Abstract
Wildlife are an important component in the vector-host-pathogen triangle of livestock diseases, as they maintain biological vectors that transmit pathogens and can serve as reservoirs for such infectious pathogens. Babesia bovis is a tick-borne pathogen, vectored by cattle fever ticks, Rhipicephalus spp., that can cause up to 90% mortality in naive adult cattle. While cattle are the primary host for cattle fever ticks, wild and exotic ungulates, including white-tailed deer (WTD), are known to be viable alternative hosts. The presence of cattle fever tick populations resistant to acaricides raises concerns regarding the possibility of these alternative hosts introducing tick-borne babesial parasites into areas free of infection. Understanding the B. bovis reservoir competence of these alternative hosts is critical to mitigating the risk of introduction. In this study, we tested the hypothesis that WTD are susceptible to infection with a B. bovis strain lethal to cattle. Two groups of deer were inoculated intravenously with either B. bovis blood stabilate or a larval extract supernatant containing sporozoites from infected R. microplus larvae. The collective data demonstrated that WTD are neither a transient host nor reservoir of B. bovis. This conclusion is supported by the failure of B. bovis to establish an infection in deer regardless of inoculum. Although specific antibody was detected for a short period in the WTD, the PCR results were consistently negative at multiple time points throughout the experiment and blood from WTD that had been exposed to parasite, transferred into naïve recipient susceptible calves, failed to establish infection. In contrast, naïve steers inoculated intravenously with either B. bovis blood stabilate or the larval extract supernatant containing sporozoites rapidly succumbed to disease. These findings provide evidence that WTD are not an epidemiological component in the maintenance of B. bovis infectivity to livestock.
Introduction
Stable enzootic foci of arthropod-borne pathogens are successfully maintained as a result of the interaction between mammalian reservoirs and competent arthropod vectors [1,2]. The ability of the pathogen to infect both vertebrate and invertebrate hosts is essential for the spread of disease [3–5]. This complex cycle is exemplified by tick-borne pathogens such as Babesia bovis and B. bigemina, the causative agents of bovine babesiosis [6,7]. In endemically stable areas, female cattle fever ticks, Rhipicephalus microplus and R. annulatus, serve as competent vectors that acquire babesial parasites from the mammalian reservoir [7,8]. The parasites infect tick midgut epithelial cells where they complete transformation into kinetes. This is a critical step in the transmission cycle, as kinetes are transmitted transovarially and the resulting tick offspring are then capable of transmitting the pathogen to naïve hosts, maintaining the endemic cycle [3,4,6]. It is increasingly clear that wildlife, especially white-tailed deer (Odocoileus virginianus; WTD), play an important epidemiological role in sustaining cattle fever tick populations in the presence or absence of livestock [9–12]. Deer serve as viable alternative hosts for cattle fever ticks, and free-ranging WTD are capable of disseminating these ticks into areas previously free of the biological vector [12,13]. This is especially important in the southern region of the United States where WTD populations have increased and are gradually expanding in distribution. Further, the frequent movement of WTD between the U.S. and Mexico increases the likelihood of transporting cattle fever ticks into the U.S. from Mexico where bovine babesiosis is endemic [8]. Should these cattle fever ticks be infected with B. bovis, they could transmit to naïve cattle resulting in significant economic losses for the livestock industry. The role of WTD as reservoirs for B. bovis and B. bigemina is unclear.
Kuttler et al (1972) reported that WTD do not develop a detectable level of Babesia parasites upon either infestation with R. annulatus larval progeny originating from female cohorts reared on a Babesia-infected calf from Mexico or inoculation with blood from an infected bovine [14]. However, the Babesia spp. was unknown, parasite load being delivered to the deer was not quantified, and sensitive detection methods were not available for monitoring the experimental animals. More recently, molecular and serological analyses of samples from free-ranging WTD in Texas and northern Mexico implicated cervids as potential reservoirs of bovine babesiosis parasites [15–18]. The results suggested either transient babesial infection or cross reactivity to parasites that are capable of establishing an infection in deer. Due to the nature of the sample acquisition, the animals could not be monitored over time to distinguish between these possibilities.
In the current study, we addressed these issues and determined that a strain of B. bovis lethal to cattle failed to infect WTD via intravenous inoculation. Blood infected with B. bovis generated in a splenectomized calf or a larval extract supernatant containing B. bovis sporozoites from infected Rhipicephalus larvae did not infect WTD. In contrast, naïve steers inoculated intravenously with either B. bovis blood stabilate or the larval extract containing sporozoites rapidly succumbed to disease. We discuss our findings in the context of epidemiology, transmission and reservoir capacity for bovine babesiosis parasites.
Materials and Methods
Pathogen, ticks and animals
In this study, we used a highly transmissible strain of B. bovis that is lethal to cattle and for which there exists a complete and annotated genome [19]. This strain of B. bovis originated from a quarantined animal in Texas. The La Minita strain of R. microplus isolated from cattle on pasture in Starr County, TX was used to produce a larval extract supernatant containing B. bovis sporozoites. Previous studies demonstrated that this R. microplus colony is efficient in acquiring and transmitting B. bovis parasites to naïve cattle [3,4].
