Abstract
Mutations of the glucocerebrosidase (GBA) gene are the most important risk factor yet discovered for Parkinson disease (PD). Homozygous GBA mutations result in Gaucher disease (GD), a lysosomal storage disorder. Heterozygous mutations have not until recently been thought to be associated with any pathological process. However, it is clear that the presence of a GBA mutation in homozygous or heterozygous form is associated with an approximately 20-fold increase in the risk for PD, with little if any difference in risk burden related to gene dose. Most studies suggest that 5–10% of PD patients have GBA mutations, although this figure is greater in the Ashkenazi population and may be an underestimate overall if the entire exome is not sequenced. GBA-associated PD is clinically indistinguishable from idiopathic PD, except for slightly earlier age of onset and a greater frequency of cognitive impairment. Pathological and imaging features, and response to pharmacotherapy are identical to idiopathic PD. GBA mutations result in reduced enzyme activity and mutant protein may become trapped in the endoplasmic reticulum (ER) leading to unfolded protein response and ER associated degradation and stress. Both mechanisms may be relevant in GD and PD pathogenesis and lead to impaired lysosomal function. Of particular relevance to PD is the interaction of glucocerebrosidase enzyme (GCase) with alpha-synuclein (SNCA). There appears to be a bi-directional reciprocal relationship between GCase levels and those of SNCA. Thus reduced GCase in GBA mutation PD brain is associated with increased SNCA, and increased SNCA deposition is associated with reduced GCase even in GBA wild-type PD brains. It is noteworthy that GBA mutations are also associated with an increase in risk for dementia with Lewy bodies, another synucleinopathy. It has been suggested that the relationship between GCase and SNCA may be leveraged to reduce SNCA levels in PD by enhancing GCase levels and activity. This hypothesis has been confirmed in GBA mutant mice, PD patient fibroblasts and cells with SNCA overexpression, and offers an important target pathway for future neuroprotection therapy in PD. This article is part of a Special Issue entitled ‘Neuronal Protein’.
Keywords: Parkinson disease, Glucocerebrosidase, Lysosome, Alpha-synuclein, Neuroprotection
1. Introduction
Parkinson disease (PD) is a multicentric neurodegenerative disease characterised pathologically by the loss of dopaminergic neurons in the substantia nigra pars compacta and other brain stem nuclei, as well as by the presence of alpha-synuclein (SNCA) aggregates in Lewy bodies and neurites. The aetiology and pathogenesis of PD have been the subject of much research for over more than a century, in the hope that this might lead to effective treatments. In part, this ambition has been achieved with the identification of dopamine deficiency in PD brain and the improvement of symptoms with the use of dopaminergic drugs. However, this strategy has limitations in terms of its effectiveness – not all PD symptoms are caused by dopamine deficiency – and a side effect profile that includes levodopa related motor complications. The most important challenge is to develop therapies that can prevent, slow or reverse the neurodegeneration associated with PD. For this, a clear understanding of the causes and biochemical pathways leading to PD needs to be defined.
There have been substantial advances in our understanding of the genetic factors associated with PD, and of the abnormal biochemistry of the PD brain (Schapira and Jenner, 2011). The accumulation of SNCA has been considered central to the pathogenesis of PD, as reflected by mutations, multiplications and polymorphisms of the SNCA gene that lead to abnormal protein, an increased generation or accumulation of wild-type protein and which are associated with PD (Lin and Farrer, 2014). Although several gene mutations have been described in familial PD, taken together these still remain relatively rare, accounting for probably <10% of all cases (Mullin and Schapira, 2015). Genome-wide association studies in PD have demonstrated a number of additional significant genetic associations with PD, confirming SNCA and tau, but adding components of the immune cascade (Nalls et al., 2014). Probably the most exciting of all genetic associations with PD is the identification that mutations of the glucocerebrosidase gene (GBA1) are a significant risk factor for the disease. This relationship was first identified in the Ashkenazi Jewish population and began to attract attention after a number of reports (Aharon-Peretz et al., 2004; Tayebi et al., 2001). This review seeks to provide an update on certain aspects of the glucocerebrosidase link with PD and the potential for the development of future therapies to target this area.
