Abstract
Although antenatal care (ANC) interventions have been in place for a long time, there is hardly any systematic evidence on the association between ANC interventions and neonatal mortality in India. The present study attempts to investigate the association between ANC interventions and neonatal mortality in India using data from the District Level Household Survey conducted in India during 2007–8. The ANC interventions included in the analysis are at least four antenatal visits, consumption of 90 or more iron–folic acid (IFA) tablets, and uptake of two or more tetanus toxoid (TT) injections. We have used discrete-time logistic regression models to investigate the association between ANC interventions and neonatal mortality. Risk of neonatal mortality was significantly lower for infants of mothers who availed four or more antenatal visits [odds ratio (OR): 0.69; 95% confidence interval (CI): 0.60–0.81], consumed 90 or more IFA tablets (OR: 0.85; 95% CI: 0.73–0.99), received two or more TT injections (OR: 0.73; 95% CI: 0.63–0.83). When we analysed different combinations of antenatal visits, IFA supplementation and TT injections, TT injections provided the main protective effect—the risk of neonatal mortality was significantly lower in newborns of women who received two or more TT injections but did not consume 90 or more IFA tablets (OR: 0.69; 95% CI: 0.60–0.78), or who received two or more TT injections but did not avail four or more antenatal visits (OR: 0.75; 95% CI: 0.66–0.86). In the statistical model, 6% (95% CI: 4–8%) of the neonatal deaths in India could be attributed to a lack of at least two TT injections during pregnancy. Indian public health programmes must ensure that every pregnant woman receives two or more TT injections during antenatal visits.
Keywords: Antenatal visits, iron–folic acid supplementation, tetanus toxoid injections, neonatal mortality, discrete-time logistic regression model, India
KEY MESSAGES.
Maternal vaccination for TT provided the main protective effect, among the ANC interventions, on neonatal mortality in India.
Six per cent of the neonatal deaths in India could be attributed to a lack of at least two TT vaccinations during pregnancy.
Introduction
Antenatal care (ANC) interventions, including ANC visits, iron–folic acid (IFA) supplementation, tetanus toxoid (TT) injections, other diagnostic tests, advice and counselling, have been recommended for a long time and have been accepted by most of the countries in the world. ANC interventions are generally thought to be effective in improving maternal and infant outcomes (Adam et al. 2005; Darmstadt et al. 2005; Coimbra et al. 2007; Wehby et al. 2009; Hollowell et al. 2011). The WHO recommends that all pregnant women should avail at least four antenatal visits, with the first antenatal visit preferably in the first trimester (WHO 2006). In India, the Government guidelines on maternal health recommend, among other things, that every pregnant woman must have at least three antenatal visits and that the first visit is preferably in the first trimester. Guidelines further suggest that, every pregnant woman must consume 90 or more IFA tablets and must receive at least two TT injections (Maternal Health Division 2005). ANC is provided free of charge in public healthcare facilities such as Primary Health Centres and Health Sub-Centres. However, women can also obtain these services from private providers. Although ANC interventions have been in the government policies and programmes for the last two to three decades, many ANC interventions have not been subjected to systematic and rigorous evaluation for their effectiveness in reducing neonatal mortality (Villar and Bergsjo 1997).
Studies carried out on the impact of ANC interventions on maternal and child health outcomes have provided inconclusive evidence. A recent systematic review in high-income countries found insufficient evidence of adequate quality to recommend implementation of any of the ANC interventions as a means of reducing neonatal or infant mortality in disadvantaged/vulnerable women (Hollowell et al. 2011). A study carried out by Carroli et al. (2001) also showed a lack of strong evidence on the effectiveness of the content, frequency and timing of visits in standard ANC programmes on maternal and child health. However, studies conducted in Finland, Indonesia and India have found significant negative association between frequency of antenatal visits and neonatal mortality (Shah et al. 2000; Raatikainen et al. 2007; Ibrahim et al. 2012).
