Abstract
Background
Glomerular filtration rate (GFR) estimating equations using the combination of creatinine and cystatin C (eGFRcr-cys) are more accurate than equations using either alone (eGFRcr or eGFRcys). New guidelines suggest measuring cystatin C as a confirmatory test when eGFRcr may be inaccurate, but do not specify demographic or clinical conditions in which eGFRcys or eGFRcr-cys are more accurate than eGFRcr nor which estimate to use in such circumstances.
Methods
We compared the performance of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations in 1119 subjects in the CKD-EPI cystatin C external validation dataset. Subgroups were defined by eGFRcr, age, sex, diabetes status and body mass index (BMI). The reference test was GFR measured using urinary or plasma clearance of exogenous filtration markers. Cystatin C and creatinine assays were traceable to primary reference materials. Accuracy was defined as the absolute difference in eGFR compared with mGFR.
Results
The mean mGFR was 70 ± 41 (SD) mL/min/1.73 m2. eGFRcys was more accurate than eGFRcr at lower BMI and less accurate at higher BMI, especially at higher levels of eGFRcr. There were small differences in accuracy in people according to the diabetes status. eGFRcr-cys was as accurate or more accurate than eGFRcr or eGFRcys in these and all other subgroups.
Conclusions
eGFRcr-cys, but not eGFRcys, is more accurate than eGFRcr in most subgroups we studied, suggesting preferential use of eGFRcr-cys when serum cystatin C is measured as a confirmatory test to obtain more accurate eGFR. Further studies are necessary to evaluate diagnostic strategies for using eGFRcys and eGFRcr-cys.
Keywords: CKD-EPI, cystatin C, diagnostic test accuracy, estimated GFR
INTRODUCTION
Glomerular filtration rate (GFR) estimates are used routinely in medical practice. In the USA, estimated GFR is reported millions of times per year using equations based on age, sex, race and serum creatinine, but their accuracy is limited by imprecision. One important cause of imprecision in GFR estimates based on serum creatinine (eGFRcr) is individual variation in non-GFR determinants of serum creatinine, such as muscle mass or diet, leading to bias in subgroups of the population.
Cystatin C is an alternative filtration marker that is less affected by muscle mass and diet, and GFR estimates based on serum cystatin C (eGFRcys) may have less bias than eGFRcr in some subgroups. However, serum cystatin C is also affected by non-GFR determinants. Recent studies have shown that eGFRcys is not more precise than eGFRcr, whereas estimated GFR based on both markers (eGFRcr-cys) is more precise than eGFR based on either marker alone, but these studies did not report differences in the performance of equations in subgroups defined by demographic and clinical characteristics across a wide range of GFRs [1–5].
Kidney Disease Improving Global Outcomes (KDIGO) has just released updated clinical practice guidelines for evaluation and management of chronic kidney disease (CKD) [6]. The guidelines recommend initial use of eGFRcr followed by a confirmatory test using cystatin C for evaluation of GFR when eGFRcr is thought to be inaccurate. However, the guidelines do not specify clinical conditions in which eGFRcys or eGFRcr-cys is more accurate than eGFRcr nor whether to use eGFRcys or eGFRcr-cys in such circumstances. Our goal in these analyses was to develop strategies for use of eGFRcys or eGFRcr-cys as confirmatory tests for eGFRcr based on easily measured clinical and demographic variables. Here, we compare eGFRcys and eGFRcr-cys with eGFRcr among subgroups with different demographic and clinical characteristics across a wide range of eGFRcr. Our results may assist clinicians in choosing when to order cystatin C and whether to use eGFRcys or eGFRcr-cys.
MATERIALS AND METHODS
We performed a cross-sectional analysis of diagnostic test accuracy, using mGFR as the reference test and eGFR computed from the CKD-Epidemiology Collaboration (CKD-EPI) 2009 creatinine equation, and the CKD-EPI 2012 cystatin C and creatinine–cystatin C equations as the index tests [1, 7]. All equations estimate GFR indexed for 1.73 m2 body surface area (BSA).
Data sources
We used the CKD-EPI cystatin C and creatinine–cystatin C external validation dataset. As previously described, collaborators provided data from research studies and clinical populations (hereafter referred to as ‘studies’) [7]. We excluded studies without measurements of cystatin C and studies of transplant patients because of large variations among such studies in the relationship of serum cystatin C to measured GFR in our preliminary analyses. GFR was measured using urinary or plasma clearance of exogenous filtration markers. In total, we included five studies with 1119 participants. Details of the studies and their measurement procedures have been previously published [1].
Laboratory methods
We used standardized serum creatinine and cystatin C for computing eGFR. Methods for calibration of serum creatinine and cystatin C have been described previously [1]. Briefly, we either calibrated serum creatinine assays or measured serum creatinine by the Roche enzymatic method, traceable to the National Institute Standardized Technology creatinine standard reference material 967 [8]. We calibrated serum cystatin C assays or measured serum cystatin C on the Siemens Dade Behring Nephelometer, with assays traceable to the International Federation for Clinical Chemists Working Group for the Standardization of Serum Cystatin C and the Institute for Reference Materials and Measurements certified reference materials [9–11].
