Abstract
Protein homeostasis (proteostasis) is essential for maintaining the functionality of the proteome. The disruption of proteostasis, due to genetic mutations or an age-related decline, leads to aberrantly folded proteins that typically lose their function. The accumulation of misfolded and aggregated protein is also cytotoxic and has been implicated in the pathogenesis of neurodegenerative diseases. Neurons have developed an intrinsic protein quality control network, of which molecular chaperones are an essential component. Molecular chaperones function to promote efficient folding and target misfolded proteins for refolding or degradation. Increasing molecular chaperone expression can suppress protein aggregation and toxicity in numerous models of neurodegenerative disease; therefore, molecular chaperones are considered exciting therapeutic targets. Furthermore, mutations in several chaperones cause inherited neurodegenerative diseases. In this review, we focus on the importance of molecular chaperones in neurodegenerative diseases, and discuss the advances in understanding their protective mechanisms.
1. Introduction
Proteins must fold to their native state in order to achieve functionality. However, in the crowded cellular environment, and under environmental and physiological stress conditions such as heat, oxidative stress and inflammation, proteins are susceptible to the formation of non-native interactions that can lead to protein misfolding and aggregation. The accumulation of misfolded and aggregated protein is considered toxic to the cell and is implicated in numerous diseases such as type 2 diabetes, cardiovascular disease and neurodegenerative diseases. Cells have therefore developed an intrinsic network of protein quality control machinery that functions to balance protein folding, misfolding, aggregation and degradation, thereby maintaining protein homeostasis (proteostasis) and the functionality of the proteome. The protein quality control machinery includes molecular chaperones, which act as the first line of defence and participate in the refolding or, alternatively, the degradation of misfolded proteins. Their role in maintaining proteostasis in neurons, which are post-mitotic cells that are particularly vulnerable to protein aggregation, is the subject of this review.
2. Molecular chaperones
Molecular chaperones are defined as proteins that interact with, stabilise or assist another protein to gain its native and functionally active conformation, without being present in the final structure [1]. Members of the molecular chaperone family are often referred to as heat-shock proteins (Hsps) due to their upregulation under stress conditions that typically destabilise proteins, such as elevated temperature and oxidative stress. Molecular chaperones are often classified according to their molecular weight and members include Hsp90, Hsp70, Hsp60, Hsp40 (DnaJ) and the small Hsps. Molecular chaperones display large functional diversity and in addition to their fundamental roles in de novo folding, and the refolding of misfolded protein, chaperones also regulate critical cellular processes such as protein trafficking, protein degradation and macromolecular complex assembly [2].
2.1 Hsp90 (HSPC) family
Hsp90 is an ATP-dependent chaperone that functions in the activation and stabilisation of client proteins; including protein kinases, cell cycle regulators, cell surface receptors and transcription factors. Therefore, Hsp90 plays a critical role in cellular processes including signal transduction, cell cycle progression, apoptosis and protein degradation [3]. The activation of Hsp90 clients is driven by a cycle of substrate binding and release mediated by a series of conformational changes in the chaperone and an ATP-induced transition between an open and a closed conformation. Hsp90 exists as a homodimer, with each subunit consisting of three domains; an N-terminal ATP-binding domain (N-domain), a middle domain that binds the substrate (M-domain) and a C-terminal dimerisation domain (C-domain). In the absence of nucleotide Hsp90 adopts a V-shaped open conformation. The binding of ATP to the N-domain induces a conformational change that closes a lid over the nucleotide binding pocket. Following lid closure, the N-domains dimerise, forming a compact structure with a closed conformation. The formation of the closed dimer induces ATP hydrolysis, subsequently promoting the N-domains to dissociate and the return of Hsp90 to the open conformation, with the release of the substrate [4].
The reaction cycle of Hsp90 is regulated by various co-chaperones. The co-chaperones exhibit specific binding preferences for different Hsp90 conformations and affect different stages of the cycle, such as client binding and ATP hydrolysis. Co-chaperones therefore usually co-operate in a sequential cycle to facilitate the maturation of Hsp90 clients.
2.2 Hsp70 (HSPA) family
Hsp70 functions in a wide array of cellular processes including the folding of newly synthesised protein, the refolding of misfolded and aggregated protein, transport of proteins across membranes, and protein degradation [3]. These functions rely on the ability of Hsp70 to interact with hydrophobic stretches exposed in client proteins and subsequently undergo an ATP-dependent cycle of substrate binding and release. Hsp70 is composed of a N-terminal ATPase domain (NBD) and a C-terminal substrate binding domain (SBD), divided into subdomains that form a hydrophobic binding pocket and a lid [2]. The NBD and SBD are connected by a flexible linker that enables the NBD to allosterically control the conformation of the SBD. In the ATP-bound state the binding pocket and lid are in an open conformation. The SBD has a low substrate affinity and fast substrate exchange rates. The hydrolysis of ATP to ADP drives the SBD into a high affinity state for substrate binding through the closing of the lid, enabling stable binding of the client protein. The release of ADP and the rebinding of ATP triggers the opening of the lid and the subsequent unloading of the bound substrate.
The Hsp70 reaction cycle contains two rate limiting steps, ATP hydrolysis, due to low basal ATPase activity, and ADP dissociation, due to the high levels of cytoplasmic ATP under physiological conditions. Therefore, Hsp70 requires the action of co-chaperones to facilitate the reaction cycle between ATP and ADP bound states. The ATPase activity of Hsp70 is stimulated by members of the DnaJ (Hsp40) family via their conserved J domain [5]. The dissociation of ADP requires the opening of the nucleotide binding cleft, a process catalysed by a range of nucleotide exchange factors (NEFs), including the Hsp70-like Hsp110 proteins, HspBP1, SIL1 and BAG family [6].
2.3 DnaJ (Hsp40) family
As mentioned above, members of the DnaJ family (also referred to as Hsp40) are important regulators of Hsp70 activity by stimulating ATP hydrolysis. All DnaJ proteins contain a J domain, a conserved region of 70 amino acids that folds into four α-helices. Helix II and helix III form an antiparallel two-helix bundle, with a loop connecting the two helices containing a histidine-proline-aspartate (HPD) motif. The HPD motif is critical for lowering the activation energy of ATP hydrolysis [7].
The human genome encodes 49 DnaJ proteins, which can be divided into three classes according to their domain composition [8]. Class I (DNAJA) proteins share all domains present in the Escherichia coli DnaJ protein, with an N-terminal J domain, a glycinephenylalanine (GF) rich region, a zinc binding domain and a C-terminal domain. Class II (DNAJB) proteins contain an N-terminal J domain and a GF rich region, whereas class III (DNAJC) proteins share only the J domain. The diversification of domains outside the J domain has enabled DnaJ proteins to adopt specialised functions. For example, domains that target DnaJ proteins to precise intracellular locations to promote the interaction of Hsp70 with specific clients. Furthermore, client binding domains enable some DnaJ proteins to deliver clients to the SBD of Hsp70. Specialised domains also facilitate the targeting of clients for degradation, thus providing an important link between Hsp70, misfolded proteins and degradation [7,8].
2.4 Hsp60 (HSPD/E and CCT) family
Hsp60, also called chaperonins, are large double ring complexes that enclose a central cavity. Substrate proteins, typically folding intermediates, are encapsulated in the central cavity, thereby shielding exposed hydrophobic residues from aggregation and allowing the substrate to fold in a protected environment. Chaperonins can be divided to two subgroups. Group I (HSPD) chaperonins are present in bacteria (GroEL) and the mitochondrial matrix (Hsp60). They contain 7 subunits per ring and require co-chaperones (HSPE) that act as lids over the complex (GroES in bacteria and Hsp10 in mitochondria). Group II chaperonins are found in archaea (thermosome) and in the cytosol of eukaryotes (CCT or TRiC). They typically have 8 subunits per ring and do not require co-chaperones.
The GroEL-GroES chaperonin in E. coli has been the most extensively studied [9]. Each subunit consists of an equatorial ATP binding domain, a hinge domain and an apical substrate binding domain. The apical domain forms the entrance to the GroEL cavity and exposes hydrophobic residues to mediate substrate binding. Following substrate binding to one of the rings, ATP binds to each of the 7 subunits, inducing conformational changes, enabling the association of GroES and the subsequent encapsulation of the substrate. Upon GroES binding, the GroEL cavity enlarges, creating an environment for productive protein folding. The hydrolysis of ATP to ADP triggers the dissociation of the GroES lid and the release of the substrate. Multiple folding cycles may be required before the substrate reaches its native state [2].
2.5 small HSP (HSPB) family
Small heat shock proteins differ from the other major molecular chaperones in that they are ATP independent. The human genome encodes 10 small Hsps and they range in size from 12-42 kDa. They share a conserved 100 amino acid α-crystallin domain that is flanked by variable N-terminal and C-terminal extensions. These extensions mediate substrate recognition as well as enabling the formation of oligomers. Small Hsps are able to bind to unfolded or misfolded protein and prevent their aggregation until the protein can be transferred to other cellular systems, such as the Hsp70-Hsp40 system [10].
3. Protein aggregates are a hallmark of neurodegenerative diseases
Neurodegenerative diseases are caused by the progressive degeneration of neurons in the brain, eye and spinal cord. The loss of specific populations of neurons defines each disease and ultimately determines the clinical manifestations of the disease. The diseases can present as sporadic or familial, which typically share common symptoms and disease progression, thus the modes of neuronal dysfunction and death are likely to be similar in the sporadic and inherited cases.
A pathological hallmark of many neurodegenerative diseases is the presence of ubiquitylated protein aggregates, indicating disturbances in proteostasis. Indeed, many of the causative mutations identified in familial cases lead to the misfolding and aggregation of the disease-related protein, for example, superoxide dismutase 1 (SOD1) in amyotrophic lateral sclerosis (ALS), huntingtin in Huntington’s disease (HD), α-synuclein in Parkinson’s disease (PD), amyloid beta (Aβ) in Alzheimer’s disease (AD), and tau in FTDP-17. Aging is considered the most significant risk factor for sporadic cases of ALS, AD and PD. It is well established that aging results in a decline in the efficiency of the protein quality control machinery [11], although the cause for this decline is currently unknown. The age-associated decline in protein quality control has been suggested to lead to an imbalance in the production of misfolded protein, which slowly overwhelms the protective capacity of the molecular chaperone machinery, ultimately leading to a vicious cycle which results in the collapse of proteostasis and causes neurodegeneration [2].
