Abstract
Focal malformations of cortical development, including focal cortical dysplasia (FCD) and hemimegalencephaly (HME), are important causes of intractable childhood epilepsy. Using targeted and exome sequencing on DNA from resected brain samples and non-brain samples from 53 patients with FCD or HME, we identified pathogenic germline and mosaic mutations in multiple PI3K/AKT pathway genes in 9 patients, and a likely pathogenic variant in 1 additional patient. Our data confirm the association of DEPDC5 with sporadic FCD but also implicate this gene for the first time in HME. Our findings suggest that modulation of the mTOR pathway may hold promise for malformation-associated epilepsy.
INTRODUCTION
Focal malformations of cortical development (MCDs), including focal cortical dysplasia (FCD) and hemimegalencephaly (HME), are important causes of intractable childhood epilepsy.1 Focal cortical dysplasia (FCD) is characterized by focal regions of abnormal cortex, whereas hemimegalencephaly (HME) is characterized by enlargement of an entire cerebral hemisphere. HME and some subtypes of FCD share pathological features with tuberous sclerosis (TSC), which is caused by mutations in TSC1 or TSC2 that abnormally activate mTOR, suggesting that hyperactivation of the mTOR pathway may be a common mechanism underlying these disorders.1
Recently, somatic activating point mutations in AKT3, PIK3CA, and MTOR have been identified in HME, and germline and somatic point mutations in AKT3, PIK3R2, and PIK3CA have been identified in the related megalencephaly-capillary malformation syndrome (MCAP) and megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome (MPPH).2-5 In addition, somatic chromosome 1q tetrasomy has been reported in HME, and de novo germline 1q43q44 trisomy in megalencephaly.4, 6, 7 A genetic etiology for FCD has been long hypothesized, and recently germline mutations in DEPDC5 have been associated with familial focal epilepsy, with some affected individuals showing FCD on imaging; additionally, somatic 1q21.1-q44 copy number increase has been associated with FCD.8, 9
Here, we studied resected brain tissue and/or blood or buccal DNA from 53 patients with FCD or HME and report pathogenic mutations and additional likely pathogenic variants in DEPDC5 in FCD, and in DEPDC5, PIK3CA, MTOR, and TSC2 in HME. Our results confirm the role of DEPDC5 in FCD and implicate it for the first time in HME. FCD, HME, and TSC appear to represent different manifestations of aberrant mTOR signaling, with complex combinations of germline and mosaic mutations, suggesting that therapies targeting this pathway may prove useful across a range of MCDs.
SUBJECTS/MATERIALS AND METHODS
Patient Cohort
The study was approved by the institutional review boards of Boston Children's Hospital, Beth Israel Deaconess Medical Center, Boston, and University of California, Los Angeles. Informed consent was obtained. 53 patients were included; 14 had FCD and 39 had HME based on MRI and neuropathology. Surgically resected brain samples and in some cases buccal or blood samples were available for 39 patients; only buccal or blood samples were available for the remaining 14 patients.
Next Generation Sequencing and Analysis for PI3K/AKT Pathway Variants
Genomic DNA was extracted from patient samples using standard methods. Whole exome sequencing (WES) was performed for 33 samples (10 FCD, 23 HME) and analyzed using standard methods.10 Molecular inversion probe sequencing (MIPS)11 was performed for 44 samples (6 FCD, 38 HME). 24 samples were analyzed using both techniques. Rare variants (minor allele frequency ≤ 1%) in genes in the PI3K/AKT3 pathway were filtered using dbSNP 137 (http://www.ncbi.nlm.nih.gov/SNP/), the 1000 Genomes Project (http://browser.1000genomes.org/index.html), and the Exome Variant Server (http://evs.gs.washington.edu/EVS/). Previously reported mutations were identified using the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php), ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/), and the Leiden Open Variation Database (http://chromium.liacs.nl/LOVD2/home.php) for TSC1/2. We used Provean (http://provean.jcvi.org/index.php), Sift (http://sift.jcvi.org/), Polyphen 2 (http://genetics.bwh.harvard.edu/pph2/), and Mutation Taster (http://www.mutationtaster.org/) to assess for pathogenicity. Variants were considered mosaic if (1) NGS showed an alternate allele frequency (AAF) <50% and we validated the AAF using ddPCR or subcloning for cases where only one tissue was available, or (2) the variant was present in brain tissue but not in non-brain tissue for some cases where multiple tissues were available.
