Abstract
Retinal degenerations are a group of clinically and genetically heterogeneous disorders characterised by progressive loss of vision due to neurodegeneration. The retina is a highly specialised tissue with a unique architecture and maintaining homeostasis in all the different retinal cell types is crucial for healthy vision. The retina can be exposed to a variety of environmental insults and stress, including light-induced damage, oxidative stress and inherited mutations that can lead to protein misfolding. Within retinal cells there are different mechanisms to cope with disturbances in proteostasis, such as the heat shock response, the unfolded protein response and autophagy. In this review, we discuss the multiple responses of the retina to different types of stress involved in retinal degenerations, such as retinitis pigmentosa, age-related macular degeneration and glaucoma. Understanding the mechanisms that maintain and re-establish proteostasis in the retina is important for developing new therapeutic approaches to fight blindness.
Introduction
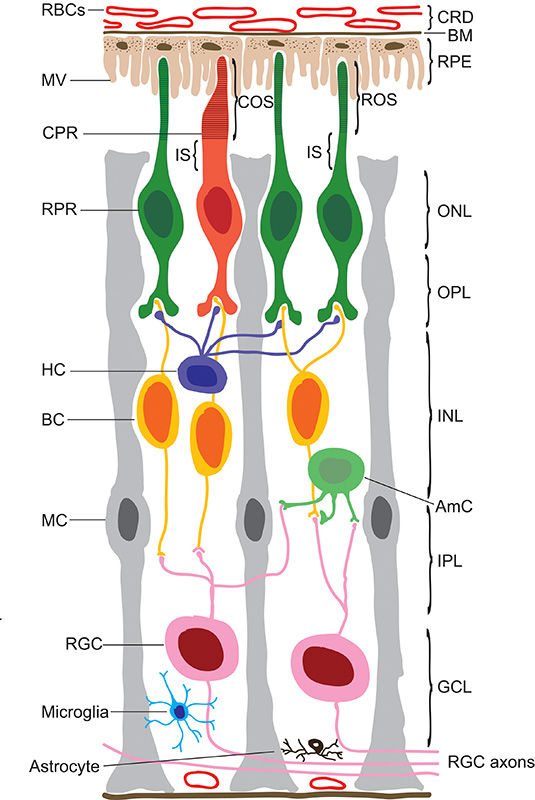
The vertebrate eye is one of the most sophisticated organs in biology and initiates our most precious sense, vision. At the back of the eye, the retina captures light and transmits electrical impulses through the optic nerve to the visual cortex in the brain. The mammalian retina is composed of eight different layers that contain a specialised network of approximately 55 neuronal types which process visual information (Figure 1) [1]. The main light-detecting cells in the retina are the photoreceptors, rods and cones. Rods are approximately 20 times more numerous than cones and are responsible for detecting low levels of light. Cones detect much higher levels of light and are responsible for colour and daytime vision. The outer nuclear layer (ONL) contains the photoreceptor cell bodies, and the photoreceptor visual pigments, rod opsin for rods and cone opsin for cones, are tightly packed into flattened membrane disks in the outer segments (OS). The inner segment (IS) of the photoreceptor contains the main biosynthetic machinery such as the endoplasmic reticulum (ER) and is rich in mitochondria [2]. The biogenesis, post-translational modifications and quality control of many OS proteins, including opsin, take place in the ER. The photoreceptor disks are continually replenished, which leads to a high turnover of proteins, and places a strain on the ER and its quality control machinery. Microvilli from the retinal pigment epithelium (RPE) cells contact the photoreceptor OS tips and phagocytose the shed material, prior to lysosomal degradation within the RPE. The RPE acts as a support for the retina, transporting nutrients and water and is important in for the recycling of 11-cis-retinal, known as the visual cycle [3]. The outer plexiform layer (OPL) consists of the processes and synaptic terminals of photoreceptors, where they contact the horizontal cell and bipolar cell dendrites. The inner nuclear layer (INL) contains horizontal, bipolar and amacrine cell bodies. Horizontal cells integrate the signal from the rods and cones by providing an inhibitory feedback to regulate photoreceptor function [4]. Bipolar cells receive the signal from photoreceptors or horizontal cells and contact the retinal ganglion cells (RGCs) [5]. The inner plexiform layer (IPL) is composed of the synapses of bipolar and RGCs and the ganglion cell layer (GCL) contains the nuclei of RGCs and their axons that form the output from the retina to the brain through the optic nerve. In addition to the neuronal cells in the retina, there are retinal glia cells; the Müller cells, microglia and astrocytes. Müller glia surround the neuronal cell bodies within the retina and are crucial for neuronal health and maintenance [6]. Due to this complex architecture and high metabolic demand, the retina needs constant maintenance to ensure the proper function of all the cellular components. During evolution retinal cells have developed adaptive responses to a variety of insults, which cooperate to restore cell homeostasis, and increase the resistance of the tissue to further damage. Cell stress can arise in many forms in the retina causing the death of several different neuronal types and leading to blindness. This review will discuss the molecular and cellular mechanisms that are present in the retina to deal with cell stress, in particular protein misfolding problems such as those caused by mutations in rhodopsin.
Figure 1. Structure of the mammalian retina.
Schematic cross section representing the different cell types and layers of the retina. The choroid (CRD) containing red blood cells (RBCs) is found at the very back of the eye and is separated from the retinal pigmented epithelia (RPE) by the Bruch’s membrane (BM). The RPE extends microvilli (MV) that facilitate the interaction between the RPE and photoreceptors (PRs). Cone (CPR) and rod (RPR) photoreceptors contain the cone and rod outer and inner segments (COS, ROS and IS respectively), the outer nuclear layer (ONL) and outer plexiform layer (OPL), which contains the synapses of the photoreceptors, horizontal cells (HCs) and bipolar cells (BCs). The inner nuclear layer (INL) contains the cell bodies of the horizontal, bipolar and amacrine cells (AmCs). The inner plexiform layer (IPL) is formed of the connections between bipolar and amacrine cells to the retinal ganglion cells (RGCs), in the ganglion cell layer (GCL).
Photoreceptor degeneration and retinitis pigmentosa
Photoreceptors are particularly susceptible to cellular stress and loss of photoreceptors due to degeneration is a major cause of blindness. Photoreceptor degenerations are possibly the most heterogeneous inherited disorder known in man, with over 140 different causative genes identified [7]. These inherited disorders can affect either rods or cones primarily, as in retinitis pigmentosa (RP) or cone dystrophy respectively, or both simultaneously, as in Leber congenital amaurosis (LCA). Electroretinogram (ERG) measurements can record the electrical response of the retina, and the measurements can be separated into rod (scotopic) or cone (photopic) responses. In this way ERGs can be used to identify the primary cell type affected in retinal degenerations. For example, RP classically involves the loss of the scotopic response, whereas in LCA, which is an early-onset, extreme retinal degeneration, both the photopic and scotopic responses are lost.
RP is one of the most common forms of inherited retinal degeneration comprising a group of retinal disorders that typically involve progressive degeneration of rod photoreceptor cells followed by secondary cone photoreceptor death. The first symptom is night blindness, due to the dysfunction of rod cells, which later advances to the loss of peripheral vision (tunnel vision). RP progresses towards the macula at the centre of the retina with the death of cone cells resulting in loss of central vision and partial or complete blindness by middle age [8]. Clinical examinations of the retinae of RP patients show abnormal fundi with pigmented bone-spicule deposits, attenuated retinal vessels and pallor of the optic nerve. The prevalence of RP is 1 in 4000 people worldwide, resulting in over 1.5 million visually impaired patients. The inheritance patterns of RP are varied; 15-25% are autosomal dominant (adRP), 5-25% are autosomal recessive (arRP), 5-15% are X-linked (XLRP), while the remaining 35-50% cannot be easily classified genetically [9]. To date, 27 adRP, 36 arRP and 6 XLRP genetic loci have been identified, often with overlap [10]. RP can also be syndromic, where other organs are also affected, such in Usher’s syndrome which is associated with hearing impairment and RP symptoms [11].
