Abstract
Background and Aims
Increasing prescription stimulant abuse among youth without diagnoses of attention deficit hyperactivity disorder (ADHD) is of concern. The most frequently cited motive for abuse is improved academic achievement via neurocognitive enhancement. Our aim in reviewing the literature was to identify neurocognitive effects of prescription stimulants in non-ADHD youth.
Methods
A systematic review was conducted for youth aged 12–25 years using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Fourteen papers were included.
Results
Modafinil appears to improve reaction time (P ≤ 0.04), logical reasoning (P ≤ 0.05) and problem-solving. Methylphenidate appears to improve performance in novel tasks and attention-based tasks (P ≤ 0.05), and reduces planning latency in more complex tasks (P ≤ 0.05). Amphetamine has been shown to improve consolidation of information (0.02 ≥ P ≤ 0.05), leading to improved recall. Across all three types of prescription stimulants, research shows improved attention with lack of consensus on whether these improvements are limited to simple versus complex tasks in varying youth populations.
Conclusions
The heterogeneity of the non-attention deficit hyperactivity disorder youth population, the variation in cognitive task characteristics and lack of replication of studies makes assessing the potential global neurocognitive benefits of stimulants among non-attention deficit hyper-activity disorder youth difficult; however, some youth may derive benefit in specific cognitive domains.
Keywords: Abuse, cognitive, enhancement, healthy, misuse, neurocognitive, non-ADHD, stimulants, youth
INTRODUCTION
A concerning trend among youth is the abuse of prescription stimulants [PS] [1], defined by the National Institute on Drug Abuse as ‘the intentional use of a medication without a prescription, in a way other than prescribed or for the experience or feeling it causes [2]’. It has been reported that 1.1 million people in the United States over the age of 12 years abuse PS [3], with 7.6% of high-school students abusing dextroamphetamine and 2.6% abusing methylphenidate [1]. There are few data to date on the prevalence of prescription stimulant abuse worldwide, but the World Health Organization has identified increasing diversion of these substances in the United States [4].
The increase in illicit use of PS has become a medical and public health concern [5,6], and is of great public interest [7–9]. Approximately 30%, or 11.4 million, of prescriptions written annually for children with attention deficit hyperactive disorder (ADHD) are diverted to those without a diagnosis of ADHD and used in ways other than intended [6]. Youth aged 12–25 years represent approximately 80% of ADHD stimulant abusers. Eighteen- to 25-year-olds are at greatest risk for misusing [10]. Data have shown that the prevalence of abuse nation-wide of PS by children and adolescents in grade school to high school in the past year is between 5 and 9% [11]. Past-year prevalence in college students may be as high as 35% [11]. Additionally, up to 10% of youth aged 12–25 years who have abused PS in the past year may meet criteria for substance dependence [10]. These numbers are distressing, as the long-term consequences of such abuse are largely unknown. Because the diversion of prescriptions occurs in such a normative context, generally at school, between friends and peers, and at such a high prevalence within certain success-driven environments, it may seem less serious than drug use stemming from more marginalized environments.
Motives frequently reported by youth who abuse stimulants include attaining a high and/or neurocognitive enhancement, where cognitive enhancement is defined as ‘the amplification or extension of core capacities of the mind through improvement or augmentation of internal or external information processing systems [12]’ through medical means without therapeutic intentions [13]. Studies have shown that of those students who abuse stimulants, approximately 60% are motivated by need to study [14], with 58% reporting need for improved concentration and 43% improved alertness [15].
Despite the growing body of literature highlighting the abuse of PS and reasons for which it occurs, there are no studies to date investigating the efficacy of PS in neurocognitive enhancement across all three types of PS in healthy [non-ADHD, no medical/psychiatric comorbidity(ies)] youth aged 12–25 years. Given this, this paper aims to review the cognitive effects of PS in healthy youth by stimulant type (amphetamine, methylphenidate and modafinil) and the methodological rigor of studies examining cognitive effects. Our aim is to assess the use of PS for the purpose of neuroenhancement, not for recreation or other non-medical purposes, as the preponderance of evidence highlights academic achievement as the primary motive for PS abuse. Off-label use in children below the age of 12 years is beyond the scope of this study, as we wish to explore the self-initiated abuse in youth of an age commensurate with greater autonomy. Additionally, this review will not delve into cognitive enhancement in youth with ADHD or those who are subsyndromal, nor will we explore the distinction between cognitive enhancement in a normative population versus those diagnosed with ADHD or a learning disability, as these topics have been addressed in other studies. Also, we will not address the multitude of ethical concerns and issues that may arise as this, too, has been addressed in previous reviews.
METHODS
This paper is a review of the current literature on the neurocognitive effects of psychostimulants on healthy youth age 12–25 years using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. We searched Embase, Medline and PsychInfo for relevant literature. Reference lists from relevant studies were further searched for papers of interest.
The sample Search String PubMed was as follows: (((((prescription stimulant abuse) OR prescription stimulant misuse) OR nonmedical prescription stimulant) AND cognitive) OR neurocognitive) AND enhancement—127.
For a study to be included in this review, the participants had to be devoid of psychiatric/medical diagnoses (including ADHD or any other substance abuse/ dependence; i.e. ‘healthy’) and must not be taking any psychotropic medications at the time of study implementation in order to clearly capture a ‘healthy’ population reflective of those who have been shown to misuse prescription stimulants for purposes of cognitive enhancement. Additionally, participants’ mean age had to be 25 years or younger to capture the population that has been shown to have the highest rates of abuse of PS. Studies that included subjects older than 25 years (up to 30 years of age), but whose mean age was under 25, were included in order to increase the breadth of data. Studies had to incorporate a validated assessment of neurocognition as an outcome measure, with the variable of interest being impact of one of three types of PS: amphetamine, methylphenidate or modafinil. Additional eligibility criteria included studies written in the English language, or with English translations.