To determine the susceptibility of WTD to B. bovis, eight WTD doe fawns were hand raised at the Knipling-Bushland U.S. Livestock Insects Research Laboratory (KBUSLIRL) in tick-free facilities. Steers were used to verify the viability and infectivity of B. bovis parasitized erythrocytes or the larval extract containing sporozoites. Prior to use, all animals tested negative for B. bovis by immunoblots and PCR amplification targeting the 18S rRNA gene [15,20].
Inoculum preparation
Babesia bovis parasitized erythrocytes were generated in a splenectomized calf [21] and used as the source of infection for the deer experiments. To prepare B. bovis blood stabilate, a splenectomized calf was infected intravenously with a lethal strain of B. bovis. When the parasitemia reached 1.1%, blood was collected, washed to remove white blood cells and cryopreserved using dimethyl sulphoxide in liquid nitrogen [21]. A larval extract supernatant containing B. bovis sporozoites was produced from infected tick larvae. Briefly, R. microplus larvae were applied under a cloth patch on a splenectomized naïve calf. When ticks molted to adults, approximately 107 B. bovis parasitized erythrocytes were inoculated intravenously into the calf. Female ticks that fed to engorgement during an ascending parasitemia were collected to rear infected larvae. Egg masses from infected engorged female ticks were pooled and one gram of tick eggs per vial was incubated at 26°C with 96% relative humidity. After hatching, larvae were incubated at 15°C with 96% relative humidity [3,4].
To stimulate the development of B. bovis sporozoites in tick salivary glands [3,4], infected larvae from one gram of eggs were placed under a cloth patch on a naïve calf Ticks were forcibly removed after four days of larval feeding. The stimulation fed, infected larvae were incubated on ice for 30 min. Approximately 300 larvae were added to cold sterile phosphate buffered saline (PBS) and ground using a tissue homogenizer. Supernatants were pooled and centrifuged at 400g, 4°C for 10 min to remove tick debris. The pellets were discarded and the larval supernatant containing B. bovis sporozoites was used to challenge WTD.
Challenge
Two groups of WTD were used to determine their susceptibility to B. bovis. The parasite levels in the inocula were determined by quantitative real time PCR [4,22]. Group 1 contained four 2-year old WTD and one intact steer as a control. Group 2 contained four 3-year old WTD and one intact steer as a control. Group 1 received B. bovis parasitized erythrocytes and group 2 received a larval extract containing B. bovis sporozoites produced from infected R. microplus larvae. The inocula were suspended in 10% homologous deer or bovine serum in Puck’s saline G and inoculated intravenously into the jugular vein.
Infection status of animals
The infection status of WTD and control cattle was determined by monitoring for clinical signs of babesiosis including alteration in body temperature and packed cell volume (PCV). The infection status was confirmed by performing molecular and serological assays at multiple time points after parasite inoculation. Deer blood samples and sera were collected daily for the first two weeks of the study and weekly up to 84 days post-challenge. Genomic DNA was isolated from whole blood with the DNeasy Blood and Tissue Kit (Qiagen) and was used as template in a nested PCR targeting 18S rRNA, a multi copy gene [15,19]. It was predicted that the 18S rRNA outer primers (Nbab-1 forward, 5′-AAG CCA TGC ATG TCT AAG TAT AAG CTT TT-3′ and Nbab-1 reverse, 5′-CTT CTC CTT CCT TTA AGT GAT AAG GTT CAC-3′) would amplify a fragment of 1,600 bp [23]. PCR was carried out under the following conditions: 95°C for 5 min; 35 cycles of 95°C for 30 sec, 50°C for 30 sec, and 72°C for 1 min; final extension at 72°C for 5 min. The reaction was conducted in 32 μl containing 2 μl of extracted genomic DNA, 2.0 mM MgCl2, 200 μM dATP, dCTP, dGTP, dTTP; 1.0 μM of each primer set, and 1.3 U of FastStart Taq (Roche). It was predicted that the 18S rRNA inner primers (forward, 5’- AATCCTGACACAGGGAGGTAGTGAC-3’ and reverse, 5’-CTAAGAATTTCACCTCTGACAGT-3’) would amplify a fragment of 390 bp. Nested PCR was carried out under the following conditions: 95°C for 5 min; 35 cycles of 95°C for 30 sec, 65°C for 30 sec, and 72°C for 30 sec; final extension at 72°C for 5 min. The reaction was conducted in 30 μl containing 0.1 μl from the first reaction, 2.0 mM MgCl2, 200 μM dATP, dCTP, dGTP, dTTP; 1.0 μM of each primer set, and 1.25 U of FastStart Taq (Roche). DNA isolated from all collection dates were screened by PCR and ten replicate amplifications were conducted per animal per sampling date to capture low parasitemia levels. To determine the sensitivity of the nested PCR, serial dilutions of B. bovis infected erythrocyte cultures were performed. Briefly, cultures with 107 infected erythrocytes per ml of blood were diluted serially 10 fold in uninfected bovine blood to a final parasitemia of 10-2 infected erythrocytes per ml of blood. Genomic DNA was extracted and nested PCR performed. Ten replicate amplifications were conducted per dilution to determine the lowest parasitemia level detected by nested PCR. All ten replicates were consistently positive with 104 infected erythrocytes per ml of blood. In contrast, only four of 10 replicates were positive with 103 infected erythrocytes per ml of blood. All replicates with 102 infected erythrocytes per ml of blood were negative. The sensitivity of our nested PCR was determined to be 103 infected erythrocytes per ml of blood which corresponded to a parasitemia as low as 0.000016%.