Statistical analysis
All measures of performance of estimating equations are computed from differences between mGFR and eGFR (mGFR − eGFR). We anticipated that clinicians would make decisions on whether to order cystatin C based on the magnitude, rather than the direction, of the differences between mGFR and eGFR computed using cystatin C versus creatinine. Hence, our primary measure of equation performance is the mean absolute value of the difference between mGFR and eGFR (mean absolute difference), rather than the mean value (bias) for each equation. Similarly, our primary measure for the comparison of equations is the difference between the absolute differences, rather than the difference between the biases. Other advantages of using the absolute difference rather than the bias are as follows: (i) it is a measure of accuracy (it includes information about bias and precision); (ii) it can be adjusted for eGFR in multivariable analysis; (iii) differences between absolute bias retain comparisons to mGFR. A larger value for absolute difference indicates a less accurate equation. Positive and negative values for the difference in absolute difference between the two equations indicate lesser or greater accuracy, respectively, of the first equation compared with the second equation. As additional measures of accuracy, we also report the proportion of participants with estimated GFR within 20 and 30% of measured GFR (P20 and P30, respectively). Finally, for descriptive purposes, we also report the bias, with a positive value for the bias indicating an underestimation of measured GFR and a negative value indicating an overestimation.
Subgroups for analyses were defined by clinical characteristics and eGFRcr. Subgroups of clinical characteristics were stratified by age (<40, 40–65 and >65 years), sex, diabetes (yes or no) and body mass index (BMI <20, 20–24, 25–30 and >30 kg/m2). We did not stratify by race because there were only 30 (2.7%) black individuals in the dataset. Since eGFRcr is the primary method used to estimate GFR in clinical practice, we used eGFRcr to categorize subgroups based on the level of GFR. The level of eGFRcr was categorized as <30, 30–59, 60–89 and ≥90 mL/min/1.73 m2. In a sensitivity analysis, we used mGFR and eGFRcys to categorize eGFR.