Studies were mined independently and manually for demographic information, study design, type of PS (amphetamine, methylphenidate or modafinil) and cognitive tasks. Assessment of bias was completed for each study based on the limitations of each investigation reviewed. Study characteristics that were evaluated included sample size, demographics (age, gender and race), use of standardized, validated or widely recognized cognitive measures and study design, all of which may have an effect on the overall conclusions proposed in this paper.
Summary measures recounted here consist of values reported by individual studies and include P-values, effect sizes, difference in means and risk ratios. Results of the studies reviewed were synthesized qualitatively, as individual statistical results (i.e. analyses used and manner of reporting data) varied among studies.
RESULTS
Search results
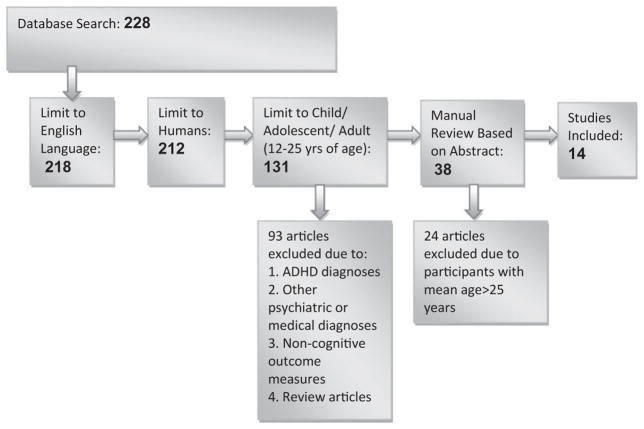
The search included all published studies, with papers from 1978 to 2012 meeting the criteria. Our initial search yielded 228 papers. Ten papers were published in non-English languages with no identifiable English translations, another six using animal models and 81 evaluating adults aged 25 years and older. The 131 remaining papers were screened manually for inclusion. Ninety-three were excluded based on inclusion of participants with ADHD diagnoses, other psychiatric or medical comorbidities, lack of cognitive outcome measures and/or review papers. Of the 38 remaining papers, 14 were selected for inclusion. The 24 that were excluded had populations outside the desired age range (Fig. 1).
Figure 1.
Methods
Expectancy
The expectation of certain drug/medication effects can elicit subjective effects without actual administration of the drug [16], and can initiate drug-seeking and consumption behaviors [17]. This has been theorized to contribute to reports of PS efficacy in healthy individuals [18]. Mitchell and colleagues explored the effect of dextroamphetamine expectancy on subjective experience in stimulant-naive youth. Those who expected PS experienced greater arousal (P < 0.05) and drug effects (P < 0.05) and liked what they felt (P < 0.01), despite receiving placebo [17]. Looby & Earlywine showed that the expectation of receiving methylphenidate is associated with subjective reports of drug effects, including dysphoria (P < 0.01), feeling more ‘high’ (P < 0.01) and ‘stimulated’ (P < 0.01) and greater intellectual energy and performance efficiency (P < 0.05) [19]. However, this did not translate into improved performance, as youth who consumed sham methylphenidate did not demonstrate any differences on cognitive indices compared to controls [19]. This indicates that there may be an element of social learning in expectancy of PS efficacy which translates to subjective effects, but not performance effects.
Utility
Much of the research conducted on the efficacy, utility and adverse effects of PS has been conducted within individuals diagnosed with ADHD receiving their medications at Food and Drug Administration (FDA)-approved doses and within the indicated clinical context. The studies that are available in non-ADHD youth tend to focus upon the cognitive areas that are targeted by PS in youth with ADHD, including attention, response inhibition and working memory.
Koelega demonstrated that the cognitive benefit of PS in those without a diagnosis of ADHD is largely an attentional one, which is mediated by improved reaction-time [20]. He found that there is no impact on response inhibition, planning or other executive functions. Additionally, both Advokat [20] and Koelega [21] found that while attending to more cognitively simple tasks PS may result in increased attention in adults. However, when engaged in a complex task stimulants might actually hinder selective attention, such that they may serve as an impediment to executive functioning. Alternatively, Greely and colleagues suggest that PS may enhance executive functioning in many healthy individuals [22]. They suggest that dextroamphetamine and methylphenidate increase the flexibility of response in tasks that require pre-frontal cortex functioning and that modafinil increases inhibitory control [22,23], and thus accuracy of response [24].
Modafinil
Studies examining the effect of modafinil on cognition included subjects in late adolescence and/or young adulthood, but not school-aged children. Marchant and colleagues [25] demonstrated that, in challenging conditions, modafinil allows for rapid shifts in constant attention on tasks with short interstimulus intervals (ISI) of 950 (P = 0.02). In tasks of alternating attention, when a working memory component was added, increasing the difficulty of the task, performance was enhanced on both short (950 ISI; P = 0.026) and long (1850 ISI; P = 0.03) intervals. Modafinil users demonstrated a trend towards decreased alertness from trials 1 to 2, so the cognitive effects of the drug were not attributed to arousal state [25]. No effect on speed of processing was found [25].
Modafinil may also improve neurocognition on selective tasks that involve the use of perceptual judgment and complex addition after periods of sleep deprivation [26]. However, it may lead to a lasting (P < 0.05) over-confident self-assessment of performance, pre-(mean overconfidence = 9.57%, P < 0.0001) and post-(mean overconfidence = 9.49%, P < 0.0001) visual–perceptual judgment task and following (mean overconfidence = 12.58%, P < 0.05) a mental additional task (with a working memory component) that does not correspond to performance accuracy [26]. Participants believed that improvements would be greater than they were, which the authors contributed to increased vigor [26]. In non-sleep-deprived participants, modafinil does not appear to induce over-confidence in cognitive abilities (although the authors found a trend towards overconfidence in post-task self-monitoring measures), while still improving cognitive performance in logical reasoning (accuracy and response times), vigilance and serial reaction time [27]. Additionally, Turner and colleagues determined that cognitive enhancement with modafinil shows a specific pattern of improvements in adaptive response inhibition [24]. In this study, the investigators used a well-validated, and oft-used, battery of tests to evaluate cognition, the Cambridge Neuropsychological Test Automated Battery (CANTAB), the Tower of London (ToL) and the Wechsler Adult Intelligence Scale–Revised (WAIS-R). Those who took modafinil demonstrated greater subjective attention and alertness, greater objective accuracy with regard to recall (digit span 0.005 > Ps < 0.05), visual memory (P = 0.010) and spatial planning (P = 0.002), and decreased latency on short-term memory tasks and a risk–reward paradigm (P = 0.038) [24]. The authors also found a dose-related improvement in response inhibition (P = 0.001) and accuracy (P = 0.027) on a stop/go task for modafinil 100 versus 200 mg [24].