2. Genetics
The lysosomal enzyme glucocerebrosidase (GCase) is encoded by the GBA1 gene on chromosome 1q21. It has 11 exons, 10 introns and is 7.6 kb in total with a nearby 5.6 kb pseudogene, 16 kb downstream (Horowitz et al., 1989). GCase metabolises glucocerebroside to glucose and ceramide and mutations of GBA1 cause the autosomal recessive lysosomal storage disorder Gaucher disease (Grabowski, 2008). Over 300 different mutations of the GBA1 gene have been described, but the N370S and L444P account for the majority of those found in both Gaucher disease and PD. Gaucher disease has an estimated frequency of 1:50,000 live births, but this rises to 1:850 in the Ashkenazi Jewish population. Both Gaucher patients and asymptomatic heterozygous gene carriers are recognised to be at almost equal risk of PD. The penetrance and lifetime risk of developing PD for those with a GBA1 mutation varies with some figures quoting up to 20% at 70 years and 30% at 80 years (Beavan and Schapira, 2013). The proportion of PD patients that carry GBA1 mutations is estimated to be between 5 and 10%, but this range may be an underestimate in some populations and also depends on whether the entire exome has been sequenced (Kumar et al., 2013; Lesage et al., 2011; McNeill et al., 2012a; Neumann et al., 2009; Sidransky et al., 2009). Certain GBA1 sequence variants e.g., E326K have been associated with PD and not Gaucher disease and this further increases the proportion of PD patients that are associated with GBA1 mutations (Duran et al., 2013). The proportion of PD cases that carry GBA1 mutations in Japan appears to be similar with 9.4% of PD cases carrying GBA1 mutations with an odds ratio of 28 compared to controls (Mitsui et al., 2009). In the Chinese PD population, 3.72% of cases had GBA1 mutations, with an odds ratio of 15 compared to controls (Huang et al., 2011).
Thus, GBA1 mutations represent the most important risk factor for PD identified to date. These mutations are substantially more common than other PD associated genes such as LRRK2 or SNCA. GBA1 has also been associated with dementia with Lewy bodies, strengthening its relationship to SNCA pathology (Goker-Alpan et al., 2006; Mata et al., 2008; Nalls et al., 2013). GBA1 mutations have not been found at increased frequency in multiple system atrophy, progressive supranuclear palsy or corticobasal degeneration (Jamrozik et al., 2010; Segarane et al., 2009; Srulijes et al., 2013).
3. Clinicopathological correlates
Individual PD patients with GBA1 mutations cannot be discriminated from idiopathic PD without GBA1 mutations on clinical or pathological grounds. There are some interesting clinical features when the PD-GBA1 group is taken as a whole. For instance, PD-GBA1 patients exhibit the classic triad of bradykinesia, rigidity and tremor, with asymmetric onset (Goker-Alpan et al., 2008). However, age of onset tends to be slightly younger and there is a greater risk for earlier and more prevalent cognitive impairment in PD-GBA1 patients (Sidransky et al., 2009; Winder-Rhodes et al., 2013). The pattern of cognitive dysfunction in GBA1 positive carriers was slightly different and present in those even without PD at the time of investigation (Zokaei et al., 2014). In contrast to other genetic causes of PD, imaging with fluorodopa positron emission tomography or single photon emission tomography with dopamine sensitive ligands in PD-GBA1 demonstrate an asymmetric pattern of abnormality indistinguishable from idiopathic PD (Goker-Alpan et al., 2012; McNeill et al., 2013b). Patients with GBA1 mutations also exhibit greater retinal abnormalities in terms of thickness as determined by optical coherence tomography compared to matched PD patients (McNeill et al., 2013a).
Of particular interest is the evidence accumulating that GBA1 mutant homozygote and heterozygote carriers without clinical evidence of PD, exhibit the prodromal features of the disease. Olfactory function and cognitive assessment were significantly reduced, and motor testing abnormal in GBA1 positive cases compared to controls (McNeill et al., 2012b). A two year follow-up showed significant deterioration in scores for depression, rapid eye movement sleep behaviour disorder, cognition, olfaction and motor scores (Beavan et al., 2015). These data indicate that individuals with GBA1 mutations exhibit identical prodromal abnormalities to those with idiopathic PD. There appears to be a relatively rapid evolution of non-motor and motor features in this cohort. Further follow up of this group will enable early diagnosis in those progressing to clinical PD and perhaps allow identification of a specific clinical or biochemical pattern that distinguishes those with GBA1 mutations who will and those who will not develop PD.