Validation of PI3K/AKT Pathway Variants
Rare and protein-altering (nonsynonymous, nonsense, splice-site, frameshift, and insertion-deletion) variants in the target genes were validated using Sanger sequencing, and parental samples were tested when available. For potential mosaic variants, the original DNA was amplified using polymerase chain reaction (PCR), subcloned into a TOPO TA vector (Invitrogen, Carlsbad, CA), and transformed into TOP10 chemically competent Escherichia coli cells (Invitrogen, Carlsbad, CA); multiple clones were then isolated and sequenced.
ddPCR Screening for PIK3CA Mutations
All samples were screened for five PIK3CA mutations (p.E545K, p.E542K, p.H1047R, p.H1047L, and p.C420R) using droplet digital PCR (ddPCR) to validate mutations identified using WES/MIPS.12 A mix of ddPCR Super Mix (Bio-Rad, Hercules, CA), mutant and reference probes (0.25 μM each), forward and reverse primers (0.9 μM each), and 30 ng of sample DNA was emulsified into 20,000 droplets using a QX100 Droplet Generator (Bio-Rad, Hercules, CA). PCR was performed using the following cycles: 10 min at 95°C, 40 cycles of 30 sec at 94°C and 60 sec at 60°C, 10 min at 98°C. Samples were analyzed using a QX200 Droplet Reader and QuantaSoft software (Bio-Rad, Hercules, CA).
RESULTS
To investigate the role of the PI3K/AKT pathway in focal malformations of cortical development, 14 patients with focal cortical dysplasia and 39 patients with hemimegalencephaly were studied using WES and/or MIPS. In total, we identified and validated 20 rare and protein-altering variants in the PI3K-AKT pathway genes DEPDC5, MTOR, PIK3CA, PIK3C2B, PIK3C2G, PIK3C3, and TSC2 in 16 patients (Supplementary Tables 1 and 3). Variants were considered pathogenic if they were loss-of-function mutations, predicted deleterious nonsynonymous mutations proven pathogenic by functional studies, or mutations previously identified in HME or related syndromes (Table 1). With the exception of one mosaic nonsynonymous mutation classified as likely pathogenic due to its somatic nature, the remaining variants were classified as variants of unknown significance (VUS) (Supplementary Table 2), though further work is likely to identify some of these as causative.
Table 1.
