Abstract
Background. Pneumococcal vaccination is recommended for human immunodeficiency virus-infected (HIV+) persons; the best timing for immunization with respect to initiation of antiretroviral therapy (ART) is unknown.
Methods. Double-blind, placebo-controlled trial in HIV+ with CD4+ T cells/µL (CD4) ≥ 200 randomized to receive the 23-valent pneumococcal polysaccharide vaccine (PPV23) or placebo at enrollment, followed by placebo or PPV23, respectively, 9–12 months later (after ≥6 months of ART). Capsular polysaccharide-specific immunoglobin (Ig) G and IgM levels to serotypes 1, 3, 4, 6B, and 23F, and opsonophagocytic killing activity (OPA) to serotypes 6B and 23F were evaluated 1 month postvaccination.
Results. One hundred seven subjects were enrolled, 72 (67.3%) were evaluable (36/group). Both groups had significant increases in pre- to 1-month postvaccination IgG levels, but negligible to IgM, and significant increases in OPA titers to serotype 6B but not to 23F. There were no significant differences between groups in serotype-specific IgM or IgG levels or OPA titers. For the combined groups, there was a significant correlation between serotype-specific IgG and OPA titers to 23F but not to 6B. There was no correlation between CD4, viral load and IgG responses.
Conclusions. In HIV+ with CD4 ≥ 200, delaying PPV23 until ≥6 months of ART does not improve responses and may lead to missed opportunities for immunization.
Keywords: antibody, HIV, pneumococcal vaccine, pneumococcal capsular polysaccharides, antiretroviral treatment
Streptococcus pneumoniae is the worldwide leading cause of bacterial pneumonia in human immunodeficiency virus (HIV)-infected adults [1]. Availability of antiretroviral treatment (ART) has more than halved the incidence of this entity; however, the residual disease burden remains more than 35-fold higher than that in age-matched HIV-uninfected people [2].
Vaccination against S. pneumoniae and influenza, use of ART, and smoking cessation are recommended for prevention of bacterial pneumonia [3]. Two types of pneumococcal vaccines are Food and Drug Administration–approved in the United States: the 23-valent pneumococcal polysaccharide vaccine (PPV23) and the 13-valent pneumococcal conjugate vaccine (PCV13). Recently, the Advisory Committee on Immunization Practices (ACIP) recommended that vaccine-naive adults with immune-compromising conditions receive an initial dose of PCV13 followed ≥8 weeks later by administration of PPV23 [4]. The ACIP also recommends that HIV-infected persons be immunized as close to HIV diagnosis as possible [5]. For HIV-infected subjects, both the type of pneumococcal vaccine and the timing of immunization may influence the effectiveness of the vaccine. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-infected adults and adolescents incorporate a CD4+ T-cell (CD4) count and/or treatment criteria to be taken into consideration for pneumococcal immunization [3]. Furthermore, these guidelines include the ACIP recommendations but make it optional to offer PPV23 after PCV13 to those with CD4 count <200/µL and suggest initiation of ART prior to immunization. Only observational studies support this latter recommendation [3].
The inability of HIV-infected persons to respond to T-cell-independent Type 2 antigens has been recognized since early in the HIV epidemic and this defect is considered to underlie their impaired pneumococcal capsular polysaccharide responses [6]. In recent years, defects in B-cell numbers, function, and subpopulation distributions have been well described [7]; and it has been recognized that B cells can be reconstituted with control of viremia [8]. Notably, evidence to suggest that T-cell-independent responses are restored as a function of CD4-cell reconstitution is scant [9, 10], whereas ample data show that ART use has led to a decrease in HIV-associated invasive pneumococcal disease [11, 12]. The latter makes it difficult to separate the effect of ART on disease pathogenesis from improved vaccine efficacy in HIV-infected persons on treatment. In addition, pneumococcal capsular polysaccharides have been shown to induce antibody responses that are highly restricted to the use of variable region heavy chain genes (VH) from the VH3 family [13–15]. Some studies indicate that the expression of VH3 family genes is decreased among HIV-infected persons [13] and one showed that ART could partially restore the VH3 response to PPV23 in HIV-infected persons [16].
It is logical to hypothesize that ART might lead to improved pneumococcal capsular polysaccharide antibody responses in HIV-infected persons. Timing of vaccine then becomes critical. Though immunizing HIV-infected patients early in the course of their disease can offer early protection, delay until viral replication is suppressed by ART might reverse the HIV-induced B-cell dysfunction [8, 17, 18]. Controlled viremia has been associated with improved antibody responses to hepatitis B [19] and influenza [20] vaccines; however, it has not yet been prospectively shown to increase responses to pneumococcal vaccines. In the present study, we compared antibody responses to pneumococcal capsular polysaccharides in HIV-infected subjects who received PPV23 prior to initiation of ART to those who received it after ≥6 months of ART.
METHODS
Study Design
This is a randomized, double blind, placebo-controlled clinical trial carried out at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) and Thomas Street Health Center (Harris Health System) in Houston, Texas, between January 2009 and December 2012. About 800 and 4000 HIV-infected patients, respectively, were followed at each of these clinics during the study period. The study was approved by the Institutional Review Board at Baylor College of Medicine, the Research and Development Committee at the MEDVAMC, and the Harris Health System. This study was monitored by a Veterans Affairs Merit Review Data Monitoring Committee.