The pathology of GBA1 mutation positive PD appears to be identical to that of idiopathic disease. In a retrospective analysis of brains exhibiting the characteristic pathology of PD, GBA1 mutations were found in almost 5% (Neumann et al., 2009; Wong et al., 2004). GCase has also been found in Lewy bodies, more frequently in those with GBA1 mutations (Goker-Alpan et al., 2010). Some studies have suggested that Lewy body deposition is more extensive in GBA1 mutant positive brains (Clark et al., 2009) but this is not universally found (Parkkinen et al., 2011).
The response to dopaminergic therapy in PD-GBA1 appears to be the same as that seen in idiopathic PD, including the development of motor complications (Ziegler et al., 2007). In one centre, retrospective genetic analysis identified GBA1 mutations in 17% of those who had undergone deep brain stimulation, and in whom clinical effect was as good as those without mutations (Angeli et al., 2013).
4. Biochemistry
The presence of a GBA1 mutation is invariably associated with a reduction in GCase enzyme activity, although the degree of this varies between mutations. Homozygous Gaucher patients may have <1% residual activity, while heterozygous carriers may have 50–60% residual activity, depending on the mutation. Peripheral GCase activity from fibroblasts has been recently studied in samples from PD patients with and without GBA1 mutations, and in Gaucher patients with various GBA1 mutations (McNeill et al., 2014). However, the mechanism by which GBA1 mutations increase the risk for PD may operate through additional or alternative mechanisms than simply GCase deficiency, and this is discussed below.
A detailed analysis of the biochemistry of the brains from PD-GBA1 patients identified a significant reduction in GCase involving substantia nigra (reduced 58%), putamen (48%), amygdala (40%) and cerebellum (47%) (Gegg et al., 2012). The loss of activity was associated with decreased protein levels of GCase, but not mRNA levels indicating this loss of activity was not induced by decreased transcription. Other lysosomal enzymes were unaffected. Mutant GCase protein has been reported to become trapped in the endoplasmic reticulum (ER) and trigger the unfolded protein response and ER-associated degradation (ERAD) (Mu et al., 2008; Ron and Horowitz, 2005). Markers of ERAD were found to be increased in the PD-GBA1 brains, supporting a role for this pathway in the pathogenesis of PD in these patients. Of particular importance was the finding of significantly reduced GCase activity in PD brains without GBA1 mutations, with a 33% decrease in substantia nigra and changes of ERAD. This observation provides a direct link between the pathogenetic pathways involved in PD-GBA1 and idiopathic PD. In addition, there is now evidence to support an age-related decline of GCase activity in the ageing brain that may act as a predisposing factor for synuclein accumulation and PD (Rocha et al., 2015).
The link between GCase and SNCA was demonstrated in vitro by the overexpression of mutant and wild-type GCase, showing that the mutant forms increased SNCA levels but the wild-type had variable effects (Cullen et al., 2011). The levels of SNCA were increased in hippocampus at 12 months in a homozygous mouse model with residual GCase activity >20%, but not at 12 weeks in Gaucher mice. Administration of the GCase inhibitor conduritol beta-epoxide (CBE) to cells or mice results in reduced GCase activity and accumulation of SNCA (Cleeter et al., 2013; Manning-Bog et al., 2009), although short-term exposure does not increase SNCA levels in rat cortical neurons (Dermentzaki et al., 2013). In addition, SNCA accumulation has been seen in cortical neuronal cultures from GBA1 knockout mice (Mazzulli et al., 2011) and in the brains of these mice (Osellame et al., 2013). Conversely, overexpression of SNCA has caused a reduction in GCase activity in SH-SY5Y cells (Gegg et al., 2012). Viral mediated expression of GBA1 in GBA1 deficient mice corrected the SNCA accumulation in brain, and reduced levels in the SNCA A53T mutant (Sardi et al., 2011, 2013). An analysis of Lewy body density load in PD brain found an inverse relationship with GCase activity (Murphy et al., 2014). There was a modest increase in GCase activity in SNCA knockout mice and when these mice were crossed with those carrying a heterozygous L444P mutation, the half-life of SNCA was increased and aggregates seen in cortical cultures (Fishbein et al., 2014). There was also an exacerbation of the motor and gastrointestinal abnormalities of these mice with the double mutation.
