of a gene expression-related programme of cytodifferentiation (Jacobson et al. 1997). Both ‘pro-death’ genes (e.g. Bax, Bad) and ‘anti-death’ genes (e.g. Bcl) are expressed in cells in varying combinations and amounts, under the influence of exogenous influences such as survival factors, for instance neurotrophins (Davies, 1994), and apoptosis-inducing factors, including proteolytic fragments of laminin (Chen and Strickland, 1997). The balance within the cell of ‘pro-death’ and ‘anti-death’ gene products determines whether a cell will survive or die.
One critical gene in the apoptotic process is CPP32, a member of the Caspase gene family which includes genes that mediate the final steps in the molecular pathway leading to programmed cell death (Salvesen and Dixit, 1997). Mice homozygous for a null mutation of CPP32 exhibit diminished apoptosis with the presence of heterotopic neuronal masses in the cerebral cortex (Kuida et al. 1996). Thus, persistence of cells that normally die during brain development can yield neuronal heterotopias. Such ‘apoptosis-related’ heterotopias may be difficult to distinguish from those arising as a result of disturbed neuronal migration, and it is unclear to what extent failure of programmed cell death should be considered a critical pathogenetic event in the origin of human NMD.
Failure of neuronal migration: defining the critical events by studies of mouse mutants
Migration of neuroblasts to form the pre-plate, and subsequently the cortical plate, involves guidance by the processes of radial glial cells which, at this stage of development, stretch from the ventricular zone to the pial surface. A growing list of genes is known to regulate this process of neuronal migration, with NMD involving faulty layering of the cerebral cortex resulting in the mutant situation (Table 1). Evidence is accumulating, from the appearance of the brain in mutant individuals, to indicate how the process of neuronal migration may be disturbed in each of the mutants. The genes listed in Table 1 appear to fall into several functionally related groups, as follows:
Table 1.
Genes regulating neuronal migration
| Gene 1 | Species 2 | Seizures? 3 | Gene identification method | References 4 |
|---|---|---|---|---|
| Pafah1b1 | H,M | Yes | Positional cloning (LIS1 in human), knockout (Pafah1b1 in Mouse) | 1 |
| Doublecortin | H | Yes | Positional cloning (X-linked lissencephaly) | 2 |
| Reelin | M | No | Positional cloning (reeler mutant) | 3 |
| Mdab1 | M | No | Positional cloning (scrambler and yotari mutants) | 4 |
| Cdk5 | M | No | Knockout | 5 |
| p35 (Cdk5r) | M | Yes | Knockout | 6 |
| MARCKS | M | No | Knockout | 7 |
| Pax6 | H, M | No | Positional cloning (aniridia in humans, small eye mutant mouse) | 8 |
| ErbB2 | M | No | Knockout | 9 |
| Neuregulin | R | - | Antibody studies in vitro | 10 |
| Astrotactin | M | - | Antibody studies in vitro | 11 |
Pafah1b1 is platelet activating factor acetylhydrolase β1 subunit; mdab1 is mouse disabled 1; Cdk5 is cyclin dependent kinase 5; MARCKS is myristoylated alanine-rich C kinase substrate.
Species in which gene has been identified or mainly studied: H, human; M, mouse; R, rat.
Seizures have been described in humans with mutations in genes affecting neuronal migration, but not in all mouse models. This may reflect insufficiently detailed study of some of the mouse mutants.
References: 1, (Hirotsune et al. 1998; Hattori et al. 1994; Reiner et al. 1993); 2, (Des Portes et al. 1998; Gleeson et al. 1998); 3, (D’Arcangelo et al. 1995); 4, (Howell et al. 1997; Sheldon et al. 1997; Ware et al. 1997); 5, (Ohshima et al. 1996); 6, (Chae et al. 1997); 7, (Blackshear et al. 1997); 8, (Schmahl et al. 1993; Caric et al. 1997); 9, 10, (Anton et al. 1997; Rio et al. 1997); 11, (Zheng et al. 1996; Fishell and Hatten, 1991).