HIV-infected patients who met the following criteria were eligible: CD4 ≥ 200/µL, no prior AIDS diagnosis (including no prior CD4 < 200/µL), no pneumococcal immunization in the prior 3 years, treatment-naive or treatment-experienced with no ART within the last year, and ready to start/restart ART. These patients were randomized in a 1:1.5 ratio to the immediate and delayed vaccinations groups (Immediate group and Delayed group, respectively) by a computer-generated random list produced by the MEDVAMC research pharmacist. The 1:1.5 ratio was chosen to account for the likelihood for increased rate of lost to follow-up in the Delayed group. Those in the Immediate group received PPV23 at the time of enrollment and placebo 9–12 months later. The Delayed group received placebo at the time of enrollment and PPV23 9–12 months post-enrollment (after ≥6 months of ART). All participants were followed up 1 month (4–6 weeks), 6 months (±1), and 12 months (±3) after each intervention.
Data Collection
Demographic and clinical data were collected from the patient records at each visit. Adherence to ART was examined by patients' self-report and by review of pharmacy records. Patients were questioned about any febrile or respiratory illness and hospitalization; records were examined for clinic visits, emergency department visits, or hospitalizations for syndromes consistent with pneumococcal infection.
Laboratory
Blood samples were obtained at each visit. Serum samples were used to measure antibodies against 5 pneumococcal capsular polysaccharides included in PPV23 (1, 3, 4, 6B, and 23F). These serotypes were included because they have been consistently included in prior studies from our laboratory [21, 22]; serotype 1 was tested because it was to be included in the 13-valent conjugate vaccine. Immunoglobin (Ig) G and IgM enzyme-linked immunosorbent assay (ELISA) was performed as previously described [23] using the 89SF reference serum as the standard. Opsonophagocytic killing activity (OPA) was evaluated for 6B and 23F. These serotypes were chosen because we have consistently found them to be intermediate to good immunogens in HIV-infected adults [24], and had a qualified OPA assay in our laboratory. OPA titers were defined as the reciprocal of the dilution of serum that killed 50% of the target bacteria (compared to the control) during 1 hour of incubation at 37°C [22]. Total IgG and IgM (in mg/dL) were measured using an endpoint radial immunodiffusion test [Radial Immunodiffusion plates, Kent Laboratories, Bellingham, Washington].
Sample Size
It was calculated based on the hypothesis that among patients with CD4 ≥200 and initiating ART, delaying immunization until after ≥6 months of ART enhances antibody responses to pneumococcal capsular polysaccharides. The variable used for sample size was defined as the average difference (post- to prevaccine titers) of the natural logarithms of the 5 serotypes studied: [log post – log pre] (Delayed group) – [log post – log pre] (Immediate group) >0.405. Sample size calculations were based on a 2-tailed, 2-sample Student t test with a type 1 error of 0.05. The standard deviation for each group was assumed to be 0.6 on the basis of previous data [21]. The power was set at 80% and the hypothesized difference is 0.405. With these parameter values, the required sample size in each group was 36 subjects [23]. Enrollment targets were set up at 43 and 64 for Immediate- and Delayed group, respectively, to account for the increased risk of loss to follow-up and protocol violations in the Delayed group (given the longer follow-up required prior to vaccination).
Statistical Analysis
Subjects' characteristics data are presented as N (percentage) for categorical values and median (interquartile range) for numerical values. Total IgG and IgM values are presented as geometric mean (95% confidence interval [CI]). Specific anti-PS IgG and IgM were determined in all samples at the specified time periods. Results are reported as IgG (µg/mL) and IgM (µg/mL) geometric mean (95% CI) and in OPA titers geometric mean (95% CI). IgG and IgM concentrations and OPA titers were natural log-transformed prior to statistical analysis. IgG and IgM antibody responses were defined as ≥2-fold increase and postvaccine levels of ≥1 µg/mL, definition that has been previously used by our laboratory and others [21, 25]. OPA responses were defined as ≥4-fold increase in the postvaccine titer. The Student t test was used to compare continuous variables between patient groups. The paired Student t test was used to compare pre- and postvaccine values. The percentages of responders from each group were evaluated by the Fisher exact test. Correlations between serotype-specific IgG and OPA, and between CD4 count, viral load, and total IgG and IgM levels at the time of vaccination, and 1-month postvaccine serotype-specific IgG or OPA were determined by the Pearson's correlation coefficients. Correlations with P values < .05 were considered significant.