Pathogenic Mutations and Likely Pathogenic Variant Detected in PI3K/AKT Pathway in Patients with FCD and HME
| Case | Diagnosis | Gene | Mutation | HGVS | Type (Alternate Allele Frequency) | Comments |
|---|---|---|---|---|---|---|
| FCD-1 | FCD IIb | DEPDC5 | Fs | p.N261Kfs*11 | Germline (55%) | Loss of function |
| FCD-2 | FCD IIb | DEPDC5 | Sp | c.624+1G>A | Germline (50%) | Loss of function |
| HME-1 | HME | DEPDC5 | Fs | p.N45Qfs*3 | Germline (61%) | Loss of function |
| HME-2 | HME | MTOR | Ms | p.C1483Y | Mosaic (14%) | Previously identified in HME2 |
| HME-3 | HME | PIK3CA | Ms | p.E542K | Mosaic (28%) | Previously identified in CLOVES15, rs121913273 |
| HME-4 | HME | PIK3CA | Ms | p.E545K | Mosaic (18%) | Previously identified in HME2 and MCAP5, rs104886003 |
| HME-5 | HME | PIK3CA | Ms | p.E545K | Mosaic (17%) | Previously identified in HME2 and MCAP5, rs104886003 |
| HME-6 | HME | PIK3CA | Ms | H1047R | Mosaic (13%) | Previously identified in CLOVES15, rs121913279 |
| HME-8 | HME | MTOR | Ms | p.A1669S1 | Mosaic (44% brain, 0% blood) | |
| HME-11 | HME | TSC2 | Ms | p.R1713H | Germline (50%) | Previously identified in TSC17, Proven pathogenic16, rs45517395 |
Ms: missense, Fs: frameshift, Sp: splicing
Likely pathogenic mutation
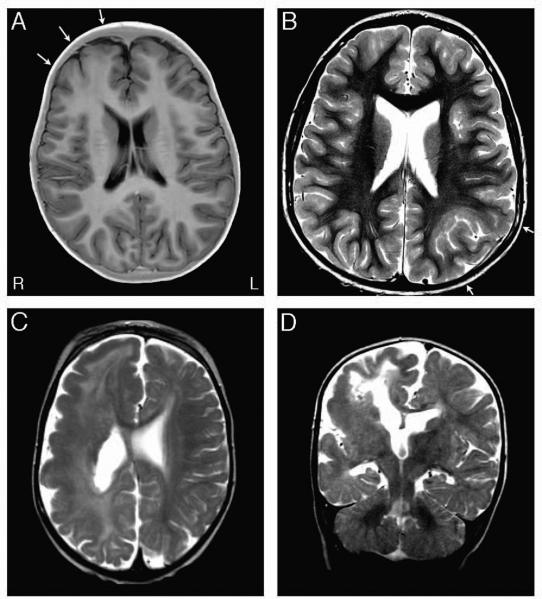
In two patients with FCD type IIb, we identified germline loss-of-function mutations in DEPDC5: a frameshift (p.N261Kfs*11) in patient FCD-1 with a right frontal FCD (Fig 1A) and a splice-site mutation (c.624+1G>A) in patient FCD-2 with a left parietal FCD (Fig 1B). We also identified a germline missense mutation (c.1355C>T, p.A452V) in FCD-3 previously reported as causative in patients with familial focal epilepsy with variable foci,13, which we conservatively classified as a VUS given recent preliminary functional studies suggesting the variant may not be pathogenic.14
Figure 1.
MRI images of FCD and HME mutation-positive patients. A. This axial inverted T2 image from the MRI of Patient FCD-1, with the germline DEPDC5 p.N261Kfs*11 frameshift mutation, shows a right frontal FCD II (arrows), seen as blurring of the gray-white matter junction and abnormal gray matter signal extending toward the ventricle. B. This T2-weighted axial image from the MRI of Patient FCD-2, harboring the germline DEPDC5 c.624+1G>A splice-altering mutation, shows the FCD II characterized by blurring of the gray-white matter junction and abnormal deep gyral configuration in the left parietal region (arrows). C&D. These T2-weighted axial (C) and coronal (D) images from Patient HME-1, with the germline DEPDC5 p.N45Qfs*3 frameshift mutation, illustrate right hemimegalencephaly, with abnormally thick gray matter, abnormal signal in the white matter, and an enlarged right ventricle. Images are shown using MRI convention (R = right, L = left).