Ninety-five percent confidence intervals (95% CIs) around the bias, mean absolute difference, difference between mean absolute difference, P20 and P30 were calculated using bootstrap methods (1000 bootstraps). For difference between mean absolute difference, 95% CIs which do not include zero were considered significant. For comparison of P20 and P30, 95% CIs which do not overlap were considered significant. A comparison of absolute difference in subgroups was performed using linear regression model, with the difference in the absolute difference as the outcome and the subgroups as dependent variables. Smooth estimates of the mean values for bias and difference in the absolute difference in graphical presentations of the data were created using the lowess function. All analyses were performed using R (version 2.15.1; Free Software Foundation Inc, www.r-project.org).
The institutional review boards of all participating institutions approved the study.
RESULTS
Table 1 shows the demographic and clinical characteristics of the participants overall and stratified by level of eGFRcr. The mean (SD) measured GFR was 69.8 (41.0) mL/min/1.73 m2; 20.7% were >65 years, 40.8% were women, 53.1% had diabetes and 7.2% had BMI <20 kg/m2. Participants with higher levels of eGFRcr were younger, and had a higher proportion with diabetes.
Table 1.
Clinical characteristics of the CKD-EPI external validation dataset
| Total | eGFRcr categories (mL/min/1.73 m2) |
P-value | ||||
|---|---|---|---|---|---|---|
| <30 | 30–59 | 60–89 | ≥90 | |||
| No. of participants | 1119 | 229 | 344 | 225 | 321 | |
| Age (years) | 50 ± 17 | 61 ± 15 | 55 ± 15 | 50 ± 15 | 36 ± 11 | <0.001 |
| <40 | 357 (31.9) | 19 (8.3) | 69 (20.1) | 60 (26.7) | 209 (65.1) | <0.001 |
| 40–65 | 530 (47.4) | 113 (49.3) | 180 (52.3) | 129 (57.3) | 108 (33.6) | |
| >65 | 232 (20.7) | 97 (42.4) | 95 (27.6) | 36 (16.0) | 4 (1.2) | |
| Sex | 0.01 | |||||
| Female | 456 (40.8) | 85 (37.1) | 124 (36.0) | 110 (48.9) | 137 (42.7) | |
| Male | 663 (59.2) | 144 (62.9) | 220 (64.0) | 115 (51.1) | 184 (57.3) | |
| Race | 0.03 | |||||
| Black | 30 (2.7) | 10 (4.4) | 13 (3.8) | 1 (0.4) | 6 (1.9) | |
| White | 1089 (97.3) | 219 (95.6) | 331 (96.2) | 224 (99.6) | 315 (98.1) | |
| Diabetes | <0.001 | |||||
| Yes | 594 (53.1) | 82 (35.8) | 144 (41.9) | 111 (49.3) | 257 (80.1) | |
| No | 525 (46.9) | 147 (64.2) | 200 (58.1) | 114 (50.7) | 64 (19.9) | |
| BMI (kg/m2) | 25 ± 4 | 26 ± 4 | 26 ± 5 | 24 ± 4 | 25 ± 4 | 0.01 |
| <20 | 81 (7.2) | 16 (7.0) | 25 (7.3) | 26 (11.6) | 14 (4.4) | 0.003 |
| 20–24 | 503 (45.0) | 92 (40.2) | 139 (40.4) | 111 (49.3) | 161 (50.2) | |
| 25–30 | 386 (34.5) | 83 (36.2) | 127 (36.9) | 64 (28.4) | 112 (34.9) | |