Studies have also examined the impact of specific components of ANC on maternal and child health outcomes. Two important ANC interventions directly linked to neonatal health outcomes are IFA and TT. IFA supplementation during pregnancy can reduce pre-term delivery, increase infant birth weight and prevent birth asphyxia (Cogswell et al. 2003; Siega-Riz et al. 2006; Zeng et al. 2008). Studies carried out in developing countries have reported that pre-term births and low infant birth weight are two prominent contributors to neonatal mortality (Yasmin et al. 2001; Lawn et al. 2005; Ngoc et al. 2006). Maternal TT injection provides passive protection against tetanus during the neonatal period (Ray et al. 2013). Neonatal tetanus is found to contribute significantly to neonatal mortality in low- and middle-income countries (Lawn et al. 2005). Studies from China and Indonesia reported significant reductions in early neonatal mortality among neonates whose mothers received IFA supplements compared with those whose mothers did not receive IFA or only received folic acid (Zeng et al. 2008; Titaley et al. 2010; Titaley et al. 2012). Although the TT vaccination of pregnant women was introduced in early mid-1970s under the WHO’s Expanded Program on Immunization (EPI), the evidence base to support mortality effect in newborns is extremely limited. A recent systematic review yielded only one randomized controlled trial and one well-controlled cohort study. The review showed that immunization of pregnant women or women of childbearing age with two doses of TT was estimated to reduce mortality from neonatal tetanus by 94% (Blencowe et al. 2010). However, the main limitation identified in the review, that could influence the resulting effect estimate, was the dearth of high-quality trials. For India, two small-scale studies examined the effect of immunization of pregnant women with TT on neonatal mortality (Kumar et al. 1988; Dutt and Srinivas 1997).
Published literature also showed a lack of robustness in research carried out in understanding the association between ANC interventions and neonatal mortality. For example, the majority of the studies did not examine the independent effect of ANC interventions on neonatal mortality. Shah et al. (2000), for example, included frequency of antenatal visits in the analysis, but did not include IFA supplementations or TT vaccinations in the analysis. Moreover, a number of studies did not include the recommended number of antenatal visits or the amount of IFA consumed in their analysis. These studies had mostly utilized information on whether or not the women had availed ANC or whether or not the women received IFA supplementation from the health system during antenatal visits.
In the Indian context, where neonatal mortality rates are high, studies on the protective effect of ANC on neonatal mortality are particularly limited. Although the neonatal mortality rates in India have declined significantly in the last two decades, from as high as 49 per 1000 live births in 1992–93, to 39 per 1000 live births in 2005–6, the rates are still very high (IIPS & ORCMacro 1995; IIPS & ORCMacro 2007). Moreover, within India there are considerable variations in neonatal mortality rates across the different states and socio-economic groups. Examining the association between ANC interventions and neonatal mortality is particularly important in a poor resource setting like India, where there is an urgent need to prioritize the interventions that yield maximum benefit in terms of neonatal and maternal health outcomes. Given the lack of systematic evidence on the impact of ANC interventions on neonatal mortality, the objective of this article is to investigate those associations using data from the 2007 to 2008 District Level Household Survey.
Data and methods
Data
We used data from the District Level Household Survey round three (DLHS-3) which was conducted in 2007–8, in 601 districts from 34 states and union territories of India (IIPS 2010). The DLHS-3 was designed to provide estimates on maternal and child health, family planning and other reproductive health indicators at the district level. The data were collected using a multistage stratified systematic sampling design, which resulted in national and state-representative samples, after applying appropriate sampling weights that were designed to control for the complex survey design (IIPS 2010). The main instrument for collection of data in DLHS-3 was a set of structured questionnaires. In all 643 944 ever married women aged 15–49 years and 166 260 unmarried women aged 15–24 years were interviewed during the survey. The household and ever married woman response rates were 94% and 89%, respectively. There were only small variations in the household and eligible woman response rates across different states of the country. The questions on ANC were asked only to those women who had live/still births in the 3 years preceding the DLHS-3 survey and were restricted to the most recent birth.
The analysis presented in this article is based on most recent singleton first births to women during the 3 years preceding the survey. This was done to reduce the residual effect of TT injections as TT injections are found to have long-term effects (Ray et al. 2013). Since DLHS-3 used a multistage sampling design, sampling weights are required to make the estimates representative. We used appropriate sampling weights for generating bivariate results presented in the article. The details of the sampling weight are given in the DLHS-3 report (IIPS 2010).
Outcome variable
The outcome variable of interest is neonatal mortality. Neonatal mortality is defined as the death of a live-born baby within 28 days of birth. The information on age at death for the live births taken place in 3 years preceding the DLHS-3 was utilized to identify neonatal deaths.
Exposure variables
We included three exposure variables in the analysis. The first exposure variable was whether or not women had availed four or more antenatal check-ups during their most recent pregnancy. This variable was created based on the new recommendations of the World Health Organization that every woman should have a minimum of four antenatal check-ups in one pregnancy (WHO 2006). The second exposure variable was whether or not women had consumed 90 or more IFA tablets. The third exposure variable was whether or not women had received two or more TT injections during their antenatal visits.