The microscopically visible inclusions seen pathologically are thought to represent the end point of the protein aggregation process, which begins with the formation of small oligomers, protofibrils and mature fibrils. The exact species that exerts cytotoxicity is unknown; however, accumulating evidence suggests that soluble oligomers and protofibrils pose the most toxic potential to the cell. In support of this hypothesis, in vivo studies have shown that the injection of rats with soluble oligomers of α-synuclein or Aβ showed increased neuronal loss compared to rats injected with fibril forms of the proteins [12,13]. The formation of large aggregates is thought to be a protective defence mechanism adopted by the cell in order to sequester the toxic oligomers and protofibrils and prevent their interaction with other proteins. Although not considered the most toxic entity, large aggregates are still likely to contribute to cytotoxicity and disease progression in neurons due to their ability to recruit other proteins and physically obstruct axonal transport and other cellular processes. Neurons are highly polarised cells and rely heavily on axonal transport between the cell body and the synaptic terminal in order to effectively sustain their function. Neurons are therefore vulnerable to protein aggregation and thus place a high demand on the molecular chaperone machinery in order to maintain proteostasis.
4. The upregulation of molecular chaperones is neuroprotective
Considering the protective nature of molecular chaperones, their upregulation is a promising therapeutic approach to combat the progression of neurodegenerative disease. Proof of principle studies have suggested this is a potential strategy for the treatment of many neurodegenerative diseases by increasing the expression of individual chaperones (Table 1) or groups of chaperones (Table 2). Some of these studies are discussed in detail below.
Table 1. Chaperones that combat neurodegeneration related protein misfolding.
| Chaperone Family | Chaperone | Disease/Protein(s) | Comments |
|---|---|---|---|
| HSP110 | HSP110 (HSPH2) | ALS/SOD1 | Improved vesicle transport deficit in SOD1G85R squid axoplasm [67]; with DNAJB1 suppressed polyQ toxicity in flies [68]. |
| HD/Htt | |||
| HSP105 (HSPH1) | AD/Tau | HSP105 knock out mouse had increased p-tau and Aβ [69]; suppressed aggregation of SOD1G93A in cells [70]. | |
| ALS/SOD1 | |||
| HSP90 | HSP86 (HSPC1) | AD/ Aβ | Reduced Aβ aggregation in vitro [22]; with Hsp60 and Hsp70 reduced Aβ mitochondrial dysfunction in cells [71]. |
| HSP70 | BiP (HSPA5) | AD/ Aβ | Bound APP and reduced Aβ secretion [72]; reduced polyQ aggregation and toxicity in cells [73]; reduced α-synuclein toxicity in rat [74]; reduced P23H rhodopsin aggregation and photoreceptor cell death [75] [76]. |
| HD/Htt | |||
| PD/α-syn | |||
| RP/Rho | |||
| Hsc70 (HSPA8) | AD/Tau | Binds tau and facilitates microtubule polymerization reducing insoluble tau [77] [78] [25]; QBP1 fusion reduced polyQ aggregation and toxicity in cells and mice [79], ATPase mutant reduced large polyQ aggregates but no effect on toxicity [80], reduced axonal transport defect in polyQ fly [81]; binds α-synuclein and reduced toxicity of fibrils [82] [83]; binding to mutant SOD1 [84]. | |
| HD/Htt | |||
| PD/α-syn | |||
| ALS/SOD1 | |||
| Hsp70 (HSPA1A*) | AD/ Aβ | Reduced Aβ aggregation in vitro [22] and in transgenic mice [85]; modest effect on R6/2 Htt mice [31] but increased aggregation on knock-down in HD flies [86]; suppression of α-synuclein toxicity in flies [17] cells and mice [16], but another report found no effect in mice [87]; reduced mutant SOD1 aggregation in cells [88] but had no effect in mice [89]; suppressed TDP-43 toxicity in fly [90]. | |
| AD/Tau | |||
| HD/Htt | |||
| PD/α-syn | |||
| ALS/SOD1 | |||
| ALS/TDP43 | |||
| HSP60 | HSP60 (HSPD1) | AD/ Aβ | Reduced Aβ aggregation in vitro [22] and improved mitochondria function in a cell model [71]. |
| HSP40/DNAJ | DNAJA1 | AD/Tau | Antagonized protective effect of Hsp70 on tau [91]; increased polyQ aggregation in some cell models [92]; increase binding of Hsp70 to α-synuclein [83]. |
| HD/Htt | |||
| PD/α-syn | |||
| DNAJB1 (Hsp40 or Hdj1) | AD/ Aβ | Reduced Aβ aggregation in vitro with Hsp70 [22]; suppressed Htt inclusion formation but did not affect toxicity in cells [93] but protective with Hsp110 in flies [68]; increase binding of Hsp70 to α-synuclein [83]. | |
| HD/Htt | |||
| PD/α-syn | |||
| DNAJB2a (HSJ1a) | HD/Htt | Reduce polyQ aggregation in vitro, in cells, in mice [37] [39] and rats [94]; reduced mutant SOD1 aggregation in cells [48] [45] and mice [45]; suppress mutant parkin aggregation and promote functional refolding in cells [95] | |
| ALS/SOD1 | |||
| PD/Parkin | |||
| DNAJB6 | AD/ Aβ | Efficient block of Aβ aggregation in vitro [21]; block polyQ aggregation and toxicity in cells and frogs [35] [96]. | |
| HD/Htt | |||
| DNAJB8 | HD/Htt | block polyQ aggregation and toxicity in cells and frogs [35] [96]. | |
| DNAJC10 | RP/Rho | Reduced P23H rhodopsin aggregation in cells [97]. | |
| small HSP | HSP27 (HSPB1) | AD/ Aβ | Reduced Aβ aggregation in vitro and toxicity on cells [98] and in mice [99]; alters tau dynamics in mice [100]; reduced polyQ aggregation and toxicity in cells [101] and by viral delivery in rats [102] but not transgenic mice [103]; reduced α-synuclein fibril formation in vitro [104] and toxicity in cells [105]; reduced SOD1 aggregation in vitro [41] but small effects in mice [106] [44]. |
| AD/Tau | |||
| HD/Htt | |||
| PD/α-syn | |||
| ALS/SOD1 | |||
| HSP22 (HSPB8) | AD/ Aβ | Reduced Aβ aggregation in vitro and toxicity on cells [98]; reduced polyQ aggregation [32]; Most effective small Hsp at reducing α-synuclein fibril formation in vitro [104]; enhanced autophagic clearance of SOD1 and TDP-43 [107]. | |
| HD/Htt | |||
| PD/α-syn | |||
| ALS/SOD1 | |||
| ALS/TDP43 | |||
| αB-crystallin (HSPB5) | AD/ Aβ | Reduced Aβ aggregation in vitro and toxicity on cells [98] [108]; reduced toxicity of α-synuclein in cells [105]; reduced α-synuclein fibril formation in vitro [104]; SOD1 aggregation in vitro [41] but does not protect in mice [109]. | |
| PD/α-syn | |||
| ALS/SOD1 | |||
| HSP20 (HSPB6) | AD/ Aβ | Reduced Aβ aggregation in vitro and toxicity on cells [98] [110] and in worms [111]; reduced α-synuclein fibril formation in vitro [104]. | |
| PD/α-syn | |||
| cvHSP (HSPB7) | HD/Htt | Most potent small Hsp against polyQ in cell model [32]. | |
| Co-chaperone | CHIP | AD/Tau | Reduced tau aggregation in cell [26] and in mice [112]; reduced polyQ (ataxin-1) aggregation and toxicity in cells [113]; enhanced ubiquitylation of α-synuclein [114]; degradation of mutant SOD1 [84]. |
| PD/α-syn | |||
| ALS/SOD1 | |||
| Cdc37 | AD/Tau | Regulates tau stability with Bag5 [115]; with Hsp90 in enhanced authophagic clearance of TDP-43 [116]. | |
| ALS/TDP43 | |||
| Bag-1 | AD/Tau | With Hsc70 to target degradation of tau [117]; protects against α-synuclein in cells and MPTP in mice [118]. | |
| PD/α-syn | |||
| Bag-3 | ALS/SOD1 | Reduction in polyQ (SCA3) with Hspb8 in cells and flies [119]; With HspB8 to stimulate autophagy of SOD1 in cells [107] [34]. | |
| Bag-5 | PD/α-syn | Enhanced ubiquitylation of α-synuclein [114]. |
Table 2. Manipulations involving networks of chaperones.
| Target | Method | Animal models showing benefit |
|---|---|---|
| HSF-1 | Overexpression or constitutively active HSF-1 | AD deficits in mice [120]; polyQ in mice [121]; mutant SOD1 in mice [122]. |
| Knock down or dominant negative HSF-1 | Enhanced neurodegeneration in polyQ flies [123]; enhanced TDP-43 toxicity in worms [124]. | |
| Other gene manipulation e.g. SIRT1 | SIRT1 mediated suppression of α-synuclein aggregation in mice [125]. | |
| Hsp90 | Hsp90 inhibitors e.g. Geldanamycin,17-AAG, 17-DMAG, HSP990, PU-H71 | Reduction of tau pathology and tau phosphorylation in mice [126, 27]; polyQ in flies [123] [127] but in mice effect was only temporary due to epigenetic changes [128]; SBMA mouse model [129]; SCA3 mouse model [130] effective on mutant SOD1 cells but effect in mice questionable [131]; α-synuclein toxicity in flies [132] [17]; rhodopsin RP in rats [133]. |
| Not defined | Arimoclomol | SOD1 mouse [134] [135] [46]; SBMA mouse model [136]; rhodopsin RP [47]. |
| Celastrol | Mouse model of Aβ [137]; SOD1 mice [138]. | |
| Geranylgeranylacetone | SBMA mice[139]; AD model mice[140]; traumatic brain injury[141] |
4.1 PD
PD is caused by the progressive loss of dopaminergic neurons in the substantia pars compacta. The disease affects around 1% of the population aged 65 or over and is characterised by bradykinesia, rigidity and tremor [14]. A pathological hallmark of PD is the presence of intracellular protein aggregates called Lewy bodies that are primarily composed of ubiquitylated α-synuclein [15]. Hsp70 is the most widely studied molecular chaperone in relation to α-synuclein aggregation, and the overexpression of Hsp70 has been shown to regulate α-synuclein aggregation both in vitro and in vivo (Table 1). In a cell culture model, the overexpression of Hsp70 reduced high molecular weight and detergent insoluble α-synuclein, as well as reducing total α-synuclein levels. This corresponded with a reduction in α-synuclein-induced toxicity [16]. Consistent with these findings, the expression of Hsp70 in an α-synuclein transgenic mouse model was also able to reduce high molecular weight and detergent insoluble species [16]. Further in vivo studies in both Drosophila melanogaster and mice showed that the Hsp70-mediated reduction in α-synuclein aggregation corresponded with an increase in dopaminergic neuron survival, enabling the preservation of striatal dopamine levels [17,18]. The mechanism by which Hsp70 suppresses α-synuclein aggregation is proposed to rely on the inhibition of fibril formation. Huang et al. (2006) showed that Hsp70 retarded the formation of α-synuclein prefibrils by binding to these species to inhibit nuclei formation [19]. Furthermore, Hsp70 was also found to bind to nuclei already present on the prefibrils to prevent their elongation.