Rhodopsin RP
Rhodopsin, the light-absorbing photopigment of rod cells, is the archetypal G-protein coupled receptor (GPCR) and therefore one of the best characterised. Rhodopsin is composed of the 348 amino acid apoprotein rod opsin, which is covalently linked with the chromophore 11-cis-retinal, an analogue of vitamin A. Rhodopsin undergoes multiple post-translational modifications such as N-linked glycosylation, disulphide bond formation, acetylation, palmitoylation, phosphorylation and ubiquitylation. Each of these post-translational modifications is either essential for the maintenance of rod OS structure or the fine-tuning of rhodopsin function [12]. Rod opsin is synthesised on the rough ER membranes before transit to the Golgi and traffic to the OS disks in rod cells or to the plasma membrane (PM) in heterologous expression systems.
Rhodopsin was the first RP gene identified [13]. To date, over 200 rhodopsin point mutations have been described which account for approximately 25% of all adRP cases. Six classes of rhodopsin mutants have been proposed, based on their cellular and biochemical characteristics [14]. Class I mutants fold normally but are not trafficked to the OS correctly, while class II mutants are misfolded and retained in the ER. The remaining mutations are classified according to their effects on endocytosis, opsin stability, increased transducin activation and constitutive activation [14]. The class II P23H mutation is the most common mutation found in North America and is the best studied. The role of the proteostasis machinery in rhodopsin biogenesis and quality control of mutant rhodopsin will be discussed in detail below.
Light-induced damage
The retina is a transparent tissue, since visible light needs to penetrate the inner retina, where the RGCs and bipolar cells reside, to reach the outer retina and the photoreceptors. While an appropriate amount of visible light is needed to initiate phototransduction, too much light entering the retina can also induce damage; therefore, the eye has developed natural mechanisms to restrict the input of light (iris and RPE pigmentation, pupil restriction). Ultraviolet (UV) light (both UV-A and UV-B) together with visible light of ~500 nm wavelength (blue light) have been linked to retinal degeneration. Acute bright light exposure can damage both the RPE and photoreceptors, even in control animals. Light-induced damage can be multifactorial and can arise from environmental insults, genetic diseases as well as aging, since increased light levels throughout life can exacerbate age-related damage [15]. Retinal topography also plays an important role in light tolerance, with the inferior hemisphere being more sensitive in light-induced damage than the superior [16]. There is growing evidence implicating the release of all-trans retinal from light-activated rhodopsin as a mediator of acute photodamage to the retina, along with the excessive build-up of (photo) toxic components in the RPE following disk phagocytosis [17;18]. All-trans retinal is a potent photosensitizer and upon photoexcitation by light, when oxygen is present, can generate reactive oxygen species such as singlet oxygen, superoxide and hydrogen peroxide, which are highly toxic leading to oxidative stress and consequently to lipid, protein and DNA damage. Light-induced damage can be more pronounced when genetic defects are associated with decreased pigmentation, increased formation or slow removal of retinoids, in efficient RPE phagocytosis, defects in the phototransduction machinery or with problems of OS stability and turnover. Studies in animal models with some of these genetic defects have shown that dark-rearing can slow or prevent retinal degeneration, suggesting that light management should be considered as part of the treatment for some patients with retinal degeneration [19].
Retinal ganglion cell related retinal degeneration
RGCs are responsible for transmitting visual information from the retina to the visual cortex in the brain. Due to the anatomic distance between the retina and the brain, the long axons of RGCs cells must transport metabolites and organelles via axonal transport, a process with a high energy demand. Mitochondria, which are more abundant in the unmyelinated parts of the axons, generate adenosine triphosphate (ATP) that is needed to propagate the action potential to the brain. Therefore, disturbances of axonal transport or mitochondrial function can have severe consequences for RGC function and viability [20;21]. Glaucoma is characterised by the progressive degeneration of RGCs and the optic nerve head leading to constricted visual fields, possibly related to increase intraocular pressure (IOP) causing pressure on the axons in the lamina cribrosa [22]. While the exact mechanisms of RGC death in glaucoma remain relatively unknown, there is a clear link between mitochondrial dysfunction and selective RGC death, since mitochondrial optic neuropathies such as Leber hereditary optic neuropathy (LHON) and autosomal dominant optic atrophy (adOA) primarily affect RGCs [23-25].
RPE-related retinal degeneration
The RPE is a monolayer of supporting epithelial cells whose main functions are maintaining the posterior blood retinal barrier (BRB), recycling of visual pigment, phagocytosis of photoreceptor OS and neurotrophic factor secretion. Because of the intimate relationship between photoreceptors and RPE cells, RPE dysfunction can have a deleterious effect on photoreceptor viability. Vision loss can occur when RPE cell death or malfunction increases photoreceptor vulnerability [3]. For example, in models of RP where RPE-specific genes are mutated, photoreceptor degeneration can occur either by accumulation of toxic by-products from the visual cycle, the loss of the essential factors, such as 11-cis-retinal, or by the loss of mechanical support that the RPE confers to photoreceptors [17]. Furthermore, the function and survival of the RPE is compromised in age-related macular degeneration (AMD), the leading cause of blindness affecting people over 65 years old. AMD results in loss of central vision due to degeneration of the macula in the central region of the retina and manifests in two forms, wet and dry. While the wet form is associated with neovascularisation, the dry form is characterised by the appearance of extracellular deposits called drusen, which accumulate between the RPE and the Bruch’s membrane leading to RPE cell death. Although it remains uncertain if drusen are the cause or consequence of RPE dysfunction, their presence blocks the diffusion of nutrients and regulatory substances, which are vital for RPE and photoreceptor survival. The accumulation of lipofuscin in the RPE, an auto-fluorescent pigment, which is implicated in several retinal dystrophies, is thought to be a risk factor for RPE degeneration in aging and in AMD [18].
Vascular defects
The retina, as part of the central nervous system (CNS), is immune-privileged and protected by the BRB, which stringently controls the exchange of molecules between the circulation and the retina [26]. Furthermore, parts of the retina, such as the outer retina that contain the RPE and photoreceptors, need to remain avascular in order to allow efficient light transmission and high acuity. Therefore, the nutritional and oxygen needs of the outer retina are met by the choriocapillaris of the adjacent choroid (choroid circulation), while in the inner retina these are met by the retinal vasculature. The requirement of high oxygen supply in the retina and the retinal vasculature can make the retina vulnerable to vascular diseases. Retinal neovascularisation is a major cause of vision loss where new blood vessels penetrate the neuroretina from the choroid or retinal vessels branch out inside the neuroretina or fuse with the choroidal cells (anastomosis), destroying underlying neuronal tissue. Neovascularisation in response to injury and disease can occur when there is an oxygen imbalance or BRB breakdown and/or deregulation of pro-angiogenic factors such as the vascular endothelial growth factor (VEGF) [27]. For example, at the late stages of wet AMD, the posterior BRB has already been compromised and choroidal vessels invade the RPE and grow into the retina. In diabetic retinopathy, hyperglycemia initiates microvascular occlusion resulting in capillary nonperfusion and hypoxia which subsequently triggers retinal neovascularisation [28].
The role of the proteostasis network in retinal degeneration
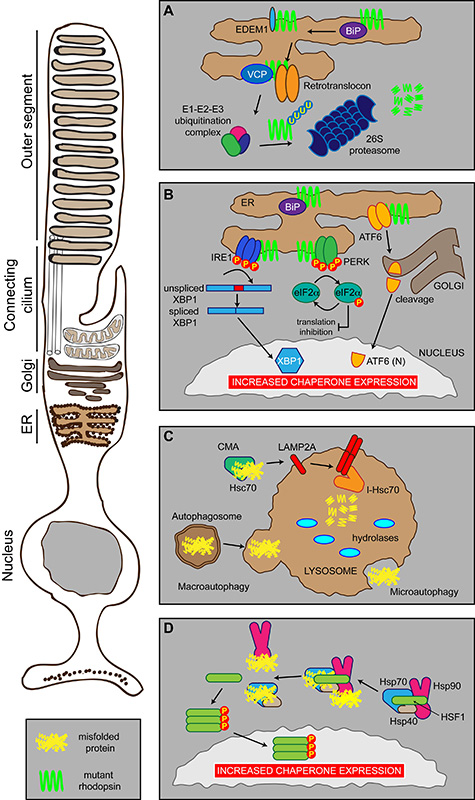
Protein folding is a complicated but imperfect process, for example as much as 30% of newly synthesised proteins are degraded before they attain their mature functional conformation [29]. To cope with misfolded proteins cells have evolved many mechanisms, including the heat shock response (HSR), the ubiquitin-proteasome system (UPS), the unfolded protein response (UPR) and ER-associated degradation (ERAD). Collectively these are referred to as the protein homeostasis (or proteostasis) network (Figure 2). The function of the proteostasis network is to generate and maintain correctly folded proteins, and to remove, sequester and destroy misfolded proteins which affect normal function [30]. Within the cell, a set of highly evolutionarily-conserved proteins known as molecular chaperones promote and maintain proper protein folding [31]. Molecular chaperones have multiple functions related to proteostasis; these include guidance of translocated proteins across membranes, disassembling oligomeric structures, facilitating degradation of damaged or misfolded proteins, vesicular trafficking and signal transduction [32-34]. Molecular chaperone proteins prevent misfolding by binding and shielding hydrophobic regions of the nascent polypeptide, which are normally buried in mature folded proteins [31;32]. Chaperone activity is frequently governed by cycles of client protein binding and release, coupled to chaperone ATP hydrolysis and nucleotide exchange. Several co-chaperone proteins regulate this process, for example DnaJ (Hsp40) co-chaperones stimulate ATP hydrolysis by Hsp70 proteins. Many molecular chaperones are also known as the heat shock proteins (Hsps), having been originally discovered as upregulated factors during the HSR. The HSR is mediated by heat shock factor transcription factors, such as heat shock factor-1 (HSF1). Under normal conditions, HSF1 is bound by the molecular chaperones Hsp70 and Hsp90 (Figure 2). In the presence of cellular stress, the molecular chaperones are recruited to misfolded proteins, thus releasing HSF1. Activation via phosphorylation and trimerisation results in HSF1 trafficking to the nucleus where it can further upregulate molecular chaperone expression [35].