Modafinil may negate the effects of sleep deprivation when sustained alertness and performance are sought [28]. In a study comparing the efficacy of dextroamphetamine, modafinil and caffeine on performance accuracy after sleep deprivation, the modafinil group demonstrated fewer perseverative errors compared to placebo, amphetamine and caffeine, exhibiting greater ability to shift appropriately from a previously reinforced strategy to a new successful strategy to solve problems, and improved ability to plan and problem-solve with less impulsivity relative to caffeine and placebo [29].
Methylphenidate
With methylphenidate, Elliott and colleagues found that accuracy increased on spatial tasks, including short-term and working memory, and planning and adaptation, but noted no improvements in verbal fluency or attentional-set shifting [30]. Relative enhancement by methylphenidate on spatial planning and working memory in the first session compared to the second led to improved performance in novel situations, but a deficit in planning latency, resulting in an increased number of incorrect responses [30]. This finding has been corroborated in several adult studies [31,32], demonstrating that methylphenidate may increase speed of response by acting on areas involved in output instead of enhancing evaluation and processing of information. In contrast, Rogers and colleagues demonstrated that healthy youth receiving methylphenidate have significantly increased response latency (P < 0.005) on both intra-(P < 0.01) and extra-dimensional (P < 0.05) shifts, which increased with evolving tasks [33]. These youth also demonstrated improved extra-dimensional discrimination flexibility (P < 0.05), such that they were better able to shift attention to novel characteristics of stimuli with fewer errors in task response [33]. However, there was a trend towards a greater number of errors on tasks that required intradimensional discriminative learning that did not require shifts in attention (P < 0.07) [33].
Youth who took methylphenidate 90–150 minutes prior to cognitive tasks were more aware of their errors (P < 0.008) and demonstrated greater response inhibition (P = 0.009) on a go/no-go task while in a magnetic resonance imaging (MRI) scanner [34]. Methylphenidate was associated with greater activity in right middle frontal, left insula and right inferior frontal brain regions. When these participants were aware of errors, there was increased activity in the dorsal anterior cingulate cortex (dACC) and left inferior parietal lobe [34]. This corresponds to the hypothesis that methylphenidate increases striatal dopamine, which transmits error signals to the dACC via the basal ganglia to promote conscious awareness of errors [18,35].
Finally, Linssen and colleagues found that methylphenidate 20 and 40 mg increased delayed recall (90–270 minutes after drug administration) of words in a verbal memory task, but not immediate recall [36]. They also demonstrated dose-dependent improvements in a set-shifting task that resulted in faster reaction time and improved performance accuracy, especially in trials requiring auditory attention, and non-reward aspects of the task. Overall, methylphenidate improved consolidation of declarative memory, attention and response inhibition in a dose-dependent fashion [36].
Amphetamine
Seminal research conducted by Rapoport and colleagues demonstrated improved vigilance and acoustic learning in a sample of school-aged children [37,38]. These youth demonstrated improved sustained attention, with a significant decrease in amount of errors of omission (P < 0.05) on a vigilance task and improved free (P = 0.025) and cued (P = 0.03) recall of previously learned information 30–150 minutes after administration of dextroamphetamine [37]. Language performance was also improved, as measured by task-directed phrases (increased; P = 0.05) and non-task-directed questions per minute (decreased; P = 0.01) on voice recordings of the children telling a story, describing a picture and instructing a listener on how to create a specific block design [37]. In comparison to young adults administered high- and low-dose amphetamine, hyperactive children demonstrated reduced vigilance (P < 0.05), as measured by commission errors and shorter mean storytelling time compared to the high-dose group [38]., With regard to omission errors, however, in the hyperactive group vigilance was comparable to age-matched ‘normal’ boys. With regard to speech communication, hyperactive boys and low-dose young men demonstrated relative improvements with decreased non-task-related speech [38].
Amphetamine may also have a positive effect on consolidation such that there is increased duration of retention of previously acquired knowledge, without a demonstrable effect on the acquisition of knowledge [39]. Differences in recall between amphetamine and placebo have been shown to increase with length of delay, such that the longer the delay (tested up to 24 hours) the greater the effect of amphetamine (P < 0.01). Amphetamine improves recall as soon as 1 hour post-administration (P < 0.05), but not immediately after drug administration [39]. Amphetamine has also been shown to improve vigilance when attending to specific tasks, with some preventative effect on natural decreases in attention that are maintained despite fatigue or prolonged task duration [20].
In a study of creativity, healthy young adults were given dextroamphetamine to evaluate the effects of stimulants on this aspect of cognition [40]. Convergent creativity was measured using the remote association task (RAT) and the group embedded figures task, which have objective correct and incorrect answers. Divergent creativity was assessed using the alternative uses task and the drawing portion of the abbreviated Torrance test and scored based on fluency, originality, elaboration, flexibility and criterion-referenced indicators of creativity. The authors found that dextroamphetamine improved convergent creativity in those with poorer baseline creative performance on remote association (P < 0.001) and group embedded figures (P = 0.03) tasks and impaired convergent creativity in those with higher baseline performance [40]. However, they also found that, regardless of baseline creativity, those who took dextroamphetamine performed better on the group embedded figures task (P = 0.027) [40].