RESULTS
Subjects
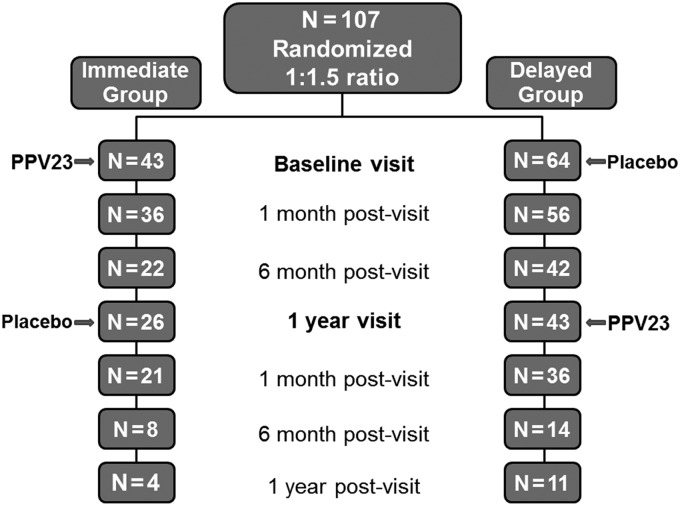
A total of 107 subjects were enrolled (Figure 1). The observed high rate of participants' attrition at 1 month and 1 year postenrollment was inherent to these clinics' patient population (high rate of missed clinic appointments) and specific efforts were made to achieve the required sample size, especially for the Delayed group. Only patients who completed the 1-month post-PPV23 visit (as per protocol) were included in the analysis (36 subjects in each group; 84% and 56% from the Immediate- and Delayed group, respectively). Both study groups had similar characteristics at enrollment (Table 1), and were not different from that of subjects lost to follow-up (data not shown). At enrollment, there were no statistically significant differences between the groups in the median CD4 count or median viral load (Table 1). Total IgG and IgM levels, markers of humoral immune activation by HIV infection, were also measured and were not significantly different between the groups. However, as expected, on the day of PPV23 administration, the Delayed group demonstrated a significant increase in the median CD4 count (from 352 to 470 cells/µL), and a significant decrease in the median viral load, from 19 795 to 48 RNA copies/mL, with 60% of subjects achieving a viral load <50 copies/mL. In addition, total IgG and total IgM geometric means (our biomarkers of B-cell immune activation) demonstrated significant decreases in the Delayed group when compared to enrollment values (P ≤ .01 for both comparisons) (Table 1).
Figure 1.
Randomization and subjects' follow-up. A total of 107 subjects were enrolled in the study and were randomized to receive 23-valent pneumococcal polysaccharide vaccine (PPV23) at baseline visit (Immediate group) or 1 year later*, and after at least 6 months of antiretroviral treatment (Delayed group). Only subjects that completed the 1 month post-PPV23 visit were included in the analytical group (n = 36 in each group). *1 year visits had a window of 9–12 months.
Table 1.
Characteristics of HIV-Infected Subjects in the Immediate- and Delayed Groups
| Category | Immediate Group (N = 36) | Delayed Group (N = 36) |
|---|---|---|
| Agea (years) | 44 (29–55) | 45 (38–50) |
| Male (%) | 32 (88.9) | 29 (80.6) |
| Race (%) | ||
| Black | 22 (68.8) | 18 (62.1) |
| Hispanic | 8 (25.0) | 7 (24.1) |
| White | 2 (6.3) | 4 (13.8) |
| Female (%) | 4 (11.1) | 7 (19.4) |
| Race (%) | ||
| Black | 2 (50.0) | 6 (85.8) |
| Hispanic | 1 (25.0) | 0 (0) |
| White | 1 (25.0) | 1 (14.3) |
| Previous PPV23 (%) | 8 (22.2) | 7 (19.4) |
| 3–5 y | 3 (8) | 4 (11) |
| >5 y | 5 (14) | 3 (8) |
| Underlying conditions (%) | ||
| Chronic liver disease | 2 (5.6) | 0 (0) |
| Hepatitis C | 6 (16.7) | 6 (16.7) |
| COPD | 3 (8.3) | 2 (5.6) |
| Diabetes | 7 (19.4) | 2 (5.6) |
| Renal insufficiency | 2 (5.6) | 2 (5.6) |
| Coronary artery disease | 3 (8.3) | 0 (0) |
| Heart failure (%) | 2 (5.6) | 0 (0) |
| Intravenous drug use (%) | 5 (13.9) | 4 (11.1) |
| Alcohol abuse (%) | 7 (19.4) | 10 (27.7) |
| Tobacco abuse (%) | 21 (58.3) | 21(58.3) |
| Current | 6 (16.7) | 8 (22.2) |
| Past | 9 (25.0) | 7 (19.4) |
| Laboratory data at enrollment | ||
| CD4+ T-cell count (cells/µL)a | 303 (238–356) | 352 (298–462) |
| Viral load (HIV-1 RNA copies/mL)a | 28 400 (10 375–94 967) | 19 795 (4403–55 164) |
| Total IgM (mg/dL)b | 139 (114–170) | 155 (123–194) |
| Total IgG (mg/dL)b | 1796 (1480–2179) | 2095 (1865–2354) |
| Laboratory data at 1 y visit (vaccination date for Delayed group) | ||
| CD4+ T-cell count (cells/µL)a | N/A | 470 (325–556)*, ** |
| Viral load (HIV-1 RNA copies/mL)a | N/A | 48 (48–368)*, ** |
| Total IgM (mg/dL)b | N/A | 112 (89–141)* |
| Total IgG (mg/dL)b | N/A | 1665 (1471–1885)* |
Immediate group and Delayed group, received PPV23 prior to starting and at least 6 months after starting antiretroviral treatment, respectively. Baseline data (at enrollment) unless otherwise specified.
Abbreviations: COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; IgG, immunoglobin G; IgM, immunoglobin M; PPV23, 23-valent pneumococcal polysaccharide vaccine.
a Data reported as median (interquartile range).
b Data reported as geometric mean (95% confidence interval).