4.2 AD
AD is the most common neurodegenerative disease, affecting over 500,000 people in the UK. The disease primarily affects the hippocampus and cerebral cortex regions of the brain, causing impairments in memory, cognitive function and difficulties with language. AD is characterised by the presence of extracellular Aβ plaques and intracellular neurofibrillary tangles of hyperphosphorylated tau. Several chaperones have been shown to modulate Aβ aggregation in vitro. Both αβ-crystallin (HSPB5), a member of the small Hsp family, and DnaJB6 bind to Aβ fibrils, inhibiting their elongation and growth [20,21]. Hsp70, Hsp40 and Hsp90 have also been shown to interact with Aβ peptides, this time acting on Aβ oligomers [22]. Recombinant Hsp70/Hsp40 and Hsp90 significantly slowed the rate of Aβ aggregation, re-directing Aβ into soluble circular structures instead of forming fibrils. However, Hsp70/40 or Hsp90 had little effect on aggregation when added to pre-formed Aβ oligomers or fibrils. Only the combination of all three chaperones was able to significantly alter the structure of pre-formed oligomers. The data supported the concept that the upregulation of a combination of chaperones, rather than individual chaperones, may be more effective in combating Aβ aggregation (Table 2). This concept has been explored through the use of the Hsp90 inhibitor, 17-AAG, which activates the transcription factor HSF1, promoting the expression of a range of chaperones including Hsp70, Hsp40 and Hsp60. The upregulation of these chaperones protected neurons against Aβ toxicity both in vitro and in vivo. In neuronal cultures, 17-AAG restored Aβ-induced damage to dendritic structures [23]. Consistent with the in vitro data, the treatment of mice with 17-AAG protected neurons against Aβ-induced synaptic damage, enhancing long term potentiation and leading to an improvement in memory function [23].
Hsp90 inhibition also protects against tau aggregation (Table 2). Tau is a client of Hsp90, binding via its aggregation prone microtubule binding repeat region [24]. The inhibition of Hsp90 prevents the stabilisation and maturation of tau, resulting in its degradation. In vitro studies have demonstrated that Hsp90 inhibition reduces the steady state levels of tau in addition to decreasing the levels of insoluble aggregated tau [25,26]. The reduction in tau aggregation was abolished in the presence of MG132, confirming that Hsp90 inhibition facilitates the proteasomal degradation of tau [27]. These findings were confirmed in vivo, with 17-AAG treatment reducing insoluble and hyperphoshorylated tau in a mouse model of tauopathy [27].
4.3 HD and polyQ diseases
HD is an autosomal dominant, genetic disease that leads to progressive degeneration in the striatum and cortex of the brain, resulting in impairments in motor, cognitive and psychiatric abilities. HD is caused by the expansion of a CAG triplet repeat region in the gene Huntingtin. The translated protein contains a long stretch of polyglutamines (polyQ), driving huntingtin aggregation. Other CAG expansion diseases also have aggregation prone polyQ expanded polypeptides and show similarities in disease pathogenesis.
The first ever report that molecular chaperones could act as potent modulators of protein aggregation in neurodegeneration was demonstrated by Cummings et al. (1998) in a cellular model of polyQ disease [28]. The overexpression of the DnaJ protein, DnaJA1, reduced the aggregation of polyglutamine expanded ataxin-1. Since this report the role of molecular chaperones in polyQ diseases has been extensively studied. Hsp70 and DnaJ proteins have been shown to suppress polyQ aggregation both in cells and in Drosophila. The expression of Hsp70 and DnaJB1 reduced the assembly of detergent insoluble polyQ fibrils, whilst increasing the formation of detergent soluble aggregates [29,30]. However, these results did not translate to a R6/2 mouse model of HD; Hsp70 overexpression had no significant effect on disease progression [31]. Increasing evidence suggests that DnaJ proteins and small Hsps are more potent suppressors of polyQ aggregation than Hsp70. Four members of the small Hsp family, HSPB6, HSPB7, HSPB8 and HSPB9, were found to significantly reduce polyQ aggregation in vitro [32]. Their protective effect was linked to stimulating the clearance of polyQ aggregates via the UPS or autophagy. For example, Hsp22 (HSPB8) forms a complex with Hsp70 and its co-chaperone Bag3 [33]. Bag3 interacts with the autophagosome receptor p62 [34], enabling the recruitment of Hsp70-Hsp22-Bag3 bound cargo into the autophagosome for degradation. The protective effect of Hsp22 overexpression was suppressed in cells in which Bag3 had been reduced, or alternatively where autophagy was inhibited, implying that Hsp22 reduces neurotoxicity by targeting polyQ for autophagy-mediated degradation [33].
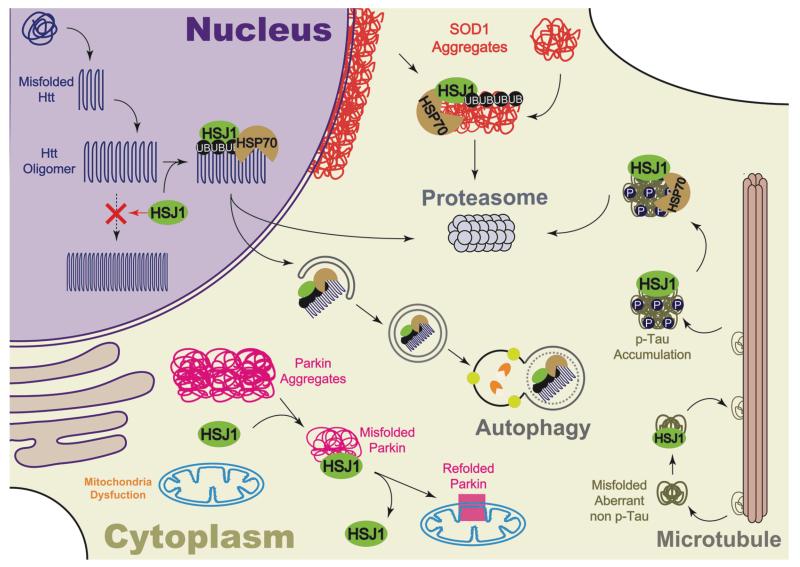
In the case of DnaJ proteins, a subfamily of Type II DnaJ proteins, including DnaJB2, DnaJB6 and DnaJB8, appear to be particularly effective at suppressing polyQ aggregation [35]. The overexpression of the neuronal DnaJ protein HSJ1 (DnaJB2) counteracts polyQ aggregation both in vitro and in vivo (Fig. 1). HSJ1 contains an N-terminal J domain, a client binding domain and two ubiquitin interacting motifs (UIMs) [36,37]. The combination of these domains enables HSJ1 to target some client proteins for proteasomal degradation. HSJ1 is alternatively spliced to produce two isoforms, HSJ1a and HSJ1b, with distinct intracellular localisations mediated by the C-terminal prenylation of the longer HSJ1b isoform [38]. Only the cytosolic and nuclear HSJ1a isoform was able to significantly reduce aggregation in a cellular model expressing polyQ huntingtin, highlighting that the localisation of chaperones is critical for their protective function [37]. The overexpression of HSJ1a was also shown to be neuroprotective in a R6/2 mouse model of HD [39]. HSJ1a expression significantly reduced the aggregation of mutant huntingtin in brain, whilst increasing the level of soluble protein. The mechanism of action was dependent on the association of HSJ1a with K63 ubiquitylated, higher order, detergent insoluble species preventing their ability to nucleate further aggregation (Fig. 1). These changes led to an improvement in neurological performance of the R6/2 mice at a late stage of the disease.
Fig. 1. HSJ1a acts restore proteostasis for several neurodegeneration proteins.
Schematic showing the effect of HSJ1a on Htt, SOD1, parkin and tau in neurons. HSJ1a can bind to ubiquitylated oligomers of Htt in the nucleus blocking the recruitment of more misfolded Htt and further aggregation, leading to increases in soluble Htt and potential autophagic clearance of cytoplasmic Htt oligomers [39]. HSJ1 also facilitates proteasomal degradation of Htt [37]. HSJ1a blocks the aggregation of mutant parkin and stimulates its refolding, so that parkin can function in mitochondrial quality control [95]. In ALS, HSJ1a reduces the aggregation of mutant SOD1 and promotes the degradation by proteasome [45]. HSJ1a can bind tau, reduce tau phosphorylation and aggregation (Novoselov and Cheetham unpublished observations).
4.4 ALS
ALS is the most common form of motor neuron disease, affecting around 2 in every 100,000 in the UK. The disease is caused by the progressive loss of upper and lower motor neurons in the brain, the brainstem and the spinal cord, and is characterised by muscle weakness and atrophy leading to progressive paralysis. Around 20% of familial cases of ALS are caused by mutations in SOD1. Transgenic mice expressing mutant SOD1 develop an ALS-like phenotype, characterised by intracellular SOD1 aggregates, and is the most widely used model to study ALS.
The protective effects of Hsp27 (HSPB1) have been investigated in both cell and animal models of ALS. The overexpression of Hsp27 in neuronal cells stably expressing either SOD1 G93A or G93R had a protective effect against mutant SOD1-induced cell death [40]. Interestingly, this anti-apoptotic effect was enhanced by the overexpression of Hsp70 in conjunction with Hsp27 [40]. In vitro studies suggest that Hsp27 directly interacts with mutant SOD1, reducing aggregation by inhibiting fibril elongation, rather than by inhibiting the formation of fibril nuclei [41]. However, it is argued that, in addition to its chaperone function, the protective effect of Hsp27 may also be attributed to its roles in negatively regulating apoptosis and maintaining redox homeostasis. This is supported by in vitro studies on primary motor neuron cultures that show the overexpression of Hsp27 makes the cells more resistant to pro-apoptotic and oxidative insults [42]. Unfortunately, the role of Hsp27 in these two processes remains poorly characterised so the exact mechanism by which Hsp27 reduces neurotoxicity is not clear. In vivo, the overexpression of Hsp27 protected motor neurons from cell death induced by nerve crush [43]. Furthermore, transgenic mice overexpressing Hsp27 and SOD1G93A showed an improvement in muscle force, reflected by an increase in motor unit numbers and an increase in motor neuron survival in the spinal cord [44]. However, these improvements were only evident at an early stage of the disease and were not sustained over the long term.
In contrast to Hsp27, the overexpression of HSJ1a was able to ameliorate late stage disease in a SOD1G93A mouse model [45]. Overexpression of HSJ1a led to a significant improvement in neuromuscular function, with a preservation of muscle force, an increase in motor unit number and enhanced motor neuron survival. HSJ1a was present in a complex of SOD1G93A and reduced its aggregation at late stages of the disease. In a cell model, HSJ1a stimulated SOD1G93A ubiquitylation and proteasomal degradation in a J domain and UIM dependent manner (Fig. 1). Altered ubiquitin immunoreactivity was observed in the double transgenic mice, suggesting this process may also be occurring in vivo.