1. Reelin and mdab1
The genes reelin (D’Arcangelo et al. 1995) and mdab1 (Howell et al. 1997; Sheldon et al. 1997; Ware et al. 1997) appear to act early in the pathway of neuronal migration since the pre-plate does not become split into deep and superficial layers in mice mutant for these genes. Subsequently, the cortical plate develops with an inverted structure, in which the early-generated neurons are located superficially and the latest-generated neurons occupy the most internal positions. This suggests that the reelin and mdab1 genes may be needed for neuroblasts to pass one another as they migrate centrifugally. Reelin is expressed mainly on Cajal Retzius cells (D’Arcangelo et al. 1997) and, since these cells are descendants of the pre-plate, it is possible that reelin expression is also necessary on the pre-plate precursors to enable the cortical plate precursors to pass through and split the pre-plate. Indeed, reelin protein is expressed on a population of early-generated neurons in the developing cerebellum (Miyata et al. 1996).
Reelin is a secreted protein with similarities to the extracellular matrix molecule tenascin, whereas mdab1 encodes a cytoplasmic protein that is likely to function as an adapter molecule, binding non-receptor tyrosine kinases including Abl and Fyn. In view of the close similarity between the mutant phenotypes of reeler and mdab1 mutants, it has been suggested that mdab1 may play a role in transducing intracellular signals arising from occupancy of the putative reelin receptor. Reelin expression is not altered in mdab1 mutant embryos, whereas mdab1 expression is up-regulated in reeler mutants (Rice et al. 1998). These findings suggest that reelin may lie upstream of mdab1 in a genetic pathway to control neuronal migration.
2. Cdk5 and p35
Mice homozygous for null mutations in Cdk5 (Ohshima et al. 1996), or in its binding protein p35 (Chae et al. 1997), display a different brain phenotype from reelin and mdab1 mutants. Here, the pre-plate appears to be split normally and the early stages of cortical plate formation are relatively unimpaired. Later-migrating neuroblasts do not appear to be able to pass these early migrating neurons, with the result that the structure of the cortical plate is disorganised (Chae et al. 1997). It seems likely, therefore, that Cdk5/p35 mutants fail at a later stage in the generation of the cerebral cortex from reelin/mdab1 mutants. Both Cdk5 and p35 are expressed on post-mitotic, migratory neuroblasts (unusually for genes of the Cdk family which are usually expressed during the cell cycle) and it seems likely that the function of these genes may be critical to the later-generated neuroblasts of the cortical plate.
3. LIS1 and doublecortin
The LIS1 and doublecortin genes have both been implicated in the aetiology of lissencephaly in humans. Mutations in LIS1 cause autosomal lissencephaly (Hattori et al. 1994; Reiner et al. 1993), whereas doublecortin mutations are responsible for X-linked lissencephaly in males and subcortical band heterotopia in females (Des Portes et al. 1998; Gleeson et al. 1998). As X-inactivation mosaics, females heterozygous for a doublecortin mutation have two populations of cortical plate neurons: those expressing the wild type allele and those expressing the mutant allele. It is argued that the subcortical band heterotopia arises from cortical plate cells that express the mutant doublecortin gene (Gleeson et al. 1998).
The lissencephaly brain phenotypes are closely similar in both autosomal and X-linked conditions pointing to a potential interaction between the LIS1 and doublecortin gene products in neuronal migration. The doublecortin gene product contains a consensus phosphorylation site for the protein kinase Abl. It could interact with the LIS1 gene product, the β-subunit of platelet activating factor acetylhydrolase (PAFAH1B1), which is also known to be bound by protein kinases. Interactions are also possible with the reelin-mdab1 molecular pathway, and this will become clearer as mouse knockouts for these genes become available (Hirotsune et al. 1998).