* Significantly different from same group baseline values.
** Significantly different from Immediate group baseline.
During the study period, 7 patients were hospitalized with a diagnosis of pneumonia. One case was confirmed as pneumococcal pneumonia (a patient randomized to the Delayed group and prior to PPV23 administration). One patient in each group had a confirmed or probable diagnosis of Pneumocystis jiroveci pneumonia. The other 4 cases (2 in each group) had no microbiologic diagnosis.
Antibody Levels to Serotypes 1, 3, 4, 6, and 23F
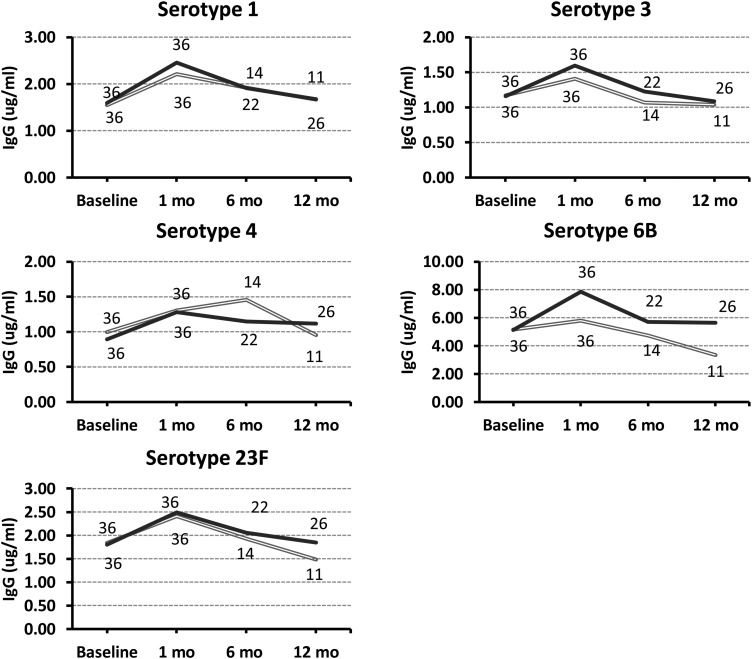
There were no significant differences between the groups in IgG or IgM baseline levels to the 5 serotypes tested (P > .05 for all serotypes; Tables 2 and 3). There were no significant changes in IgG or IgM levels after placebo administration (data not shown). IgG and IgM levels 1-month post-PPV23 were not significantly different between the groups. IgG levels 1-month post-PPV23 compared to prevaccine levels were significantly higher for all 5 serotypes studied in the Immediate group, and for 4 in the Delayed group (Table 2). Pre- to 1-month post-PPV23 changes in IgM levels were minimal, with significant increases to only 2 (3 and 6B) and 1 (6B) of the serotypes studied in the Immediate- and the Delayed group, respectively (Table 3). IgG levels returned to baseline values among the subjects that completed the 1-year post-PPV23 evaluation (Figure 2). The percentage of subjects that responded to any serotype (response defined as ≥2-fold increase and ≥1 µg/mL in IgG or IgM level) was low and similar between both groups (Table 4). Excluding from the analysis those that had received prior immunization (8 in the Immediate group and 7 in the Delayed group) did not affect the IgG results, but the P value became nonsignificant for 1 serotype in the Delayed group, likely due to decreased sample size.
Table 2.
Geometric Mean Concentrations and 95% Confidence Intervals (CIs) of IgG (µg/mL) to Indicated Serotypes in HIV-Infected Subjects That Completed the 1-Month Post-PPV23 Visit
| Serotype 1 Geometric Mean (95% CI) | Serotype 3 Geometric Mean (95% CI) | Serotype 4 Geometric Mean (95% CI) | Serotype 6B Geometric Mean (95% CI) | Serotype 23F Geometric Mean (95% CI) | |
|---|---|---|---|---|---|
| Immediate group | |||||
| Prevaccine (n = 36) | 1.6 (1.22–2.09) | 1.16 (.91–1.48) | 0.89 (.71–1.12) | 5.12 (3.92–6.7) | 1.80 (1.39–2.33) |
| 1-mo post-PPV23 (n = 36) | 2.45 (1.84–3.27)* | 1.59 (1.19–2.13)* | 1.28 (1.02–1.61)* | 7.85 (6.25–9.86)* | 2.50 (1.9–3.27)* |
| Delayed group | |||||
| Prevaccine (n = 36) | 1.54 (1.12–2.13) | 1.17 (.91–1.5) | 1 (.81–1.23) | 5.17 (4.1–6.52) | 1.83 (1.56–2.16) |
| 1-mo post-PPV23 (n = 36) | 2.21 (1.55–3.14)* | 1.41 (1.08–1.84)* | 1.3 (.99–1.72)* | 5.79 (4.66–7.2) | 2.42 (1.99–2.92)* |
Immediate group and Delayed group received PPV23 prior to starting and at least 6 months after starting antiretroviral treatment, respectively.
Abbreviations: HIV, human immunodeficiency virus; IgG, immunoglobin G; PPV23, 23-valent pneumococcal polysaccharide vaccine.
* P < .05 compared to prevaccine level.
Table 3.