Figure 2. Photoreceptor proteostasis networks.
Schematic showing the proteostasis networks in a rod photoreceptor that deal with misfolded proteins, such as P23H rod opsin (mutant rhodopsin in green), or cell stress. (A) ERAD. Misfolded proteins are detected by the ER quality control machinery (including BiP, EDEM1, VCP) and shuttled to the cytoplasm by the retrotranslocon where they are ubiquitylated before being degraded by the proteasome. (B) The UPR. Misfolded proteins, such as mutant rod opsin, in the ER are recognised by three sensors: IRE1, PERK and ATF6 that inhibit protein synthesis and stimulate the production of chaperones and the ERAD machinery (see text for details). (C) Autophagy. Misfolded proteins can be degraded by three modes of autophagy: macroautophagy, microautophagy or CMA. (D) The HSR. Molecular chaperones Hsp70, Hsp40 and Hsp90 can exist in a complex in the cytosol with their transcription factor HSF1. Upon binding misfolded proteins, Hsp70, Hsp40 and Hsp90 dissociate from HSF1, which can trimerise and activate via phosphorylation. This results in traffic to the nucleus leading to increased chaperone expression.
The cell keeps a tight regulation of the protein folding capacity in the ER by the activation of intracellular signal transduction pathways that together constitute the UPR (Figure 2). The accumulation of unfolded proteins in the lumen of the ER initiates the UPR, which responds to alleviate stress by upregulating the protein folding and degradation capacity and inhibiting protein synthesis, eventually restoring the balance. At least three distinct branches of the UPR regulate the expression of numerous genes that maintain homeostasis in the ER or induce apoptosis if ER stress remains unchanged. Three ER-resident transmembrane proteins have been identified: the kinase and endoribonuclease inositol requiring enzyme-1 (IRE1), the double-stranded RNA-activated protein kinase (PKR)-like ER kinase (PERK) and the basic leucine-zipper activating transcription factor 6 (ATF6). The activation of the three branches depends on BiP, an Hsp70 molecular chaperone located in the lumen of the ER that binds newly synthesized proteins as they are translocated into the ER and maintains them in a state competent for subsequent folding and oligomerization. Luminal domains of IRE1, ATF6 and PERK interact with BiP in unstressed cells, whereas perturbation of protein folding titrates BiP away from association with these sensors, freeing them for activation and induction of the UPR [36-38].
Under stress conditions PERK phosphorylates the eukaryotic translation initiation factor 2 subunit a (eIF2a), reducing protein synthesis to prevent further increase of nascent proteins. ATF6 activation leads to the expression of target genes encoding chaperones [39]. IRE1 activation initiates the nonconventional splicing of XBP-1 (X-box DNA binding protein), a UPR specific b-ZIP transcription factor that binds to ER-stress responsive genes (ERSE). Many of these genes are part of the ERAD mechanism, thereby stimulating efficient protein degradation [40].
Degradation of misfolded proteins by ERAD can be divided into three steps: recognition of misfolded proteins in the ER, retrotranslocation into the cytosol and ubiquitin dependent-degradation via the UPS (Figure 2) [41]. For glycoproteins, recognition is achieved via UDP-glucose glycoprotein:glucosyltransferase (UGGT) which detects changes in the glycan structure, such as immature glycans and allows proteins to switch from the calnexin (Cnx)/calreticulin (Crt) cycle to degradation via ER degradation enhancing mannosidase α-like 1 (EDEM1) [42]. Since the UPS is located in the cytosol, ERAD substrates have to pass through a protein channel (retrotranslocon channel) found in the ER membrane for transport to the cytoplasm. While the exact proteins that form the translocon remain unknown, the protein Sec61 complex is a possible candidate since it is the major component of the translocation channel that imports polypeptides into the ER [43]. Another possibility is the protein Derlin-1 since studies have shown that it interacts with several ERAD substrates and that its depletion can induce the UPR [44;45]. Finally, as nascent polypeptides enter the ER in an unfolded conformation, this would suggest that dislocation of ERAD substrates requires unfolding, including reduction of disulphide bonds [46]. While in the retrotranslocon, the protein needs a driving force in order to be pulled through the channel which is provided by the Cdc48p/p97/VCP ATPase using the energy from ATP hydrolysis for substrate extraction [47]. Once the substrate reaches the cytoplasmic face of the ER it needs to be targeted for degradation by the addition of four or more molecules of ubiquitin by ubiquitin ligases. The ubiquitylated protein is then degraded by the proteasome.
An alternative degradation pathway is autophagy, which involves the disposal of damaged proteins or entire organelles by the hydrolases present in high concentrations in lysosomes (Figure 2). Three pathways of autophagy exist, macroautophagy, microautophagy and chaperone-mediated autophagy (CMA) [48]. Macroautophagy involves the sequestration of substrates or organelles with a double-membrane structure called the autophagosome that is transported through the cytoplasm to the lysosome. Fusion of the two membranes allows the autophagosome to enter the lysosome [48]. Microautophagy involves the direct sequestration of substrate material via the invagination of their membranes. CMA involves the selective recognition of cytosolic proteins by the Hsc70 complex and their targeting to the lysosome for degradation [49].
The disruption of proteostasis has been implicated in several retinal diseases, such as retinitis pigmentosa, age-related macular degenerations and glaucoma.
Proteostasis mechanisms and RP
The majority of rod opsin mutations, including P23H, lead to the misfolding of rod opsin and to its accumulation within the cell, with detrimental effects on photoreceptor function and viability. Therefore, the role of molecular chaperones in rod opsin biogenesis, quality control and aggregation are potentially important for disease.
Studies of Drosophila rhodopsin1 (Rh1) identified NinaA, a photoreceptor specific ER integral membrane glycoprotein, which acts as a specialised molecular chaperone and is required for Rh1 maturation [50;51]. Furthermore, Cnx, an ER resident lectin chaperone, was also required for Rh1 maturation as Cnx mutations led to severe defects in Rh1 expression and disturbance of calcium regulation [52]. More recently, XPORT (exit protein of rhodopsin and TRP) was identified as a molecular chaperone that is important for the biosynthesis and transport of the transient receptor potential (TRP) channel and Rh1 [53]. While mammalian orthologues of NinaA and XPORT remain to be identified, the cellular factors that facilitate the folding, quality control and degradation of mammalian rod opsin have started to be identified.
In mammalian cells, overexpression of Cnx in combination with the pharmacological chaperone 11-cis-retinal partially enhanced the folding of P23H rod opsin and partially inhibited its degradation [54]. However, Cnx is not required for the maturation of control wild-type (WT) rod opsin and in cells that lack Cnx, the degradation of P23H rod opsin was unaffected [55]. Additionally, Cnx deficiency in mice did not affect rod opsin expression or traffic [56]. Therefore, unlike the situation for fly Rh1, Cnx is not essential for mammalian rod opsin folding or quality control.