Overall the genetic manipulation of molecular chaperones, in particular Hsp70 and Hsp27, has shown limited success in combating ALS disease progression in vivo. However, a different approach that utilises pharmacological agents to modulate the proteostasis network, rather than just one specific chaperone, was more effective in targeting protein aggregation and restoring proteostasis (Table 2). Arimoclomol, a hydroxylamine derivate, amplifies Hsp expression by stabilising the binding of HSF1 to heat shock elements, leading to the upregulation of chaperones including Hsp90, Hsp70 and Hsp60. The treatment of SOD1G93A mice with arimoclomol at pre-symptomatic or early symptomatic stages of the disease delayed disease progression, with a decrease in ubiquitin positive aggregates in the spinal cord and an improvement in muscle function and motor neuron survival at late stages of the disease [46]. This was accompanied by a 22% increase in lifespan. In addition to potentiating the heat shock response, arimoclomol can also enhance the unfolded protein response, potentially expanding the range of applicable diseases [47].
5. Mutations in molecular chaperones cause neurodegenerative disease
The importance of molecular chaperones in maintaining neuronal proteostasis is further highlighted by the identification of mutations in molecular chaperones in familial cases of neurodegenerative disease (Table 3). Interestingly, mutations have been identified in chaperones that are also protective against neurotoxicity when overexpressed in models of disease, such as HSJ1, Hsp22 and Hsp27.
Table 3. Chaperones mutations identified in neurodegenerative diseases.
| Chaperone family | Chaperone | Inheritance | Disease | Reference |
|---|---|---|---|---|
| Hsp70 | HSPA9 (Mortalin) | Dominant | Parkinson’s disease | [142,143] |
| Hsp60 | HSP60 (HSPD) | Dominant | Spastic paraplegia | [144,145] |
| Recessive | Hypomyelinating leukodystrophy | [146] | ||
| CCT4 | Recessive | Hereditary sensory neuropathy | [147] | |
| CCT5 | Recessive | Sensory neuropathy with spastic paraplegia | [148] | |
| Hsp40 (DnaJ) | DNAJB2 (HSJ1) | Recessive | Distal hereditary motor neuropathy, Charcot Marie Tooth disease | [48,49] |
| DNAJB6 (Mrj) | Dominant | Limb-girdle muscular dystrophy | [149-151] | |
| DNAJC3 (ERdj6) | Recessive | Diabetes and multisystemic neurodegeneration | [152] | |
| DNAJC5 (CSPα) | Dominant | Neuronal ceroid lipofusinosis | [153,154] | |
| DNAJC6 (Auxilin) | Recessive | Juvenile Parkinsonism | [155,156] | |
| DNAJC13 (RME-8) | Dominant | Parkinson’s disease | [157] | |
| DNAJC19 (TIM14) | Recessive | Dilated cardiomyopathy and cerebellar ataxia | [158,159] | |
| DNAJC29 (Sacsin) | Recessive | Spastic ataxia of Charlevoix-Saguenay | [160,161] | |
| Small Hsp | HSPB1 (Hsp27) | Dominant and recessive | Distal hereditary motor neuropathy, Charcot Marie Tooth type 2 | [50-56] |
| HSPB3 (HspL27) | Dominant | Hereditary motor neuropathy | [162] | |
| HSPB5 (αβ-crystallin) | Recessive | Infantile muscular dystrophy | [163] | |
| HSPB8 (Hsp22) | Dominant | Distal hereditary motor neuropathy, Charcot Marie Tooth disease | [57] | |
| Chaperone co-factors | SIL1 | Recessive | Cerebellar ataxia | [164] |
| VCP | Dominant | Amyotrophic lateral sclerosis | [165,166] | |
| BAG3 | Dominant | Muscular dystrophy. Giant axonal neuropathy |
[167,168] |
5.1 HSJ1 (DnaJB2)
Distal hereditary motor neuropathies (dHMN) are a clinically and genetically heterogeneous group of disorders caused by the progressive degeneration of the lower motor neurons in the spinal cord. The disease is characterised by muscle weakness and atrophy of the lower limbs. Symptom onset typically occurs in the first two decades of life, and patients typically present with gait abnormalities, paralysis of foot and toe extension and lack of ankle and knee-jerk reflexes. In 2012, Blumen et al. reported mutations in HSJ1 in a consanguineous Moroccan family with dHMN [48]. Recently, a second mutation was identified in a Turkish family also with dHMN [49]. Both mutations are homozygous and are located at donor splice sites, leading to the loss of HSJ1 protein expression. A third mutation in HSJ1 has also been identified recently, this time in a family with Charcot-Marie-Tooth type 2 (CMT2) [49]. CMT2 closely resembles dHMN but patients have sensory abnormalities in addition to motor involvement. The mutation is a substitution of a tyrosine for a cysteine at reside 5 (Y5C). Residue 5 of HSJ1 is located in the J domain and is highly conserved in HSJ1 orthologs across multiple species. Our lab has confirmed the pathogenicity of the HSJ1 Y5C mutation (Heather Smith, unpublished observations). All three mutations identified thus far lead to a loss of HSJ1 function, suggesting that HSJ1 is important for motor neuron survival. Further investigations are required to reveal how the loss of HSJ1 chaperone function leads to motor neuron degeneration.
5.2 Hsp27 (HSPB1) and Hsp22 (HSPB8)
Mutations in Hsp27 (HSPB1) and Hsp22 (HSPB8) have been reported in families with both dHMN and CMT2. 16 mutations in Hsp27 have been identified to date and the majority of mutations identified are missense mutations in the α-crystallin domain [50-57]. The mutations share the common property of inducing protein instability and aggregation. All mutations reported, apart from one, were inherited in an autosomal dominant manner. The dominant inheritance might result from a gain of toxic function, but it is also likely that the loss of chaperone function contributes to the disease mechanism, possibly through dominant negative effects on chaperone oligomers. In addition to their chaperone function, some HSPB proteins can modulate the dynamics of the cytoskeleton by regulating the stabilisation of microtubules and intermediate filaments. Investigations suggest that this regulatory function is impaired in Hsp27 mutants. The expression of mutant Hsp27 in cells was found to result in the sequestration of intermediate filaments into Hsp27 aggregates, leading to the destabilisation of the cytoskeletal network [50,58]. Furthermore, transgenic mice expressing either Hsp27 S135F or P182L exhibit impaired axonal transport of mitochondria, with the mice developing a distal motor neuropathy [59]. The evidence therefore suggests that Hsp27-mediated disruption of axonal transport is an important underlying mechanism of motor neuron degeneration.
Two mutations in Hsp22 have been identified in five separate families with dominant inheritance. The mutations (K141E and K141N) affect lysine residue 141 in the α-crystallin domain and cause aggregation [57]. Interestingly, when expressed in cells, mutant Hsp22 was found to sequester Hsp27 in its aggregates [57]. The loss of Hsp27 may contribute to the degeneration process. It has also been proposed that mutations in Hsp22 cause motor neuron death due to dysfunctions in the removal of misfolded proteins by autophagy [33], a reduction in the ability to remove misfolded protein could be a critical determinant in neuronal toxicity.
5.3 Sacsin
Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) is an early onset recessive neurodegenerative disease caused by the progressive loss of Purkinje cells in the cerebellum, followed by motor neurons in the spinal cord [60]. The disease is caused by mutations in the DnaJ protein sacsin (DnaJC29), with over 170 mutations identified throughout the 520 kDa protein. Sacsin is composed of a N-terminal ubiquitin-like domain that binds to the proteasome [61], followed by three large sacsin repeat regions, suggested to have Hsp90-like chaperone function [62,63], a XPCB domain that binds to protein ligases [64], a C-terminal J domain and a HEPN domain that mediates sacsin dimerisation [61,62,65]. The combination of these domains implies a role in protein quality control. The mutations identified are proposed to lead to loss of sacsin function, however, a full understanding of sacsin function is currently lacking. Sacsin is predominantly localised in the cytoplasm but also shows localisation to the mitochondria. Sacsin interacts with dynamin-related protein 1 (DRP1), a GTPase that mediates mitochondrial fission [66]. Interestingly, in both patient fibroblasts and a sacsin knockout mouse, the mitochondria appear overly fused and show a reduction in mobility [66]. This suggests that sacsin may participate, either directly or indirectly, in mitochondrial fission. Mitochondrial dysfunction is a common feature in many neurodegenerative diseases and is likely to be a key mechanism underlying ARSACS.
6. Conclusions
Neurodegenerative diseases are characterised by disturbances in neuronal proteostasis caused by genetic mutations or alternatively an age-related decline in the proteostasis network. The upregulation of molecular chaperones has been demonstrated to suppress the neurotoxicity associated with protein misfolding and restore proteostasis both in vitro and in vivo. Therefore, molecular chaperones represent important therapeutic targets and their manipulation could potentially slow disease progression. A better understanding of specialised chaperone functions in neurons will assist in the design of new potential therapies based on restoring proteostasis. In addition, it is likely that the study of chaperone mutations in disease will provide important insights into how chaperones function in neurons and will be important in understanding the specific challenges of neuronal proteostasis.
Acknowledgments
Research in the Cheetham lab is supported by the Wellcome Trust, MRC, RP Fighting Blindness and Fight for Sight. WL is a China Scholarship Council (CSC) PhD student. We are grateful to all of the researchers that have contributed to this area and apologize for any missing citations due to limits on article length.