Geometric Mean Concentrations and 95% Confidence Intervals (CIs) of IgM (µg/mL) to Indicated Serotypes in HIV-Infected Subjects That Completed the 1-Month post-PPV23 Visit
| Serotype 1 Geometric Mean (95% CI) | Serotype 3 Geometric Mean (95% CI) | Serotype 4 Geometric Mean (95% CI) | Serotype 6B Geometric Mean (95% CI) | Serotype 23F Geometric Mean (95% CI) | |
|---|---|---|---|---|---|
| Immediate group | |||||
| Prevaccine (n = 36) | 0.96 (0.68–1.36) | 1.32 (1.07–1.63) | 0.82 (0.64–1.04) | 1.89 (1.47–2.44) | 0.59 (0.42–0.82) |
| 1-mo post-PPV23 (n = 36) | 1.04 (0.75–1.45) | 1.67 (1.35–2.07)* | 1 (0.8–1.25) | 2.18 (1.73–2.75)* | 0.65 (0.48–0.88) |
| Delayed group | |||||
| Prevaccine (n = 36) | 1.40 (1.14–1.73) | 1.43 (1.14–1.8) | 0.79 (0.64–0.98) | 1.85 (1.54–2.22) | 0.78 (0.61–1) |
| 1-mo post-PPV23 (n = 36) | 1.51 (1.21–1.88) | 1.51 (1.2–1.91) | 0.92 (0.76–1.11) | 2.26 (1.84–2.77)* | 0.84 (0.67–1.04) |
Immediate group and Delayed group received PPV23 prior to starting and at least 6 months after starting antiretroviral treatment, respectively.
Abbreviations: HIV, human immunodeficiency virus; IgM, immunoglobin M; PPV23, 23-valent pneumococcal polysaccharide vaccine.
* P < .05 compared to prevaccine level.
Figure 2.
Immunoglobin G (IgG) levels to 5 pneumococcal serotypes included in the 23-valent pneumococcal polysaccharide vaccine, 1, 3, 4, 6B and 23F, were measured at baseline, and at 1, 6 and 12 months postvaccination. The number of subjects is indicated at each time point. Gray: Delayed group. Black: Immediate group.
Table 4.
Number (Percentage) of IgG and IgM Responders to Pneumococcal Capsular Polysaccharides in HIV-Infected Subjects That Completed the 1-Month Post-23-Pneumococcal Polysaccharide Vaccine Visit
| Number of Responses | Immediate Group (N = 36) Number (%) | Delayed Group (N = 36) Number (%) |
|---|---|---|
| IgG | ||
| 0 | 16 (44.4) | 23 (63.9) |
| 1 | 13 (36.1) | 6 (16.7) |
| 2 | 2 (5.6) | 4 (11.1) |
| 3 | 2 (5.6) | 2 (5.6) |
| 4 | 1 (2.8) | 1 (2.8) |
| 5 | 2 (5.6) | 0 (0) |
| IgM | ||
| 0 | 24 (66.7) | 24 (66.7) |
| 1 | 8 (22.2) | 8 (22.2) |
| 2 | 3 (8.3) | 2 (5.6) |
| 3 | 0 (0) | 2 (5.6) |
| 4 | 1 (2.8) | 0 (0) |
| 5 | 0 (0) | 0 (0) |
Immediate group and Delayed group received PPV23 prior to starting and at least 6 months after starting antiretroviral treatment, respectively. Responses were defined as ≥2-fold increases in IgG (top panel) or IgM levels (bottom panel) to at least 1 µg/mL 1 month after vaccination.
P ≥ .05 for all comparisons between Immediate- and Delayed groups.
Abbreviations: HIV, human immunodeficiency virus; IgG, immunoglobin G; IgM, immunoglobin M; PPV23, 23-valent pneumococcal polysaccharide vaccine.
OPA Responses to Serotypes 6B and 23F
All subjects in the Delayed group and 23 in the Immediate group had serum samples available for this analysis. Significant increases in OPA titers were observed for both groups against 6B (Immediate group, P = .0002; Delayed group, P = .02), but not against 23F (Immediate group, P = .09; Delayed group, P = .56) (Table 5). One-month post-PPV23, OPA titers were not significantly different between the groups. Furthermore, there were no significant differences in the percentage of responders (defined as 4-fold increases) to 6B:13/23 (56.5%) in the Immediate group, and 15/36 (41.7%) in the Delayed group (P = .3) or in the percentage of responders to serotype 23F: 6/23 (26.1%) in the Immediate group, and 5/36 (13.9%) in the Delayed group (P = .31). It is worth noting that in both groups and for both serotypes, we observed OPA responses in some subjects who did not show IgG responses. For the combined groups, there was a significant correlation between 1-month postvaccine serotype-specific IgG levels and OPA titers to serotype 23F (r = 0.3, P = .01); but not for serotype 6B (r = 0.2, P = .07).
Table 5.