Studies in pluripotent stem cells showed that partial loss of GCase impaired lysosomal protein degradation and increased SNCA levels (Mazzulli et al., 2011). Pluripotential stem cells (iPS) derived from individuals with GBA1 mutations recapitulated some of the biochemical abnormalities seen in other systems in terms of reduced GCase activity, impaired lysosomal function and increased sphingolipids (Panicker et al., 2012). Another iPS model has demonstrated abnormal calcium homeostasis and lysosomal dysfunction in cells derived from GBA1 mutant individuals (Schondorf et al., 2014).
The molecular and biochemical basis for the interaction between GCase and SNCA has yet to be elucidated. Some have proposed a direct interaction (Yap et al., 2011) that may be mediated at membrane sites (Yap et al., 2013). Others propose a disturbance of GCase trafficking (Cooper et al., 2006; Thayanidhi et al., 2010) for which there is indirect support in PD brain with and without GBA1 mutations (Chung et al., 2013; Gegg et al., 2012).
The data from PD brains, with and without GBA1 mutations, and from the various models of GBA1 mutation mice highlight the importance of lysosomal dysfunction in PD (Tofaris, 2012). SNCA is predominantly metabolised through chaperone mediated autophagy (CMA) and abnormalities of CMA have been identified in PD brain (Alvarez-Erviti et al., 2010; Cuervo et al., 2004). Impaired lysosomal function enhances the exosomal release of SNCA (Alvarez-Erviti et al., 2011) and decreased GCase activity promotes propagation of SNCA aggregates (Bae et al., 2014). Lysosomes also play an important role in calcium homeostasis (Kilpatrick et al., 2013) and calcium dysregulation has been implicated in PD pathogenesis (Schapira, 2013). The finding of altered calcium homeostasis in GBA1 iPS cells noted above is therefore of significance (Schondorf et al., 2014).
5. Therapeutic implications
There are several candidate pathways through which GCase deficiency may promote the pathogenesis of PD including the reciprocity with SNCA levels, lysosomal dysfunction, ERAD, calcium dysregulation and also mitochondrial abnormalities (see Fig. 1). The latter have been seen in the CBE toxin model (Cleeter et al., 2013) cell models (Gegg et al., 2012) and in the GBA1 knockout mouse (Osellame et al., 2013). Thus there are several potential pathways to target to influence the effect of GBA1 mutations on the pathogenesis of PD and importantly, this strategy would have relevance to idiopathic PD given the evidence for the GCase–SNCA interaction that might promote SNCA pathology (Schapira and Gegg, 2013).
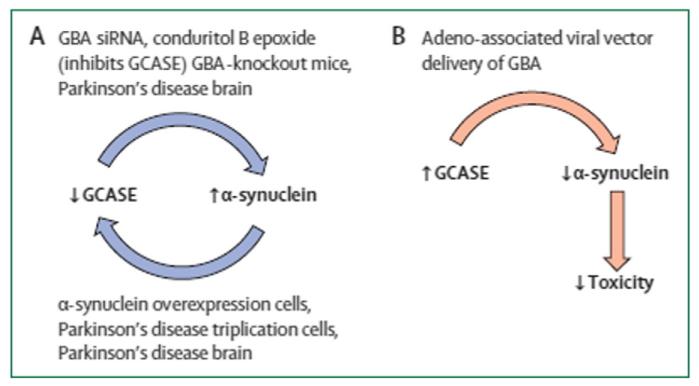
Fig. 1.
The reciprocal relationship between GCase and alpha-synuclein as supported by in vitro, in vivo and post-mortem studies. Reproduced with permission from Lancet (2014; 384:545).