Ilieva and colleagues examined the effects of mixed amphetamine salts alone and catechol O-methyltransferase (COMT) genotype alone and with amphetamine in university students on 13 domains of cognition; scholastic achievement, intelligence, memory (episodic, working and the ability to maintain and update information in working memory despite interference), creativity and inhibitory control [41]. There was no overall enhancing effect of amphetamine on cognition; however, amphetamine improved word recall (P = 0.02), convergent creativity (P = 0.01) and non-verbal intelligence (P = 0.03) scores in those with low baseline scores [41]. There was a tendency towards worse performance on cognitive tasks for those with high baseline scores. Additionally, valine–valine COMT genotype was associated with improvements in the scholastic assessment test (SAT) mathematics score (P < 0.02) when taking amphetamine [41]. COMT has been shown to metabolize endogenous dopamine, thus affecting levels of synaptic dopamine and influencing the effects of amphetamine on the brain [42] (Table 1).
Table 1.
Results.
| Study | Design, a medication and dose | Participants (mean age) | b Cognitive tasks | Results of stimulant administration |
|---|---|---|---|---|
| Modafinil Baranski & Pigeau 1997 [26] | Double-blind, placebo-controlled; Ml 300 mg versus DA 20 mg versus placebo | 39 M, 2 F (24 years) | Judgment accuracy (JA) and self-monitored cognitive performance (SMCP):
|
|
| Baranski et al. 2004 [27] | Double-blind, placebo-controlled, within-subjects; MI 4 mg/kg versus placebo | 18 M (24.2 ± 6.4 years) | 1. Four-choice serial reaction time; 2. MA, 3; Detection of repeated numbers vigilance task; 4. Logical reasoning; 5. PCT; 6. Self-monitoring |
|
| Killgore et al. 2009 [29] | Double-blind, placebo-controlled; MI 400 mg versus DA 20 mg versus caffeine 600 mg versus placebo | 29 M, 25 F (23.5 ± 4 years) |
|
|
| Marchant et al. 2009 [25] | Double-blind, placebo-controlled, between-subjects; MI 200 mg versus placebo | 7 M, 17 F (22.48 ± 0.65 years): MI (12), mean age = 22.4 ± 3.13; placebo (12), mean age = 22.5 ± 2.94 |
|
Attention shift: (a) constant ↑ target detection at 950 ISI (P = 0.02); (b) alternating ↑ target detection at 950 ISI (P = 0.026) and 1850 ISI (P = 0.03) |
| Turner et al. 2003 [24] | Double-blind, placebo-controlled, between-subjects; Ml 100 mg versus Ml 200 mg versus placebo | 60 M; placebo (20), mean age = 25.30 ± 5.09 years; Ml 100 mg (20), mean age = 24.35 ± 3.28 years; Ml 200 mg (20), mean age = 25.10 ± 4.61 years |
|
MI versus placebo: alertness (P = 0.001), subjective attention (P = 0.020), forwards (P = 0.004) and backwards (P = 0.047) DS, forwards DS score (P = 0.026), DMTS (P = 0.031), PRM % correct (P = 0.010), ToL mean attempts (P = 0.002), gambling deliberation time (P = 0.038) 100 versus 200 mg: stop trial RT (P = 0.001), go trial accuracy (P = 0.027) |
| Methylphenidate Elliott et al. 1997 [30] | Placebo-controlled, within-subjects, counter-balanced cross-over; 1st test session: MP 20 mg versus placebo 2nd test session: placebo versus MP 40 mg |
28 M (21.25 ± 1.84 years) |
|
↑ Subjective alertness F(1,26) = 6.5, P < 0.05; ↓ subjective tiredness F(1,26) = 13.4, P < 0.001; spatial span: session order × drug F(1,26) = 4.87, P < 0.05; spatial working memory: session order × drug F(1,26) = 7.45, P < 0.01; ↓ RVIP latency (P = 0.001); drug × ToL difficulty level (session 1) F(3,78) = 4.34, P < 0.01; ↑ ToL accuracy (1st session new and old; P < 0.01); ↑ ToL accuracy (1st session new; P < 0.01); ↓ planning latency (2nd session; P < 0.05); ↑ sequence generation (P < 0.01) |
| Hester et al. 2012 [34] | Randomized, placebo-controlled, cross-over design; MP 30 mg versus ATM 60 mg versus CIT 30 mg versus placebo | 27 M (22 years) |
|
MP versus placebo: (a) ↑ awareness of errors (P < 0.008); (b) ↑ response inhibition (P = 0.009); (c) ↑ activity right middle frontal, left insula and right inferior frontal; (d) ↑ activity left inferior parietal (aware errors); (e) ↑ activity dACC (unaware errors) |
| Linssen et al. 2012 [36] | Double-blind, placebo-controlled, four-way cross-over; MP 10 mg versus 20 mg versus 40 mg versus placebo | 19 M (23.4 ± 5.4 years) |
|
|
| Rogers et al. 1999 [33] | Double-blind, between-subjects; MP 40 mg versus Cl 1.5 μg/kg versus Lo-Trp | 79 MP (16), 20.4 ± 0.4 years; Cl (8), 24.5 ± 2.0 years; Lo-Trp (15), 27.8 ± 1.6 years, placebo (40), 23.7 ± 0.7 years | Intra-/extra-dimensional shift (ID/ED) task | MP: ↓ ED shift errors (compared to placebo and lo-Trp) F(3,67) = 4.4, P < 0.05; compared to all other groups, MP: no ↑ ED shift errors compared to ID errors F(3,67) = 4, P < 0.05, ↑ response latencies F(3,65) = 4.9, P < 0.005; MP ↑ response latencies over ID and ED shifts F(1,29) = 10.4, P < 0.005 |