Geometric Mean of OPA Titers and 95% Confidence Intervals (CIs) to Serotypes 6B and 23F in the Immediate- and Delayed Groups
| Serotype 6B Geometric Mean (95% CI) | Serotype 23F Geometric Mean (95% CI) | |
|---|---|---|
| Immediate group (N = 23)a | ||
| Prevaccine | 4.38 (2.24–8.57) | 2.87 (2.08–3.97) |
| 1-mo post-PPV23 | 23 (9.57–55.14) | 4.65 (2.61–8.29) |
| Delayed group (N = 36) | ||
| Prevaccine | 5.99 (3.4–10.57) | 2.94 (2.12–4.08) |
| 1-mo post-PPV23 | 13.72 (7.29–25.81) | 3.3 (2.47–4.4) |
Immediate group and Delayed group received PPV23 prior to starting and at least 6 months after starting antiretroviral treatment, respectively.
Abbreviations: OPA, opsonophagocytic killing activity; PPV23, 23-valent pneumococcal polysaccharide vaccine.
a Sample available only for 23 of the 36 patients. Pre- to postvaccine titers increases in the Immediate- and Delayed groups were significant (P < .05) for serotype 6B but not for serotype 23F. All comparisons between patient groups were not significant.
Correlation Between Total IgG and IgM, CD4 Cell Count and HIV-1 Viral Load at Vaccination and IgG Antibody Responses
When the 72 patients from the 2 groups were combined, there were no correlations between HIV-1 viral load at time of PPV23 administration and 1-month postvaccine antiserotype-specific IgG. In addition, there were no correlations between CD4 count and postvaccine antiserotype IgG levels (except for serotype 4, r = 0.3, P = .006), and total IgG and total IgM levels and antiserotype IgG responses. Similarly, there were no correlations in the combined group of subjects that underwent OPA testing between CD4 count, HIV-1 viral load, or total IgG and IgM levels and OPA titers.
DISCUSSION
The results from this double-blind placebo-controlled, randomized trial indicate that in HIV-infected subjects with CD4 ≥200 cells/µL, delaying PPV23 until receipt of ≥6 months of ART does not increase responses measured by OPA and ELISA, and may lead to missed opportunities for immunization, and unnecessary risk of developing pneumococcal disease among those that may derive protection from immediate vaccination. In the Delayed group, 6–12 months of ART led to nondetectable viral load in 60% of subjects, significant decrease in hypergammaglobulinemia (a hallmark of HIV induced B-cell immune hyperactivity) [26], and a significant increase in CD4 cell count; however, the immune responses were not improved compared to those immunized prior to ART. The reasons underlying this phenomenon are likely related to B-cell dysfunction that was not reverted by short course of ART [7, 26].
IgM-memory B cells (IgM+IgD-CD27+ B lymphocytes) have been implicated in responses to capsular polysaccharides. This subset of lymphocytes are absent in children <2 years, and reduced in asplenic, older individuals (>65), and HIV-infected subjects, all populations with increased susceptibility to infection with encapsulated bacteria [7, 8, 27]. In HIV-infected patients, decreased numbers of IgM-memory B cells have been associated with decreased responses to pneumococcal capsular polysaccharides [9]. In addition, a recent study showed that among elderly subjects, decreased proportion of IgM-memory B cells was associated with decreased IgM, IgG, and OPA responses to pneumococcal capsular polysaccharides [28]. Switched-memory B cells (IgM-IgD-CD27+ B lymphocytes), which traditionally have been associated with responses to T-cell–dependent antigens, may also play a role in the IgG responses to pneumococcal antigens [27, 29]. These B-cell subsets are decreased in subjects with chronic HIV infection and do not seem to be restored with ART [9, 10, 29]. Moir et al showed that patients started on ART during chronic HIV infection yield worst antibody response to influenza antigens than those started on ART shortly after seroconversion [7], suggesting that there are certain abnormalities in B-cell function that occur in chronic HIV infection that are not readily reversible.
Serotype-specific antibody levels and OPA are associated with protection against invasive pneumococcal disease, but there are no clear threshold concentrations that accurately predict protection [30]. Thus, the poor responses observed by measuring these parameters in HIV-infected subjects does not necessarily translate to poor vaccine efficacy, underscoring that correlates of vaccine protection against invasive pneumococcal disease are greatly needed [31]. Protection against invasive pneumococcal disease from PPV23 has been established [32], and some observational studies indicate that a reduction in all-cause pneumonia in HIV-infected patients receiving ART is associated with PPV23 vaccination [33, 34]. Furthermore, it is unknown whether OPA is an adequate test for vaccine response to PPV23 in immunocompromised patients. Nonopsonic antibodies have been shown recently to be highly protective in murine models and are not measured by OPA [35]. In our study, we observed a correlation between IgG and OPA responses for serotype 23F but none for 6B. We also observed OPA responses among subjects with no IgG responses (as defined in methods) for both serotypes. Poor correlations between OPA responses and IgG titers have been described to some serotypes (including 6B), in the elderly, and in immunocompromised populations [36, 37]; when there is discrepancy between these assays, OPA correlates better with protection against S. pneumoniae because it directly measures the capacity of antibodies to opsonize pneumococci [36, 37]. Taken together, it is possible that vaccine-induced in vivo protection is occurring in these patients, even though currently available biomarkers of immunogenicity (or definitions used for responses) do not indicate it.