Control variables
A number of other socio-economic, demographic- and residence-related variables have also been shown to have a significant impact on mortality during the neonatal period (Singh et al. 2012a). Accordingly, we included mother’s age at the birth of the newborn (<20 years; 20–24 years; >24 years), mother’s schooling (<5 years; 5–8 years; 9–12 years; >12 years), sex of the newborn, wealth status, religion (Hindu; Muslim; others), caste (scheduled castes; scheduled tribes; other backward classes; others), urban–rural residence and region of residence (north; central; east; northeast; west; south) as control variables in the statistical models. We used asset-based wealth quintiles to measure the wealth status of households. The wealth quintiles, which are already computed in the DLHS dataset, were generated through a principal components analysis conducted on a set of variables based on the ownership of household assets as has been used in similar studies (Filmer and Pritchett 2001; Vyas and Kumaranayake 2006; Rutstein 2008; Howe et al. 2009). Indian states can be broadly classified into six geographic regions namely north, central, east, northeast, west and south. Accordingly, we classified different states into their geographic regions and created ‘region of residence’ (for details see IIPS & ORCMacro 2007).
A number of earlier studies have also included ‘type of supervision at delivery (skilled/unskilled)’ and ‘mode of delivery (vaginal/caesarean)’ to explain neonatal mortality (Pradhan and Arokiasamy 2006; Titaley et al. 2010; Singh et al. 2012e). Skilled supervision at the time of delivery is known to have a protective effect (WHO 2005). In contrast, caesarean deliveries in Asia are known to be associated with increased risk of neonatal mortality (Lumbiganon et al. 2010). Accordingly, we also controlled for type of supervision at delivery and mode of delivery in the statistical models.
Methods
We used discrete-time logistic regression models to examine the association between the outcome variable and the exposure variables after adjusting for selected control variables. The outcome variable for the discrete-time logistic model was the binary indicator of occurrence of neonatal death. Discrete-time logistic regression analysis treats ‘time’ not as a continuous variable, but as being divided into intervals of the same length. Then hazard associated with any specific time interval is taken to be of the form of a logistic function including a number of covariates (Maul 1994). The discrete-time logistic regression model can be mathematically expressed as
The covariates 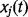 can be constant over time or time-varying.
can be constant over time or time-varying. 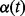 is a function of time, called the logit of the baseline hazard function (Singer and Willett 1993; Allison 1995; Steele 2007) and
is a function of time, called the logit of the baseline hazard function (Singer and Willett 1993; Allison 1995; Steele 2007) and 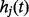 is the hazard function. A couple of recent studies have used this approach to model neonatal mortality (Singh et al. 2012a,b).
is the hazard function. A couple of recent studies have used this approach to model neonatal mortality (Singh et al. 2012a,b).
We estimated two separate sets of models. Model I included only the frequency of antenatal visits along with other control variables to examine the association between the frequency of antenatal visits and neonatal mortality in India. In Model II, we included TT injections and IFA supplementation along with other control variables to examine the association of these two interventions with neonatal mortality. We estimated two separate models because the information on TT injections and IFA supplementation were collected only from those women who availed ANC for their most recent birth in the 3 years preceding the DLHS-3. Finally, we examined the effect of different combinations of antenatal visits, IFA supplementation and TT injections on neonatal mortality.
Having found a significant relationship between TT injections during pregnancy and risk of neonatal mortality, we further estimated the population attributable risk (PAR). The PAR is given by
where w = proportion of women who did not receive ≥2 tt.
aHR = adjusted hazard ratio for neonatal death in neonates born to women who did not receive ≥2 tt.
All the control variables were tested for possible multicollinearity before putting in the regression models. We used STATA 11.0 for statistical computations.
Results
Table 1 presents the frequency of antenatal visits, the amount of IFA supplements consumed and the frequency of TT injections in 3 years preceding DLHS-3. Findings suggest that only 48% of the mothers of infants born in 3 years preceding DLHS-3 had made four or more antenatal visits. Only 33% of the mothers consumed 90 or more IFA tablets. TT injections were more prevalent compared with four or more antenatal visits or IFA supplements. Approximately, 78% of the mothers reported receiving two or more TT injections during pregnancy. About 17% of mothers reported that they did not receive any TT injection during their recent pregnancy. Skilled supervision at birth was far from universal; only 65% of the recent first births were delivered under the supervision of a skilled birth attendant. A majority of deliveries were normal vaginal deliveries (84%). Around 51% of the first births were delivered by mothers in the age of 20–24 years, and 32% of the births were delivered by mothers who were <20 years of age.