4. Genes required for termination of neuronal migration
A further type of gene function is essential to ensure that late-generated neuroblasts terminate their migration at the boundary between the most superficial layer of the cortical plate (layer II) and the marginal zone. The protein kinase C substrate MARCKS appears to be needed to define the pial basement membrane that serves as the external anchoring point for radial glial end feet. Homozygotes for a null mutation in MARCKS exhibit aberrant localisation of various extracellular matrix molecules, for instance laminin and collagens, at the pial limiting membrane. Without correct function of these molecules, layer II neurons are found heterotopically in the marginal zone or in the subarachnoid space (Blackshear et al. 1997). We recently found similar abnormalities in mice homozygous for the dreher mutation (Costa, Harding and Copp, unpublished), in which heterotopic neurons are observed in the marginal zone of homozygotes (Sekiguchi et al. 1994).
5. Molecules mediating the interaction between neuroblasts and radial glia
A number of molecules have been shown to play a role in mediating the interaction between neuroblasts and radial glia. These molecules, for instance astrotactin and neuregulin (glial growth factor) have mainly been studied in cerebellar culture systems and their role in neuronal migration within the developing cerebral cortex is less well understood. Astrotactin is a heterophilic cell adhesion protein expressed on migrating neuroblasts that enables their adhesion to the radial glia and, in addition, regulates the adoption of an extended cell phenotype by the glia (Zheng et al. 1996). Neuregulin is also expressed on migrating neuroblasts and, similarly, has a role in modulating radial glial cell shape, possibly via inducing the expression of brain lipid-binding protein (BLBP). While the glial receptor for astrotactin is yet to be determined, erbB receptors are known to transduce signals from neuregulin. Recent studies demonstrate a neuronal migration disorder in mice homozygous for a knockout mutation of the erbB2 gene (Rio et al. 1997).
Conclusion
This review has demonstrated the range of clinical phenotypes encompassed by the term ‘neuronal migration disorder’, and has highlighted the role played by faulty migration, differentiation and apoptosis of neuroblasts in the generation of these malformations. The recent development, in the mouse, of several genetic model systems of neuronal migration disorder, promises to reveal important information on the underlying molecular and cellular control mechanisms. It should be borne in mind, however, that brain corticogenesis in the mouse exhibits certain differences from the corresponding process in humans, for instance as exemplified by the absence of gyri even in the normal adult mouse brain. Hence, it may be not always be straightforward to model specific human malformations (e.g. lissencephaly) using the mouse. Clearly the direct study of humans must proceed in parallel with the analysis of mouse models, if we are to gain a thorough understanding of neuronal migration disorders and their functional consequences.
References
- Acampora D, Mazan S, Lallemand Y, Avantaggiato V, Maury M, Simeone A, Brûlet P. Forebrain and midbrain regions are deleted in Otx2-/- mutants due to a defective anterior neuroectoderm specification during gastrulation. Development. 1995;121:3279–3290. doi: 10.1242/dev.121.10.3279. [DOI] [PubMed] [Google Scholar]
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: Its role in the development of connections between thalamus and cortex. Annu. Rev. Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- Blackshear PJ, Silver J, Nairn AC, Sulik KK, Squier MV, Stumpo DJ, Tuttle JS. Widespread neuronal ectopia associated with secondary defects in cerebrocortical chondroitin sulfate proteoglycans and basal lamina in MARCKS-deficient mice. Exp. Neurol. 1997;145:46–61. doi: 10.1006/exnr.1997.6475. [DOI] [PubMed] [Google Scholar]