BiP is important for rod opsin biogenesis as its depletion or dysregulation results in aggregation of WT rod opsin in the ER. Furthermore, BiP overexpression improved P23H rod opsin ER mobility, even though it did not improve mutant opsin traffic. These findings suggest that chaperone manipulation can be used as a tool to reduce rod opsin aggregation and highlight a role for BiP in maintaining rod opsin solubility in the ER [57]. Furthermore, EDEM1 can bind P23H rod opsin and promote its degradation (Figure 2A). Importantly, EDEM1 overexpression also enhanced the folding and traffic of a fraction of P23H rod opsin, and EDEM1 was present in a complex with WT rod opsin in the ER of photoreceptor cells suggesting that is important for both WT and mutant rod opsin biogenesis and quality control [58]. Consistent with these findings, homologues of human EDEM2 and EDEM3 reduced mutant Rh1 levels and suppressed degeneration in flies [59].
P23H rod opsin is a substrate of the ERAD effector valosin-containing protein (VCP) [60]. Similarly, studies in Drosophila showed that Ter94, the VCP homolog, was essential for the degradation of mutant Rh1P37H, the fly equivalent of P23H. However, inhibition of VCP activity suppressed retinal degeneration, suggesting that ERAD of Rh1P37Hmight contribute to cell death [61].
If misfolded opsin escapes degradation, it can form inclusions of aggregated protein. Like many other intracellular inclusions of aggregated protein, P23H rod opsin inclusions can recruit Hsp70 [62]. However, Hsp70 overexpression did not reduce P23H rod opsin inclusion incidence [63]. The neuronal DnaJ (Hsp40) protein HSJ1b (DNAJB2b) is a molecular chaperone that is targeted to the cytoplasmic face of the ER and can also associate with mutant rod opsin inclusions. In photoreceptors, HSJ1b is enriched at sites of rod opsin synthesis and may be part of a cytoplasmic chaperone network that participates in rod opsin folding or quality control of the cytoplasmic domains as HSJ1b overexpression can lead to the retention in the ER of WT rod opsin [63].
Other components of the proteostasis machinery are likely to be involved in rod opsin quality control and may be upregulated by cells to deal with rod opsin misfolding. For example, chemical-genetic activation of IRE1 in mammalian cells promoted the degradation of mutant misfolded class II rhodopsin [64]. Furthermore, activation of ATF6 also resulted in reduced levels of class II rod opsin mutants, whereas specific PERK activation led to global reductions in protein synthesis. Surprisingly, ATF6 activation also led to reductions in the class I rod opsin mutant S334ter, which is more often associated with problems of traffic to the OS, not protein folding [65].
Several studies have examined the potential involvement of ER stress in retinal degeneration by examining UPR activation (Figure 2B). For example, alterations in UPR related genes like BiP and C/EBP-homologous protein (CHOP) potentially corresponding to a persistent ER stress state were observed in P23H expressing transgenic rats [66]. Furthermore, overexpression of BiP alleviated ER stress and enhanced photoreceptor survival in the P23H rat model, suggesting that ER stress might be involved in adRP progression [67]. Moreover, ER stress was observed in the retina of hT17M Rho mice [68]. Interestingly, more recent findings raise the possibility that ER stress may be involved in retinal degeneration arising from causes besides protein misfolding. For example, ER stress markers and ERAD associated genes were upregulated in the S334ter rod opsin class I mutant transgenic rat model [69] and classic UPR markers were upregulated in retina dystrophies with completely different aetiologies distinct from mutations in rhodopsin [70]. Therefore, UPR induction might be a common pathway that is activated in retinal degeneration in different conditions, either as part of the degenerative process or as a cellular response to photoreceptor cell death.
Glaucoma
The most common form of glaucoma is primary open angle glaucoma (POAG) and is frequently associated with elevated intraocular pressure [71]. Three candidate genes, myocilin [72], optineurin [73] and WD repeat domain 36 [74] have been identified for familial glaucoma. Myocilin is normally secreted into the aqueous humour of the eye and may have a role in the trabecular meshwork, a tissue in the anterior segment of the eye that controls aqueous humour outflow and helps regulate intraocular pressure [75]. Mutations in myocilin lead to the accumulation of mutant myocilin in the ER of the trabecular meshwork cells and activate ER stress-induced cell death [76]. The ER protein quality control program fails to degrade mutant myocilin because of an anomalous interaction with Grp94, which leads to pathogenic consequences. It is not understood why myocilin is not cleared by ERAD but it has been shown that manipulation of ER chaperones could have a therapeutic effect, promoting the clearance of aggregated proteins through the autophagic route [77]. Optineurin interacts with Rab8, myosin VI and transferrin receptor (TfR), suggesting a role in protein traffic. In normal homeostatic situations the turnover of endogenous optineurin involves mainly the ubiquitin-proteasome pathway. When optineurin is upregulated or mutated, the UPS function is compromised, and autophagy is activated [78].
In experimental glaucoma models, activation of the retinal UPS has been observed, especially in ganglion cells where ubiquitin accumulates [79]. Hsp70 is upregulated in a rat model of glaucoma, suggesting activation of the HSR and a potential response to RGC stress [80;81]. After retinal insult (N-methyl-d-aspartate (NMDA)-induced cell death) in rats, levels of HSF1 and Hsp70 increase in RGCs suggesting a stress response [82]. In zebrafish, which can regenerate their RGCs, Hsp70 and HSF1 were both upregulated soon after optic nerve transaction [83]. Furthermore, treatment with heat shock protein-inducer geranylgeranylacetone (GGA) protected against RGC death in a glaucoma model [84].
Similarly, the small heat shock protein Hsp27 is highly expressed in RGCs [85] and throughout the retina, particularly in the photoreceptors and RPE cells [86]. Hsp27 has been shown to be specifically upregulated in RGCs in response to retinal ischemia [87]. Furthermore, Hsp27 expression was upregulated in RGCs in rats after optic nerve injury [88] and similarly in the increased intraocular pressure DBA/J2 mouse model of glaucoma [89]. In human eyes with POAG or normal pressure glaucoma Hsp27 levels were elevated in RGCs compared to normal age-matched eyes [90].
AMD
Oxidative stress has been implicated as a factor in the pathogenesis of AMD [91]. Other studies suggest that a decline in autophagy and lysosomal activity is associated with retinal aging and AMD [92;93]. The role of molecular chaperones in RPE and AMD is not well understood. Hsc70 is associated with lysosomes in RPE cells, suggesting a role for chaperone-mediated autophagy in these cells [94]. However, a proteomic analysis of RPE-derived mitochondria from AMD patients showed a decrease in the mitochondrial Hsp70 (mtHsp70), suggesting a decrease in mitochondrial protein import. This could potentially lead to dysregulation of mitochondrial function [95]. An oxidative stress model in ARPE19 human RPE cells showed increased Hsp27 and Hsp70 expression after stress. Furthermore, an increase in heat shock protein expression correlated with cell differentiation and enhanced resistance to oxidative stress [96]. The small Hsps, alpha-crystallins, are significantly upregulated in the RPE in response to heat shock and oxidative stress [97;98]. Similarly, alpha crystallin is upregulated in AMD-affected eyes [99]. In a light-induced model of degeneration, alpha-crystallins were found to be upregulated in the RPE [100]. Recent work has shown that uptake of alpha-crystallin ‘mini-chaperones’ by cultured RPE cells could reduce apoptotic cell death after oxidative stress [101]. The build up of aggregated material in RPE and drusen may play a role in the activation of inflammatory responses, such as the inflammasome, that are central to the risk of developing AMD. The interaction between UPS and lysosomal proteolytic pathway could provide an opportunity to manipulate the activities of these degradation pathways for AMD prevention or treatment.