An immunoflurecence assay (IFA) was performed to detect B. bovis antibodies in deer sera. Briefly, cultures with 5% parasitized bovine erythrocytes were prepared by washing four times at 400xg in PBS. Infected erythrocyte pellets were suspended in PBS containing 1% bovine serum albumin (Fraction V) and thin smear microscope slides of infected erythrocytes prepared. The slides were air dried, covered, and stored at -80°C. Prior to performing the IFA, slides were warmed at room temperature in a desiccator jar for 30 min and fixed in cold acetone for 1 min. Sera were diluted 1:50 in PBS and applied to the antigen. The slides were incubated at 37°C in a humidity chamber for 30 min. After three successive washings in PBS, the slides were dried and exposed for 30 min with fluorescein conjugated Protein G (Rockland) diluted 1:5,000 in PBS to detect specific bovine and deer IgG. Protein G binds WTD immunoglobulin [24]. Slides were washed in PBS, dried and mounted in glycerol/PBS (1:1) and examined with an epifluorescence microscope.
Immunoblots were performed to identify B. bovis proteins recognize by specific antibodies in deer sera. Briefly, cultures with 30% parasitized bovine erythrocytes were washed with PBS and the pellets suspended in lysis buffer containing 50 mM Tris [pH 8], 5 mM EDTA, 1% Nonidet P-40, and Complete Protease Inhibitor (Roche) [20]. Babesia bovis or normal bovine erythrocyte antigens were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and blocked with a solution containing 150 mM NaCl, 10 mM Tris-HCl, 1% polyvinylpyrrolidone, 0.2% Tween-20 and 5% non-fat milk (blocking solution). The membrane was cut into strips and incubated in sera from experimental WTD or control steers diluted 1:100 in blocking solution. The membrane strips were washed and incubated with a 1:20,000 dilution of Protein G conjugated with horseradish peroxidase in blocking solution. After washing, reactivity was detected by using HyGlo Quick Spray chemiluminescent substrate solution (Denville Scientific) and the membrane exposed to x-ray film.
Positive control steers were examined daily to determine when signs of bovine babesiosis occurred including alteration in body temperature, anorexia, anemia, weakness and depression. The infection of the steers was confirmed by 18S rRNA nested PCR amplification of DNA isolated from whole blood [15].
Transfer of deer blood to susceptible recipient calves: Whole blood (50 ml) from each WTD was collected in citrate phosphate dextrose anticoagulant and shipped on ice overnight to the U.S. Department of Agriculture-Agricultural Research Service-Animal Disease Research Unit (USDA-ARS-ADRU) facility in Pullman, WA. Deer blood was sub-inoculated into a susceptible splenectomized calf to determine if B. bovis had established an infection in WTD. To minimize the number of recipient animals, the blood from each WTD was inoculated separately into a single splenectomized recipient calf. Recipient calf #1 received blood from WTD challenged with B. bovis blood stabilate and calf #2 received blood from WTD challenged with the larval extract containing B. bovis sporozoites. Recipient animals were monitored daily for clinical signs of babesiosis for 35 days post-blood inoculation. Upon completion of this observation period, the susceptibility of the calves was tested by intravenous inoculation with B. bovis.
Ethics Statement
This study was approved by the Institutional Animal Care and Use Protocol Committees of the University of Idaho, Moscow, Idaho and the Institutional Animal Care and Use Protocol Committees of the Knipling-Bushland United States Livestock Insects Research Laboratory, Kerrville, Texas, in accordance with institutional guidelines based on the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All splenectomy procedures were performed under sedation with xylazine and isoflurane inhalation, and all efforts were made to minimize suffering. All bovine and deer exposed with an exotic pathogen were sacrificed by injection intravenously of sodium pentobarbitone formulated for euthanasia, and the resulting sera and whole blood stored at −80°C.
Results
In this study we tested the hypothesis that WTD are susceptible to infection and are competent reservoirs for B. bovis. Deer challenged with either B. bovis blood stabilate or larval supernatant extract containing sporozoites showed no evidence of transient or long-term infection, as determined by molecular and serological techniques. This was confirmed by the failure to cause infection upon transfer of blood from B. bovis-challenged WTD into susceptible, naïve calves.