| >30 | 149 (13.3) | 38 (16.6) | 53 (15.4) | 24 (10.7) | 34 (10.6) | |
| Weight (kg) | 74 ± 15 | 73 ± 16 | 75 ± 16 | 72 ± 15 | 75 ± 14 | 0.02 |
| <60 | 184 (16.4) | 45 (19.7) | 51 (14.8) | 48 (21.3) | 40 (12.5) | 0.05 |
| 60–90 | 793 (70.9) | 156 (68.1) | 241 (70.1) | 153 (68.0) | 243 (75.7) | |
| >90 | 142 (12.7) | 28 (12.2) | 52 (15.1) | 24 (10.7) | 38 (11.8) | |
| BSA (m2) | 1.85 ± 0.21 | 1.82 ± 0.22 | 1.87 ± 0.21 | 1.83 ± 0.21 | 1.88 ± 0.20 | 0.004 |
| Scr (mg/dL) | 1.61 ± 1.10 | 3.33 ± 1.23 | 1.64 ± 0.34 | 1.03 ± 0.18 | 0.78 ± 0.14 | <0.001 |
| Scys (mg/dL) | 1.49 ± 0.79 | 2.67 ± 0.73 | 1.59 ± 0.40 | 1.06 ± 0.21 | 0.84 ± 0.18 | <0.001 |
| Measured GFR (mL/min/1.73 m2) | 69.8 ± 41.0 | 21.8 ± 8.5 | 47.1 ± 14.7 | 82.1 ± 18.4 | 119.9 ± 23.1 | <0.001 |
| ≥120 | 172 (15.4) | 0 (0) | 0 (0) | 4 (1.8) | 168 (52.3) | |
| 90–119 | 199 (17.8) | 0 (0) | 2 (0.58) | 71 (31.6) | 126 (39.3) | |
| 60–89 | 215 (19.2) | 0 (0) | 66 (19.2) | 124 (55.1) | 25 (7.8) | |
| 30–59 | 316 (28.2) | 47 (20.5) | 241 (70.1) | 26 (11.6) | 2 (0.6) | |
| 15–29 | 166 (14.8) | 131 (57.2) | 35 (10.2) | 0 (0) | 0 (0) | |
| <15 | 51 (4.6) | 51 (22.3) | 0 (0) | 0 (0) | 0 (0) | |
Date are presented as mean ± SD and number (%). GFR is categorized by eGFR based on creatinine.
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFRcr, estimated glomerular filtration rate based on serum creatinine; BMI, body mass index; BSA, body surface area; Scr, serum creatinine; Scys, serum cystatin C.
To convert GFR from mL/min/1.73 m2 to mL/s/1.73 m2, multiply by 0.0167. To convert Scr from mg/dL to μmol/L, multiply by 88.4.
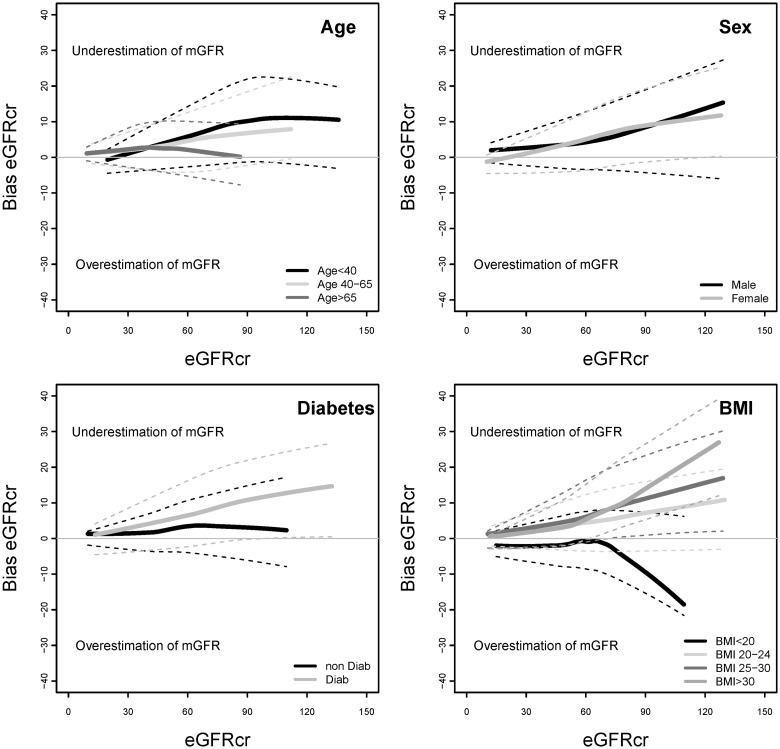
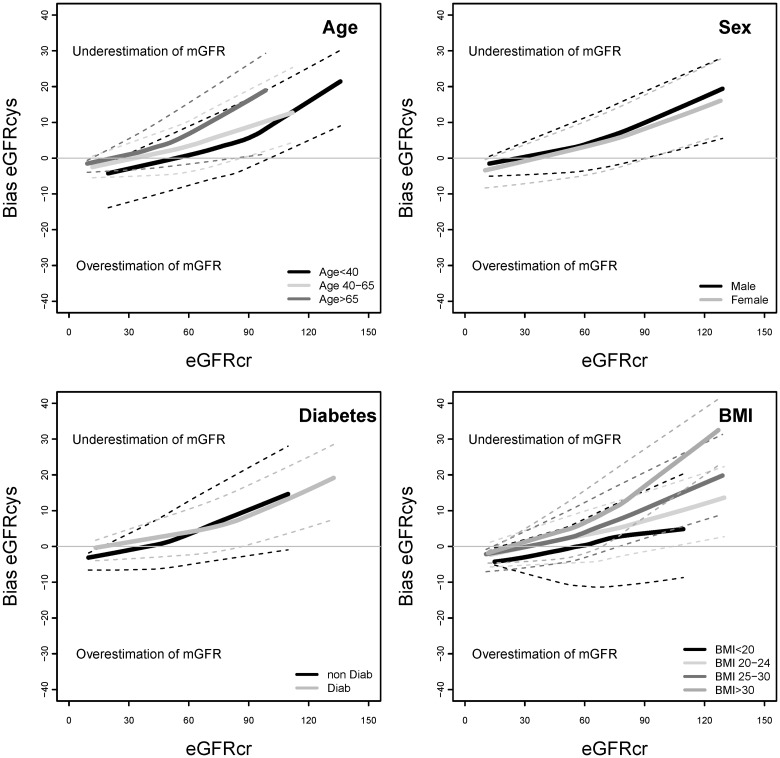
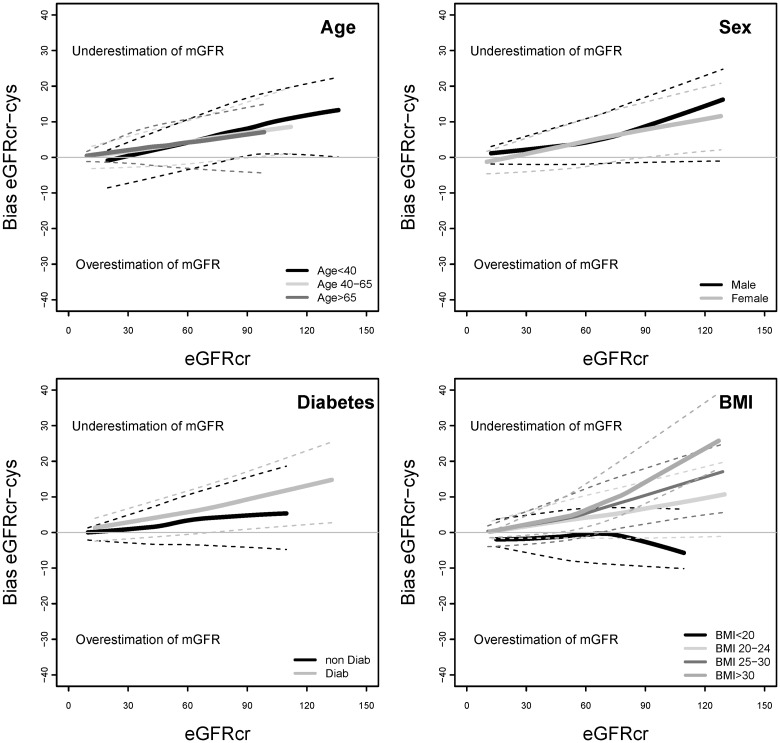
Table 2 compares the bias and absolute difference of eGFRcr, eGFRcys and eGFRcr-cys overall and stratified by the level of eGFRcr. Overall, bias was similar for the three equations. For all the three equations, bias was higher at higher levels of eGFRcr, and differences among subgroups appeared to be greater at higher eGFRcr. eGFRcr underestimated mGFR more for younger versus older people and in people with versus without diabetes (Figure 1). eGFRcr underestimated mGFR more for people with higher BMI and overestimated mGFR for people with BMI <20 kg/m2. In contrast, eGFRcys underestimated mGFR more for older versus younger people and in people with higher BMI (Figure 2). For eGFRcr-cys, differences among subgroups in bias were intermediate between those observed for eGFRcr and eGFRcys (Figure 3).
Table 2.