Genomic Response to Retina Degeneration
Converging evidence from many studies highlight the central role of the Müller cells in retina degeneration. Müller cells undergo reactive gliosis in all forms of retinal injury and disease, which may contribute to neurodegeneration and impede the regenerative processes in retinal tissue by the formation of glial scars [102]. A microarray-based study of the retina transcriptome following different types of photoreceptor insults (genetically determined, light induced and induced retina detachment) showed a characteristic genomic response, with upregulation of a small number of genes that overlap in the different models [103]. In particular, among the induced transcripts, upregulation of endothelin (Edn2) and fibroblast growth factor 2 (Fgf2) was found in all the models of photoreceptor degeneration tested. The concomitant induction of endothelin receptor B (ENDRB) in Müller cells suggests the existence of a cross talk between photoreceptors and glia, where damaged photoreceptors signal to Müller cells, which in turn produce Fgf2, a potent neurotrophic factor [103]. An analogous mechanism driven by Edn2/ENDRB upregulation in the CNS following mechanical trauma has been observed in various rodent models [104]. Edn2 signalling is not necessarily protective. In fact, early stage expression changes in Edn2 were found also in a model of glaucoma at a stage where the eyes were indistinguishable from control eyes based on optic nerve damage. Compromising the Edn2 pathway at this stage was found to be significantly protective from glaucoma in DBA/2J mice [105].
Another active player in the retina response to damage, linked to the Edn2 pathway, is leukaemia inhibitory factor (LIF). This molecule was strongly induced in the retina of P23H transgenic models [106]. Photoreceptor injury induced LIF expression in a subset of Müller glia cells in the INL of the retina. Ablation of LIF in this model prevented upregulation of genes like Fgf2 and Edn2 in the photoreceptor cells. Collectively, the data support the role of neuronal survival factors, like LIF, initiating an intrinsic protective mechanism that includes Edn2 signalling to support photoreceptor cell survival.
Therapeutic approaches and concluding remarks
At present there are no effective treatments for most retinal degenerations. The need for an effective treatment for retinal diseases, coupled to the development of appropriate animal models has stimulated a variety of potential therapeutic directions. These include preventive strategies that aim to counteract the underlying disease mechanisms, either by manipulating cellular pathways or genetic modification by gene silencing and/or gene replacement; strategies that do not deal with the cause of the disease, such as cell death prevention with neurotrophic factors; or cell replacement by transplantation and artificial vision. This section will discuss the strategies that target the cell stress machinery in order to alleviate the cell stress response in retinal degenerations.
Several compounds that can alter either the cellular milieu or target directly the protein structure have been shown to protect cells from the toxic effects of aberrant misfolded proteins, such as those associated with retinal degenerations. These compounds include pharmacological chaperones, kosmotropes, molecular chaperone inducers and those that manipulate autophagic pathways. For instance, pharmacological chaperones often share structural similarities with natural receptor ligands (agonists, antagonists, inverse agonists) and can stabilise native states. They can penetrate the cell, diffuse to the ER and bind to newly synthesised proteins, shifting the folding equilibrium in favour of native or folded conformation. 11-cis retinal and 9-cis retinal can act as pharmacological chaperones for rod opsin and improve P23H folding in cells [54;62;107-109].
Kosmotropes, also known as chemical chaperones, are small, low molecular weight, compounds that stabilise proteins and hydrophobic aggregates. A range of chemical chaperones such as trimethylamine N-oxide (TMAO) and 4-phenylbutyric acid (4-PBA) reduced aggregation and cell death associated with P23H rod opsin in cells [108]. In glaucoma, TMAO facilitated the folding and secretion of myocilin in vitro [110], while PBA reduced aggregation and apoptosis associated with myocilin mutation in mice [111] and in cells [112]. When tauroursodeoxcholic acid (TUDCA), a major component of bear bile that can act as a chemical chaperone, was tested in several animal models of retinal degeneration, it prevented apoptosis and preserved the structure and function of photoreceptor cells [113-115]. Interestingly, TUDCA has been found to be protective in other ocular cell types including RGCs and lens epithelial cells which are compromised in cataract [116;117].
Barbe and colleagues (1988) showed that inducing a pre-conditioning hyperthermia in rats could reduce or prevent light-induced photoreceptor cell loss, suggesting that increasing Hsp and molecular chaperone production as a potential treatment for retinal disorders [118]. Indeed, chaperone inducers such as radicicol, geldanamycin, and its analogue 17-allylamino-17-demethoxygeldanamycin (17-AAG) were effective in reducing aggregation and/or cell death in a P23H rod opsin cell model [108]. In another study, administration of 17-AAG, coupled to manipulation of the BRB by claudin-5 siRNA, in an adRP (RP10) mouse model protected photoreceptor cells by inducing Hsp expression and reducing protein aggregation associated with the disease [119]. Additionally, geranylgeranylacetone and canavanine have been found to be protective in a rat glaucoma model and streptozotocin-diabetic retinopathy rat model respectively [120;121]. Furthermore, the progress in gene-replacement strategies using viral-mediated delivery to treat retinal degenerations with known mutations has paved the way for viral-mediated manipulation of molecular chaperones to treat retinal degeneration associated with protein misfolding. For example, viral-mediated overexpression of BiP reduced photoreceptor cell death and improved visual function in a P23H rat model [67].
Another approach to alleviate retinal cell death is to manipulate the mTOR signalling pathway and stimulate autophagy. One such compound, rapamycin, has been shown to enhance the clearance of aggregation-prone proteins and enhance the degradation of P23H rod opsin [108;122]. Administration of rapamycin to a rat model with some AMD-like pathology suppressed retinal degeneration [123], and reduced choroidal and retinal neovascularisation in photocoagulation- and hyperoxia/hypoxia-induced mice [124].
Alternative therapeutic approaches include the deacetylase inhibitor valproic acid, which is currently in moving towards clinical trials but its applicability is questioned due to its side effects, calcium channel blockers, antioxidants and neurotrophic factors which can be used to slow retinal degeneration [54;125-128].
Despite encouraging results from the above treatments, research is still on-going and currently the only recommended treatment for RP is vitamin A supplementation alone or in combination with docosahexaenoic acid to modestly delay photoreceptor degeneration [129]. However, high dose vitamin A could in itself be problematic and this needs to be carefully considered. The advantages of investigating the most accessible part of the CNS have opened up a variety of therapeutic directions for retinal degeneration. A thorough understanding of the molecular mechanisms underlying retinal degenerations as well as identification of all the genetic causes of these disorders will improve the prospect of therapies.
Acknowledgments
Research in the Cheetham lab on cell stress in the retina is supported by The Wellcome Trust, RP Fighting Blindness, the BIG Lottery Fund (BLF) and Fight for Sight.