- Brunelli S, Faiella A, Capra V, Nigro V, Simeone A, Cama A, Boncinelli E. Germline mutations in the homeobox gene EMX2 in patients with severe schizencephaly. Nature Genet. 1996;12:94–96. doi: 10.1038/ng0196-94. [DOI] [PubMed] [Google Scholar]
- Caric D, Gooday D, Hill RE, McConnell SK, Price DJ. Determination of the migratory capacity of embryonic cortical cells lacking the transcription factor Pax-6. Development. 1997;124:5087–5096. doi: 10.1242/dev.124.24.5087. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmincatalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen S-C, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM. The role of neurotrophins in the developing nervous system. J. Neurobiol. 1994;25:1334–1348. doi: 10.1002/neu.480251103. [DOI] [PubMed] [Google Scholar]
- Des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald Netter Y, Catala M, Kahn A, Beldjord C, Chelly J. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Dobyns WB, Reiner O, Carrozzo R, Ledbetter DH. Lissencephaly: A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA. 1993;270:2838–2842. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- Eksioglu YZ, Scheffer IE, Cardenas P, Knoll J, DiMario F, Ramsby G, Berg M, Kamuro K, Berkovic SF, Duyk GM, Parisi J, Huttenlocher PR, Walsh CA. Periventricular heterotopia: An X-linked dominant epilepsy locus causing aberrant cerebral cortical development. Neuron. 1996;16:77–87. doi: 10.1016/s0896-6273(00)80025-2. [DOI] [PubMed] [Google Scholar]
- Fishell G, Hatten ME. Astrotactin provides a receptor system for CNS neuronal migration. Development. 1991;113:755–765. doi: 10.1242/dev.113.3.755. [DOI] [PubMed] [Google Scholar]
- Gillies K, Price DJ. The fates of cells in the developing cerebral cortex of normal and methylazoxymethanol acetate-lesioned mice. Eur. J. Neurosci. 1993;5:73–84. doi: 10.1111/j.1460-9568.1993.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Green AJ, Smith M, Yates JRW. Loss of heterozygosity on chromosome 16p13.3 in hamartomas from tuberous sclerosis patients. Nature Genet. 1994;6:193–196. doi: 10.1038/ng0294-193. [DOI] [PubMed] [Google Scholar]
- Grove EA, Williams BP, Li D-Q, Hajihosseini M, Friedrich A, Price J. Multiple restricted lineages in the embryonic rat cerebral cortex. Development. 1993;117:553–561. doi: 10.1242/dev.117.2.553. [DOI] [PubMed] [Google Scholar]
- Harding BN, Copp AJ. Congenital malformations. In: Graham D.l., Lantos PL., editors. Greenfield’s Neuropathology. Arnold; London: 1997. pp. 397–533. [Google Scholar]
- Hattori M, Adachi H, Tsujimoto M, Arai H, Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor. Nature. 1994;370:216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ, Wynshaw-Boris A. Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nature Genet. 1998;19:333–339. doi: 10.1038/1221. [DOI] [PubMed] [Google Scholar]
- Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- knudson a.j. Genetics of human cancer. Annu. Rev. Genet. 1986;20:231–251. doi: 10.1146/annurev.ge.20.120186.001311. [DOI] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na SQ, Kuan CY, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Labosky PA, Winnier GE, Jetton TL, Hargett L, Ryan AK, Rosenfeld MG, Parlow AF, Hogan BLM. The winged helix gene, Mf3, is required for normal development of the diencephalon and midbrain, postnatal growth and the milk-ejection reflex. Development. 1997;124:1263–1274. doi: 10.1242/dev.124.7.1263. [DOI] [PubMed] [Google Scholar]
- Matsuo I, Kuratani S, Kimura C, Takeda N, Aizawa S. Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev. 1995;9:2646–2658. doi: 10.1101/gad.9.21.2646. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- Miyata T, Nakajima K, Aruga J, Takahashi S, Ikenaka K, Mikoshiba K, Ogawa M. Distribution of a reeler gene-related antigen in the developing cerebellum: An immunohistochemical study with an allogeneic antibody CR-50 on normal and reeler mice. J. Comp. Neurol. 1996;372:215–228. doi: 10.1002/(SICI)1096-9861(19960819)372:2<215::AID-CNE5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter FD, Drago J, Xu Y, Cheema SS, Wassif C, Huang SP, Lee E, Grinberg A, Massalas JS, Bodine D, Alt F, Westphal H. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development. 1997;124:2935–2944. doi: 10.1242/dev.124.15.2935. [DOI] [PubMed] [Google Scholar]