Challenge with Babesia bovis blood stabilate
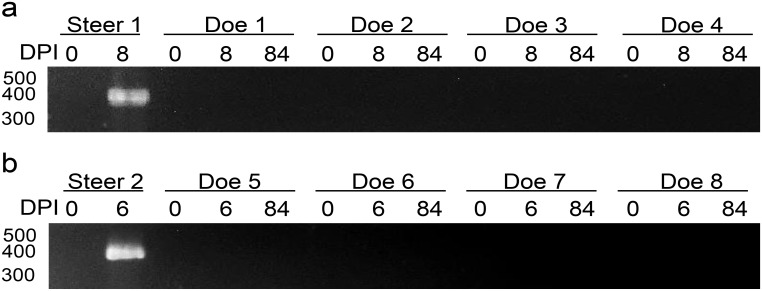
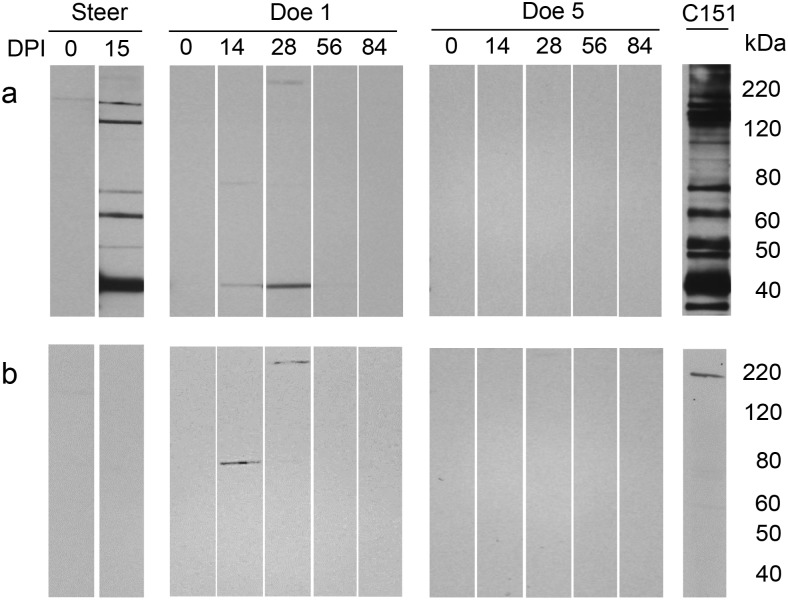
Deer challenged with 109 B. bovis infected erythrocytes showed no clinical signs of babesiosis throughout the experiment. Body temperature and PCV of the WTD remained within normal ranges of 38.4°C (±0.13) and 38.7% (± 2.1), respectively. Blood collected at multiple time points demonstrated that B. bovis failed to establish infection in deer, as determined by nested PCR (Fig 1a, Table 1). Immunoflurecence showed that three of four deer challenged with B. bovis blood stabilate developed antibodies against B. bovis and bovine erythrocytes. The same sera were tested by immunoblots and revealed that WTD developed antibody responses against proteins of 40, 70 and 220 kDa. Reactivity to the 40 kDa protein was observed only on immunoblots using B. bovis infected erythrocyte antigen (Fig 2a), but not on immunoblots using normal bovine erythrocyte antigen (Fig 2b). In contrast, reactivity to the 70 and 220 kDa proteins was also detected on immunoblots using normal bovine erythrocyte antigens (Fig 2b). Antibody responses against the inoculum were no longer detectable after 56 days post-inoculation.
Fig 1. Evaluation of Babesia bovis infection by nested PCR.
Amplicons were separated in 2% agarose by electrophoresis. White-tailed deer challenged with either B. bovis a) blood stabilate or b) a larval extract containing B. bovis sporozoites. Steer 1 and 2 control for the inocula. DPI: day post-inoculation. Molecular size is indicated on the left.
Table 1. Evaluation of the white-tailed deer challenged with Babesia bovis.
| Week post-inoculation | |||||||||||||
| Group 1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Doe 1 | N | n | n | n | n | n | n | n | n | n | n | n | n |
| Doe 2 | n | n | n | n | n | n | n | n | n | n | n | n | n |
| Doe 3 | n | n | n | n | n | n | n | n | n | n | n | n | n |
| Doe 4 | n | n | n | n | n | n | n | n | n | n | n | n | n |
| Steer 1 | n | N | P SD * | ||||||||||
| Week post-inoculation | |||||||||||||
| Group 2 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Doe 5 | n | n | n | n | n | n | n | n | n | n | n | n | n |
| Doe 6 | n | n | n | n | n | n | n | n | n | n | n | n | n |
| Doe 7 | n | n | n | n | n | n | n | n | n | n | n | n | n |
| Doe 8 | n | n | n | n | n | n | n | n | n | n | n | n | n |
| Steer 2 | n | P SD * | |||||||||||
White tailed deer challenged with B. bovis. Group 1: blood stabilate and group 2: a larval extract containing sporozoites. Nested PCR targeting 18S ribosomal RNA. N: negative; P: positive,
SD: Severe Clinical Disease and *: steers were euthanized.
Fig 2. Detection of serum antibody reactivity to B. bovis infected erythrocyte and normal erythrocyte antigens after inoculation with a blood stabilate.