Table 1.
The prevalence of ANC services and other birth-related characteristics of most recent singleton first births in 3 years preceding DLHS-3, India, 2007–8
| Pregnancy healthcare service | N (weighted) (%) |
|---|---|
| ANC | |
| <4 ANC visits | 30 297 (52.4) |
| Four or more ANC visits | 27 488 (47.6) |
| IFA supplements | |
| Consumed <90 IFA tablets | 39 377 (67.2) |
| Consumed 90 or more IFA tablets | 19 232 (32.8) |
| TT injections | |
| No injection | 9873 (16.7) |
| 1 injection | 2706 (4.6) |
| ≥2 injections | 46 026 (78.5) |
| Type of supervision at delivery | |
| Unskilled | 20 649 (35.2) |
| Skilled | 37 951 (64.8) |
| Mode of delivery | |
| Vaginal | 49 482 (84.4) |
| C-section | 9111 (15.6) |
| Mother’s age at the birth of the baby | |
| <20 years | 18 561 (31.7) |
| 20–24 years | 29 988 (51.2) |
| >24 years | 10 053 (17.1) |
Note: Total number varies between categories because some values are missing.
Discrete-time logistic regression results are shown in Table 2. Results adjusted for the specified control variables suggest that newborns whose mothers availed four or more antenatal visits were significantly less likely to die during the neonatal period compared with newborns whose mothers did not avail four or more antenatal visits [odds ratio (OR): 0.69; 95% confidence interval (CI): 0.60–0.81]. Likewise, newborns whose mothers received two or more TT injections were only 0.73 (95% CI: 0.63–0.83) times as likely as newborns whose mothers did not receive two or more TT injections to die during the neonatal period. Consumption of 90 or more IFA tablets was also significantly associated with neonatal mortality—neonatal mortality reduced significantly in newborns of women who consumed 90 or more IFA tablets (OR: 0.85; 95% CI: 0.73–0.99). To check the robustness of the regression results, we also estimated two more regression models without controlling for the intra-partum control variables (i.e. type of supervision at delivery and mode of delivery). The results from these two regressions were exactly similar to those reported above. For example, the OR for four or more antenatal visits was 0.70 (95% CI: 0.60–0.81). Similarly, the OR for two or more TT injections was 0.74 (95% CI: 0.65–0.85).
Table 2.
Discrete-time logistic regression results for neonatal mortality, India, 2007–8
| Pregnancy health- care service | OR (95% CI) |
|
|---|---|---|
| Model I | Model II | |
| ANC | ||
| <4 ANC visits (reference) | ||
| Four or more ANC visits | 0.69 (0.60–0.81)* | |
| IFA supplements | ||
| Consumed <90 IFA tablets (reference) | ||
| Consumed 90 or more IFA tablets | 0.85 (0.73–0.99)* | |
| TT injections | ||
| No injection (reference) | ||
| 1 injection | 1.19 (0.94–1.50) | |
| ≥2 injections | 0.73 (0.63–0.83)* | |
*P < 0.05.
Control variables include type of supervision at delivery, mode of delivery, mother’s age at birth of the newborn, mother’s schooling, sex of the newborn, wealth status, religion, caste, urban–rural residence, region of residence.
When we analysed a combination of IFA supplementation, TT injections and antenatal visits, TT injections provided the main protective effect (Table 3). The risk of neonatal mortality was significantly lower in newborns of women who received two or more TT injections but did not consume 90 or more IFA tablets (OR: 0.69; 95% CI: 0.60–0.78). But there was no significant reduction in the risk of neonatal mortality in newborns of women who consumed 90 or more IFA tablets but did not receive two or more TT injections. Again the risk of neonatal mortality was significantly lower in newborns of women who received two or more TT injections but did not avail four or more antenatal visits (OR: 0.75; 95% CI: 0.66–0.86). By comparison, there was no significant reduction in the risk of neonatal mortality in newborns of mothers who availed four or more antenatal visits but did not receive two or more TT injections. The risk reduction was found to be highest among those mothers whose antenatal visits had also included two or more TT injections (OR: 0.57; 95% CI: 0.47–0.68).
Table 3.