In seven patients with HME, we identified loss-of-function or damaging missense mutations in DEPDC5, MTOR, PIK3CA, and TSC2. HME-1 has a germline frameshift in DEPDC5 (p.N45Qfs*3) (Fig 1C-D), and imaging shows right hemimegalencephaly with abnormally thick gray matter, abnormal signal in the white matter, and an enlarged right ventricle. HME-7, who has generalized tonic-clonic seizures, speech delay, and mild right hemiparesis, has a germline inherited missense variant in DEPDC5 (c.1265G>A, p.R422Q), and imaging shows left hemimegalencephaly with blurring of the gray-white matter junction and cortical irregularity most striking in the left parietal lobe (Sup Fig 1). Two patients showed mosaic missense mutations in PIK3CA, E542K (c.1624G>A) in HME-3 and H1047R (c.3140A>G) in HME-6, both previously identified in CLOVES syndrome.15 HME-6 also harbors a germline missense variant in PIK3CA (c.1432G>T, p.D478Y). HME-8, who has complex partial seizures, harbors a mosaic missense variant in MTOR (c.5005G>T, p.A1669S) present at an AAF of 44% in the brain but not detectable in blood. HME-11 harbors a germline missense mutation in TSC2 (c.5138G>A, p.R1713H) previously shown to be pathogenic.16, 17 In an additional three patients, we detected the same mosaic mutations in PIK3CA (c.1633G>A, p.E545K) and MTOR (c.4448G>A, p.C1483Y) that had been previously reported.2, 5
DISCUSSION
Our data show that FCD and HME are allelic disorders, reflecting activating mutations in the PI3K/AKT pathway. DEPDC5 mutations have only recently been shown to be associated with FCD, originally reported in familial focal epilepsies with FCD in a few family members9; our data confirm this association and extend it to sporadic FCD, while implicating DEPDC5 mutations for the first time in HME.
The mTOR pathway is critical for sensing nutrients and other metabolic cues and regulating protein synthesis and cell growth18 (Fig 2). Activating mutations in positive regulators of the pathway, including MTOR, PIK3CA, and PIK3R2, lead to excessive mTOR signaling. DEPDC5 encodes a member of the GATOR1 complex, and, along with TSC1 andTSC2, acts as a negative regulator of the mammalian target of rapamycin complex 1 (mTORC1)18, 19. In fact, we observed one pathogenic mutation and two additional potentially pathogenic variants in TSC2 in HME patients that we conservatively classified as VUS (Supplementary Table 2). Thus, the loss-of-function and damaging nonsynonymous mutations identified here are all predicted to result in hyperactivation of the mTOR pathway.
Figure 2.
Schematic of the mTOR pathway annotated with pathogenic mutations and likely pathogenic variant identified in this study; mosaic mutations are bolded.
Both TSC1 and TSC2 provide critical regulation of mTORC1 through the GTPase-activating protein (GAP) activity of the TSC protein complex towards the RHEB GTPase.18 Similarly, the GATOR1 complex provides critical regulation of mTORC1 through its GAP activity on the Rag GTPases.9 Loss of either of these protein complexes through loss of any one of their critical protein components leads to high-level activation of mTORC1, and downstream effects on anabolic processes, including synthesis of all components needed for organelle synthesis, protein translation, and an increase in cell size. Hence it is not surprising that mutation in any one of TSC1, TSC2, or DEPDC5 could cause a neurologic syndrome in which giant cells are a primary feature.
Several of the variants identified here were germline, but the focal nature of both HME and FCD suggest the possibility of a somatic “second hit,” either in the other allele of the gene with a germline mutation or in another gene in the same pathway.20 In fact, given the previously identified patients with familial DEPDC5 mutations, most of whom lack cortical malformations, we strongly suspect a somatic second hit giving rise to the FCDs in a few family members. For example, in one case with an inherited DEPDC5 variant (p.R422Q), it is possible that the variant-carrying parent, who is phenotypically unaffected, represents non-penetrance and that the patient carries a second mutation. Similar to a “second hit” in TSC giving rise to cortical tubers in the presence of a germline TSC1 or TSC2 mutation21, a somatic mutation in a neural progenitor at a different developmental timepoint could give rise to either FCD or HME in combination with a germline mutation or on its own. However, identification of such somatic mutations will require very high coverage next generation sequencing, ideally of affected brain tissue, given that the mutation may be present in only a small fraction of the cells.22 Both WES and MIPS analysis are also not sensitive to genic deletions, which would be one plausible cause of such second hits. Moving forward, it will be critical to perform such ultra-deep sequencing, ideally using a targeted list of known and candidate genes, for FCD, HME, TSC, and related disorders.