Serum antibody from representative deer challenged with either B. bovis blood stabilate (Doe 1) or a larval extract containing B. bovis sporozoites (Doe 5) was evaluated by immunoblot. a) B. bovis infected erythrocyte antigen and b) normal bovine erythrocyte antigen. Steer: challenged with B. bovis blood stabilate and C151: bovine persistently infected with B. bovis. DPI: days post-inoculation.
Consistent with previous studies, the blood stabilate readily infected the control steer by 9 days post-inoculation as determined by PCR (Fig 1a) and severe clinical signs of bovine babesiosis including elevation of body temperature (40.7°C) and a decline in the PCV to 29%. The steer was euthanized to minimize animal suffering. Immunoflurecence demonstrated that the steer had an antibody response to B. bovis antigens. Immunoblots demonstrated that the steer responded to multiple B. bovis antigens ranging from 40 kDa to 120 kDa (Fig 2a and 2b).
Challenge with larval extract supernatant containing Babesia bovis sporozoites
Infectivity of a larval extract containing 105 B. bovis sporozoites was confirmed by intravenous inoculation of a control steer with the same inoculum used for the WTD challenge. The steer was PCR positive at 6 days post-sporozoite inoculation (Fig 1b). The larval extract containing B. bovis sporozoites caused rapid severe clinical signs of bovine babesiosis in the steer including elevation of body temperature (40.8°C) and a decline in the PCV to 23%. To minimize animal suffering, the steer was euthanized.
WTD challenged with larval extract containing 105 B. bovis sporozoites showed no evidence of infection. Body temperature as well as PCV of the deer remained within normal ranges of 38.9°C (± 0.09) and 38.3% (±3.5), respectively. PCR amplification of blood collected weekly from the deer indicated that B. bovis sporozoites within the larval extract failed to establish infection (Table 1). Throughout the experiment, none of the deer in this group showed evidence of an antibody response against B. bovis by either IFA or immunoblots using B. bovis infected erythrocyte antigen (Fig 2a) or normal bovine erythrocyte antigen (Fig 2b).
Blood transfer from deer into susceptible splenectomized cattle
Blood from WTD challenged with either B. bovis infected erythrocytes or a larval extract containing sporozoites failed to infect susceptible cattle. Clinical signs of babesiosis were not observed in the splenectomized calves throughout the experiment (data not shown). PCR results during the five weeks post-inoculation showed no evidence of infection (Fig 3). To test the susceptibility of the calves, B. bovis stabilate was inoculated intravenously and severe clinical signs of bovine babesiosis were observed at day 10 post-inoculation including body temperature exceeding 40.6°C. During acute infection, parasites were detected by Giemsa blood smear or PCR amplification and calves were euthanized to minimized animal suffering.
Fig 3. Analysis of naïve susceptible bovines that received blood transferred from deer challenged with B. bovis by nested PCR.
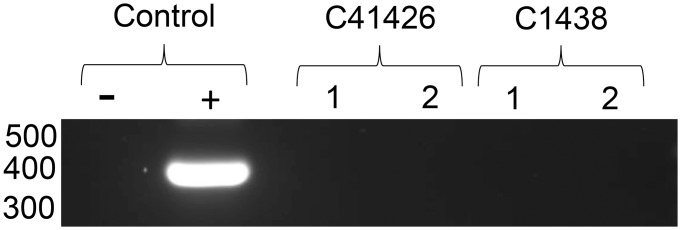
Calves 41426 and 1438 received blood from deer challenged with either B. bovis blood stabilate or a larval extract supernatant containing B. bovis sporozoites, respectively. Amplicons were separated in 2% agarose by electrophoresis for visualization. Genomic DNA from 1: pre-inoculation and 2: five weeks post-inoculation. Molecular size is indicated on the left.
Discussion
The wildlife-livestock interface is a restriction to the success of controlling and eradicating diseases of domestic animals [25–29] highlighting the necessity to understand the importance of wildlife in the epidemiology and maintenance of infectious diseases of livestock. This is illustrated by the incidence of tick-transmitted bovine theileriosis in Africa. The disease is caused by Theileria parva and is typically transmitted from infected to susceptible cattle via ticks. However, Cape buffalo (Syncerus caffer) are known competent reservoirs for T. parva and occupies overlapping habitats with cattle, providing a continuous source of parasite for tick transmission [25,28,30]. Biological vectors that acquire infection while feeding on buffalo can transmit the pathogen to either buffalo or cattle causing significant economic loss to the livestock industry.
It is critical to understand if wildlife can be a source of pathogens for transmission to livestock. The lack of knowledge regarding pathogens that infect wildlife and the difficulties with disease surveillance of wildlife create obstacles to detection and monitoring for organisms that can cause significant disease in livestock. Recent reports suggested that free-ranging WTD commingling with livestock could be a source of tick-borne pathogens that cause bovine babesiosis [15–17]. However, it remained unclear whether WTD were indeed competent reservoirs. In this study, we tested the hypothesis that B. bovis blood stabilate or a larval extract supernatant containing sporozoites were infective to WTD. The collective data does not support an epidemiological role for WTD in the maintenance of B. bovis. This conclusion is supported by the failure of B. bovis to establish an infection in WTD by either B. bovis blood or tick stages. Nested PCR results were consistently negative at multiple time points post-inoculation, suggesting there was no transient infection. Blood from WTD challenged with B. bovis failed to infect naïve susceptible recipient calves, confirming that WTD are not a reservoir for B. bovis.