The effect of different combinations of ANC, IFA supplementation and TT injections on neonatal mortality, India, 2007–8
| Variable | N (weighted) (%) | Adjusted ORsa (95% CI) |
|---|---|---|
| Combination of IFA and TT | ||
| <90 IFA tablets and <2 TT | 11 573 (19.8) | |
| ≥90 IFA tablets and <2 TT | 1006 (1.7) | 0.65 (0.38–1.11) |
| <90 IFA tablets and ≥2 TT | 27 802 (47.4) | 0.69 (0.60–0.78)* |
| ≥90 IFA tablets and ≥2 TT | 18 224 (31.1) | 0.60 (0.50–0.73)* |
| Combination of ANC and TT | ||
| <4 ANC and <2 TT (reference) | 10 894 (18.8) | |
| 4 or more ANC and <2 TT | 1533 (2.7) | 0.72 (0.47–1.11) |
| <4 ANC and ≥2 TT | 19 402 (33.6) | 0.75 (0.66–0.86)* |
| 4 or more ANC and ≥2 TT | 25 952 (44.9) | 0.57 (0.47–0.68)* |
*P < 0.05.
aControl variables include type of supervision at delivery, mode of delivery, mother’s age at birth of the newborn, mother’s schooling, sex of the newborn, wealth status, religion, caste, urban–rural residence, region of residence.
Considering the plausible association between TT injections during pregnancy and neonatal mortality, we computed the PAR. The PAR indicates that 6% of the neonatal deaths in India could be attributed to a lack of at least two doses of TT injection during pregnancy (PAR: 0.06; 95% CI: 0.04–0.08).
Discussion
Our findings suggest that among the three ANC interventions considered (four or more antenatal visits, IFA supplementation and TT injections), TT injections provided the main protective effect on neonatal mortality in India. The findings show that 6% of the neonatal deaths in India could be attributed to a lack of at least two doses of TT injections. These findings, perhaps for the first time, have demonstrated the impact of recommended TT injections to pregnant women on the survival of their newborns during the neonatal period, using a large-scale household survey. The findings are consistent with various small-scale studies that documented protective effect of maternal TT vaccinations on neonatal mortality.
The findings related to maternal TT vaccinations are not at all surprising, given that a significant number of neonatal deaths in India occur because of neonatal infections. Recent estimates from the Million Death Study revealed that 32 000 neonatal deaths in India in 2005 were from tetanus (Million Death Study Collaborators 2010). These figures, when converted to percentages, suggest that ∼3% of the neonatal deaths in India in 2005 were because of tetanus. Two studies conducted in rural Uttar Pradesh attributed 4% and 6% of the total of neonatal deaths to tetanus (Nandan et al. 2005; Baqui et al. 2006). Globally, around 7% of neonatal deaths are due to neonatal tetanus (Lawn et al. 2006). In the light of the evidence presented above, our estimate of PAR (6%) seems plausible.
In India, the main mortality burden is concentrated in the northern states (Roper et al. 2007; Singh et al. 2011). Estimates from 2008 (www.indiastat.com) suggest that 56% of neonatal deaths in India due to neonatal tetanus occurred in the northern states of Rajasthan, Uttarakhand, Uttar Pradesh, Madhya Pradesh, Chattisgarh and Jharkhand. Of these, the state of Uttar Pradesh alone contributed 28% of the deaths followed by Rajasthan 16% (www.indiastat.com). In the northern states, uptake of two or more TT injections is far below the national average (IIPS 2010). Clearly, there is a need to provide TT injections to pregnant women in these states.
In complete contrast to the finding of Titaley et al. (2010), we found a lower protective effect of IFA supplements compared with TT injections on neonatal mortality. The lower protective effect of IFA on neonatal mortality in our study may be affected by the omission of important variables such as prevalence of maternal infections and dietary nutrient intake in our analysis, due to non-availability of such data. Dietary intake and maternal infections are particularly relevant in poor rural settings, where maternal education is generally low (Kawai et al. 2011). Further, an apparent bias during antenatal visits in India is observed towards medical interventions as opposed to nutritional supplementation (Pallikadavath et al. 2004; Singh et al. 2012c). Further research is required to examine the role of IFA on neonatal health outcomes in settings that bear a higher burden of poor maternal nutrition and infections.
The findings of this study have important implications for the maternal and child health programmes being run in India and other countries with similar social contexts and maternal and child health services. As the Government of India’s guidelines suggest that every pregnant woman must avail three or more antenatal visits, we re-estimated all the models presented in this article using three or more antenatal visits to examine whether or not the results vary according to the number of antenatal visits. The OR and the 95% CIs for three or more antenatal visits were very close to those obtained for four or more antenatal visits. The two CIs overlapped, indicating that the protective effect of three or more antenatal visits is similar to that obtained for four or more antenatal visits. Results further show that the maximum protective effect is obtained when women received two or more TT injections during antenatal visits. Thus, this study clearly demonstrates that Government of India’s policy of minimum three antenatal visits provides similar protective effect on neonatal mortality as four or more ANC visits would have.