References
- [1].Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Masland RH. The fundamental plan of the retina. Nat. Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- [3].Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- [4].Herrmann R, Heflin SJ, Hammond T, Lee B, Wang J, Gainetdinov RR, Caron MG, Eggers ED, Frishman LJ, McCall MA, Arshavsky VY. Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron. 2011;72:101–110. doi: 10.1016/j.neuron.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kamermans M, Spekreijse H. The feedback pathway from horizontal cells to cones. A mini review with a look ahead. Vision Res. 1999;39:2449–2468. doi: 10.1016/s0042-6989(99)00043-7. [DOI] [PubMed] [Google Scholar]
- [6].Lindqvist N, Liu Q, Zajadacz J, Franze K, Reichenbach A. Retinal glial (Muller ) cells: sensing and responding to tissue stretch. Invest Ophthalmol. Vis. Sci. 2010;51:1683–1690. doi: 10.1167/iovs.09-4159. [DOI] [PubMed] [Google Scholar]
- [7].Wright AF, Chakarova CF, bd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev.Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- [8].Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- [9].Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch. Ophthalmol. 2007;125:151–158. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Churchill JD, Bowne SJ, Sullivan LS, Lewis RA, Wheaton DK, Birch DG, Branham KE, Heckenlively JR, Daiger SP. Mutations in the X-linked retinitis pigmentosa genes RPGR and RP2 found in 8.5% of families with a provisional diagnosis of autosomal dominant retinitis pigmentosa. Invest Ophthalmol. Vis. Sci. 2013;54:1411–1416. doi: 10.1167/iovs.12-11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Keats BJ, Savas S. Genetic heterogeneity in Usher syndrome. Am. J. Med. Genet. A. 2004;130A:13–16. doi: 10.1002/ajmg.a.30052. [DOI] [PubMed] [Google Scholar]
- [12].Murray AR, Fliesler SJ, Al-Ubaidi MR. Rhodopsin: the functional significance of asn-linked glycosylation and other post-translational modifications. Ophthalmic Genet. 2009;30:109–120. doi: 10.1080/13816810902962405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dryja TP, McGee TL, Hahn LB, Cowley GS, Olsson JE, Reichel E, Sandberg MA, Berson EL. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N. Engl. J. Med. 1990;323:1302–1307. doi: 10.1056/NEJM199011083231903. [DOI] [PubMed] [Google Scholar]
- [14].Mendes HF, van der SJ, Chapple JP, Cheetham ME. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol. Med. 2005;11:177–185. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- [15].Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog. Retin. Eye Res. 2010;29:113–134. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schwartz L, Boelle PY, D’hermies F, Ledanois G, Virmont J. Blue light dose distribution and retinitis pigmentosa visual field defects: an hypothesis. Med. Hypotheses. 2003;60:644–649. doi: 10.1016/s0306-9877(02)00391-2. [DOI] [PubMed] [Google Scholar]
- [17].Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu Y, Fishkin NE, Pande A, Pande J, Sparrow JR. Novel lipofuscin bisretinoids prominent in human retina and in a model of recessive Stargardt disease. J. Biol. Chem. 2009;284:20155–20166. doi: 10.1074/jbc.M109.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Paskowitz DM, LaVail MM, Duncan JL. Light and inherited retinal degeneration. Br. J. Ophthalmol. 2006;90:1060–1066. doi: 10.1136/bjo.2006.097436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog. Retin. Eye Res. 2004;23:53–89. doi: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- [21].Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J. Cell Sci. 2012;125:2095–2104. doi: 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Quigley HA, Hohman RM, Addicks EM, Massof RW, Green WR. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am. J. Ophthalmol. 1983;95:673–691. doi: 10.1016/0002-9394(83)90389-6. [DOI] [PubMed] [Google Scholar]
- [23].Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, starie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- [24].Lee S, Van Bergen NJ, Kong GY, Chrysostomou V, Waugh HS, O’Neill EC, Crowston JG, Trounce IA. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp. Eye Res. 2011;93:204–212. doi: 10.1016/j.exer.2010.07.015. [DOI] [PubMed] [Google Scholar]
- [25].Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- [26].Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog. Retin. Eye Res. 2008;27:622–647. doi: 10.1016/j.preteyeres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- [27].Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- [28].Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr. Diabetes Rev. 2006;2:71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- [29].Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- [30].Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- [31].Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- [32].Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- [33].Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- [34].Outeiro TF, Tetzlaff J. Mechanisms of disease II: cellular protein quality control. Semin. Pediatr. Neurol. 2007;14:15–25. doi: 10.1016/j.spen.2006.11.005. [DOI] [PubMed] [Google Scholar]
- [35].Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb. Perspect. Biol. 2010;2:a004390. doi: 10.1101/cshperspect.a004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu CY, Schroder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 2000;275:24881–24885. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- [37].Liu CY, Xu Z, Kaufman RJ. Structure and intermolecular interactions of the luminal dimerization domain of human IRE1alpha. J. Biol. Chem. 2003;278:17680–17687. doi: 10.1074/jbc.M300418200. [DOI] [PubMed] [Google Scholar]
- [38].Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- [39].Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev. Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- [40].Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Taylor SC, Thibault P, Tessier DC, Bergeron JJ, Thomas DY. Glycopeptide specificity of the secretory protein folding sensor UDP-glucose glycoprotein:glucosyltransferase. EMBO Rep. 2003;4:405–411. doi: 10.1038/sj.embor.embor797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–669. doi: 10.1038/nature06384. [DOI] [PubMed] [Google Scholar]
- [44].Okuda-Shimizu Y, Hendershot LM. Characterization of an ERAD pathway for nonglycosylated BiP substrates, which require Herp. Mol. Cell. 2007;28:544–554. doi: 10.1016/j.molcel.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- [46].Tsai B, Rodighiero C, Lencer WI, Rapoport TA. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell. 2001;104:937–948. doi: 10.1016/s0092-8674(01)00289-6. [DOI] [PubMed] [Google Scholar]
- [47].Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S. AAA ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol. Cell Biol. 2002;22:626–634. doi: 10.1128/MCB.22.2.626-634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ravikumar B, Futter M, Jahreiss L, Korolchuk VI, Lichtenberg M, Luo S, Massey DCO, Menzies FM, Narayanan U, Renna M, Jimenez-Sanchez M, Sarkar S, Underwood B, Winslow A, Rubinsztein DC. Mammalian macroautophagy at a glance. Journal of Cell Science. 2009;122:1707–1711. doi: 10.1242/jcs.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol. Cell Biol. 2008;28:5747–5763. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stamnes MA, Shieh BH, Chuman L, Harris GL, Zuker CS. The cyclophilin homolog ninaA is a tissue-specific integral membrane protein required for the proper synthesis of a subset of Drosophila rhodopsins. Cell. 1991;65:219–227. doi: 10.1016/0092-8674(91)90156-s. [DOI] [PubMed] [Google Scholar]
- [51].Colley NJ, Cassill JA, Baker EK, Zuker CS. Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3070–3074. doi: 10.1073/pnas.92.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rosenbaum EE, Hardie RC, Colley NJ. Calnexin is essential for rhodopsin maturation, Ca2+ regulation, and photoreceptor cell survival. Neuron. 2006;49:229–241. doi: 10.1016/j.neuron.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rosenbaum EE, Brehm KS, Vasiljevic E, Liu CH, Hardie RC, Colley NJ. XPORT-dependent transport of TRP and rhodopsin. Neuron. 2011;72:602–615. doi: 10.1016/j.neuron.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Noorwez SM, Sama RR, Kaushal S. Calnexin improves the folding efficiency of mutant rhodopsin in the presence of pharmacological chaperone 11-cis-retinal. J. Biol. Chem. 2009;284:33333–33342. doi: 10.1074/jbc.M109.043364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kosmaoglou M, Cheetham ME. Calnexin is not essential for mammalian rod opsin biogenesis. Mol. Vis. 2008;14:2466–2474. [PMC free article] [PubMed] [Google Scholar]