Bias and absolute difference of the CKD-EPI eGFRcr, eGFRcys and eGFRcr-cys equations by eGFRcr
| eGFRcr (mL/min/1.73 m2) | N | eGFRcr |
eGFRcys |
eGFRcr-cys |
|||
|---|---|---|---|---|---|---|---|
| Bias (95% CI) | Mean absolute difference (95% CI) | Bias (95% CI) | Mean absolute difference (95% CI) | Bias (95% CI) | Mean absolute difference (95% CI) | ||
| Overall | 1119 | 5.3 (4.5, 6.1) | 11.0 (10.4, 11.7) | 5.2 (4.4, 6.0) | 10.8 (10.2, 11.4) | 5.4 (4.8, 6.1) | 9.4 (8.9, 9.9) |
| ≥90 | 229 | 9.1 (7.0, 11.5) | 17.6 (16.0, 19.1) | 14.3 (12.4, 16.2) | 17.5 (16.2, 18.9) | 10.8 (9.0, 12.4) | 15.4 (14.2, 16.6) |
| 60–89 | 344 | 6.8 (4.8, 8.7) | 13.2 (11.8, 14.6) | 5.2 (3.5, 7.2) | 11.5 (10.4, 12.9) | 6.3 (4.7, 7.8) | 10.4 (9.4, 11.5) |
| 30–59 | 225 | 2.7 (1.6, 3.9) | 8.0 (7.3, 8.8) | 0.8 (−0.4, 2.1) | 8.4 (7.6, 9.3) | 2.5 (1.7, 3.5) | 7.0 (6.4, 7.6) |
| <30 | 321 | 2.3 (1.6, 2.9) | 4.3 (3.8, 4.8) | −1.0 (−1.7, −0.3) | 4.3 (3.9, 4.8) | 1.4 (0.8, 2.0) | 3.6 (3.2, 4.0) |
Bias is calculated as the mean value of (mGFR – eGFR). Absolute difference is calculated as |mGFR – eGFR|. GFR is categorized by eGFR based on creatinine.
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; mGFR, measured GFR; eGFRcr, estimated GFR based on serum creatinine; eGFRcys, estimated GFR based on serum cystatin C; eGFRcr-cys, estimated GFR based on serum creatinine and cystatin C.
FIGURE 1:
Bias of eGFRcr by demographic and clinical characteristics of subgroups. Bias is calculated as the mean value of (mGFR − eGFRcr). eGFRcr, estimated glomerular filtration rate based on serum creatinine; mGFR, measured glomerular filtration rate.
FIGURE 2:
Bias of eGFRcys by demographic and clinical characteristics of subgroups. Bias is calculated as the mean value of (mGFR − eGFRcys). eGFRcys, estimated glomerular filtration rate based on serum cystatin C; eGFRcr, estimated glomerular filtration rate based on serum creatinine; mGFR, measured glomerular filtration rate.
FIGURE 3:
Bias of eGFRcr-cys by demographic and clinical characteristics of subgroups. Bias is calculated as the mean value of (mGFR − eGFRcr-cys). eGFRcr-cys, estimated glomerular filtration rate based on serum creatinine and cystatin C; eGFRcr, estimated glomerular filtration rate based on serum creatinine; mGFR, measured glomerular filtration rate.
The mean absolute difference was smaller for eGFRcr-cys than for eGFRcr and eGFRcys at all levels of eGFRcr (Table 2). Table 3 (Column 1) compares the difference in absolute difference of eGFRcys with eGFRcr in the overall dataset and in subgroups; comparisons between subgroups stratified by eGFRcr are shown in Supplementary Table S1 and Figure S1. Overall, eGFRcr and eGFRcys had a similar absolute difference [difference of 0.2 (−0.4, 0.8) mL/min/1.73 m2]; however, there were differences among some subgroups. eGFRcys had a smaller absolute difference than eGFRcr in people with diabetes [difference of 1.7 (0.9, 2.5)] and a larger absolute difference than eGFRcr in people without diabetes [difference of −1.4 (−2.3, −0.5) mL/min/1.73 m2] (P < 0.001). Across BMI groups, there was a non-significant trend (P = 0.08) toward a smaller absolute difference for eGFRcys than eGFRcr at lower BMI and a larger absolute difference for eGFRcys than eGFRcr at higher BMI. The interaction of BMI with eGFRcr was significant (P = 0.03), so that among people with eGFRcr >90 mL/min/1.73 m2, eGFRcys had a smaller absolute difference than eGFRcr for the subgroup with BMI <20 kg/m2 [n = 14, difference of 11.1 (0.4, 21.8) mL/min/1.73 m2] but larger absolute difference than eGFRcr for the subgroup with BMI >30 kg/m2 [n = 34, difference of −4.9 (−9.8, −0.1) mL/min/1.73 m2] (Supplementary Table S1).
Table 3.