| Amphetamine Farah et al. 2009 [40] | Double-blind, placebo-controlled, within-subject; AS 10 mg versus placebo | 16 (21.25 ± 0.45 years) |
|
Improvement on GEF (P = 0.027); significant improvement on RAT (P < 0.001) and GEF (P = 0.003) in lower placebo performance/lower baseline creativity cohort |
| Ilieva et al. 2013 [41] | Double-blind, placebo-controlled, cross-over; AS 20 mg versus placebo | 22 M, 24 F (24 ± 2.88 years) |
|
|
| Rapoport et al. 1978 [37] | Double-blind, placebo-controlled, cross-over; DA 0.5 m/kg (10–23 mg) versus placebo | 14 M (10 years, 1 month ± 2 years, 1 month) |
|
↓ Omission errors (↑ vigilance; P < 0.05), ↑ task-related descriptive speech (P = 0.05), ↓ non-task-related speech (P = 0.01) ↑ free (P = 0.25) and total (P = 0.03) recall |
| Rapoport et al. 1980 [38] | Double-blind, placebo-controlled, cross-over; DA children, 0.5 mg/kg: ‘normal’, 15.80 ± 3.90, ‘hyperactive’, 16.17 ± 4.60; DA young adults, 0.23 ± 0.02–0.45 ± 0.04 mg/kg: high dose, 34.0 ± 2.00; low dose,17.00 ± 0.99 | 14 ‘normal’ M (10.1 ± 2.1 years) and 15 ‘hyperactive’ M (9.44 ± 2.12) versus 15 high-dose M (22.50 ± 2.80) and 16 low-dose M (22.20 ± 3.7) |
|
Compared to placebo:
|
| Soetens et al. 1995c [39] | Double-blind, counter-balanced, within-subjects; Exp. 1: 3 sessions, (a) no pill, (b) DA 10 mg, (c) placebo Exp. 2: 4 (a,b) DA 10 mg pre- (a) and post- (b) learning, (c,d) placebo Exp. 3: 2, (a) DA 10 mg, (b) placebo Exp. 4: 4, (a,b) DA 10 mg with 1 s (a) word presentation time or 4 s (b), (c,d) placebo Exp. 5: 2-DA 10 mg versus placebo |
18 M (19–25 years) | Exp 1: IFR, FRF, DFR Exp 2: IFR, FRF, FRH, DFR Exp 3: IFR, FRT, DFR Exp 4: IFR, FRF, DFR, DFR2, DFR3 Exp 5: 3 recognition tests with differing words; IMF, DFR, FRW |
Exp. 1: ↑ recall F(2,24) = 4.68, P < 0.02, no effect on IFR, trend towards sig ↑ FFR, ↑DFR F(2,34) = 5.34, P < 0.01; drug × delay for 1st 16/20 words F(2,34) = 5.09, P < 0.02 Exp. 2: DA pre-learning-F(1,10) = 8.12, P < 0.02: ↑ FRH-t(13) = 2.43, P < 0.05, ↑ DFR-t(13) = 2.53, P < 0.05 ↑ FRH and DFR; word recall: DA (1 h 260, 24 h 218) versus placebo (1 h 219, 24 h 182) Exp. 3: drug × delay F(1,10) = 6.48, P < 0.03, ↑ DFR F(1,11) = 13.11, P < 0.005; word recall: DA (20 m 226, 24 h 205) versus placebo (20 m 199, 24 h 143) Exp. 4: ↑ FR in 4 s versus 1 s; ↑ FRF (in 1 s) F(1,11) = 5.87, P < 0.05; ↑ DFR in 4 s (1 day) F(1,11) = 14.29, P < 0.003 (2 days) F(1,110) = 8.78, P = 0.013 and (3 days) F(1,11) = 5.93, P = 0.033 Exp. 5: DA: ↓ false (+) in FRW-t(11) = 2.363, P < 0.05 |
Medications (alphabetical order): AS = amphetamine salts; ATM = atomoxetine; CIT = citalopram; Cl = clonidine; DA = dextroamphetamine; Lo-Trp = low-dose tryptophan; Ml = modafinil; MP = methylphenidate.
Cognitive tests and what they measure (in order of appearance in table): perceptual comparison task (PCT): visual perceptual judgment; mental addition (MA): memory; psychomotor vigilance task: reaction time, psychomotor speed; Tower of London (ToL): spatial planning, sequencing, visuospatial working memory; Tower of Hanoi (ToH): spatial planning; Wisconsin Card Sorting Task (WCST): ability to form abstract concepts, learn from feedback and shift mental set; National Adult Reasoning Test (NART)-IQ, digit symbol substitution task (DSST): processing speed; attention shift task: constant and alternating visual and auditory attention; Weschler Adult Intelligence Scale–Revised (WAIS-R)-IQ; Cambridge Neuropsychological Test Automated Battery (CANTAB); (a) pattern recognition (PRM): visual memory, (b) paired associates learning; (c) delayed matching to sample (DMTS); (d) spatial working memory; (e) spatial span task-planning; (f) rapid visual information processing: sustained attention; (g) itntradimensional/extra-dimensional (ID/ED) paradigm: attentional set-shifting; (h) sequence generation; Gambling Task: decision-making; stop signal/go-no-go response inhibition, motor impulsivity; error awareness task (EAT): conscious error recognition; Rey’s auditory learning test: declarative memory; set-shifting task: cognitive flexibility and influence of reward; object relocation: spatial working memory; alternate uses: divergent thinking; remote association task (RAT): convergent thinking (insightful problem solving); group embedded figures (GEF): convergent thinking; abbreviated Torrance drawing task: divergent thinking; face memory: episodic memory; digit span: working memory; object-2-back: ability to maintain and update working memory despite interference; Hanker-response inhibition; Raven’s advanced progressive matrices (RAPM): non-verbal intelligence; Rosvold’s continuous performance task (RCPT): vigilance.