Finally, the growing evidence that the shared pathology of FCD, HME, and TSC reflects shared genetic etiology suggests that modulators of the mTOR pathway, currently in clinical trials for patients with TSC, may also apply to the refractory epilepsy associated with FCD and HME, for which patients currently rely on surgical resection to alleviate seizures.19
Supplementary Material
Acknowledgements
We thank June Goto for sample preparation, Matthew Warman for reagents and helpful discussion, and Peter Black, Joseph Madsen, and Elizabeth Engle for patient recruitment. A.M.D. was supported by the NIGMS (T32GM007753) and NRSA (5T32 GM007226-39). D.J.K was supported by the European Commission 602391-2. J.S. was supported by the National Cancer Institute (1R21CA160080). G.W.M was supported by the NIN (R01 NS38992 and NS083823). A.P was supported by the NINDS (K23 NS069784). C.A.W. was supported by the National Institute of Mental Health (R01MH083565 and 1RC2MH089952), the National Institute of Neurological Disorders and Stroke (R01NS032457, R01NS079277 and R01NS035129), the Simons Foundation, the Paul G. Allen Family Foundation, and the Manton Center for Orphan Disease Research. C.A.W. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Potential Conflicts of Interest:
E.A.B. has a patent pending on Molecular Inversion Probe design. D.J.K. reports personal fees form Novartis, outside the submitted work. H.V.V. owns stock in and receives dividends from 3M, GE, Becton-Dickinson, Teva Pharma, Glaxo-SmithKline Beecham, and Pfizer, receives book royalties from Mosby Publishers, and receives honoraria for CME lectures (none related to the subject of this paper).
Authorship:
A.M.D., Y.G., C.A.W. and A.P. designed the study, and C.A.W. and A.P. supervised the study. A.M.D., Y.G., J.A.C, B.M., E.A.B, C.M.L., A.H. and N.E.H. performed experiments and analyzed data. B.B. coordinated sample collection and phenotyping. D.J.K., H.V.V. and G.W.M. recruited patients and collected and prepared tissue samples. J.S. designed and supervised MIPS experiments. Y.G., A.J.B., G.W.M., C.A.W., and A.P. interpreted brain imaging data. A.M.D., C.A.W. and A.P. wrote the manuscript and all coauthors edited the manuscript.
REFERENCES
- 1.Mirzaa GM, Poduri A. Megalencephaly and hemimegalencephaly: breakthroughs in molecular etiology. American journal of medical genetics Part C, Seminars in medical genetics. 2014 Jun;166C(2):156–72. doi: 10.1002/ajmg.c.31401. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Huynh M, Silhavy JL, et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nature genetics. 2012 Aug;44(8):941–5. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura K, Kato M, Tohyama J, et al. AKT3 and PIK3R2 mutations in two patients with megalencephaly-related syndromes: MCAP and MPPH. Clinical genetics. 2014 Apr;85(4):396–8. doi: 10.1111/cge.12188. [DOI] [PubMed] [Google Scholar]
- 4.Poduri A, Evrony GD, Cai X, et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron. 2012 Apr 12;74(1):41–8. doi: 10.1016/j.neuron.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riviere JB, Mirzaa GM, O'Roak BJ, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nature genetics. 2012 Aug;44(8):934–40. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai X, Evrony GD, Lehmann HS, et al. Single-Cell, Genome-wide Sequencing Identifies Clonal Somatic Copy-Number Variation in the Human Brain. Cell reports. 2014 Sep 11;8(5):1280–9. doi: 10.1016/j.celrep.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Zeesman S, Tarnopolsky MA, Nowaczyk MJ. Duplication of AKT3 as a cause of macrocephaly in duplication 1q43q44. American journal of medical genetics Part A. 2013 Aug;161A(8):2016–9. doi: 10.1002/ajmg.a.35999. [DOI] [PubMed] [Google Scholar]