- [56].Kraus A, Groenendyk J, Bedard K, Baldwin TA, Krause KH, Dubois-Dauphin M, Dyck J, Rosenbaum EE, Korngut L, Colley NJ, Gosgnach S, Zochodne D, Todd K, Agellon LB, Michalak M. Calnexin deficiency leads to dysmyelination. J. Biol. Chem. 2010;285:18928–18938. doi: 10.1074/jbc.M110.107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Athanasiou D, Kosmaoglou M, Kanuga N, Novoselov SS, Paton AW, Paton JC, Chapple JP, Cheetham ME. BiP prevents rod opsin aggregation. Mol. Biol. Cell. 2012;23:3522–3531. doi: 10.1091/mbc.E12-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kosmaoglou M, Kanuga N, Aguila M, Garriga P, Cheetham ME. A dual role for EDEM1 in the processing of rod opsin. J. Cell Sci. 2009;122:4465–4472. doi: 10.1242/jcs.055228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kang MJ, Ryoo HD. Suppression of retinal degeneration in Drosophila by stimulation of ER-associated degradation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17043–17048. doi: 10.1073/pnas.0905566106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Griciuc A, Aron L, Piccoli G, Ueffing M. Clearance of Rhodopsin(P23H) aggregates requires the ERAD effector VCP. Biochim. Biophys. Acta. 2010;1803:424–434. doi: 10.1016/j.bbamcr.2010.01.008. [DOI] [PubMed] [Google Scholar]
- [61].Griciuc A, Aron L, Roux MJ, Klein R, Giangrande A, Ueffing M. Inactivation of VCP/ter94 suppresses retinal pathology caused by misfolded rhodopsin in Drosophila. PLoS. Genet. 2010;6 doi: 10.1371/journal.pgen.1001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Saliba RS, Munro PM, Luthert PJ, Cheetham ME. The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J. Cell Sci. 2002;115:2907–2918. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- [63].Chapple JP, Cheetham ME. The chaperone environment at the cytoplasmic face of the endoplasmic reticulum can modulate rhodopsin processing and inclusion formation. J. Biol. Chem. 2003;278:19087–19094. doi: 10.1074/jbc.M212349200. [DOI] [PubMed] [Google Scholar]
- [64].Chiang WC, Messah C, Lin JH. IRE1 directs proteasomal and lysosomal degradation of misfolded rhodopsin. Mol. Biol. Cell. 2012;23:758–770. doi: 10.1091/mbc.E11-08-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chiang WC, Hiramatsu N, Messah C, Kroeger H, Lin JH. Selective activation of ATF6 and PERK endoplasmic reticulum stress signaling pathways prevent mutant rhodopsin accumulation. Invest Ophthalmol. Vis. Sci. 2012;53:7159–7166. doi: 10.1167/iovs.12-10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, LaVail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gorbatyuk MS, Knox T, LaVail MM, Gorbatyuk OS, Noorwez SM, Hauswirth WW, Lin JH, Muzyczka N, Lewin AS. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kunte MM, Choudhury S, Manheim JF, Shinde VM, Miura M, Chiodo VA, Hauswirth WW, Gorbatyuk OS, Gorbatyuk MS. ER stress is involved in T17M rhodopsin-induced retinal degeneration. Invest Ophthalmol. Vis. Sci. 2012;53:3792–3800. doi: 10.1167/iovs.11-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shinde VM, Sizova OS, Lin JH, LaVail MM, Gorbatyuk MS. ER stress in retinal degeneration in S334ter Rho rats. PLoS. One. 2012;7:e33266. doi: 10.1371/journal.pone.0033266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kroeger H, Messah C, Ahern K, Gee J, Joseph V, Matthes MT, Yasumura D, Gorbatyuk MS, Chiang WC, LaVail MM, Lin JH. Induction of endoplasmic reticulum stress genes, BiP and chop, in genetic and environmental models of retinal degeneration. Invest Ophthalmol. Vis. Sci. 2012;53:7590–7599. doi: 10.1167/iovs.12-10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N. Engl. J. Med. 2009;360:1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- [73].Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- [74].Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Heon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum. Mol. Genet. 2005;14:725–733. doi: 10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- [75].Tamm ER. Myocilin and glaucoma: facts and ideas. Prog. Retin. Eye Res. 2002;21:395–428. doi: 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- [76].Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem. Biophys. Res. Commun. 2003;312:592–600. doi: 10.1016/j.bbrc.2003.10.162. [DOI] [PubMed] [Google Scholar]
- [77].Suntharalingam A, Abisambra JF, O’Leary JC, III, Koren J, III, Zhang B, Joe MK, Blair LJ, Hill SE, Jinwal UK, Cockman M, Duerfeldt AS, Tomarev S, Blagg BS, Lieberman RL, Dickey CA. Glucose-regulated protein 94 triage of mutant myocilin through endoplasmic reticulum-associated degradation subverts a more efficient autophagic clearance mechanism. J. Biol. Chem. 2012;287:40661–40669. doi: 10.1074/jbc.M112.384800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Shen X, Ying H, Qiu Y, Park JS, Shyam R, Chi ZL, Iwata T, Yue BY. Processing of optineurin in neuronal cells. J. Biol. Chem. 2011;286:3618–3629. doi: 10.1074/jbc.M110.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Calandrella N, Scarsella G, Pescosolido N, Risuleo G. Degenerative and apoptotic events at retinal and optic nerve level after experimental induction of ocular hypertension. Mol. Cell Biochem. 2007;301:155–163. doi: 10.1007/s11010-006-9407-0. [DOI] [PubMed] [Google Scholar]
- [80].Schallenberg M, Prokosch V, Thanos S. Regulation of retinal proteome by topical antiglaucomatous eye drops in an inherited glaucoma rat model. PLoS. One. 2012;7:e33593. doi: 10.1371/journal.pone.0033593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Park KH, Cozier F, Ong OC, Caprioli J. Induction of heat shock protein 72 protects retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol. Vis. Sci. 2001;42:1522–1530. [PubMed] [Google Scholar]
- [82].Ahn J, Piri N, Caprioli J, Munemasa Y, Kim SH, Kwong JM. Expression of heat shock transcription factors and heat shock protein 72 in rat retina after intravitreal injection of low dose N-methyl-D-aspartate. Neurosci. Lett. 2008;433:11–16. doi: 10.1016/j.neulet.2007.12.045. [DOI] [PubMed] [Google Scholar]
- [83].Nagashima M, Fujikawa C, Mawatari K, Mori Y, Kato S. HSP70, the earliest-induced gene in the zebrafish retina during optic nerve regeneration: its role in cell survival. Neurochem. Int. 2011;58:888–895. doi: 10.1016/j.neuint.2011.02.017. [DOI] [PubMed] [Google Scholar]
- [84].Ishii Y, Kwong JM, Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol. Vis. Sci. 2003;44:1982–1992. [PubMed] [Google Scholar]
- [85].Dean DO, Tytell M. Hsp25 and -90 immunoreactivity in the normal rat eye. Invest Ophthalmol. Vis. Sci. 2001;42:3031–3040. [PubMed] [Google Scholar]
- [86].Strunnikova N, Baffi J, Gonzalez A, Silk W, Cousins SW, Csaky KG. Regulated heat shock protein 27 expression in human retinal pigment epithelium. Invest Ophthalmol. Vis. Sci. 2001;42:2130–2138. [PubMed] [Google Scholar]
- [87].Li Y, Roth S, Laser M, Ma JX, Crosson CE. Retinal preconditioning and the induction of heat-shock protein 27. Invest Ophthalmol. Vis. Sci. 2003;44:1299–1304. doi: 10.1167/iovs.02-0235. [DOI] [PubMed] [Google Scholar]
- [88].Krueger-Naug AM, Emsley JG, Myers TL, Currie RW, Clarke DB. Injury to retinal ganglion cells induces expression of the small heat shock protein Hsp27 in the rat visual system. Neuroscience. 2002;110:653–665. doi: 10.1016/s0306-4522(01)00453-5. [DOI] [PubMed] [Google Scholar]
- [89].Huang W, Fileta JB, Filippopoulos T, Ray A, Dobberfuhl A, Grosskreutz CL. Hsp27 phosphorylation in experimental glaucoma. Invest Ophthalmol. Vis. Sci. 2007;48:4129–4135. doi: 10.1167/iovs.06-0606. [DOI] [PubMed] [Google Scholar]
- [90].Tezel G, Hernandez R, Wax MB. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch. Ophthalmol. 2000;118:511–518. doi: 10.1001/archopht.118.4.511. [DOI] [PubMed] [Google Scholar]
- [91].Beatty S, Koh HH, Phil M, Henson D, Boulton M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Survey of Ophthalmology. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- [92].Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS. One. 2009;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mitter SK, Rao HV, Qi X, Cai J, Sugrue A, Dunn WA, Jr, Grant MB, Boulton ME. Autophagy in the retina: a potential role in age-related macular degeneration. Adv. Exp. Med. Biol. 2012;723:83–90. doi: 10.1007/978-1-4614-0631-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ryhanen T, Hyttinen JM, Kopitz J, Rilla K, Kuusisto E, Mannermaa E, Viiri J, Holmberg CI, Immonen I, Meri S, Parkkinen J, Eskelinen EL, Uusitalo H, Salminen A, Kaarniranta K. Crosstalk between Hsp70 molecular chaperone, lysosomes and proteasomes in autophagy-mediated proteolysis in human retinal pigment epithelial cells. J. Cell Mol. Med. 2009;13:3616–3631. doi: 10.1111/j.1582-4934.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Nordgaard CL, Karunadharma PP, Feng X, Olsen TW, Ferrington DA. Mitochondrial proteomics of the retinal pigment epithelium at progressive stages of age-related macular degeneration. Invest Ophthalmol. Vis. Sci. 2008;49:2848–2855. doi: 10.1167/iovs.07-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, Cheetham ME. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol. Vis. Sci. 2004;45:675–684. doi: 10.1167/iovs.03-0351. [DOI] [PubMed] [Google Scholar]