Abbreviations
- 17-AAG
17-Allylamino-17-demethoxygeldanamycin
- 17-DMAG
17-Dimethylaminoethylamino-17-demethoxygeldanamycin
- Aβ
amyloid beta
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- ARSACS
Autosomal recessive spastic ataxia of Charlevoix-Saguenay
- α-syn
α-synuclein
- CMT2
Charcot-Marie-Tooth type 2
- dHMN
distal hereditary motor neuropathies
- DRP1
dynamin-related protein 1
- FTDP-17
Frontotemporal dementia with parkinsonism linked to chromosome 17
- GF
glycine-phenylalanine
- HD
Huntington’s disease
- HPD
histidine-proline-aspartate
- Hsps
Heat shock proteins
- Htt
huntingtin
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NBD
Nucleotide binding domain
- NEFs
nucleotide exchange factors
- PD
Parkinson’s disease
- polyQ
polyglutamine
- p-tau
hyperphosphorylated tau
- Rho
rhodopsin
- SBD
substrate binding domain
- SOD1
superoxide dismutase
- UIMs
ubiquitin interacting motifs
References
- [1].Ellis J. Proteins as molecular chaperones. Nature. 1987;328:378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- [2].Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–333. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- [3].Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- [4].Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Ann. Rev. Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- [5].Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc. Natl. Acad. Sci. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bracher A, Verghese J. GrpE, Hsp110/Grp170, HspBP1/Sil1 and BAG domain proteins: nucleotide exchange factors for Hsp70 molecular chaperones. Subcell. Biochem. 2015;78:1–33. doi: 10.1007/978-3-319-11731-7_1. [DOI] [PubMed] [Google Scholar]
- [7].Kampinga HH, Craig EA. The Hsp70 chaperone machinery; J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cheetham ME, Caplan AJ. Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chap. 1998;3:28–36. doi: 10.1379/1466-1268(1998)003<0028:sfaeod>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nature Struct. Mol. Biol. 2009;16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- [10].Carra S, Crippa V, Rusmini P, Boncoraglio A, Minoia M, Giogetti E, et al. Alterations of protein folding and degradation in motor neuron disease: Implications and protective functions of small heat shock proteins. Prog. Neurobiol. 2011;1159:1–18. doi: 10.1016/j.pneurobio.2011.09.009. [DOI] [PubMed] [Google Scholar]
- [11].Brehme M, Voisine C, Rolland T, Wachi S, Soper JH, Zhu Y, et al. A chaperone subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 2014;9:1135–1150. doi: 10.1016/j.celrep.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, et al. In vivo demonstration that α-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- [14].Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur. J. Epidemiol. 2011;26:S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- [15].Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 reduces α-synuclein aggregation and toxicity. J. Biol. Chem. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- [17].Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- [18].Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J. Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- [19].Huang C, Cheng H, Hao S, Zhou H, Zhang X, Gao J, et al. Heat shock protein 70 inhibits α-synuclein fibril formation via interactions with diverse intermediates. J. Mol. Biol. 2006;364:323–336. doi: 10.1016/j.jmb.2006.08.062. [DOI] [PubMed] [Google Scholar]
- [20].Shammas SL, Waudby CA, Wang S, Buell AK, Knowles TP, Ecroyd H, et al. Binding of the molecular chaperone αβ-crystallin to Aβ amyloid fibrils inhibits fibril elongation. Biophys. J. 2011;101:1681–1689. doi: 10.1016/j.bpj.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mansson C, Arosio P, Hussein R, Kampinga HH, Hashem RM, Boelens WC, et al. Interaction of the molecular chaperone DNAJB6 with growing amyloid-beta 42 (Aβ42) aggregates leads to sub-stoichiometric inhibition of amyloid formation. J. Biol. Chem. 2014;289:31066–31076. doi: 10.1074/jbc.M114.595124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Evans CG, Wisén S,m, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J. Biol. Chem. 2006;281:33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- [23].Chen Y, Wang B, Liu D, Li JJ, Xue Y, Sakata K, et al. Hsp90 chaperone inhibitor 17-AAG attenuates Aβ-induced synaptic toxicity and memory impairment. J. Neurosci. 2014;34:2464–2470. doi: 10.1523/JNEUROSCI.0151-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Karagöz GE, Duarte AM, Akoury E, Ippel H, Biernat J, Morán Luengo T, et al. Hsp90-Tau complex reveals molecular basis for specificity in chaperone action. Cell. 2014;156:963–974. doi: 10.1016/j.cell.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, et al. Chaperones increase association of tau protein with microtubules. Proc. Natl. Acad. Sci. USA. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, et al. CHIP and Hsp70 regulate tau ubiquitylation, degradation and aggregation. Hum. Mol. Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- [27].Luo W, Dou F, Rodina A, Chip S, Kim J, Zhao Q, et al. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc. Natl. Acad. Sci. USA. 2007;104:9511–9516. doi: 10.1073/pnas.0701055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat. Genet. 1998;19:148–154. doi: 10.1038/502. [DOI] [PubMed] [Google Scholar]
- [29].Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. Hsp70 and Hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc. Natl. Acad. Sci. USA. 2000;97:7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kazemi-Esfarjani P, Benzer S. Genetic suppression of polyglutamine toxicity in Drosophila. Science. 2000;287:1837–1840. doi: 10.1126/science.287.5459.1837. [DOI] [PubMed] [Google Scholar]
- [31].Hansson O, Nylandsted J, Castilho RF, Leist M, Jäättelä M, Brundin P. Overexpression of heat shock prptein 70 in R6/2 Huntington’s disease mice has only modest effects on disease progression. Brain Res. 2003;970:47–57. doi: 10.1016/s0006-8993(02)04275-0. [DOI] [PubMed] [Google Scholar]
- [32].Vos MJ, Zijlstra MP, Kanon B, Van Waarde-Verhagen MA, Brunt ER, Oosterveld-Hut HM, et al. HSPB7 is the most potent polyQ aggregation suppressor within the HSPB family of molecular chaperones. Hum. Mol. Genet. 2010;19:4677–4693. doi: 10.1093/hmg/ddq398. [DOI] [PubMed] [Google Scholar]
- [33].Carra S, Sequin SJ, Lambert H, Landry J. HspB8 chaperone activity towards polyQ-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J. Biol. Chem. 2008;283:1437–1444. doi: 10.1074/jbc.M706304200. [DOI] [PubMed] [Google Scholar]
- [34].Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009;28:889–901. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hageman J, Rujano MA, van Waarde M.A, Kakkar, V., Dirks RP, Govorukhina N, et al. A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol. Cell. 2010;37:355–369. doi: 10.1016/j.molcel.2010.01.001. [DOI] [PubMed] [Google Scholar]
- [36].Cheetham ME, Brion JP, Anderton BH. Human homologues of the bacterial heat-shock protein DnaJ are preferentially expressed in neurons. Biochem. J. 1992;284:469–476. doi: 10.1042/bj2840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Westhoff B, Chapple JP, van der Spuy J, Höhfeld J, Cheetham ME. HSJ1 is a neuronal shuttling factor for the sorting of chaperone clients to the proteasome. Curr. Biol. 2005;15:1058–1064. doi: 10.1016/j.cub.2005.04.058. [DOI] [PubMed] [Google Scholar]
- [38].Chapple JP, Cheetham ME. The chaperone environment at the cytoplasmic face of the endoplasmic reticulum can modulate rhodopsin processing and inclusion formation. J. Biol. Chem. 2003;278:19087–19094. doi: 10.1074/jbc.M212349200. [DOI] [PubMed] [Google Scholar]
- [39].Labbadia J, Novoselov SS, Bett JS, Weiss A, Paganetti P, Bates GP, et al. Suppression of protein aggregation by chaperone modification of high molecular weight complexes. Brain. 2012;135:1180–1196. doi: 10.1093/brain/aws022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Patel YJ, Payne Smith MD, de Belleroche J, Latchman DS. Hsp27 and Hsp70 administered in combination have a potent protective effect against fALS-associated SOD1-mutant-induced cell death in mammalian neuronal cells. Brain Res. Mol. Brain Res. 2005;134:256–274. doi: 10.1016/j.molbrainres.2004.10.028. [DOI] [PubMed] [Google Scholar]
- [41].Yerbury JJ, Gower D, Vanags L, Roberts K, Lee JA, Ecroyd H. The small heat shock proteins αβ-crystallin and Hsp27 suppress SOD1 aggregation in vitro. Cell Stress Chaperones. 2013;18:251–257. doi: 10.1007/s12192-012-0371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kalmar B, Kieran D, Greensmith L. Molecular chaperones as therapeutic targets in amyotrophic lateral sclerosis. Biochem. Soc. Trans. 2005;33:551–552. doi: 10.1042/BST0330551. [DOI] [PubMed] [Google Scholar]
- [43].Sharp P, Krishnan M, Pullar O, Navarrete R, Wells D, de Belleroche J. Heat shock protein 27 rescues motor neurons following nerve injury and preserves muscle function. Exp. Neurol. 2006;198:511–518. doi: 10.1016/j.expneurol.2005.12.031. [DOI] [PubMed] [Google Scholar]
- [44].Sharp PS, Akbar MT, Bouri S, Senda A, Joshi K, Chen HJ, et al. Protective effects of heat shock protein 27 in a model of ALS occur in the early stages of disease progression. Neurobiol. Dis. 2008;1:42–55. doi: 10.1016/j.nbd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- [45].Novoselov S, Mustill W, Gray AL, Dick JR, Kanuga N, Kalmar B, et al. Molecular chaperone mediated late-stage neuroprotection in the SOD1 (G93A) mouse model of amyotrophic lateral sclerosis. PLoS One. 2013;8:e73944. doi: 10.1371/journal.pone.0073944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat. Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- [47].Parfitt DA, Aguila M, McCulley CH, Bevilacqua D, Mendes HF, Athanasiou D, et al. The heat-shock response co-inducer arimoclomol protects against retinal degeneration in rhodopsin retinitis pigmentosa. Cell Death Dis. 2014;5:e1236. doi: 10.1038/cddis.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Blumen S, Astord S, Robin V, Vignaud L, Toumi N, Cieslik A, et al. A rare recessive distal hereditary motor neuropathy with HSJ1 chaperone mutation. Ann. Neurol. 2012;71:509–519. doi: 10.1002/ana.22684. [DOI] [PubMed] [Google Scholar]
- [49].Gess B, Auer-Grumbach M, Schirmacher A, Strom T, Zitzelsberger M, Rudnik-Schöneborn S, et al. HSJ1-related hereditary neuropathies: novel mutations and an extended clinical spectrum. Neurology. 2014;83:1–7. doi: 10.1212/WNL.0000000000000966. [DOI] [PubMed] [Google Scholar]