Comparison of the performance of eGFRcr, eGFRcys and eGFRcr-cys equations
| No. | eGFRcr − eGFRcys (Column 1) |
eGFRcr − eGFRcr-cys (Column 2) |
eGFRcys − eGFRcr-cys (Column 3) |
||||
|---|---|---|---|---|---|---|---|
| ΔAbs difference | P-value | ΔAbs difference | P-value | ΔAbs difference | P-value | ||
| Overall | 1119 | 0.2 (−0.4, 0.8) | 1.6 (1.3, 2.0) | 1.4 (1.1, 1.8) | |||
| eGFRcr | 0.1 | 0.001 | 0.04 | ||||
| <30 | 229 | −0.0 (−0.6, 0.6) | 0.7 (0.4, 1.0) | 0.8 (0.4, 1.2) | |||
| 30–59 | 344 | −0.3 (−1.3, 0.7) | 1.0 (0.5, 1.6) | 1.4 (0.8, 2.0) | |||
| 60–89 | 225 | 1.6 (−0.0. 3.3) | 2.7 (1.8, 3.7) | 1.1 (0.2, 2.0) | |||
| ≥90 | 321 | 0.0 (−1.4, 1.4) | 2.2 (1.2, 3.1) | 2.1 (1.4, 2.9) | |||
| Age (years) | 0.3 | 0.1 | 0.1 | ||||
| <40 | 357 | 0.7 (−0.5, 1.9) | 2.1 (1.3, 2.9) | 1.4 (0.7, 2.1) | |||
| 40–65 | 530 | 0.3 (−0.6, 1.2) | 1.7 (1.1, 2.2) | 1.4 (0.8, 1.9) | |||
| >65 | 232 | −0.7 (−1.7, 0.4) | 0.9 (0.2, 1.6) | 1.6 (1.0, 2.2) | |||
| Sex | 0.2 | 0.3 | 0.2 | ||||
| Female | 456 | −0.3 (−1.3, 0.7) | 1.4 (0.8, 2.0) | 1.7 (1.1, 2.3) | |||
| Male | 663 | 0.6 (−0.2, 1.4) | 1.8 (1.3, 2.3) | 1.2 (0.0, 1.6) | |||
| Diabetes | <0.001 | 0.02 | 0.02 | ||||
| Yes | 594 | 1.7 (0.9, 2.5) | 2.1 (1.6, 2.6) | 0.4 (−0.1, 0.8) | |||
| No | 525 | −1.4 (−2.3, −0.5) | 1.2 (0.6, 1.8) | 2.6 (2.1, 3.1) | |||
| BMI (kg/m2) | 0.08 | 0.002 | 0.1 | ||||
| <20 | 81 | 2.3 (−0.5, 5.0) | 4.0 (1.7, 6.3) | 1.7 (0.3, 3.1) | |||
| 20–25 | 503 | 0.1 (−0.8, 0.9) | 1.7 (1.2, 2.3) | 1.7 (1.2, 2.1) | |||
| 25–30 | 386 | 0.6 (−0.6, 1.7) | 1.4 (0.8, 2.0) | 0.8 (0.2, 1.5) | |||
| >30 | 149 | −1.3 (−3.0, 0.4) | 0.6 (−0.4, 1.6) | 1.9 (1.0, 2.8) | |||
eGFRcr is better eGFRcys is better eGFRcr-cys is better no difference.
Data are presented as mean (95% CI). Abs difference is calculated as the mean of the absolute value of the difference (mGFR − eGFR). ΔAbs difference is the difference in absolute difference between eGFRs. A positive value indicates a larger absolute difference for the first equation than the second equation. P-value indicates the comparison among subgroups. The unit of GFR is mL/min/1.73 m2. The unit for conversion for GFR in mL/min/1.73 m2 to mL/s/m2, ×0.0167.
Table 3 (Column 2), Supplementary Table S2 and Figure S2 compare the difference in absolute difference of eGFRcr-cys with eGFRcr. eGFRcr-cys had smaller absolute difference than eGFRcr overall [difference of 1.6 (1.3, 2.0) mL/min/1.73 m2], and in all subgroups defined by eGFRcr, age, sex, diabetes status or BMI (except >30 kg/m2). By comparing the difference in absolute difference between eGFRcr minus eGFRcr-cys (column 2) versus eGFRcr minus eGFRcys (Column 1), as well as in Supplementary Tables S2 versus S1, we found that eGFRcr-cys had smaller absolute difference than eGFRcr in the subgroups in which eGFRcys had smaller absolute difference than eGFRcr (people with diabetes and people with eGFRcr > 90 mL/min/1.73 m2 and BMI < 20 kg/m2)
Table 3 (Column 3), Supplementary Table S3 and Figure S3 show that eGFRcr-cys had a smaller absolute difference than eGFRcys in the overall dataset [difference of 1.4 (1.1, 1.8) mL/min/1.73 m2], and in all subgroups defined by eGFRcr categories, age, sex or BMI and in people without diabetes. eGFRcr-cys and eGFRcys were similar in people with diabetes. By comparing the difference in absolute difference between eGFRcys minus eGFRcr-cys (column 3) versus eGFRcr minus eGFRcys (column 1) and Supplementary Tables S3 versus S1, we found that eGFRcr-cys had a smaller absolute difference than eGFRcys in the subgroups in which eGFRcys had a larger absolute difference than eGFRcr (people without diabetes and people with eGFR > 90 mL/min/1.73 m2 and BMI > 30 kg/m2).
Analyses using P20 and P30 as measures of accuracy revealed similar results (Supplementary Tables S4 and S5). Sensitivity analyses using mGFR (Supplementary Tables S6–S9) and eGFRcys rather than eGFRcr to categorize eGFR did not reveal substantially different results.