We currently lack tools to predict which HIV-infected subjects will respond to pneumococcal immunization. Testing serotype-specific memory B-cell numbers (as recently shown in the elderly) [28] may be a better predictor of vaccine response than CD4 cell count or viremia; however, this test is not readily available and it is unlikely to be in the near future. T-cell–independent responses are characterized by IgM responses; but specific postvaccine IgM titers are not consistently evaluated in most pneumococcal vaccine studies. In our study, the responses to IgM were generally low. This can be partly explained by the timing of blood sampling, as IgM responses tend to peak at 2 weeks and we obtained the 1-month postvaccine sample at 4–6 weeks. To further investigate this question, for our current vaccine studies investigating immune correlates of response to PPV23 and PCV13, we are obtaining 1-week postvaccine samples.
Currently, a new vaccination schedule is recommended for HIV-infected persons, PCV13 followed by PPV23 [4], with additional recommendations based on CD4 count and/or prior vaccine status [4]. Conjugation of polysaccharides to protein antigens has led to marginal increases in antibody responses among those with HIV infection [38], including those on ART and controlled viremia [21, 25, 39]. In one study, PCV7 yielded protection among HIV-infected subjects with prior episodes of invasive pneumococcal disease [40]; however, the effect was markedly reduced after the first year. In the United States, with the introduction of PCV13, a declined in the incidence of invasive pneumococcal disease caused by serotypes contained in PCV13 (that were not included in PCV7) has already been observed among children and adults [41, 42]. However, there is concern for an increased incidence of non-PCV13 serotypes as a cause of disease in the general population, and more so among immunosuppressed individuals [43]; hence, regardless of the availability of PCV vaccines with expanded serotype coverage, PPV23 is indicated among immunosuppressed subjects following PCV13 administration to protect against the real possibility of disease caused by non-PCV13 serotypes [4]; and for the time being, strategies to improve responses to pneumococcal polysaccharides are worth pursuing.
This study's main strength is that it followed a prospective, randomized, double-blind placebo-control design to evaluate the question of best timing of pneumococcal immunization for HIV-infected subjects initiating ART, and included both quantitative and qualitative evaluation of responses. Some limitations include the high attrition rate among the initially enrolled study subjects (for inability to comply with strict follow-up schedule; these subjects were not significantly different than those included in the analysis); the inclusion of subjects that had previously received pneumococcal immunization (>3 years prior); and inclusion of subjects that although not on ART, had been previously exposed to ART (>1 year prior). Given the consistency of our results, it is unlikely that any of the above issues would have significantly affected the overall conclusion of this study.
Our data support vaccination of HIV-infected patients with CD4 ≥200 cells/µL against S. pneumoniae without delaying for the initiation of ART. Further research to understand the mechanisms that elicit immune responses to polysaccharide-based vaccines and to identify biomarkers that can measure protection in this population should be explored.
Notes
Acknowledgments. The authors are indebted to the patients at the Michael E. DeBakey Veterans Administration Medical Center and the Thomas Street Health Center (Harris Health System) in Houston, Texas.
Financial support. This work was supported by the Department of Veterans Affairs through the Merit Review Program (M. C. R.-B.) and by National Institutes of Health grants R01-AI045459 and R01-AI044374 (L. P.). This work is registered in Clinical Trials.gov under “Immune Responses to Pneumococcal Vaccination Among HIV-Infected Subjects” (INDA-002-08S).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis 2004; 4:445–55. [DOI] [PubMed] [Google Scholar]
- 2.Yin Z, Rice BD, Waight P, et al. Invasive pneumococcal disease among HIV-positive individuals, 2000–2009. AIDS 2012; 26:87–94. [DOI] [PubMed] [Google Scholar]
- 3.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America, 2013. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed August 2014.
- 4.Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–9. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:1–24. [PubMed] [Google Scholar]
- 6.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med 1983; 309:453–8. [DOI] [PubMed] [Google Scholar]
- 7.Moir S, Buckner CM, Ho J, et al. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood 2010; 116:5571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moir S, Malaspina A, Ho J, et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis 2008; 197:572–9. [DOI] [PubMed] [Google Scholar]
- 9.Hart M, Steel A, Clark SA, et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol 2007; 178:8212–20. [DOI] [PubMed] [Google Scholar]
- 10.Titanji K, De Milito A, Cagigi A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 2006; 108:1580–7. [DOI] [PubMed] [Google Scholar]
- 11.Jordano Q, Falco V, Almirante B, et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis 2004; 38:1623–8. [DOI] [PubMed] [Google Scholar]
- 12.Heffernan RT, Barrett NL, Gallagher KM, et al. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis 2005; 191:2038–45. [DOI] [PubMed] [Google Scholar]
- 13.Scamurra RW, Miller DJ, Dahl L, et al. Impact of HIV-1 infection on VH3 gene repertoire of naive human B cells. J Immunol 2000; 164:5482–91. [DOI] [PubMed] [Google Scholar]
- 14.Chang Q, Abadi J, Alpert P, Pirofski L. A pneumococcal capsular polysaccharide vaccine induces a repertoire shift with increased VH3 expression in peripheral B cells from human immunodeficiency virus (HIV)-uninfected but not HIV-infected persons. J Infect Dis 2000; 181:1313–21. [DOI] [PubMed] [Google Scholar]