Recent estimates in India suggest that only 50% of currently married women in the age group of 15–49 years availed three or more antenatal visits (IIPS 2010). Moreover, only 67% of the currently married women in the age group of 15–49 received two or more doses of TT injections. There is, therefore, a need to ensure that every pregnant woman must avail the recommended antenatal visits, and receive two or more TT injections. Women can be provided with TT and other ANC interventions only when they have made antenatal visits. Therefore, increasing ANC attendance remains an important priority for the primary health-care system in India, to increase the uptake of various antenatal services. Providing at least two TT injections to pregnant women should be a priority in northern states, where neonatal deaths are disproportionally high. Innovative methods, including private–public partnerships, need to be explored to deliver TT injections to pregnant women in India, particularly in areas and population groups that under-represent ANC users.
The strengths and limitations of our study must also be noted. One of the key strengths of our study is the use of a large-scale population-based dataset particularly in a diverse and large country like India. The availability of DLHS-3 provided us with a great opportunity to examine the association between specified ANC interventions and neonatal mortality in a detailed manner. The information collected in DLHS-3 are of optimal quality and have been widely used for examining maternal and child health issues in India (Singh et al. 2012d,e). The DLHS-3 is also widely used for monitoring the reproductive and child health programme sponsored by the Government of India. Furthermore, DLHS-3 for the first time provided us with better measure of IFA supplementation in India. Earlier studies on a similar topic have relied heavily on whether or not the pregnant women received IFA supplementation during their antenatal visits. In complete contrast to the earlier studies, we have utilized information on the amount of IFA consumed during the pregnancy. Limitations of our study must also be noted. An obvious limitation of our study is that we could not control for birth weight or size of the newborn at birth in regression analysis. This was due to the unavailability of information on birth weight and size of the newborn at birth in DLHS-3. A second limitation could be of reporting or recall bias on the part of mothers. However, the chances of reporting or recall bias are low in DLHS-3 as information on ANC interventions were collected only for most recent birth in 3 years preceding survey date. The chances of such bias are also low because the survey teams receive specialized training of 3 weeks duration, and quality control measures are in place.
To conclude, this research showed that TT injections provided the main protective effect, among the ANC interventions, on neonatal mortality in India. Provision of at least two TT injections to pregnant women has the potential to avert 6% of neonatal deaths in India. Further research is needed to investigate the reasons for lower protective effect of IFA supplementation on neonatal mortality in India. More research is also required to establish whether or not four or more ANC visits have any impact on other maternal and child health outcomes.
Conflict of interest
None declared.
References
- Adam T, Lim SS, Mehta S, et al. Cost effectiveness analysis of strategies for maternal and neonatal health in developing countries. British Medical Journal. 2005;331:1–6. doi: 10.1136/bmj.331.7525.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PD. Survival Analysis Using SAS System: a Practical Guide. Cary, NC: SAS Institute; 1995. [Google Scholar]
- Baqui AH, Darmstadt GL, Williams EK, et al. Rates, timing and causes of neonatal deaths in rural India: implications for neonatal health programmes. Bulletin of World Health Organization. 2006;84:706–13. doi: 10.2471/blt.05.026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Lawn J, Vandelaer J, Roper M, Cousens S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. International Journal of Epidemiology. 2010;39:i102–i9. doi: 10.1093/ije/dyq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroli G, Villar J, Piaggio G, et al. WHO systematic review of randomized controlled trials of routine antenatal care. The Lancet. 2001;357:1565–70. doi: 10.1016/S0140-6736(00)04723-1. [DOI] [PubMed] [Google Scholar]
- Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. American Journal of Clinical Nutrition. 2003;78:773–81. doi: 10.1093/ajcn/78.4.773. [DOI] [PubMed] [Google Scholar]