- [97].Klemenz R, Frohli E, Steiger RH, Schafer R, Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Alge CS, Priglinger SG, Neubauer AS, Kampik A, Zillig M, Bloemendal H, Welge-Lussen U. Retinal pigment epithelium is protected against apoptosis by alpha B-crystallin. Invest Ophthalmol. Vis. Sci. 2002;43:3575–3582. [PubMed] [Google Scholar]
- [99].Nakata K, Crabb JW, Hollyfield JG. Crystallin distribution in Bruch’s membrane-choroid complex from AMD and age-matched donor eyes. Exp. Eye Res. 2005;80:821–826. doi: 10.1016/j.exer.2004.12.011. [DOI] [PubMed] [Google Scholar]
- [100].Lee H, Chung H, Lee SH, Jahng WJ. Light-induced phosphorylation of crystallins in the retinal pigment epithelium. Int. J. Biol. Macromol. 2011;48:194–201. doi: 10.1016/j.ijbiomac.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Sreekumar PG, Chothe P, Sharma KK, Baid R, Kompella U, Spee C, Kannan N, Manh C, Ryan SJ, Ganapathy V, Kannan R, Hinton DR. Antiapoptotic Properties of alpha-Crystallin-Derived Peptide Chaperones and Characterization of Their Uptake Transporters in Human RPE Cells. Invest Ophthalmol. Vis. Sci. 2013;54:2787–2798. doi: 10.1167/iovs.12-11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bringmann A, Wiedemann P. Involvement of Muller glial cells in epiretinal membrane formation. Graefes Arch. Clin. Exp. Ophthalmol. 2009;247:865–883. doi: 10.1007/s00417-009-1082-x. [DOI] [PubMed] [Google Scholar]
- [103].Rattner A, Nathans J. The genomic response to retinal disease and injury: evidence for endothelin signaling from photoreceptors to glia. J. Neurosci. 2005;25:4540–4549. doi: 10.1523/JNEUROSCI.0492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Peters CM, Rogers SD, Pomonis JD, Egnaczyk GF, Keyser CP, Schmidt JA, Ghilardi JR, Maggio JE, Mantyh PW. Endothelin receptor expression in the normal and injured spinal cord: potential involvement in injury-induced ischemia and gliosis. Exp. Neurol. 2003;180:1–13. doi: 10.1016/s0014-4886(02)00023-7. [DOI] [PubMed] [Google Scholar]
- [105].Howell GR, Macalinao DG, Sousa GL, Walden M, Soto I, Kneeland SC, Barbay JM, King BL, Marchant JK, Hibbs M, Stevens B, Barres BA, Clark AF, Libby RT, John SW. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Invest. 2011;121:1429–1444. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Joly S, Lange C, Thiersch M, Samardzija M, Grimm C. Leukemia inhibitory factor extends the lifespan of injured photoreceptors in vivo. J. Neurosci. 2008;28:13765–13774. doi: 10.1523/JNEUROSCI.5114-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Krebs MP, Holden DC, Joshi P, Clark CL, III, Lee AH, Kaushal S. Molecular mechanisms of rhodopsin retinitis pigmentosa and the efficacy of pharmacological rescue. J. Mol. Biol. 2010;395:1063–1078. doi: 10.1016/j.jmb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- [108].Mendes HF, Cheetham ME. Pharmacological manipulation of gain-of-function and dominant-negative mechanisms in rhodopsin retinitis pigmentosa. Hum. Mol. Genet. 2008;17:3043–3054. doi: 10.1093/hmg/ddn202. [DOI] [PubMed] [Google Scholar]
- [109].Noorwez SM, Kuksa V, Imanishi Y, Zhu L, Filipek S, Palczewski K, Kaushal S. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J. Biol. Chem. 2003;278:14442–14450. doi: 10.1074/jbc.M300087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jia LY, Gong B, Pang CP, Huang Y, Lam DS, Wang N, Yam GH. Correction of the disease phenotype of myocilin-causing glaucoma by a natural osmolyte. Invest Ophthalmol. Vis. Sci. 2009;50:3743–3749. doi: 10.1167/iovs.08-3151. [DOI] [PubMed] [Google Scholar]
- [111].Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J. Clin. Invest. 2011;121:3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yam GH, Gaplovska-Kysela K, Zuber C, Roth J. Sodium 4-phenylbutyrate acts as a chemical chaperone on misfolded myocilin to rescue cells from endoplasmic reticulum stress and apoptosis. Invest Ophthalmol. Vis. Sci. 2007;48:1683–1690. doi: 10.1167/iovs.06-0943. [DOI] [PubMed] [Google Scholar]
- [113].Fernandez-Sanchez L, Lax P, Pinilla I, Martin-Nieto J, Cuenca N. Tauroursodeoxycholic acid prevents retinal degeneration in transgenic P23H rats. Invest Ophthalmol. Vis. Sci. 2011;52:4998–5008. doi: 10.1167/iovs.11-7496. [DOI] [PubMed] [Google Scholar]
- [114].Oveson BC, Iwase T, Hackett SF, Lee SY, Usui S, Sedlak TW, Snyder SH, Campochiaro PA, Sung JU. Constituents of bile, bilirubin and TUDCA, protect against oxidative stress-induced retinal degeneration. J. Neurochem. 2011;116:144–153. doi: 10.1111/j.1471-4159.2010.07092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Phillips MJ, Walker TA, Choi HY, Faulkner AE, Kim MK, Sidney SS, Boyd AP, Nickerson JM, Boatright JH, Pardue MT. Tauroursodeoxycholic acid preservation of photoreceptor structure and function in the rd10 mouse through postnatal day 30. Invest Ophthalmol. Vis. Sci. 2008;49:2148–2155. doi: 10.1167/iovs.07-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Boatright JH, Nickerson JM, Moring AG, Pardue MT. Bile acids in treatment of ocular disease. J. Ocul. Biol. Dis. Infor. 2009;2:149–159. doi: 10.1007/s12177-009-9030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mulhern ML, Madson CJ, Kador PF, Randazzo J, Shinohara T. Cellular osmolytes reduce lens epithelial cell death and alleviate cataract formation in galactosemic rats. Mol. Vis. 2007;13:1397–1405. [PubMed] [Google Scholar]
- [118].Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science. 1988;241:1817–1820. doi: 10.1126/science.3175623. [DOI] [PubMed] [Google Scholar]
- [119].Tam LC, Kiang AS, Campbell M, Keaney J, Farrar GJ, Humphries MM, Kenna PF, Humphries P. Prevention of autosomal dominant retinitis pigmentosa by systemic drug therapy targeting heat shock protein 90 (Hsp90) Hum. Mol. Genet. 2010;19:4421–4436. doi: 10.1093/hmg/ddq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Caprioli J, Ishii Y, Kwong JM. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Trans. Am. Ophthalmol. Soc. 2003;101:39–50. [PMC free article] [PubMed] [Google Scholar]
- [121].Mihaly K, Toth S, Szlavik L, Toth A, Csermely P. Attenuation of diabetic retinopathy by the molecular chaperone-inducer amino acid analogue canavanine in streptozotocin-diabetic rats. Cell Mol. Life Sci. 1998;54:1154–1160. doi: 10.1007/s000180050243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kaushal S. Effect of rapamycin on the fate of P23H opsin associated with retinitis pigmentosa (an American Ophthalmological Society thesis) Trans. Am. Ophthalmol. Soc. 2006;104:517–529. [PMC free article] [PubMed] [Google Scholar]
- [123].Kolosova NG, Muraleva NA, Zhdankina AA, Stefanova NA, Fursova AZ, Blagosklonny MV. Prevention of age-related macular degeneration-like retinopathy by rapamycin in rats. Am. J. Pathol. 2012;181:472–477. doi: 10.1016/j.ajpath.2012.04.018. [DOI] [PubMed] [Google Scholar]
- [124].Dejneka NS, Kuroki AM, Fosnot J, Tang W, Tolentino MJ, Bennett J. Systemic rapamycin inhibits retinal and choroidal neovascularization in mice. Mol. Vis. 2004;10:964–972. [PubMed] [Google Scholar]
- [125].Clemson CM, Tzekov R, Krebs M, Checchi JM, Bigelow C, Kaushal S. Therapeutic potential of valproic acid for retinitis pigmentosa. Br. J. Ophthalmol. 2011;95:89–93. doi: 10.1136/bjo.2009.175356. [DOI] [PubMed] [Google Scholar]
- [126].Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- [127].Sandberg MA, Rosner B, Weigel-DiFranco C, Berson EL. Lack of scientific rationale for use of valproic acid for retinitis pigmentosa. Br. J. Ophthalmol. 2011;95:744. doi: 10.1136/bjo.2010.198176. [DOI] [PubMed] [Google Scholar]
- [128].Sisk RA. Valproic acid treatment may be harmful in non-dominant forms of retinitis pigmentosa. Br. J. Ophthalmol. 2012;96:1154–1155. doi: 10.1136/bjophthalmol-2012-301950. [DOI] [PubMed] [Google Scholar]
- [129].Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Moser A, Brockhurst RJ, Hayes KC, Johnson CA, Anderson EJ, Gaudio AR, Willett WC, Schaefer EJ. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Arch. Ophthalmol. 2004;122:1297–1305. doi: 10.1001/archopht.122.9.1297. [DOI] [PubMed] [Google Scholar]