- [50].Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, et al. Mutant small heat-shock protein 27 causes axonal Charcot–Marie–Tooth disease and distal hereditary motor neuropathy. Nat. Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- [51].Houlden H, Laura M, Wavrant-De Vrieze F, Blake J, Wood N, Reilly MM. Mutations in the HSP27 (HSPB1) gene cause dominant, recessive, and sporadic distal HMN/CMT type 2. Neurology. 2008;71:1660–1668. doi: 10.1212/01.wnl.0000319696.14225.67. [DOI] [PubMed] [Google Scholar]
- [52].Ikeda Y, Abe A, Ishida C, Takahashi K, Hayasaka K, Yamada M. A clinical phenotype of distal hereditary motor neuronopathy type II with a novel HSPB1 mutation. J. Neurol. Sci. 2009;277:9–12. doi: 10.1016/j.jns.2008.09.031. [DOI] [PubMed] [Google Scholar]
- [53].James PA, Rankin J, Talbot K. Asymmetrical late onset motor neuropathy associated with a novel mutation in the small heat shock protein HSPB1 (HSP27) J. Neurol. Neurosurg. Psychiatry. 2008;79:461–463. doi: 10.1136/jnnp.2007.125179. [DOI] [PubMed] [Google Scholar]
- [54].Kijima K, Numakura C, Goto T, Takahashi T, Otagiri T, Umetsu K, et al. Small heat shock protein 27 mutation in a Japanese patient with distal hereditary motor neuropathy. J.Hum. Genet. 2005;50:473–476. doi: 10.1007/s10038-005-0280-6. [DOI] [PubMed] [Google Scholar]
- [55].Luigetti M, Fabrizi GM, Madia F, Ferrarini M, Conte A, Del Grande A, et al. A novel HSPB1 mutation in an Italian patient with CMT2/dHMN phenotype. J. Neurol. Sci. 2010;298:114–117. doi: 10.1016/j.jns.2010.09.008. [DOI] [PubMed] [Google Scholar]
- [56].Mandich P, Grandis M, Varese A, Geroldi, Acquaviva M, Ciotti P, et al. Severe neuropathy after diphtheria-tetanus-pertussis vaccination in a child carrying a novel frame-shift mutation in the small heat-shock protein 27 gene. J. Child. Neurol. 2010;25:107–109. doi: 10.1177/0883073809334387. [DOI] [PubMed] [Google Scholar]
- [57].Irobi J, Van Impe K, Seeman P, Jordanova A, Dierick I, Verpoorten N, et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat. Genet. 2004;36:597–601. doi: 10.1038/ng1328. [DOI] [PubMed] [Google Scholar]
- [58].Zhai J, Lin H, Julien JP, Schlaepfer WW. Disruption of neurofilament network with aggregation of light neurofilament protein: a common pathway leading to motor neuron degeneration due to Charcot Marie Tooth disease-linked mutations in NFL and HSPB1. Hum. Mol. Genet. 2007;16:3103–3116. doi: 10.1093/hmg/ddm272. [DOI] [PubMed] [Google Scholar]
- [59].d’Ydewalle C, Krishnan J, Chiheb DM, Van Damme P, Irobi J, Kozikowski AP, et al. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot Marie Tooth disease. Nat. Med. 2011;17:968–974. doi: 10.1038/nm.2396. [DOI] [PubMed] [Google Scholar]
- [60].Bouchard JP, Barbeau A, Bouchard R, Bouchard RW. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Can. J. Neurol. Sci. 1978;5:61–69. [PubMed] [Google Scholar]
- [61].Parfitt DA, Michael GJ, Vermeulen EG, Prodromou NV, Webb TR, Gallo JM, et al. The ataxia protein sacsin is a functional co-chaperone that protects against polyglutamine-expanded ataxin-1. Hum. Mol. Genet. 2009;18:1556–1565. doi: 10.1093/hmg/ddp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Anderson JF, Siller E, Barral JM. The sacsin repeating region (SRR): A novel Hsp90-related supra-domain associated with neurodegeneration. J. Mol. Biol. 2010;400:665–674. doi: 10.1016/j.jmb.2010.05.023. [DOI] [PubMed] [Google Scholar]
- [63].Anderson JF, Siller E, Barral JM. The neurodegenerative-disease-related protein sacsin is a molecular chaperone. J. Mol. Biol. 2011;411:870–880. doi: 10.1016/j.jmb.2011.06.016. [DOI] [PubMed] [Google Scholar]
- [64].Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kozlov G, Denisov AY, Girard M, Dicaire M, Hamlin J, McPherson PS, et al. Structural basis of defects in the sacsin HEPN domain responsible for autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) J. Biol. Chem. 2011;286:20407–20412. doi: 10.1074/jbc.M111.232884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Girard M, Larivière R, Parfitt DA, Deane EC, Gaudet R, Nossova N, et al. Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) Proc. Natl. Acad. Sci. USA. 2012;109:1661–1666. doi: 10.1073/pnas.1113166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Song Y, Nagy M, Ni W, Tyagi NK, Fenton WA, Lopez-Giraldez F, et al. Molecular chaperone Hsp110 rescues a vesicle transport defect produced by an ALS-associated mutant SOD1 protein in squid axoplasm. Proc. Natl. Acad. Sci. U S A. 2013;110:5428–5433. doi: 10.1073/pnas.1303279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kuo Y, Ren S, Lao U, Edgar BA, Wang T. Suppression of polyglutamine protein toxicity by co-expression of a heat-shock protein 40 and a heat-shock protein 110. Cell Death Dis. 2013;4:e833. doi: 10.1038/cddis.2013.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Eroglu B, Moskophidis D, Mivechi NF. Loss of Hsp110 leads to age-dependent tau hyperphosphorylation and early accumulation of insoluble amyloid beta. Mol. Cell. Biol. 2010;30:4626–4643. doi: 10.1128/MCB.01493-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yamashita H, Kawamata J, Okawa K, Kanki R, Nakamizo T, Hatayama T, et al. Heat-shock protein 105 interacts with and suppresses aggregation of mutant Cu/Zn superoxide dismutase: clues to a possible strategy for treating ALS. J. Neurochem. 2007;102:1497–1505. doi: 10.1111/j.1471-4159.2007.04534.x. [DOI] [PubMed] [Google Scholar]
- [71].Veereshwarayya V, Kumar P, Rosen KM, Mestril R, Querfurth HW. Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular beta-amyloid-induced inhibition of complex IV and limit apoptosis. J. Biol. Chem. 2006;281:29468–29478. doi: 10.1074/jbc.M602533200. [DOI] [PubMed] [Google Scholar]
- [72].Yang Y, Turner RS, Gaut JR. The chaperone BiP/GRP78 binds to amyloid precursor protein and decreases Abeta40 and Abeta42 secretion. J. Biol. Chem. 1998;273:25552–25555. doi: 10.1074/jbc.273.40.25552. [DOI] [PubMed] [Google Scholar]
- [73].Jiang Y, Lv H, Liao M, Xu X, Huang S, Tan H, et al. GRP78 counteracts cell death and protein aggregation caused by mutant huntingtin proteins. Neurosci. Lett. 2012;516:182–187. doi: 10.1016/j.neulet.2012.03.074. [DOI] [PubMed] [Google Scholar]
- [74].Gorbatyuk MS, Shabashvili A, Chen W, Meyers C, Sullivan LF, Salganik M, et al. Glucose regulated protein 78 diminishes alpha-synuclein neurotoxicity in a rat model of Parkinson disease. Mol. Ther. 2012;20:1327–1337. doi: 10.1038/mt.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Athanasiou D, Kosmaoglou M, Kanuga N, Novoselov SS, Paton AW, Paton JC, et al. BiP prevents rod opsin aggregation. Mol. Biol. Cell. 2012;23:3522–3531. doi: 10.1091/mbc.E12-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gorbatyuk MS, Knox T, LaVail MM, Gorbatyuk OS, Noorwez SM, Hauswirth WW, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc. Natl. Acad. Sci. U S A. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jinwal UK, O’Leary JC, Borysov SI, Jones JR, Li Q, Koren J, et al. Hsc70 rapidly engages tau after microtubule destabilization. J. Biol. Chem. 2010;285:16798–16805. doi: 10.1074/jbc.M110.113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sarkar M, Kuret J, Lee G. Two motifs within the tau microtubule-binding domain mediate its association with the hsc70 molecular chaperone. J. Neurosci. Res. 2008;86:2763–2773. doi: 10.1002/jnr.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bauer PO, Goswami A, Wong HK, Okuno M, Kurosawa M, Yamada M, et al. Harnessing chaperone-mediated autophagy for the selective degradation of mutant huntingtin protein. Nat. Biotechnol. 2010;28:256–263. doi: 10.1038/nbt.1608. [DOI] [PubMed] [Google Scholar]
- [80].Wong SL, Chan WM, Chan HY. Sodium dodecyl sulfate-insoluble oligomers are involved in polyglutamine degeneration. FASEB J. 2008;22:3348–3357. doi: 10.1096/fj.07-103887. [DOI] [PubMed] [Google Scholar]
- [81].Gunawardena S, Her LS, Brusch RG, Laymon RA, Niesman IR, Gordesky-Gold B, et al. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron. 2003;40:25–40. doi: 10.1016/s0896-6273(03)00594-4. [DOI] [PubMed] [Google Scholar]
- [82].Redeker V, Pemberton S, Bienvenut W, Bousset L, Melki R. Identification of protein interfaces between alpha-synuclein, the principal component of Lewy bodies in Parkinson disease, and the molecular chaperones human Hsc70 and the yeast Ssa1p. J. Biol. Chem. 2012;287:32630–32639. doi: 10.1074/jbc.M112.387530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pemberton S, Madiona K, Pieri L, Kabani M, Bousset L, Melki R. Hsc70 protein interaction with soluble and fibrillar alpha-synuclein. J. Biol. Chem. 2011;286:34690–34699. doi: 10.1074/jbc.M111.261321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Urushitani M, Kurisu J, Tateno M, Hatakeyama S, Nakayama K, Kato S, et al. CHIP promotes proteasomal degradation of familial ALS-linked mutant SOD1 by ubiquitinating Hsp/Hsc70. J. Neurochem. 2004;90:231–244. doi: 10.1111/j.1471-4159.2004.02486.x. [DOI] [PubMed] [Google Scholar]
- [85].Hoshino T, Murao N, Namba T, Takehara M, Adachi H, Katsuno M, et al. Suppression of Alzheimer’s disease-related phenotypes by expression of heat shock protein 70 in mice. J. Neurosci. 2011;31:5225–5234. doi: 10.1523/JNEUROSCI.5478-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].McLear JA, Lebrecht D, Messer A, Wolfgang WJ. Combinational approach of intrabody with enhanced Hsp70 expression addresses multiple pathologies in a fly model of Huntington’s disease. FASEB J. 2008;22:2003–2011. doi: 10.1096/fj.07-099689. [DOI] [PubMed] [Google Scholar]
- [87].Shimshek DR, Mueller M, Wiessner C, Schweizer T, van der Putten PH. The HSP70 molecular chaperone is not beneficial in a mouse model of alpha-synucleinopathy. PLoS One. 2010;5:e10014. doi: 10.1371/journal.pone.0010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Koyama S, Arawaka S, Chang-Hong R, Wada M, Kawanami T, Kurita K, et al. Alteration of familial ALS-linked mutant SOD1 solubility with disease progression: its modulation by the proteasome and Hsp70. Biochem. Biophys. Res. Commun. 2006;343:719–730. doi: 10.1016/j.bbrc.2006.02.170. [DOI] [PubMed] [Google Scholar]
- [89].Liu J, Shinobu LA, Ward CM, Young D, Cleveland DW. Elevation of the Hsp70 chaperone does not effect toxicity in mouse models of familial amyotrophic lateral sclerosis. J. Neurochem. 2005;93:875–882. doi: 10.1111/j.1471-4159.2005.03054.x. [DOI] [PubMed] [Google Scholar]