DISCUSSION
Accurate eGFR is important for detection and staging of CKD, drug dosing and decisions on administration of intravenous contrast. The recent KIDGO guidelines on CKD recommended using eGFRcr for the initial evaluation and then measuring cystatin C and using eGFRcys or eGFRcr-cys for confirmation in the clinical settings in which eGFRcr is less accurate. The difficulty in implementing this recommendation is that without the gold standard mGFR, physicians do not know when eGFRcr is inaccurate. Our analyses seek to provide clinicians with tools to know when to measure cystatin C based on the readily available clinical and demographic information and whether to report eGFRcys or eGFRcr-cys. Our results showed that eGFRcr and eGFRcys had similar accuracy in the total population, but differed across subgroups according to diabetes status and BMI at higher eGFRcr. However, eGFRcr-cys was as accurate or more accurate than eGFRcr and eGFRcys in most subgroups, including the subgroups in which eGFRcys was more accurate than eGFRcr. These results have important implications for future studies on endogenous filtration markers and use of GFR estimating equations in clinical practice.
GFR estimating equations use the serum level of the endogenous filtration markers, combined with demographic variables, such as age, sex and race, to account for unmeasured non-GFR determinants of the filtration markers that affect their serum levels (generation, tubular secretion or reabsorption, and extra-renal elimination). In principle, differences in bias between eGFRcr and eGFRcys may reflect differences in the non-GFR determinants of each marker that are not accounted for by the demographic variables in the estimating equations, and therefore, the improved accuracy of eGFRcr-cys over eGFRcr and eGFRcys reflects the smaller effects of the non-GFR determinants of each marker when they are used in combination [2–5]. Neither BMI nor diabetes is included as a variable in the CKD-EPI estimating equations, so it is not unexpected that eGFRcr or eGFRcys may have differential bias across subgroups defined by these variables. Some of our findings can be accounted for by known non-GFR determinants in serum creatinine which are affected by these variables. The non-GFR determinants of serum cystatin C have not been carefully evaluated; more study will be required to determine how factors associated with the non-GFR determinants may affect our results.
Higher BMI is associated with higher muscle and fat mass. Muscle is the primary determinant of creatinine generation, and variation in muscle mass can affect serum creatinine concentration independently of GFR, leading to bias in eGFRcr compared with mGFR (overestimation at low BMI and underestimation at high BMI), as observed (Figure 1). Some studies suggest that fat mass may be a primary determinant of cystatin C generation, and if so, then variation in fat mass among individuals could affect serum cystatin C concentration independently of GFR, leading to bias in eGFRcys compared with mGFR [12–16]. We observed differences in bias in both eGFRcr and eGFRcys across BMI groups (Figures 1 and 2), but a trend toward greater accuracy for eGFRcys than eGFRcr at low BMI and a lesser accuracy for eGFRcys than eGFRcr at higher BMI (Table 3), which was significant at higher eGFRcr (Supplementary Table S1). However, this finding must be interpreted with caution because of the small number of people in these subgroups.
Diabetes is not known to be directly associated with the non-GFR determinants of serum creatinine. In this study, we observed small differences in the bias of eGFRcr between people according to the diabetes status, but not for eGFRcys (Figures 1 and 2 and Table 3). Prior studies by CKD-EPI and others have suggested some differences according to the diabetes status in the relationships of serum creatinine and cystatin C concentrations to mGFR, even after adjustment for age, sex and race, but efforts to incorporate diabetes as a variable in GFR estimating equations using either creatinine or cystatin C have not led to improved equation performance [1, 7, 14, 17, 18]. Possibly, these differences reflect confounding by other variables. Possibly, differentiation of Type 1 from Type 2 diabetes may provide some insight.
These findings lead to specific suggestions for implementation of the KDIGO recommendations in practice. First, our findings indicate that eGFRcr-cys, but not eGFRcys, was more accurate than eGFRcr in most subgroups defined by age, sex, diabetes status, BMI or eGFRcr. They suggest that clinicians could measure cystatin C in most patients when more accurate eGFR is required, that clinical laboratories should report both eGFRcys and eGFRcr-cys when cystatin C is measured, and that clinicians should generally use eGFRcr-cys, rather than eGFRcys alone, for clinical decision making. Second, the findings suggest that cystatin C should be measured in patients with low BMI (<20 kg/m2), especially if eGFRcr is high (>90 mL/min/1.73 m2). eGFRcr may be substantially less accurate than eGFRcys in this subgroup and eGFRcys was as accurate as eGFRcr-cys. In clinical practice, this may be applicable to patients with low muscle mass (anorexia nervosa, malnutrition, neuromuscular disorders, limb amputation), although the accuracy of eGFRcys and eGFRcr-cys has not been established in these patients [19]. eGFRcr may also be less accurate than eGFRcys in patients with diabetes, and eGFRcys was as accurate as eGFRcr-cys in this subgroup, but we are uncertain of the mechanism or the clinical relevance of these findings.