A previous attempt also failed to infect WTD with bovine babesial parasites upon inoculation of spleen-intact or splenectomized deer with infected blood from a naturally infected bovine [14]. However, the level of parasitemia used in the challenge study was undetectable by light microscopy of stained thin smears. With our challenge strain, a minimum dose sufficient to infect cattle and cause severe disease is ten B. bovis infected erythrocytes [21]. In order to definitively determine whether or not WTD are susceptible to B. bovis infection, we challenged the animals with >108 more parasites needed to sufficiently infect cattle. Although we challenged WTD with a high dose, the deer showed no evidence of infection suggesting that WTD are not susceptible to B. bovis infection. Our results shed light on the inability of blood and serum from WTD to support in vitro culture of B. bovis [31].
Attempts to transmit babesial parasites using infected ticks harvested from a natural infected animal failed to infect deer [14]. Persistently infected calves harbor dramatically lower levels of parasites in peripheral blood, resulting in low larval infection rates from eggs of R. microplus females allowed to acquisition feed [3]. The infected larvae used by Kuttler et al (1972) to infest WTD were obtained from female R. annulatus fed on a clinically negative animal naturally infected with babesial parasites. Further, larval feeding rates of cattle fever ticks on WTD can be variable depending on the level of resistance to tick infestation and grooming [13,32]. To overcome these issues, we produced a larval extract supernatant containing B. bovis sporozoite from infected R. microplus larvae and inoculated them into WTD. This approach allowed us to quantify the amount of B. bovis and ensure a high dose was delivered to each animal. The results provide additional evidence that WTD are not susceptible to B. bovis infection.
A salient distinction between the current study and previous Babesia spp. surveillance studies is our use of tick-naïve WTD. Deer challenged with a single exposure responded to a single B. bovis protein, but because there was not continuous stimulation by an established infection, antibodies against B. bovis were no longer detected after 6 weeks. In contrast, acute infection in cattle resulted in a response to multiple B. bovis proteins. A single inoculum of 105 B. bovis parasites contained within a larval extract supernatant failed to stimulate a deer immune response demonstrating that B. bovis did not establish infection in deer. In contrast, free-ranging WTD captured at a single time point likely had multiple exposures to various tick species, each providing opportunities for exposure to the parasite delivered via tick saliva during feeding. Such parasite exposure would elicit an immune response in WTD, explaining the detection of anti-B. bovis and B. bigemina antibodies in these samples. Tick salivary proteins can modulate the mammalian immune response to the feeding tick, enabling larvae to feed for days [33]. Thus, the parasite can be continuously inoculated into the blood stream via saliva. Detection of DNA isolated from peripheral blood may be possible without an infection necessarily being established due to the accumulation of parasites in the blood. Indeed, Cantu et al (2009) observed that the frequency of Babesia spp. detection by serology or PCR was elevated in scenarios where exposure to cattle ticks was increased [17]. Pharmacologically bioactive tick proteins may enhance Babesia transmission. However, the results from the current study using a larval extract containing B. bovis sporozoites and a previous study performed by Kuttler et al (1972) that attempted to transmit parasites via on-animal infestation with infected Rhipicephalus larvae demonstrated no evidence of Babesia infection in WTD. Together, these collective results indicate that these free-ranging WTD do not present a risk for B. bovis transmission.
In conclusion, we demonstrated that WTD are not transiently infected with and are not a reservoir of B. bovis. These findings provide evidence that WTD are not an epidemiological threat for maintenance of bovine babesiosis to livestock. However, it is possible that co-evolution between the mammalian host-vector-pathogen may influence parasite adaptation to wildlife and these animals could become potential reservoirs of parasites. Therefore, it is important for the United States to maintain continuous surveillance of wildlife, including WTD, to provide control measurement for preventing bovine babesiosis emerging in areas free of infectious disease.