- Price DJ, Aslam S, Tasker L, Gillies K. Fates of the earliest generated cells in the developing murine neocortex. J. Comp. Neurol. 1997;377:414–422. [PubMed] [Google Scholar]
- Qiu MS, Bulfone A, Martinez S, Meneses JJ, Shimamura K, Pedersen RA, Rubenstein JLR. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 1995;9:2523–2538. doi: 10.1101/gad.9.20.2523. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 1972;145:61–84. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Raymond AA, Fish DR, Stevens JM, Sisodiya SM, Alsanjari N, Shorvon SD. Subependymal heterotopia: a distinct neuronal migration disorder associated with epilepsy. J. Neurol. Neurosurg. Psychiatry. 1994;57:1195–1202. doi: 10.1136/jnnp.57.10.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Rice DS, Sheldon M, D’Arcangelo G, Nakajima K, Goldowitz D, Curran T. Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development. 1998;125:3719–3729. doi: 10.1242/dev.125.18.3719. [DOI] [PubMed] [Google Scholar]
- Rio C, Rieff HI, Qi PM, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- Rorke LB. The role of disordered genetic control of neurogenesis in the pathogenesis of migration disorders. J. Neuropathol. Exp. Neurol. 1994;53:105–117. doi: 10.1097/00005072-199403000-00001. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM. Caspases: Intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- Schmahl W, Knoedlseder M, Favor J, Davidson D. Defects of neuronal migration and the pathogenesis of cortical malformations are associated with Small eye (Sey) in the mouse, a point mutation at the Pax-6-locus. Acta Neuropathol. (Berl. ) 1993;86:126–135. doi: 10.1007/BF00334879. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Abe H, Shimai K, Huang G, Inoue T, Nowakowski RS. Disruption of neuronal migration in the neocortex of the dreher mutant mouse. Dev. Brain Res. 1994;77:37–43. doi: 10.1016/0165-3806(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Sheldon M, Rice DS, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell BW, Cooper JA, Goldowitz D, Curran T. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature. 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Szucsik JC, Witte DP, Li H, Pixley SK, Small KM, Potter SS. Altered forebrain and hindbrain development in mice mutant for the Gsh-2 homeobox gene. Dev. Biol. 1997;191:230–242. doi: 10.1006/dbio.1997.8733. [DOI] [PubMed] [Google Scholar]
- Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J. Neurol. Neurosurg. Psychiatry. 1971;34:369–387. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinters HV, Fisher RS, Cornford ME, Mah V, Secor DL, De Rosa MJ, Comair YG, Peacock WJ, Shields WD. Morphological substrates of infantile spasms: studies based on surgically resected cerebral tissue. Childs. Nerv. Syst. 1992;8:8–17. doi: 10.1007/BF00316556. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Subplate neurons - missing link in brain injury of the premature infant. Pediatrics. 1996;97:112–113. [PubMed] [Google Scholar]
- Ware ML, Fox JW, González JL, Davis NM, De Rouvroit CL, Russo CJ, Chua SC, Jr., Goffinet AM, Walsh CA. Aberrant splicing of a mouse disabled homolog, mdab1, in the scrambler mouse. Neuron. 1997;19:239–249. doi: 10.1016/s0896-6273(00)80936-8. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Suda Y, Matsuo I, Miyamoto N, Takeda N, Kuratani S, Aizawa S. Emx1 and Emx2 functions in development of dorsal telencephalon. Development. 1997;124:101–111. doi: 10.1242/dev.124.1.101. [DOI] [PubMed] [Google Scholar]
- Zheng C, Heintz N, Hatten ME. CNS gene encoding astrotactin, which supports neuronal migration along glial fibers. Science. 1996;272:417–419. doi: 10.1126/science.272.5260.417. [DOI] [PubMed] [Google Scholar]