Small molecule chaperones for GCase have been developed for the potential treatment of Gaucher disease on the basis that the mutant protein is trapped in the ER and that trafficking to the lysosome will not only reduce ERAD, but also improve lysosomal function (Bendikov-Bar et al., 2013; Lieberman et al., 2009). Studies in fibroblasts derived from homozygous and heterozygous GBA1 mutation carriers, with and without PD, as well as PD patients without GBA1 mutations revealed the expected range of reduction in GCase activity — severe in Gaucher patients, intermediate in carriers and unaffected in PD patients without mutations (McNeill et al., 2014). The cells were then exposed to the GCase chaperone ambroxol for 5 days. There was a significant increase in GCase activity in Gaucher patients, although in absolute terms this was small. There was a significant increase of GCase activity and protein levels in carriers both with and without PD. One case had the E326K mutation and had low GCase activity, again increased by ambroxol. Of particular interest was the increase in activity in PD patient cells without mutations. This effect was also seen in controls. There was also evidence of ERAD and free radical mediated damage in the GBA1 mutant cells, again improved by ambroxol. The authors showed that some of the effect of ambroxol was mediated via activation of the transcription factor EB (TFEB) which controls coordinated lysosomal expression and regulation (CLEAR) pathway. Ambroxol reduced SNCA levels in a SH-SY5Y over-expression line.
These results are of obvious interest to strategies that are intended to influence lysosomal function and SNCA deposition in PD, as a mechanism of neuroprotection (Schapira et al., 2014). The value of the GCase–lysosomal–SNCA pathway as a target for drug intervention is that it links to several other potential contributors to PD pathogenesis (see Fig. 2). Improving the trafficking of mutant GCase by chaperones will reduce ERAD, but also in the case of ambroxol, may enhance GCase not only by lysosomal localisation, but also by upregulation. The latter mechanism is relevant to patients without GBA1 mutations. Reducing the interaction of GCase and SNCA and improving lysosomal function will increase the turnover of SNCA by CMA, thereby reducing its propensity to aggregate. Enhancing lysosomal function and reducing SNCA levels will reduce the release and spread of SNCA and its related pathology. Importantly, these effects will not be restricted to dopaminergic neurons, an essential feature of a successful neuroprotective agent (Olanow and Schapira, 2013).
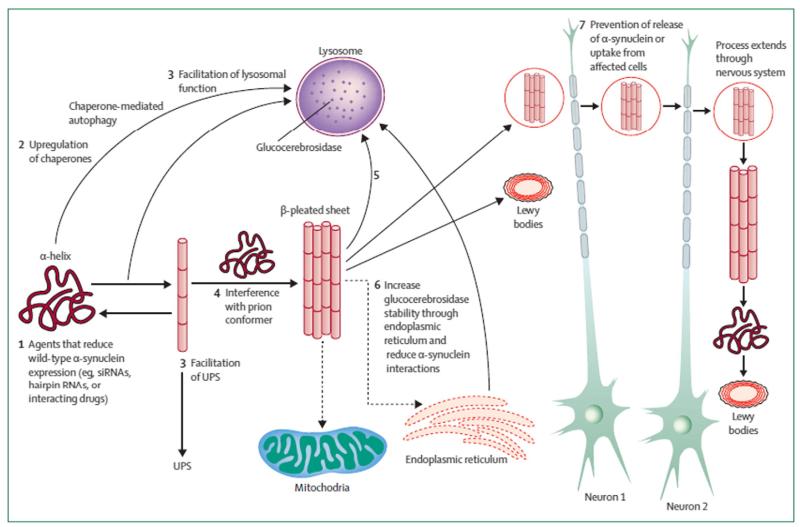
Fig. 2.
Pathways for potential interventions in the treatment of aberrant α-synuclein metabolism are shown. Neurodegeneration is thought to be associated with conversion of the normal α-helix protein structure to a β-sheet-rich configuration and the formation of toxic oligomers or aggregates. Potential treatments could include: (1) agents that reduce expression of wild-type α-synuclein and thus reduce the natural substrate for a prion or templating reaction; (2) upregulation of chaperones that promote refolding or clearance of abnormal proteins; (3) facilitation of UPS or autophagy/lysosomal function to promote clearance of unwanted proteins; (4) interference with the prion conformer whereby misfolded α-synuclein act as template to promote the conversion of wild-type α-synuclein; (5) agents or immune approaches targeted to remove toxic α-synuclein oligomers or aggregates; (6) increased glucocerebrosidase stability or trafficking through the endoplasmic reticulum to normalise α-synuclein metabolism and lysosomal function. These interventions (1–6) are designated to prevent or reduce the toxic effects of α-synuclein oligomers or aggregates on vital cell processes (e.g., mitochondrial function and axonal transport). Intervention (7) represents agents that prevent release of α-synuclein from affected cells and/or the uptake of α-synuclein into healthy unaffected cells whereby the process might extend throughout the nervous system. Dashed arrows represent inhibition and solid arrows represent pathways of progression. UPS = ubiquitin proteasome system.