Acknowledgments
We appreciate the excellent technical assistance of Sara Davis and Kathy Mason of the USDA-ARS-ADRU and Greta Buckmeier of the KBUSLIRL. We also thank Ralph Horn and James Allison of the USDA-ARS-ADRU and Keith Shelley, Larry Camarillo, and Evelio Gonzalez of the KBUSLIRL for excellent animal care.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by USDA-ARS CRIS project number 5348-32000-034-00D and 6205-32000-034D-00D. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. De Moraes CM, Stanczyk NM, Betz HS, Pulido H, Sim DG, Read AF, et al. Malaria-induced changes in host odors enhance mosquito attraction. Proc Natl Acad Sci U S A. 2014; 111: 11079–11084. 1405617111 [pii]; 10.1073/pnas.1405617111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ueti MW, Tan Y, Broschat SL, Castaneda Ortiz EJ, Camacho-Nuez M, Mosqueda JJ, et al. Expansion of variant diversity associated with a high prevalence of pathogen strain superinfection under conditions of natural transmission. Infect Immun. 2012; 80: 2354–2360. IAI.00341-12 [pii]; 10.1128/IAI.00341-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howell JM, Ueti MW, Palmer GH, Scoles GA, Knowles DP. Persistently infected calves as reservoirs for acquisition and transovarial transmission of Babesia bovis by Rhipicephalus (Boophilus) microplus . J Clin Microbiol. 2007; 45: 3155–3159. JCM.00766-07 [pii]; 10.1128/JCM.00766-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howell JM, Ueti MW, Palmer GH, Scoles GA, Knowles DP. Transovarial transmission efficiency of Babesia bovis tick stages acquired by Rhipicephalus (Boophilus) microplus during acute infection. J Clin Microbiol. 2007; 45: 426–431. JCM.01757-06 [pii]; 10.1128/JCM.01757-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Futse JE, Brayton KA, Dark MJ, Knowles DP Jr., Palmer GH. Superinfection as a driver of genomic diversification in antigenically variant pathogens. Proc Natl Acad Sci U S A. 2008; 105: 2123–2127. 0710333105 [pii]; 10.1073/pnas.0710333105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suarez CE, Noh S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet Parasitol. 2011; 180: 109–125. S0304-4017(11)00385-2 [pii]; 10.1016/j.vetpar.2011.05.032 [DOI] [PubMed] [Google Scholar]
- 7. Bock R, Jackson L, de VA, Jorgensen W. Babesiosis of cattle. Parasitol. 2004; 129 Suppl: S247–S269. [DOI] [PubMed] [Google Scholar]
- 8. Lopez M, Figueroa JV, Ramos JA, Mosqueda JJ, Rojas E, Vega CA, et al. Infection and seroconversion of susceptible animals introduced into a babesiosis endemic area. Ann N Y Acad Sci. 2008; 1149: 131–135. NYAS1149072 [pii]; 10.1196/annals.1428.072 [DOI] [PubMed] [Google Scholar]
- 9. Phillips PL, Welch JB, Kramer M. Development of a spatially targeted field sampling technique for the southern cattle tick, Rhipicephalus microplus, by mapping white tailed deer, Odocoileus virginianus, habitat in South Texas. J Insect Sci. 2014; 14: 88 14.1.88 [pii]; 10.1093/jis/14.1.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Busch JD, Stone NE, Nottingham R, Araya-Anchetta A, Lewis J, Hochhalter C, et al. Widespread movement of invasive cattle fever ticks (Rhipicephalus microplus) in southern Texas leads to shared local infestations on cattle and deer. Parasit Vectors. 2014; 7: 188 1756-3305-7-188 [pii]; 10.1186/1756-3305-7-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lohmeyer KH, Pound JM, May MA, Kammlah DM, Davey RB. Distribution of Rhipicephalus (Boophilus) microplus and Rhipicephalus (Boophilus) annulatus (Acari: Ixodidae) infestations detected in the United States along the Texas/Mexico border. J Med Entomol. 2011; 48: 770–774. [DOI] [PubMed] [Google Scholar]
- 12. Pound JM, George JE, Kammlah DM, Lohmeyer KH, Davey RB. Evidence for role of white-tailed deer (Artiodactyla: Cervidae) in epizootiology of cattle ticks and southern cattle ticks (Acari: Ixodidae) in reinfestations along the Texas/Mexico border in south Texas: a review and update. J Econ Entomol. 2010; 103: 211–218. [DOI] [PubMed] [Google Scholar]
- 13. Cooksey LM, Davey RB, Ahrens EH, George JE. Suitability of white-tailed deer as hosts for cattle fever ticks (Acari: Ixodidae). J Med Entomol. 1989; 26: 155–158. [DOI] [PubMed] [Google Scholar]
- 14. Kuttler KL, Graham OH, Johnson SR, Trevino JL. Unsuccessful attempts to establish cattle Babesia infections in white-tailed deer. J Wildl Dis. 1972; 8: 63–66. [DOI] [PubMed] [Google Scholar]
- 15. Holman PJ, Carroll JE, Pugh R, Davis DS. Molecular detection of Babesia bovis and Babesia bigemina in white-tailed deer (Odocoileus virginianus) from Tom Green County in central Texas. Vet Parasitol. 2011; 177: 298–304. S0304-4017(10)00702-8 [pii]; 10.1016/j.vetpar.2010.11.052 [DOI] [PubMed] [Google Scholar]