Soetens et al. DFR = delayed free recall (24 hours after; 2, after 2 days; 3, after 3 days); FRF = free recall after 5 minutes; FRH = free recall after 1 hour; FRt = free recall after 20 minutes; FRW = free recall after 1 week; IFR = immediate free recall; ToL = Tower of London (spatial planning); RT = reaction time; WAIS-R = Weschler Adult Intelligence Scale–Revised; M = male; F =female.
DISCUSSION
Although PS are touted for neurocognitive enhancement, translating to enhanced academic performance, the expectations and perceptions of performance of those who abuse these drugs may exceed the actual efficacy. Modafinil appears to have some effect on complex learning during both sleep-deprived and alert states. Modafinil users may, more efficiently, plan, sequence and engage working memory and improve decision-making skills and adaptive response inhibition. On tasks of complex reasoning, modafinil demonstrates efficacy in decreasing perseverative errors, improving ability to form abstract concepts and learn from feedback in order to make appropriate shifts in behavioral responses. Modafinil may also reduce impulsivity by increasing motor response latency in simple tasks. However, it may lead to overconfident assessment of cognitive capabilities such that users may be unable to self-monitor actual achievement accurately.
Methylphenidate also appears to have some effect on higher-order cognitive processes; however, there seem to be environmental and task limitations. Declarative memory, cognitive flexibility and increased response time and accuracy on auditory tasks show improvements for up to 4.5 hours after methylphenidate ingestion. Also, improvements in spatial tasks utilizing skills of planning and adaptation and memory have been shown in novel situations. Methylphenidate appears to have a dual but contradictory effect on cognitive enhancement such that it improves performance in unfamiliar tasks, but results in a deficit in planning latency and increased impulsivity leading to poorer performance in familiar tasks. Indeed, novelty appears to influence cognitive effect, as those who take methylphenidate may be better able to shift attention to unfamiliar characteristics of stimuli with fewer errors in task response. Additionally, there may be up to a 10% improvement in conscious error awareness without a concomitant change in response speed. This has been confirmed neurophysiologically, with demonstrated activation differences between the dACC and the inferior parietal lobe in conscious errors versus unaware errors.
Some studies suggest that while amphetamine may have a small effect on certain aspects of cognition, which may also be limited by stimuli and temporal characteristics, it does not have an overall robust cognitive enhancing effect. Additionally, the effect of amphetamine-based stimulants in children may differ from that of older adolescents and young adults due to relative developmental immaturity. Children/pre-adolescents taking amphetamine demonstrate greater improvements in attention-based cognitive tasks with increased reaction time, vigilance, memory and ability to remain on-task. However, caution must be taken when interpreting these findings, as the child cohort examined included children who were ‘hyperactive’, such that they may have had subsyndromal or as-yet undiagnosed ADHD, and thus an inappropriate comparison group for a young adult cohort without psychiatric symptomatology.
Amphetamine may enhance knowledge acquisition and coding of information, as well as ability to retrieve information. However, these processes may, again, be limited by stimuli characteristics and medication half-life. Studies have shown that acoustic and semantic information may be encoded and accessed more easily with amphetamine. Temporally, amphetamine should be taken prior to learning; the hour after knowledge acquisition may be the most crucial for consolidation; recall may be most noticeably improved 1–3 days following the initial learning event; and recognition of previously learned information may be maximized 1 week following learning. Those with lower baseline functioning in insightful problem-solving, semantic retrieval and non-verbal intelligence may be aided by amphetamine in these domains. Finally, the valine–valine COMT genotype in combination with amphetamine use may confer some advantage in mathematical problem-solving.
All three stimulants demonstrate an effect on arousal, such that participants felt increased alertness after taking a stimulant. This may be misinterpreted as enhanced cognition. This theory is consistent with the finding that, in amphetamine and modafinil, participants overestimated their cognitive performance in anticipation of, or following, stimulant ingestion (Table 2).
Table 2.
Summary of neurocognitive effects of stimulant use.
| Source | Improvements | Deficits | Inconclusive |
|---|---|---|---|
| Advokat 2010 [21] |
|
Selective attention during complex tasks | No improvement shown in acquisition or retention of information or working memory |
| Baranski & Pigeau 1997 [26] |
|
||
| Baranski et al. 2004 [27] |
|
||
| Caldwell et al. 2000 [28] |
|
||
| Dyme et al. 1982 [43] | Task speed and performance | Response errors on tasks requiring executive functioning | |
| Elliott et al. 1997 [30] |
|
In familiar situations: poor response latency/impulsivity in response → response errors | No effects on non-spatial tasks related to frontal lobe function including: 1. Verbal fluency 2. Attention shifting |
| Farah et al. 2009 [40] | Convergent creativity (tasks with objective corrective answers) in those with lower baseline creativity | Convergent creativity in those who are highly creative at baseline | Effects on divergent (subjective) creativity |
| Greely et al. 2008 [22] |
|
||
| Hester et al. 2012 [34] | Conscious performance error detection | ||
| Ilieva et al. 2013 [41] |
|
|
Potential enhanced performance on novel tasks |
| Izquierdo et al. 2008 [44] | Duration of information retention/short-term memory | ||
| Killgore et al. 2009 [29] | During sleep deprivation:
|
||
| Koelega 1993 [20] |
|
|
|
| Marchant et al. 2009 [25] | Cognitive performance in challenging tasks which require several switches in attention | ||
| Pigeau et al. 1995 [45] |
|
||
| Rapoport et al. 1980 [38] |
|
||
| Rogers et al. 1999 [33] | In evolving tasks …
|
Selective attention with greater response latency and errors | |
| Smith & Farah 2011 [46] |
|
Decreased response latency → increased impulsive responses | Unknown effect on tasks requiring reasoning |
| Soetens et al. 1995 [39] |
|
Knowledge acquisition | |
| Turner et al. 2003 [24] | Adaptive inhibitory control → response accuracy |
Limitations
Clinicians’ ability to confidently predict results of PS use in children is limited, as there are few studies in a pre-adolescent population and results in young adults should not be extrapolated to younger populations, as brain development is not as advanced. Many of the included papers have small sample sizes, and are thus limited in power and ability to detect small–medium effects. However, studies employing cross-over designs were able to increase power. Additionally, in the majority of studies, participants were mainly/solely male, limiting the generalizability of the findings due to gender bias [24,27,30,33,34,36–39]. Some studies evaluate subpopulations, such as those that are sleep-deprived [27,29] or those who are from families with ‘superior intellectual intelligence’ [37,38], further limiting generalizability.