Reproduced with permission from Lancet (2014; 384:545).
In conclusion, the discovery of the connection between GBA1 mutations and PD has provided invaluable insights into the pathogenesis of this disease and provides opportunities to target the GCase–lysosomal pathway for the development of neuroprotective drugs in PD.
Acknowledgements
The work described by the author’s research group in this review has been supported by the MRC/Wellcome Trust (WT089698), MRC (MR/J009660/1 and MR/L501499/1), the UK Parkinson’s Disease (G-1104), the Kattan Trust and the Javon Trust. AHVS is a NIHR Senior Investigator NF-SI-0611-10237 and is supported by the NIHR UCLH BRC.
References
- Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N. Engl. J. Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch. Neurol. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol. Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli A, et al. Genotype and phenotype in Parkinson’s disease: lessons in heterogeneity from deep brain stimulation. Mov. Disord. 2013;28:1370–1375. doi: 10.1002/mds.25535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae EJ, et al. Glucocerebrosidase depletion enhances cell-to-cell transmission of alpha-synuclein. Nat. Commun. 2014;5:4755. doi: 10.1038/ncomms5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavan MS, Schapira AH. Glucocerebrosidase mutations and the pathogenesis of Parkinson disease. Ann. Med. 2013;45:511–521. doi: 10.3109/07853890.2013.849003. [DOI] [PubMed] [Google Scholar]
- Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AH. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol. 2015;72:201–208. doi: 10.1001/jamaneurol.2014.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendikov-Bar I, Maor G, Filocamo M, Horowitz M. Ambroxol as a pharmacological chaperone for mutant glucocerebrosidase. Blood Cells Mol. Dis. 2013;50:141–145. doi: 10.1016/j.bcmd.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, et al. Identification and rescue of alpha-synuclein toxicity in Parkinson patient-derived neurons. Science. 2013;342:983–987. doi: 10.1126/science.1245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LN, et al. Association of glucocerebrosidase mutations with dementia with Lewy bodies. Arch. Neurol. 2009;66:578–583. doi: 10.1001/archneurol.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeter MW, Chau KY, Gluck C, Mehta A, Hughes DA, Duchen M, Wood NW, Hardy J, Mark Cooper J, Schapira AH. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem. Int. 2013;62(1):1–7. doi: 10.1016/j.neuint.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA, et al. Alpha-synuclein blocks ER–Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Cullen V, et al. Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter alpha-synuclein processing. Ann. Neurol. 2011;69:940–953. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- Dermentzaki G, Dimitriou E, Xilouri M, Michelakakis H, Stefanis L. Loss of beta-glucocerebrosidase activity does not affect alpha-synuclein levels or lysosomal function in neuronal cells. PLoS One. 2013;8:e60674. doi: 10.1371/journal.pone.0060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran R, et al. The glucocerobrosidase E326K variant predisposes to Parkinson’s disease, but does not cause Gaucher’s disease. Mov. Disord. 2013;28:232–236. doi: 10.1002/mds.25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein I, Kuo YM, Giasson BI, Nussbaum RL. Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a Gaucher mutation. Brain. 2014;137:3235–3247. doi: 10.1093/brain/awu291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, Schapira AH. Glucocerebrosidase deficiency in substantia nigra of Parkinson disease brains. Ann. Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker-Alpan O, Giasson BI, Eblan MJ, Nguyen J, Hurtig HI, Lee VM, Trojanowski JQ, Sidransky E. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- Goker-Alpan O, Lopez G, Vithayathil J, Davis J, Hallett M, Sidransky E. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch. Neurol. 2008;65:1353–1357. doi: 10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 2010;120:641–649. doi: 10.1007/s00401-010-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker-Alpan O, et al. The neurobiology of glucocerebrosidase-associated parkinsonism: a positron emission tomography study of dopamine synthesis and regional cerebral blood flow. Brain. 2012;135:2440–2448. doi: 10.1093/brain/aws174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372:1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Wilder S, Horowitz Z, Reiner O, Gelbart T, Beutler E. The human glucocerebrosidase gene and pseudogene: structure and evolution. Genomics. 1989;4:87–96. doi: 10.1016/0888-7543(89)90319-4. [DOI] [PubMed] [Google Scholar]