- 16. Ramos CM, Cooper SM, Holman PJ. Molecular and serologic evidence for Babesia bovis-like parasites in white-tailed deer (Odocoileus virginianus) in south Texas. Vet Parasitol. 2010; 172: 214–220. S0304-4017(10)00283-9 [pii]; 10.1016/j.vetpar.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 17. Cantu C, Ortega-S JA, Garcia-Vazquez Z, Mosqueda J, Henke SE, George JE. Epizootiology of Babesia bovis and Babesia bigemina in free-ranging white-tailed deer in northeastern Mexico. J Parasitol. 2009; 95: 536–542. GE-1648 [pii]; 10.1645/GE-1648.1 [DOI] [PubMed] [Google Scholar]
- 18. Cantu A, Ortega-S JA, Mosqueda J, Garcia-Vazquez Z, Henke SE, George JE. Immunologic and molecular identification of Babesia bovis and Babesia bigemina in free-ranging white-tailed deer in northern Mexico. J Wildl Dis. 2007; 43: 504–507. 43/3/504 [pii]; 10.7589/0090-3558-43.3.504 [DOI] [PubMed] [Google Scholar]
- 19. Brayton KA, Lau AO, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ, et al. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007; 3: 1401–1413. 07-PLPA-RA-0191 [pii]; 10.1371/journal.ppat.0030148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Awinda PO, Mealey RH, Williams LB, Conrad PA, Packham AE, Reif KE, et al. Serum antibodies from a subset of horses positive for Babesia caballi by competitive enzyme-linked immunosorbent assay demonstrate a protein recognition pattern that is not consistent with infection. Clin Vaccine Immunol. 2013; 20: 1752–1757. CVI.00479-13 [pii]; 10.1128/CVI.00479-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goff WL, Johnson WC, Cluff CW. Babesia bovis immunity. In vitro and in vivo evidence for IL-10 regulation of IFN-gamma and iNOS. Ann N Y Acad Sci. 1998; 849: 161–180. [DOI] [PubMed] [Google Scholar]
- 22. Sondgeroth KS, McElwain TF, Ueti MW, Scoles GA, Reif KE, Lau AO. Tick passage results in enhanced attenuation of Babesia bovis . Infect Immun. 2014; 82: 4426–4434. IAI.02126-14 [pii]; 10.1128/IAI.02126-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oosthuizen MC, Zweygarth E, Collins NE, Troskie M, Penzhorn BL. Identification of a novel Babesia sp. from a sable antelope (Hippotragus niger Harris, 1838). J Clin Microbiol. 2008; 46: 2247–2251. JCM.00167-08 pii]; 10.1128/JCM.00167-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kramsky JA, Manning EJ, Collins MT. Protein G binding to enriched serum immunoglobulin from nondomestic hoofstock species. J Vet Diagn Invest. 2003; 15: 253–261. [DOI] [PubMed] [Google Scholar]
- 25. Walker JG, Klein EY, Levin SA. Disease at the wildlife-livestock interface: acaricide use on domestic cattle does not prevent transmission of a tick-borne pathogen with multiple hosts. Vet Parasitol. 2014; 199: 206–214. S0304-4017(13)00599-2 [pii]; 10.1016/j.vetpar.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 26. Caron A, Miguel E, Gomo C, Makaya P, Pfukenyi DM, Foggin C, et al. Relationship between burden of infection in ungulate populations and wildlife/livestock interfaces. Epidemiol Infect. 2013;141: 1522–1535. S0950268813000204 [pii]; 10.1017/S0950268813000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berggoetz M, Schmid M, Ston D, Wyss V, Chevillon C, Pretorius AM, et al. Tick-borne pathogens in the blood of wild and domestic ungulates in South Africa: interplay of game and livestock. Ticks Tick Borne Dis. 2014; 5: 166–175. S1877-959X(13)00121-0 [pii]; 10.1016/j.ttbdis.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 28. Berggoetz M, Schmid M, Ston D, Wyss V, Chevillon C, Pretorius AM, et al. Protozoan and bacterial pathogens in tick salivary glands in wild and domestic animal environments in South Africa. Ticks Tick Borne Dis. 2014; 5: 176–185. S1877-959X(13)00116-7 [pii]; 10.1016/j.ttbdis.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 29. Githaka N, Konnai S, Bishop R, Odongo D, Lekolool I, Kariuki E, et al. Identification and sequence characterization of novel Theileria genotypes from the waterbuck (Kobus defassa) in a Theileria parva-endemic area in Kenya. Vet Parasitol. 2014; 202: 180–193. S0304-4017(14)00147-2 [pii]; 10.1016/j.vetpar.2014.02.056 [DOI] [PubMed] [Google Scholar]
- 30. Odongo DO, Ueti MW, Mwaura SN, Knowles DP, Bishop RP, Scoles GA. Quantification of Theileria parva in Rhipicephalus appendiculatus (Acari: Ixodidae) confirms differences in infection between selected tick strains. J Med Entomol. 2009; 46: 888–894. [DOI] [PubMed] [Google Scholar]
- 31. Holman PJ, Waldrup KA, Droleskey RE, Corrier DE, Wagner GG. In vitro growth of Babesia bovis in white-tailed deer (Odocoileus virginianus) erythrocytes. J Parasitol. 1993; 79: 233–237. [PubMed] [Google Scholar]
- 32. Davey RB. Failure of white-tailed deer, Odocoileus virginianus L., to sustain a population of cattle ticks, Boophilus annulatus (Say), through successive generations. J Parasitol. 1990; 76: 356–359. [PubMed] [Google Scholar]
- 33. Wikel S. Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front Microbiol. 2013; 4: 337 10.3389/fmicb.2013.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.