Also, there are few studies examining the effects of PS on measures of academic performance such as grades and/or standardized tests; however, there was a wide array of cognitive abilities tested that would impact ability to achieve academically. Additionally, many studies utilized the same or similar cognitive tests allowing for comparisons across studies and stimulant types. While not all studies accounted for drug half-life, and none of the studies measured bioavailability, this may mimic more accurately real-life scenarios of PS use in youth who probably do not take these pharmacological/ biological factors into account when using PS. Several studies did, however, administer the same PS at different doses to evaluate for any dose-dependent cognitive effects [24,30,36,38]. The methodology employed appears rigorous, as all the studies reviewed were randomized, double-blind, placebo-controlled trials, many employed within- and between-subject comparisons and were applicable to real-world scenarios, and the construct of ‘healthy’ (individuals without medical or psychiatric comorbidities) was consistent across studies. The quality of the studies is difficult to ascertain as, for many of the studies, effect sizes and precision of results (confidence intervals) were not reported.
There are several limitations of this review itself. While we did not limit our search to American youth, we did not encounter published studies examining youth PS abuse in international populations, probably due in part to the exclusion of papers not in English or with an English translation and varying rates of PS abuse world-wide. Study design was not evaluated as a part of eligibility criteria, so all studies that met our inclusion criteria were reviewed. Finally, given the limited data on populations that fell within the 12–25-year age range, we included studies that had participants up to 30 years of age (as long as the mean age of the sample or the intervention group was ≤ 25 years). This may limit the generalizability of our findings to younger, pre-adolescent and/or adolescent populations.
CONCLUSIONS
The research indicates improvement in certain neurocognitive domains, including realms of executive functioning, with use of prescription stimulants in a non-ADHD population across arousal states. However, use may lead to overconfident self-assessment of neurocognitive abilities and the benefits conferred may be limited by task and user characteristics, including novelty of task, type of sensory information presented, level of baseline abilities and genotype of user.
Footnotes
Declaration of interests
Dr Bagot reports no financial support or conflicts of interest. Dr Kaminer receives financial support from the National Institute on Drug Abuse, National Institute on Alcoholism and Alcohol Abuse and royalties for books from Hazelden, Routledge and APPI.
References
- 1.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings, 2011. Ann Arbor, MI: The University of Michigan, Institute for Social Research; 2012. [Google Scholar]
- 2.National Institute on Drug Abuse (NIDA) Topics in Brief: Prescription Drug Abuse. Bethesda, MD: NIDA; 2011. [Google Scholar]
- 3.Substance Abuse and Mental Health Services (SAMHSA) Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: SAMHSA; 2011. [Google Scholar]
- 4.Swanson JM, Volkow ND. Increasing use of stimulants warns of potential abuse. Nature. 2008;453:586. doi: 10.1038/453586a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaminer Y, Winters KC. Clinical Manual of Adolescent Substance Abuse Treatment. Arlington, VA: American Psychiatric Publishing; 2010. [Google Scholar]
- 6.Swanson JM, Wigal TL, Volkow ND. Contrast of medical and nonmedical use of stimulant drugs, basis for the distinction, and risk of addiction: comment on Smith and Farah (2011) Psychol Bull. 2011;137:742–8. doi: 10.1037/a0024898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz A. Risky rise of the good-grade pill. [accessed 16 January 2014];New York Times. 2012 Jun 10; Available at: http://www.nytimes.com/2012/06/10/education/seeking-academic-edge-teenagers-abuse-stimulants.html?pagewanted=all. (Archived at http://www.webcitation.org/6Mg2VI375 on 16 January 2014)
- 8.Schwarz A. Drowned in a stream of prescriptions. [accessed 16 January 2014];New York Times. 2013 Feb 3; Available at: http://www.nytimes.com/2013/02/03/us/concerns-about-adhd-practices-and-amphetamine-addiction.html. (Archived at http://www.webcitation.org/6Mg2lKXDA on 16 January 2014)
- 9.Schwarz A. Attention disorder or not, pills to help in school. [accessed 16 January 2014];New York Times. 2012 Oct 9; Available at: http://www.nytimes.com/2012/10/09/health/attention-disorder-or-not-children-prescribed-pills-to-help-in-school.html?pagewanted=all. (Archived at http://www.webcitation.org/6Mg2sfdv4 on 16 January 2014)
- 10.Kroutil LA, Van Brunt DL, Herman-Stahl MA, Heller DC, Bray RM, Penne MA. Nonmedical use of prescription stimulants in the United States. Drug Alcohol Depend. 2006;84:135–43. doi: 10.1016/j.drugalcdep.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- 12.Bostrom N, Sandberg A. Cognitive enhancement: methods, ethics, regulatory challenges. Sci Eng Ethics. 2009;15:311–41. doi: 10.1007/s11948-009-9142-5. [DOI] [PubMed] [Google Scholar]
- 13.Heinz A, Kipke R, Heimann H, Wiesing U. Cognitive neuroenhancement: false assumptions in the ethical debate. J Med Ethics. 2012;38:372–5. doi: 10.1136/medethics-2011-100041. [DOI] [PubMed] [Google Scholar]
- 14.Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–10. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teter CJ, McCabe SE, Cranford JA, Boyd CJ, Guthrie SK. Prevalence and motives for illicit use of prescription stimulants in an undergraduate student sample. J Am Coll Health. 2005;53:253–62. doi: 10.3200/JACH.53.6.253-262. [DOI] [PubMed] [Google Scholar]