- [90].Estes PS, Boehringer A, Zwick R, Tang JE, Grigsby B, Zarnescu DC. Wild-type and A315T mutant TDP-43 exert differential neurotoxicity in a Drosophila model of ALS. Hum. Mol. Genet. 2011;20:2308–2321. doi: 10.1093/hmg/ddr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Abisambra JF, Jinwal UK, Suntharalingam A, Arulselvam K, Brady S, Cockman M, et al. DnaJA1 antagonizes constitutive Hsp70-mediated stabilization of tau. J. Mol. Biol. 2012;421:653–661. doi: 10.1016/j.jmb.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wyttenbach A, Carmichael J, Swartz J, Furlong RA, Narain Y, Rankin J, et al. Effects of heat shock, heat shock protein 40 (HDJ-2), and proteasome inhibition on protein aggregation in cellular models of Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2000;97:2898–2903. doi: 10.1073/pnas.97.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ormsby AR, Ramdzan YM, Mok YF, Jovanoski KD, Hatters DM. A platform to view huntingtin exon 1 aggregation flux in the cell reveals divergent influences from chaperones hsp40 and hsp70. J. Biol. Chem. 2013;288:37192–37203. doi: 10.1074/jbc.M113.486944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Howarth JL, Kelly S, Keasey MP, Glover CP, Lee YB, Mitrophanous K, et al. Hsp40 molecules that target to the ubiquitin-proteasome system decrease inclusion formation in models of polyglutamine disease. Mol. Ther. 2007;15:1100–1105. doi: 10.1038/sj.mt.6300163. [DOI] [PubMed] [Google Scholar]
- [95].Rose JM, Novoselov SS, Robinson PA, Cheetham ME. Molecular chaperone-mediated rescue of mitophagy by a Parkin RING1 domain mutant. Hum. Mol. Genet. 2011;20:16–27. doi: 10.1093/hmg/ddq428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gillis J, Schipper-Krom S, Juenemann K, Gruber A, Coolen S, van den Nieuwendijk R, et al. The DNAJB6 and DNAJB8 protein chaperones prevent intracellular aggregation of polyglutamine peptides. J. Biol. Chem. 288:17225–17237. doi: 10.1074/jbc.M112.421685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Athanasiou D, Bevilacqua D, Aguila M, McCulley C, Kanuga N, Iwawaki T, et al. The co-chaperone and reductase ERdj5 facilitates rod opsin biogenesis and quality control. Hum. Mol. Genet. 2014;23:6594–6606. doi: 10.1093/hmg/ddu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, de Waal RM, Verbeek MM. Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res. 2006;1089:67–78. doi: 10.1016/j.brainres.2006.03.058. [DOI] [PubMed] [Google Scholar]
- [99].Toth ME, Szegedi V, Varga E, Juhasz G, Horvath J, Borbely E, et al. Overexpression of Hsp27 ameliorates symptoms of Alzheimer’s disease in APP/PS1 mice. Cell Stress Chaperones. 2013;18:759–771. doi: 10.1007/s12192-013-0428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Abisambra JF, Blair LJ, Hill SE, Jones JR, Kraft C, Rogers J, et al. Phosphorylation dynamics regulate Hsp27-mediated rescue of neuronal plasticity deficits in tau transgenic mice. J. Neurosci. 2010;30:15374–15382. doi: 10.1523/JNEUROSCI.3155-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wyttenbach A, Sauvageot O, Carmichael J, Diaz-Latoud C, Arrigo AP, Rubinsztein DC. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum. Mol. Genet. 2002;11:1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- [102].Perrin V, Regulier E, Abbas-Terki T, Hassig R, Brouillet E, Aebischer P, et al. Neuroprotection by Hsp104 and Hsp27 in lentiviral-based rat models of Huntington’s disease. Mol. Ther. 2007;15:903–911. doi: 10.1038/mt.sj.6300141. [DOI] [PubMed] [Google Scholar]
- [103].Zourlidou A, Gidalevitz T, Kristiansen M, Landles C, Woodman B, Wells DJ, et al. Hsp27 overexpression in the R6/2 mouse model of Huntington’s disease: chronic neurodegeneration does not induce Hsp27 activation. Hum. Mol. Genet. 2007;16:1078–1090. doi: 10.1093/hmg/ddm057. [DOI] [PubMed] [Google Scholar]
- [104].Bruinsma IB, Bruggink KA, Kinast K, Versleijen AA, Segers-Nolten IM, Subramaniam V, et al. Inhibition of alpha-synuclein aggregation by small heat shock proteins. Proteins. 2011;79:2956–2967. doi: 10.1002/prot.23152. [DOI] [PubMed] [Google Scholar]
- [105].Outeiro TF, Klucken J, Strathearn KE, Liu F, Nguyen P, Rochet JC, et al. Small heat shock proteins protect against alpha-synuclein-induced toxicity and aggregation. Biochem. Biophys. Res. Commun. 2006;351:631–638. doi: 10.1016/j.bbrc.2006.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Krishnan J, Vannuvel K, Andries M, Waelkens E, Robberecht W, Van Den Bosch L. Over-expression of Hsp27 does not influence disease in the mutant SOD1(G93A) mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2008;106:2170–2183. doi: 10.1111/j.1471-4159.2008.05545.x. [DOI] [PubMed] [Google Scholar]
- [107].Crippa V, Sau D, Rusmini P, Boncoraglio A, Onesto E, Bolzoni E, et al. The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS) Hum. Mol. Genet. 2010;19:3440–3456. doi: 10.1093/hmg/ddq257. [DOI] [PubMed] [Google Scholar]
- [108].Wilhelmus MM, de Waal RM, Verbeek MM. Heat shock proteins and amateur chaperones in amyloid-Beta accumulation and clearance in Alzheimer’s disease. Mol. Neurobiol. 2007;35:203–216. doi: 10.1007/s12035-007-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Xu G, Fromholt S, Ayers JI, Brown H, Siemienski Z, Crosby KW, et al. Substantially elevating the levels of alphaB-crystallin in spinal motor neurons of mutant SOD1 mice does not significantly delay paralysis or attenuate mutant protein aggregation. J. Neurochem. 2014 doi: 10.1111/jnc.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Cameron RT, Quinn SD, Cairns LS, MacLeod R, Samuel ID, Smith BO, et al. The phosphorylation of Hsp20 enhances its association with amyloid-beta to increase protection against neuronal cell death. Mol. Cell Neurosci. 2014;61:46–55. doi: 10.1016/j.mcn.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wu Y, Cao Z, Klein WL, Luo Y. Heat shock treatment reduces beta amyloid toxicity in vivo by diminishing oligomers. Neurobiol. Aging. 2010;31:1055–1058. doi: 10.1016/j.neurobiolaging.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sahara N, Murayama M, Mizoroki T, Urushitani M, Imai Y, Takahashi R, et al. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J. Neurochem. 2005;94:1254–1263. doi: 10.1111/j.1471-4159.2005.03272.x. [DOI] [PubMed] [Google Scholar]
- [113].Al-Ramahi I, Lam YC, Chen HK, de Gouyon B, Zhang M, Perez AM, et al. CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J. Biol. Chem. 2006;281:26714–26724. doi: 10.1074/jbc.M601603200. [DOI] [PubMed] [Google Scholar]
- [114].Kalia LV, Kalia SK, Chau H, Lozano AM, Hyman BT, McLean PJ. Ubiquitinylation of alpha-synuclein by carboxyl terminus Hsp70-interacting protein (CHIP) is regulated by Bcl-2-associated athanogene 5 (BAG5) PLoS One. 2011;6:e14695. doi: 10.1371/journal.pone.0014695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Jinwal UK, Trotter JH, Abisambra JF, Koren J, 3rd, Lawson LY, Vestal GD, et al. The Hsp90 kinase co-chaperone Cdc37 regulates tau stability and phosphorylation dynamics. J. Biol. Chem. 2011;286:16976–16983. doi: 10.1074/jbc.M110.182493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jinwal UK, Abisambra JF, Zhang J, Dharia S, O’Leary JC, Patel T, et al. Cdc37/Hsp90 protein complex disruption triggers an autophagic clearance cascade for TDP-43 protein. J. Biol. Chem. 2012;287:24814–24820. doi: 10.1074/jbc.M112.367268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Elliott E, Tsvetkov P, Ginzburg I. BAG-1 associates with Hsc70.Tau complex and regulates the proteasomal degradation of Tau protein. J. Biol. Chem. 2007;282:37276–37284. doi: 10.1074/jbc.M706379200. [DOI] [PubMed] [Google Scholar]
- [118].Kermer P, Kohn A, Schnieder M, Lingor P, Bahr M, Liman J, et al. BAG1 is Neuroprotective in In Vivo and In Vitro Models of Parkinson’s Disease. J. Mol. Neurosci. 2015;55:587–595. doi: 10.1007/s12031-014-0396-2. [DOI] [PubMed] [Google Scholar]
- [119].Carra S, Boncoraglio A, Kanon B, Brunsting JF, Minoia M, Rana A, et al. Identification of the Drosophila ortholog of HSPB8: implication of HSPB8 loss of function in protein folding diseases. J. Biol. Chem. 2010;285:37811–37822. doi: 10.1074/jbc.M110.127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Pierce A, Podlutskaya N, Halloran JJ, Hussong SA, Lin PY, Burbank R, et al. Over-expression of heat shock factor 1 phenocopies the effect of chronic inhibition of TOR by rapamycin and is sufficient to ameliorate Alzheimer’s-like deficits in mice modeling the disease. J. Neurochem. 2013;124:880–893. doi: 10.1111/jnc.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Fujimoto M, Takaki E, Hayashi T, Kitaura Y, Tanaka Y, Inouye S, et al. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J. Biol. Chem. 2005;280:34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- [122].Lin PY, Simon SM, Koh WK, Folorunso O, Umbaugh CS, Pierce A. Heat shock factor 1 over-expression protects against exposure of hydrophobic residues on mutant SOD1 and early mortality in a mouse model of amyotrophic lateral sclerosis. Mol. Neurodegener. 2013;8:43. doi: 10.1186/1750-1326-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Fujikake N, Nagai Y, Popiel HA, Okamoto Y, Yamaguchi M, Toda T. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J. Biol. Chem. 2008;283:26188–26197. doi: 10.1074/jbc.M710521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ash PE, Zhang YJ, Roberts CM, Saldi T, Hutter H, Buratti E, et al. Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum. Mol .Genet. 2010;19:3206–3218. doi: 10.1093/hmg/ddq230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Donmez G, Arun A, Chung CY, McLean PJ, Lindquist S, Guarente L. SIRT1 protects against alpha-synuclein aggregation by activating molecular chaperones. J. Neurosci. 2012;32:124–132. doi: 10.1523/JNEUROSCI.3442-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [126].Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Agrawal N, Pallos J, Slepko N, Apostol BL, Bodai L, Chang LW, et al. Identification of combinatorial drug regimens for treatment of Huntington’s disease using Drosophila. Proc. Natl. Acad. Sci. USA. 2005;102:3777–3781. doi: 10.1073/pnas.0500055102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Labbadia J, Cunliffe H, Weiss A, Katsyuba E, Sathasivam K, Seredenina T, et al. Altered chromatin architecture underlies progressive impairment of the heat shock response in mouse models of Huntington disease. J. Clin. Invest. 2011;121:3306–3319. doi: 10.1172/JCI57413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Tokui K, Adachi H, Waza M, Katsuno M, Minamiyama M, Doi H, et al. 17-DMAG ameliorates polyglutamine-mediated motor neuron degeneration through well-preserved proteasome function in an SBMA model mouse. Hum. Mol. Genet. 2009;18:898–910. doi: 10.1093/hmg/ddn419. [DOI] [PubMed] [Google Scholar]