The findings of our study must be interpreted in light of its strengths and limitations. Strengths include the large study population, measurements of creatinine and cystatin C using standardized assays, and rigorous statistical analysis including point estimates and 95% CIs for subgroups defined by demographic and clinical characteristics and eGFRcr. However, there are also several limitations. We pooled studies of different populations which may have differed by characteristic or methods for measuring GFR, and we cannot rule out that some of the findings may reflect the characteristics of a particular study. In our previous work [1, 7], we did not find that differences among studies affected our results. The study population did not include transplant recipients, a substantial number of blacks or patients with extremes of factors associated with non-GFR determinants of creatinine. We did not have data on a large number of variables and did not apply multivariable analysis to characterize factors that affect non-GFR determinants of creatinine versus cystatin C. We did not have data of albuminuria, a marker of kidney damage, but our prior work has not shown differences in equation performance by level of proteinuria [7]. We could not differentiate Type 1 from Type 2 diabetes. Other studies with a more diverse study population and with data on more variables may be better suited for identification of likely factors most closely associated with non-GFR determinants of cystatin C. Indexing of mGFR and eGFR by BSA has been questioned in low- and high-BMI groups. In principle, differences between mGFR and eGFR may differ between indexed and non-indexed measures if the BSA is associated with non-GFR determinants of the endogenous filtration marker. Finally, measurement error in mGFR may contribute to imprecision in eGFR, but should have a smaller effect on differences between mGFR and eGFR based on different filtration markers.
In conclusion, we have demonstrated that eGFRcr-cys, but not eGFRcys, is more accurate than eGFRcr in most subgroups that we studied, suggesting preferential use of eGFRcr-cys when serum cystatin C is measured to obtain more accurate eGFR than can be obtained from eGFRcr alone. eGFRcys may be as accurate as eGFRcr-cys in patients with low BMI. Further studies are necessary to evaluate diagnostic strategies for using eGFRcys and eGFRcr-cys.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
M.F. has been employed by Amgen (Europe) GmbH since 1 January 2011, but was a full-time academic associate professor during the time of study conception and data collection.
Supplementary Material
ACKNOWLEDGEMENTS
The NephroTest CKD cohort study is supported by the following grants: INSERM GIS-IReSP AO 8113LS TGIR, French Ministry of Health AOM 09114 and 10, Agence de la Biomédecine R0 8156LL, Roche 2009-152-447G and AURA.
REFERENCES
- 1.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma YC, Zuo L, Chen JH, et al. Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int. 2007;72:1535–1542. doi: 10.1038/sj.ki.5002566. [DOI] [PubMed] [Google Scholar]
- 3.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksen BO, Mathisen UD, Melsom T, et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int. 2010;78:1305–1311. doi: 10.1038/ki.2010.321. [DOI] [PubMed] [Google Scholar]
- 5.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157:471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 9.Grubb A, Blirup-Jensen S, Lindstrom V, et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48:1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 10.Blirup-Jensen S, Grubb A, Lindstrom V, et al. Standardization of cystatin C: development of primary and secondary reference preparations. Scand J Clin Lab Invest Suppl. 2008;241:67–70. doi: 10.1080/00365510802150067. [DOI] [PubMed] [Google Scholar]
- 11.Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew-Harris JS, Florkowski CM, George PM, et al. The relative effects of fat versus muscle mass on cystatin C and estimates of renal function in healthy young men. Ann Clin Biochem. 2013;50:39–46. doi: 10.1258/acb.2012.011241. [DOI] [PubMed] [Google Scholar]
- 13.Vupputuri S, Fox CS, Coresh J, et al. Differential estimation of CKD using creatinine- versus cystatin C-based estimating equations by category of body mass index. Am J Kidney Dis. 2009;53:993–1001. doi: 10.1053/j.ajkd.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naour N, Fellahi S, Renucci JF, et al. Potential contribution of adipose tissue to elevated serum cystatin C in human obesity. Obesity (Silver Spring) 2009;17:2121–2126. doi: 10.1038/oby.2009.96. [DOI] [PubMed] [Google Scholar]
- 16.Taleb S, Cancello R, Clement K, et al. Cathepsin s promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology. 2006;147:4950–4959. doi: 10.1210/en.2006-0386. [DOI] [PubMed] [Google Scholar]
- 17.Stevens LA, Schmid CH, Zhang YL, et al. Development and validation of GFR-estimating equations using diabetes, transplant and weight. Nephrol Dial Transplant. 2010;25:449–457. doi: 10.1093/ndt/gfp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rigalleau V, Lasseur C, Perlemoine C, et al. Estimation of glomerular filtration rate in diabetic subjects: Cockcroft formula or modification of diet in renal disease study equation? Diabetes Care. 2005;28:838–843. doi: 10.2337/diacare.28.4.838. [DOI] [PubMed] [Google Scholar]
- 19.Inker LA, Okparavero A. Cystatin C as a marker of glomerular filtration rate: prospects and limitations. Curr Opin Nephrol Hypertens. 2011;20:631–639. doi: 10.1097/MNH.0b013e32834b8850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.