- 15.Abadi J, Friedman J, Mageed RA, Jefferis R, Rodriguez-Barradas MC, Pirofski L. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of V(H)3 gene segment usage. J Infect Dis 1998; 178:707–16. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniam KS, Segal R, Lyles RH, Rodriguez-Barradas MC, Pirofski LA. Qualitative change in antibody responses of human immunodeficiency virus-infected individuals to pneumococcal capsular polysaccharide vaccination associated with highly active antiretroviral therapy. J Infect Dis 2003; 187:758–68. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson MA, Khayam-Bashi H, Martin JN, Black D, Ng V. Effect of long-term highly active antiretroviral therapy in restoring HIV-induced abnormal B-lymphocyte function. J Acquir Immune Defic Syndr 2002; 31:472–7. [DOI] [PubMed] [Google Scholar]
- 18.Redgrave BE, Stone SF, French MA, Krueger R, James IR, Price P. The effect of combination antiretroviral therapy on CD5 B- cells, B-cell activation and hypergammaglobulinaemia in HIV-1-infected patients. HIV Med 2005; 6:307–12. [DOI] [PubMed] [Google Scholar]
- 19.Overton ET, Sungkanuparph S, Powderly WG, Seyfried W, Groger RK, Aberg JA. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005; 41:1045–8. [DOI] [PubMed] [Google Scholar]
- 20.Kroon FP, Rimmelzwaan GF, Roos MT, et al. Restored humoral immune response to influenza vaccination in HIV-infected adults treated with highly active antiretroviral therapy. Aids 1998; 12:F217–23. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Barradas MC, Alexandraki I, Nazir T, et al. Response of human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy to vaccination with 23-valent pneumococcal polysaccharide vaccine. Clin Infect Dis 2003; 37:438–47. [DOI] [PubMed] [Google Scholar]
- 22.Musher DM, Rueda AM, Nahm MH, Graviss EA, Rodriguez-Barradas MC. Initial and subsequent response to pneumococcal polysaccharide and protein-conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. J Infect Dis 2008; 198:1019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wernette CM, Frasch CE, Madore D, et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol 2003; 10:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Barradas MC, Groover JE, Lacke CE, et al. IgG antibody to pneumococcal capsular polysaccharide in human immunodeficiency virus-infected subjects: persistence of antibody in responders, revaccination in nonresponders, and relationship of immunoglobulin allotype to response. J Infect Dis 1996; 173:1347–53. [DOI] [PubMed] [Google Scholar]
- 25.Falco V, Jordano Q, Cruz MJ, et al. Serological response to pneumococcal vaccination in HAART-treated HIV-infected patients: one year follow-up study. Vaccine 2006; 24:2567–74. [DOI] [PubMed] [Google Scholar]
- 26.Moir S, Fauci AS. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev 2013; 254:207–24. [DOI] [PubMed] [Google Scholar]
- 27.Moens L, Wuyts M, Meyts I, De Boeck K, Bossuyt X. Human memory B lymphocyte subsets fulfill distinct roles in the anti-polysaccharide and anti-protein immune response. J Immunol 2008; 181:5306–12. [DOI] [PubMed] [Google Scholar]
- 28.Leggat DJ, Thompson RS, Khaskhely NM, Iyer AS, Westerink MA. The immune response to pneumococcal polysaccharides 14 and 23F among elderly individuals consists predominantly of switched memory B cells. J Infect Dis 2013; 208:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johannesson TG, Sogaard OS, Tolstrup M, et al. The impact of B-cell perturbations on pneumococcal conjugate vaccine response in HIV-infected adults. PLOS One 2012; 7:e42307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedlund J, Ortqvist A, Konradsen HB, Kalin M. Recurrence of pneumonia in relation to the antibody response after pneumococcal vaccination in middle-aged and elderly adults. Scand J Infect Dis 2000; 32:281–6. [DOI] [PubMed] [Google Scholar]
- 31.Watera C, Nakiyingi J, Miiro G, et al. 23-Valent pneumococcal polysaccharide vaccine in HIV-infected Ugandan adults: 6-year follow-up of a clinical trial cohort. AIDS 2004; 18:1210–3. [DOI] [PubMed] [Google Scholar]
- 32.Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008; CD000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Barradas MC, Goulet J, Brown S, et al. Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the Veterans Aging Cohort 5-Site Study. Clin Infect Dis 2008; 46:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teshale EH, Hanson D, Flannery B, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine on pneumonia in HIV-infected adults in the United States, 1998–2003. Vaccine 2008; 26:5830–4. [DOI] [PubMed] [Google Scholar]
- 35.Tian H, Weber S, Thorkildson P, Kozel TR, Pirofski LA. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect Immun 2009; 77:1502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero-Steiner S, Musher DM, Cetron MS, et al. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis 1999; 29:281–8. [DOI] [PubMed] [Google Scholar]
- 37.Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother 2013; 19:412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis 2011; 52:633–40. [DOI] [PubMed] [Google Scholar]
- 39.Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001; 20:545–53. [DOI] [PubMed] [Google Scholar]
- 40.French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feikin DR, Kagucia EW, Loo JD, et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med 2013; 10:e1001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011(1.). Emerg Infect Dis 2013; 19:1074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flannery B, Heffernan RT, Harrison LH, et al. Changes in invasive pneumococcal disease among HIV-infected adults living in the era of childhood pneumococcal immunization. Ann Intern Med 2006; 144:1–9. [DOI] [PubMed] [Google Scholar]