- Huang CL, Wu-Chou YH, Lai SC, Chang HC, Yeh TH, Weng YH, Chen RS, Huang YZ, Lu CS. Contribution of glucocerebrosidase mutation in a large cohort of sporadic Parkinson’s disease in Taiwan. Eur. J. Neurol. 2011;18:1227–1232. doi: 10.1111/j.1468-1331.2011.03362.x. [DOI] [PubMed] [Google Scholar]
- Jamrozik Z, Lugowska A, Slawek J, Kwiecinski H. Glucocerebrosidase mutations p.L444P and p.N370S are not associated with multisystem atrophy, progressive supranuclear palsy and corticobasal degeneration in Polish patients. J. Neurol. 2010;257:459–460. doi: 10.1007/s00415-009-5363-4. [DOI] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Schapira AH, Futter CE, Patel S. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J. Cell Sci. 2013;126:60–66. doi: 10.1242/jcs.118836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KR, et al. Glucocerebrosidase mutations in a Serbian Parkinson’s disease population. Eur. J. Neurol. 2013;20:402–405. doi: 10.1111/j.1468-1331.2012.03817.x. [DOI] [PubMed] [Google Scholar]
- Lesage S, et al. Large-scale screening of the Gaucher’s disease-related glucocerebrosidase gene in Europeans with Parkinson’s disease. Hum. Mol. Genet. 2011;20:202–210. doi: 10.1093/hmg/ddq454. [DOI] [PubMed] [Google Scholar]
- Lieberman RL, D’aquino JA, Ringe D, Petsko GA. Effects of pH and iminosugar pharmacological chaperones on lysosomal glycosidase structure and stability. Biochemistry. 2009;48:4816–4827. doi: 10.1021/bi9002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MK, Farrer MJ. Genetics and genomics of Parkinson’s disease. Genome Med. 2014;6:48. doi: 10.1186/gm566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Bog AB, Schule B, Langston JW. Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology. 2009;30:1127–1132. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Mata IF, et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch. Neurol. 2008;65:379–382. doi: 10.1001/archneurol.2007.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, Duran R, Hughes DA, Mehta A, Schapira AH. A clinical and family history study of Parkinson’s disease in heterozygous glucocerebrosidase mutation carriers. J. Neurol. Neurosurg. Psychiatry. 2012a;83:853–854. doi: 10.1136/jnnp-2012-302402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, Duran R, Proukakis C, Bras J, Hughes D, Mehta A, Hardy J, Wood NW, Schapira AH. Hyposmia and cognitive impairment in Gaucher disease patients and carriers. Mov. Disord. 2012b;27:526–532. doi: 10.1002/mds.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, Roberti G, Lascaratos G, Hughes D, Mehta A, Garway-Heath DF, Schapira AH. Retinal thinning in Gaucher disease patients and carriers: results of a pilot study. Mol. Genet. Metab. 2013a;109:221–223. doi: 10.1016/j.ymgme.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, et al. Dopaminergic neuronal imaging in genetic Parkinson’s disease: insights into pathogenesis. PLoS One. 2013b;8:e69190. doi: 10.1371/journal.pone.0069190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A, et al. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain. 2014;137:1481–1495. doi: 10.1093/brain/awu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui J, et al. Mutations for Gaucher disease confer high susceptibility to Parkinson disease. Arch. Neurol. 2009;66:571–576. doi: 10.1001/archneurol.2009.72. [DOI] [PubMed] [Google Scholar]
- Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR, III, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin S, Schapira AH. Pathogenic mechanisms of neurodegeneration in Parkinson disease. Neurol. Clin. 2015;33:1–17. doi: 10.1016/j.ncl.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, Cooper A, Garner B, Halliday GM. Reduced glucocerebrosidase is associated with increased alpha-synuclein in sporadic Parkinson’s disease. Brain. 2014;137:834–848. doi: 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 2013;70:727–735. doi: 10.1001/jamaneurol.2013.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Schapira AH. Therapeutic prospects for Parkinson disease. Ann. Neurol. 2013;74:337–347. doi: 10.1002/ana.24011. [DOI] [PubMed] [Google Scholar]
- Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S, Waddington SN, Schapira AH, Duchen MR. Mitochondria and quality control defects in a mouse model of Gaucher disease—links to Parkinson’s disease. Cell Metab. 2013;17:941–953. doi: 10.1016/j.cmet.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker LM, et al. Induced pluripotent stem cell model recapitulates pathologic hallmarks of Gaucher disease. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18054–18059. doi: 10.1073/pnas.1207889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen L, Neumann J, O’Sullivan SS, Holton JL, Revesz T, Hardy J, Lees AJ. Glucocerebrosidase mutations do not cause increased Lewy body pathology in Parkinson’s disease. Mol. Genet. Metab. 2011;103:410–412. doi: 10.1016/j.ymgme.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Rocha EM, Smith GA, Park E, Cao H, Brown E, Hallett P, Isacson O. Progressive decline of glucocerebrosidase in aging and Parkinson’s disease. Ann. Clin. Transl. Neurol. 2015 doi: 10.1002/acn3.177. http://dx.doi.org/10.1002/acn3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum. Mol. Genet. 2005;14:2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- Sardi SP, et al. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi SP, et al. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3537–3542. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH. Calcium dysregulation in Parkinson’s disease. Brain. 2013;136:2015–2016. doi: 10.1093/brain/awt180. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Gegg ME. Glucocerebrosidase in the pathogenesis and treatment of Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3214–3215. doi: 10.1073/pnas.1300822110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov. Disord. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Olanow CW, Greenamyre JT, Bezard E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: future therapeutic perspectives. Lancet. 2014;384:545–555. doi: 10.1016/S0140-6736(14)61010-2. [DOI] [PubMed] [Google Scholar]
- Schondorf DC, et al. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat. Commun. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- Segarane B, et al. Glucocerebrosidase mutations in 108 neuropathologically confirmed cases of multiple system atrophy. Neurology. 2009;72:1185–1186. doi: 10.1212/01.wnl.0000345356.40399.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srulijes K, et al. No association of GBA mutations and multiple system atrophy. Eur. J. Neurol. 2013;20:e61–e62. doi: 10.1111/ene.12086. [DOI] [PubMed] [Google Scholar]
- Tayebi N, Callahan M, Madike V, Stubblefield BK, Orvisky E, Krasnewich D, Fillano JJ, Sidransky E. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol. Genet. Metab. 2001;73:313–321. doi: 10.1006/mgme.2001.3201. [DOI] [PubMed] [Google Scholar]
- Thayanidhi N, Helm JR, Nycz DC, Bentley M, Liang Y, Hay JC. Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs. Mol. Biol. Cell. 2010;21:1850–1863. doi: 10.1091/mbc.E09-09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris GK. Lysosome-dependent pathways as a unifying theme in Parkinson’s disease. Mov. Disord. 2012;27:1364–1369. doi: 10.1002/mds.25136. [DOI] [PubMed] [Google Scholar]
- Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T, Duran R, Mencacci NE, Sawcer SJ, Barker RA. Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain. 2013;136:392–399. doi: 10.1093/brain/aws318. [DOI] [PubMed] [Google Scholar]
- Wong K, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol. Genet. Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Yap TL, Gruschus JM, Velayati A, Westbroek W, Goldin E, Moaven N, Sidransky E, Lee JC. Alpha-synuclein interacts with Glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. J. Biol. Chem. 2011;286:28080–28088. doi: 10.1074/jbc.M111.237859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TL, Velayati A, Sidransky E, Lee JC. Membrane-bound alpha-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Mol. Genet. Metab. 2013;108:56–64. doi: 10.1016/j.ymgme.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SG, Eblan MJ, Gutti U, Hruska KS, Stubblefield BK, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in Chinese subjects from Taiwan with sporadic Parkinson disease. Mol. Genet. Metab. 2007;91:195–200. doi: 10.1016/j.ymgme.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zokaei N, et al. Visual short-term memory deficits associated with GBA mutation and Parkinson’s disease. Brain. 2014;137:2303–2311. doi: 10.1093/brain/awu143. [DOI] [PMC free article] [PubMed] [Google Scholar]