- 8.Conti V, Pantaleo M, Barba C, et al. Focal dysplasia of the cerebral cortex and infantile spasms associated with somatic 1q21.1-q44 duplication including the AKT3 gene. Clinical genetics. 2014 Aug 5; doi: 10.1111/cge.12476. [DOI] [PubMed] [Google Scholar]
- 9.Scheffer IE, Heron SE, Regan BM, et al. Mutations in mammalian target of rapamycin regulator DEPDC5 cause focal epilepsy with brain malformations. Annals of neurology. 2014 May;75(5):782–7. doi: 10.1002/ana.24126. [DOI] [PubMed] [Google Scholar]
- 10.Yu TW, Chahrour MH, Coulter ME, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013 Jan 23;77(2):259–73. doi: 10.1016/j.neuron.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiatt JB, Pritchard CC, Salipante SJ, O'Roak BJ, Shendure J. Single molecule molecular inversion probes for targeted, high-accuracy detection of low-frequency variation. Genome research. 2013 May;23(5):843–54. doi: 10.1101/gr.147686.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luks VL, Kamitaki N, Vivero MP, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. doi: 10.1016/j.jpeds.2014.12.069. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dibbens LM, de Vries B, Donatello S, et al. Mutations in DEPDC5 cause familial focal epilepsy with variable foci. Nature genetics. 2013 May;45(5):546–51. doi: 10.1038/ng.2599. [DOI] [PubMed] [Google Scholar]
- 14.van Kranenburg M, Hoogeveen-Westerveld M, Nellist M. Preliminary Functional Assessment and Classification of DEPDC5 Variants Associated with Focal Epilepsy. Human mutation. 2014 Nov 4; doi: 10.1002/humu.22723. [DOI] [PubMed] [Google Scholar]
- 15.Kurek KC, Luks VL, Ayturk UM, et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. American journal of human genetics. 2012 Jun 8;90(6):1108–15. doi: 10.1016/j.ajhg.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoogeveen-Westerveld M, Wentink M, van den Heuvel D, et al. Functional assessment of variants in the TSC1 and TSC2 genes identified in individuals with Tuberous Sclerosis Complex. Human mutation. 2011 Apr;32(4):424–35. doi: 10.1002/humu.21451. [DOI] [PubMed] [Google Scholar]
- 17.Hirfanoglu T, Gupta A. Tuberous sclerosis complex with a single brain lesion on MRI mimicking focal cortical dysplasia. Pediatric neurology. 2010 May;42(5):343–7. doi: 10.1016/j.pediatrneurol.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012 Apr 13;149(2):274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poduri A. DEPDC5 does it all: shared genetics for diverse epilepsy syndromes. Annals of neurology. 2014 May;75(5):631–3. doi: 10.1002/ana.24160. [DOI] [PubMed] [Google Scholar]
- 20.Poduri A, Evrony GD, Cai X, Walsh CA. Somatic mutation, genomic variation, and neurological disease. Science. 2013 Jul 5;341(6141):1237758. doi: 10.1126/science.1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin W, Chan JA, Vinters HV, et al. Analysis of TSC cortical tubers by deep sequencing of TSC1, TSC2 and KRAS demonstrates that small second-hit mutations in these genes are rare events. Brain Pathol. 2010 Nov;20(6):1096–105. doi: 10.1111/j.1750-3639.2010.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamuar SS, Lam AT, Kircher M, et al. Somatic mutations in cerebral cortical malformations. The New England journal of medicine. 2014 Aug 21;371(8):733–43. doi: 10.1056/NEJMoa1314432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.