- 16.Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychol Bull. 2004;130:324–40. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell SH, Laurent CL, de Wit H. Interaction of expectancy and the pharmacological effects of d-amphetamine: subjective effects and self-administration. Psychopharmacology (Berl) 1996;125:371–8. doi: 10.1007/BF02246020. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–18. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- 19.Looby A, Earleywine M. Expectation to receive methylphenidate enhances subjective arousal but not cognitive performance. Exp Clin Psychopharmacol. 2011;19:433–44. doi: 10.1037/a0025252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koelega HS. Stimulant drugs and vigilance performance: a review. Psychopharmacology (Berl) 1993;111:1–16. doi: 10.1007/BF02257400. [DOI] [PubMed] [Google Scholar]
- 21.Advokat C. What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/ hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2010;34:1256–66. doi: 10.1016/j.neubiorev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456:702–5. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- 23.Thaler DS. Improving introspection to inform free will regarding the choice by healthy individuals to use or not use cognitive enhancing drugs. Harm Reduct J. 2009;6:10. doi: 10.1186/1477-7517-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl) 2003;165:260–9. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- 25.Marchant NL, Kamel F, Echlin K, Grice J, Lewis M, Rusted JM. Modafinil improves rapid shifts of attention. Psychopharmacology (Berl) 2009;202:487–95. doi: 10.1007/s00213-008-1395-1. [DOI] [PubMed] [Google Scholar]
- 26.Baranski JV, Pigeau RA. Self-monitoring cognitive performance during sleep deprivation: effects of modafinil, d-amphetamine and placebo. J Sleep Res. 1997;6:84–91. doi: 10.1111/j.1365-2869.1997.00032.x. [DOI] [PubMed] [Google Scholar]
- 27.Baranski JV, Pigeau R, Dinich P, Jacobs I. Effects of modafinil on cognitive and meta-cognitive performance. Hum Psychopharmacol. 2004;19:323–32. doi: 10.1002/hup.596. [DOI] [PubMed] [Google Scholar]
- 28.Caldwell JA, Jr, Caldwell JL, Smythe NK, III, Hall KK. A double-blind, placebo-controlled investigation of the efficacy of modafinil for sustaining the alertness and performance of aviators: a helicopter simulator study. Psychopharmacology (Berl) 2000;150:272–82. doi: 10.1007/s002130000450. [DOI] [PubMed] [Google Scholar]
- 29.Killgore WD, Kahn-Greene ET, Grugle NL, Killgore DB, Balkin TJ. Sustaining executive functions during sleep deprivation: a comparison of caffeine, dextroamphetamine, and modafinil. Sleep. 2009;32:205–16. doi: 10.1093/sleep/32.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl) 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- 31.Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–8. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 32.Naylor H, Halliday R, Callaway E. The effect of methylphenidate on information processing. Psychopharmacology (Berl) 1985;86:90–5. doi: 10.1007/BF00431690. [DOI] [PubMed] [Google Scholar]
- 33.Rogers RD, Blackshaw AJ, Middleton HC, Matthews K, Hawtin K, Crowley C, et al. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology (Berl) 1999;146:482–91. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- 34.Hester R, Nandam LS, O’Connell RG, Wagner J, Strudwick M, Nathan PJ, et al. Neurochemical enhancement of conscious error awareness. J Neurosci. 2012;32:2619–27. doi: 10.1523/JNEUROSCI.4052-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 36.Linssen AM, Vuurman EF, Sambeth A, Riedel WJ. Methylphenidate produces selective enhancement of declarative memory consolidation in healthy volunteers. Psychopharmacology (Berl) 2012;221:611–9. doi: 10.1007/s00213-011-2605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rapoport JL, Buchsbaum MS, Zahn TP, Weingartner H, Ludlow C, Mikkelsen EJ. Dextroamphetamine: cognitive and behavioral effects in normal prepubertal boys. Science. 1978;199:560–3. doi: 10.1126/science.341313. [DOI] [PubMed] [Google Scholar]
- 38.Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine. Its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry. 1980;37:933–43. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- 39.Soetens E, Casaer S, D’Hooge R, Hueting JE. Effect of amphetamine on long-term retention of verbal material. Psychopharmacology (Berl) 1995;119:155–62. doi: 10.1007/BF02246156. [DOI] [PubMed] [Google Scholar]
- 40.Farah MJ, Haimm C, Sankoorikal G, Smith ME, Chatterjee A. When we enhance cognition with dextroamphetamine, do we sacrifice creativity? A preliminary study. Psychopharmacology (Berl) 2009;202:541–7. doi: 10.1007/s00213-008-1369-3. [DOI] [PubMed] [Google Scholar]
- 41.Ilieva I, Boland J, Farah MJ. Objective and subjective cognitive enhancing effects of mixed amphetamine salts in healthy people. Neuropharmacology. 2013;64:496–505. doi: 10.1016/j.neuropharm.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 42.Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyme IZ, Sahakian BJ, Golinko BE, Rabe EF. Perseveration induced by methylphenidate in children: preliminary findings. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:269–73. doi: 10.1016/s0278-5846(82)80177-2. [DOI] [PubMed] [Google Scholar]
- 44.Izquierdo I, Bevilaqua LR, Rossato JI, Lima RH, Medina JH, Cammarota M. Age-dependent and age-independent human memory persistence is enhanced by delayed posttraining methylphenidate administration. Proc Natl Acad Sci U S A. 2008;105:19504–7. doi: 10.1073/pnas.0810650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pigeau R, Naitoh P, Buguet A, McCann C, Baranski J, Taylor M, et al. Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work. I. Effects on mood, fatigue, cognitive performance and body temperature. J Sleep Res. 1995;4:212–28. doi: 10.1111/j.1365-2869.1995.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 46.Smith ME, Farah MJ. Are prescription stimulants ‘smart pills’? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychol Bull. 2011;137:717–41. doi: 10.1037/a0023825. [DOI] [PMC free article] [PubMed] [Google Scholar]