- [130].Silva-Fernandes A, Duarte-Silva S, Neves-Carvalho A, Amorim M, Soares-Cunha C, Oliveira P, et al. Chronic treatment with 17-DMAG improves balance and coordination in a new mouse model of Machado-Joseph disease. Neurotherapeutics. 2014;11:433–449. doi: 10.1007/s13311-013-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cha JR, St Louis KJ, Tradewell ML, Gentil BJ, Minotti S, Jaffer ZM, et al. A novel small molecule HSP90 inhibitor, NXD30001, differentially induces heat shock proteins in nervous tissue in culture and in vivo. Cell Stress Chaperones. 2014;19:421–435. doi: 10.1007/s12192-013-0467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Auluck PK, Meulener MC, Bonini NM. Mechanisms of Suppression of alpha-Synuclein Neurotoxicity by Geldanamycin in Drosophila. J. Biol. Chem. 2005;280:2873–2878. doi: 10.1074/jbc.M412106200. [DOI] [PubMed] [Google Scholar]
- [133].Aguila M, Bevilacqua D, McCulley C, Schwarz N, Athanasiou D, Kanuga N, et al. Hsp90 inhibition protects against inherited retinal degeneration. Hum. Mol. Genet. 2014;23:2164–2175. doi: 10.1093/hmg/ddt613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kalmar B, Novoselov S, Gray A, Cheetham ME, Margulis B, Greensmith L. Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1 mouse model of ALS. J. Neurochem. 2008;107:339–350. doi: 10.1111/j.1471-4159.2008.05595.x. [DOI] [PubMed] [Google Scholar]
- [135].Kalmar B, Lu CH, Greensmith L. The role of heat shock proteins in Amyotrophic Lateral Sclerosis: The therapeutic potential of Arimoclomol. Pharmacol. Ther. 2014;141:40–54. doi: 10.1016/j.pharmthera.2013.08.003. [DOI] [PubMed] [Google Scholar]
- [136].Malik B, Nirmalananthan N, Gray AL, La Spada AR, Hanna MG, Greensmith L. Co-induction of the heat shock response ameliorates disease progression in a mouse model of human spinal and bulbar muscular atrophy: implications for therapy. Brain. 2013;136:926–943. doi: 10.1093/brain/aws343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Paris D, Ganey NJ, Laporte V, Patel NS, Beaulieu-Abdelahad D, Bachmeier C, et al. Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer’s disease. J. Neuroinflammation. 2010;7:17. doi: 10.1186/1742-2094-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener. Dis. 2005;2:246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- [139].Katsuno M, Sang C, Adachi H, Minamiyama M, Waza M, Tanaka F, et al. Pharmacological induction of heat-shock proteins alleviates polyglutamine-mediated motor neuron disease. Proc. Natl. Acad. Sci. USA. 2005;102:16801–16806. doi: 10.1073/pnas.0506249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Hoshino T, Suzuki K, Matsushima T, Yamakawa N, Suzuki T, Mizushima T. Suppression of Alzheimer’s disease-related phenotypes by geranylgeranylacetone in mice. PLoS One. 2013;8:e76306. doi: 10.1371/journal.pone.0076306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zhao Z, Faden AI, Loane DJ, Lipinski MM, Sabirzhanov B, Stoica BA. Neuroprotective effects of geranylgeranylacetone in experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 2013;33:1897–1908. doi: 10.1038/jcbfm.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].De Mena L, Coto E, Sánchez-Ferrero E, Ribacoba R, Guisasola LM, Salvador C, et al. Mutational screening of the mortalin gene (HSPA9) in Parkinson’s disease. J. Neural. Transm. 2009;116:1289–1293. doi: 10.1007/s00702-009-0273-2. [DOI] [PubMed] [Google Scholar]
- [143].Freimann K, Zschiedrich K, Brüggemann N, Grünewald A, Pawlack H, Hagenah J, et al. Mortalin mutations are not a frequwnt cause of early-onset Parkinson disease. Neurobiol. Aging. 2013;34:e19–20. doi: 10.1016/j.neurobiolaging.2013.05.021. [DOI] [PubMed] [Google Scholar]
- [144].Hansen JJ, Dürr A, Cournu-Rebeix I, Georgopoulos C, Ang D, Nielsen MN, et al. Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. Am. J. Hum. Genet. 2002;70:1328–1332. doi: 10.1086/339935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Bross P, Naundrup S, Hansen J, Nielsen MN, Christensen JH, Kruhoffer M, et al. The Hsp60-(p.V98I) mutation associated with hereditary spastic paraplegia SPG13 compromises chaperonin function both in vitro and in vivo. J. Biol. Chem. 2008;283:15694–15700. doi: 10.1074/jbc.M800548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Magen D, Georgopoulos C, Bross P, Ang D, Segev Y, Goldsher D, et al. Mitochondrial hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am. J. Hum. Genet. 2008;83:30–42. doi: 10.1016/j.ajhg.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lee MJ, Stephenson DA, Groves MJ, Sweeney MG, Davis MB, An SF, et al. Hereditary sensory neuropathy is caused by a mutation in the delta subunit of the cytosolic chaperonin-containing t-complex peptide-1 (Cct4) gene. Hum. Mol. Genet. 2003;12:1917–1925. doi: 10.1093/hmg/ddg198. [DOI] [PubMed] [Google Scholar]
- [148].Bouhouche A, Benomar A, Bouslam N, Chkili T, Yahyaoui M. Mutation in the epsilon subunit of the cytosolic chaperonin-containing t-complex peptide-1 (Cct5) gene causes autosomal recessive mutilating sensory neuropathy with spastic paraplegia. J. Med. Genet. 2006;43:441–443. doi: 10.1136/jmg.2005.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Harms MB, Sommerville RB, Allred P, Bell S, Ma D, Cooper P, et al. Exome sequencing reveals DNAJB6 mutations in dominantly-inherited myopathy. Ann. Neurol. 2012;71:407–416. doi: 10.1002/ana.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Sarparanta J, Jonson PH, Golzio C, Sandell S, Lugue H, Screen M, et al. Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-gridle muscular dystrophy. Nat. Genet. 2012;44:450–455. doi: 10.1038/ng.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Sato T, Hayashi YK, Oya Y, Kondo T, Sugie K, Kaneda D, et al. DNAJB6 myopathy in an Asian cohort and cytoplasmic/nuclear inclusions. Neuromuscul. Disord. 2013;23:269–276. doi: 10.1016/j.nmd.2012.12.010. [DOI] [PubMed] [Google Scholar]
- [152].Synofzik M, Haack TB, Kopajtich R, Gorza M, Rapaport D, Greiner M, et al. Absence of BiP co-chaperone DNAJC3 causes diabetes mellitus and multisystemic neurodegeneration. Am. J. Hum. Genet. 2014;95:689–697. doi: 10.1016/j.ajhg.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Benitez BA, Alvarado D, Cai Y, Mayo K, Chakraverty S, Norton J, et al. Exome-sequencing confirms DNAJC5 mutations as cause of adult neuronal-ceroidlipofuscinosis. PLoS One. 2011;6:e26741. doi: 10.1371/journal.pone.0026741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Nosková L, Stranecky V, Hartmannova H, Pristoupilova A, Baresova V, Ivanek R, et al. Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. Am. J. Hum. Genet. 2011;89:241–252. doi: 10.1016/j.ajhg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim Y, Zenvirt S, et al. A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS one. 2012;7:e36458. doi: 10.1371/journal.pone.0036458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Köroğlu C, Baysal L, Cetinkaya M, Karasoy H, Tolun A. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Parkinsonism Relat. Disord. 2013;19:320–324. doi: 10.1016/j.parkreldis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- [157].Vilariño-Güell C, Rajput A, Milnerwood AJ, Shah B, Szu-Tu C, Trinh J, et al. DNAJC13 mutations in Parkinson’s disease. Hum. Mol. Genet. 2014;23:1794–1801. doi: 10.1093/hmg/ddt570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Davey KM, Parboosingh JS, McLeod DR, Chan A, Casey R, Ferreira P, et al. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, cause DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. J. Med. Genet. 2006;43:385–393. doi: 10.1136/jmg.2005.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Ojala T, Polinati P, Manninen T, Hiippala A, Rajantie J, Karikoski R, et al. New mutation of mitochondrial DNAJC19 causing dilated and noncompaction cardiomyopathy, anemia, ataxia and male genital anomalies. Pediatr. Res. 2012;72:432–437. doi: 10.1038/pr.2012.92. [DOI] [PubMed] [Google Scholar]
- [160].Engert JC, Berube P, Mercier J, Dore C, Lepage P, Ge B, et al. ARSACS, a spastic ataxia common in northeastern Quebec, is caused by mutations in a new gene encoding an 11.5 kb ORF. Nat. Genet. 2000;24:12–125. doi: 10.1038/72769. [DOI] [PubMed] [Google Scholar]
- [161].Thiffault I, Dicaire MJ, Tetreault M, Huang KN, Demer-Lamarche J, Bernard G, et al. Diversity of ARSACS mutations in French-Canadians. Can. J. Neurol. Sci. 2013;40:61–66. doi: 10.1017/s0317167100012968. [DOI] [PubMed] [Google Scholar]
- [162].Kolb SJ, Snyder PJ, Poi EJ, Renard EA, Bartlett A, Gu S, et al. Mutant small heat shock protein B3 causes motor neuropathy: utility of a candidate gene approach. Neurology. 2010;74:502–506. doi: 10.1212/WNL.0b013e3181cef84a. [DOI] [PubMed] [Google Scholar]
- [163].Del Bigio MR, Chudley AE, Sarnet HB, Campbell C, Goobie S, Chodirker BN, et al. Infantile muscular dystrophy in Canadian aboriginals is an αβ-crystallinopathy. Ann. Neurol. 2011;69:866–871. doi: 10.1002/ana.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Senderek J, Krieger M, Stendel C, Bergmann C, Moser M, Breitbach-Faller N, et al. Mutations in SIL1 cause Marinesco-Sjögren syndrome, a cerebellar ataxia with cateract and myopathy. Nat. Genet. 2005;37:1312–1314. doi: 10.1038/ng1678. [DOI] [PubMed] [Google Scholar]
- [165].Johnson JO, Mandrioli J, Benatar Y, Abramzon VM, Van Deerlin JQ, Trojanowski JR, et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–864. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Gonzalez-Perez P, Cirulli VE, Drory R, Dabby P, Nisipeanu RL, Carasso M, et al. Novel mutation in VCP gene caused atypical amyotrophic lateral sclerosis. Neurology. 2012;79:2201–2208. doi: 10.1212/WNL.0b013e318275963b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV, et al. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann. Neuol. 2009;65:83–89. doi: 10.1002/ana.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Jaffer F, Murphy SM, Scoto M, Healy E, Rossor AM, Brandner S, et al. BAG3 mutations: another cause of giant axonal neuropathy. J. Peripher. Nerv. Syst. 2012;17:210–216. doi: 10.1111/j.1529-8027.2012.00409.x. [DOI] [PubMed] [Google Scholar]