- Coimbra LC, Figueiredo FP, Silva AA, et al. Inadequate utilization of prenatal care in two Brazilian birth cohorts. Brazilian Journal of Medical and Biological Research. 2007;40:1195. doi: 10.1590/s0100-879x2006005000116. [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Bhutta ZA, Cousens S, et al. Evidence-based, cost-effective interventions: how many newborn babies can we save? The Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- Dutt D, Srinivas DK. Impact of maternal and child health strategy on child survival in a rural community of Pondicherry. Indian Pediatrics. 1997;34:785–92. [PubMed] [Google Scholar]
- Filmer D, Pritchett L. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–32. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- Hollowell J, Oakley L, Kurinczuk JJ, Brocklehurst P, Gray R. The effectiveness of antenatal care programmes to reduce infant mortality and preterm birth in socially disadvantaged and vulnerable women in high- income countries: a systematic review. BMC Pregnancy Childbirth. 2011;11:11–13. doi: 10.1186/1471-2393-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe LD, Hargreaves JR, Gabrysch S, et al. Is the wealth index a proxy for consumption expenditure? A systematic review. Journal of Epidemiology and Community Health. 2009;63:871–7. doi: 10.1136/jech.2009.088021. [DOI] [PubMed] [Google Scholar]
- Ibrahim JA, Yorifuji TA, Tsuda TB, Kashima SC, Doi HA. Frequency of antenatal care visits and neonatal mortality in Indonesia. Journal of Tropical Pediatrics. 2012;58:184–8. doi: 10.1093/tropej/fmr067. [DOI] [PubMed] [Google Scholar]
- IIPS, MacroInternational. National Family Health Survey (NFHS-1), 1992-93: India. Mumbai: International Institute for Population Sciences; 1995. [Google Scholar]
- IIPS, MacroInternational. National Family Health Survey (NFHS-3), 2005-06: India. Vol. I. Mumbai: International Institute for Population Sciences; 2007. [Google Scholar]
- Indiastat.com. Statewise Number of Cases and Deaths Due to Neonatal Tetanus in India for 2008. www.indiastat.com, accessed 8 February 2012.
- IIPS. District Level Household Survey (DLHS-3), 2007-08: India. Mumbai: International Institute for Population Sciences; 2010. [Google Scholar]
- Kawai K, Spiegelman D, Shankar AH, Fawzi WW. Maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries: meta-analysis and meta-regression. Bulletin of the World Health Organization. 2011;89:402–411B. doi: 10.2471/BLT.10.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Kumar R, Mathur VN, et al. Neonatal tetanus mortality in a rural community of Haryana. Indian Pediatrics. 1988;25:167–9. [PubMed] [Google Scholar]
- Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Katarzyna W, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. International Journal of Epidemiology. 2006;35:706–18. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- Lumbiganon P, Laopaiboon M, Gulmezoglu AM, et al. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007-08. The Lancet. 2010;375:490–9. doi: 10.1016/S0140-6736(09)61870-5. [DOI] [PubMed] [Google Scholar]
- Maternal Health Division. Guidelines for Antenatal Care and Skilled Attendance at Birth by ANMs and LHVs. New Delhi: Maternal Health Division, Department of Family Welfare, Ministry of Health & Family Welfare, Government of India; 2005. [Google Scholar]
- Maul A. A discrete time logistic regression model for analyzing censored survival data. Environmetrics. 1994;5:145–57. [Google Scholar]
- Million Death Study Collaborators. Causes of neonatal and child mortality in India: a nationally representative mortality survey. The Lancet. 2010;376:1853–60. doi: 10.1016/S0140-6736(10)61461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandan D, Mishra SK, Jain M, et al. Social audit for community actions: a tool to initiate community action for reducing child mortality. Indian Journal of Community Medicine. 2005;30:78–80. [Google Scholar]
- Ngoc NT, Merialdi M, Abdel-Aleem H, et al. Causes of stillbirths and early neonatal deaths: data from 7993 pregnancies in six developing countries. Bulletin of World Health Organization. 2006;84:699–705. doi: 10.2471/blt.05.027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallikadavath S, Foss M, Stones RW. Antenatal care: provision and inequality in rural north India. Social Science and Medicine. 2004;59:1147–58. doi: 10.1016/j.socscimed.2003.11.045. [DOI] [PubMed] [Google Scholar]
- Pradhan J, Arokiasamy P. High infant and child mortality rates in Orissa: an assessment of major reasons. Population, Space and Place. 2006;12:187–200. [Google Scholar]
- Raatikainen K, Heiskanen N, Heinonen S. Under-attending free antenatal care is associated with adverse pregnancy outcomes. BMC Public Health. 2007;7:268. doi: 10.1186/1471-2458-7-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B, Balmer P, Roper MH. Immunological Basis for Immunization – Module 3: Tetanus (revision) 2013 http://www.who.int/immunization/documents/ISBN 9789241595551/en/index.html, accessed 4 May 2013. [Google Scholar]
- Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. The Lancet. 2007;370:1947–59. doi: 10.1016/S0140-6736(07)61261-6. [DOI] [PubMed] [Google Scholar]
- Rutstein SO. The DHS Wealth Index: Approaches for Rural and Urban Areas. Calverton, MD: Macro International, USA; 2008. [Google Scholar]
- Shah D, Shroff S, Ganla K. Factors affecting perinatal mortality in India. Prenatal and Neonatal Medicine. 2000;5:288–302. doi: 10.1016/s0020-7292(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Siega-Riz AM, Hartzema AG, Turnbull C, et al. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. American Journal of Obstetrics and Gynecology. 2006;194:512–9. doi: 10.1016/j.ajog.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. It’s about time: using discrete-time survival analysis to study duration and the timing of events. Journal of Educational and Behavioural Statistics. 1993;18:155–95. [Google Scholar]
- Singh A, Pathak PK, Chauhan R, Pan W. Infant and child mortality in India in the last two decades: a Geospatial analysis. PLoS One. 2011;6:e26856. doi: 10.1371/journal.pone.0026856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Chalasani S, Koenig M, Mahapatra B. The consequences of unintended births for maternal and child health in India. Population Studies. 2012a;66:223–39. doi: 10.1080/00324728.2012.697568. [DOI] [PubMed] [Google Scholar]
- Singh A, Singh A, Mahapatra B. The consequences of unintended pregnancy for maternal and child health in rural India: evidence from prospective data. Maternal and Child Health Journal. 2012b;17:493–500. doi: 10.1007/s10995-012-1023-x. [DOI] [PubMed] [Google Scholar]
- Singh A, Pallikadavadh S, Ram F, Ogollah R. Inequalities in advice provided by public health workers to women during antenatal sessions in rural India. PLoS One. 2012c;7:e44931. doi: 10.1371/journal.pone.0044931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Padmadas SS, Mishra US, et al. Socioeconomic inequalities in the use of postnatal care in India. PLoS One. 2012d;7:e37037. doi: 10.1371/journal.pone.0037037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Yadav A, Singh A. Utilization of postnatal care for newborns and its association with neonatal mortality in India: an analytical appraisal. BMC Pregnancy and Childbirth. 2012e;12:33. doi: 10.1186/1471-2393-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele F. Multilevel Discrete-Time Event History Analysis. Bristol: University of Bristol; 2007. www.bristol.ac.uk/cmm/software/support/workshops/…/eha.ppt, accessed 31 August 2012. [Google Scholar]
- Titaley CR, Dibley MJ, Roberts CL, Hall J, Agho K. Iron and folic acid supplements and reduced early neonatal deaths in Indonesia. Bulletin of the World Health Organization. 2010;88:500–8. doi: 10.2471/BLT.09.065813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titaley CR, Dibley MJ. Antenatal iron/folic acid supplements, but not postnatal care, prevents neonatal deaths in Indonesia: analysis of Indonesia Demographic and Health Surveys 2002/2003-2007 (a retrospective cohort study) BMJ Open. 2012;2:e001399. doi: 10.1136/bmjopen-2012-001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Bergsjo P. Scientific basis for the content of routine antenatal care I. Philosophy, recent studies, and power to eliminate or alleviate adverse maternal outcomes. Acta Obstetricia et Gynecologica Scandinavica. 1997;76:1–14. doi: 10.3109/00016349709047778. [DOI] [PubMed] [Google Scholar]
- Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy and Planning. 2006;21:459–68. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- Wehby GL, Murray JC, Castilla EE, Lopez-Camelo JS, Ohsfeldt RL. Prenatal care effectiveness and utilization in Brazil. Health Policy and Planning. 2009;24:175–88. doi: 10.1093/heapol/czp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. The World Health Report: 2005: Make Every Mother and Child Count. Geneva: World Health Organization (WHO); 2005. [Google Scholar]
- WHO. Provision of Effective Antenatal Care: Integrated Management of Pregnancy and Child Birth (IMPAC) Geneva, Switzerland: Standards for Maternal and Neonatal care (1.6), Department of Making Pregnancy Safer, World Health Organization; 2006. http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/effective_antenatal_care.pdfS, accessed 14 March 2012. [Google Scholar]
- Yasmin S, Osrin D, Paul E, et al. Neonatal mortality of low-birth-weight infants in Bangladesh. Bulletin of World Health Organization. 2001;79:608–14. [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Dibley MJ, Cheng Y, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomized controlled trial. British Medical Journal. 2008;337:a2522. doi: 10.1136/bmj.a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


