Abstract
This study was designed to develop and validate a short-term in vivo protocol termed the Fetal Phthalate Screen (FPS) to detect phthalate esters (PEs) and other chemicals that disrupt fetal testosterone synthesis and testis gene expression in rats. We propose that the FPS can be used to screen chemicals that produce adverse developmental outcomes via disruption of the androgen synthesis pathway more rapidly and efficiently, and with fewer animals than a postnatal one-generation study. Pregnant rats were dosed from gestational day (GD) 14 to 18 at one dose level with one of 27 chemicals including PEs, PE alternatives, pesticides known to inhibit steroidogenesis, an estrogen and a potent PPARα agonist and ex vivo testis testosterone production (T Prod) was measured on GD 18. We also included some chemicals with “unknown” activity including DMEP, DHeP, DHEH, DPHCH, DAP, TOTM, tetrabromo-diethyl hexyl phthalate (BrDEHP), and a relatively potent environmental estrogen BPAF. Dose-response studies also were conducted with this protocol with 11 of the above chemicals to determine their relative potencies. CD-1 mice also were exposed to varying dose levels of DPeP from GD 13 to 17 to determine if DPeP reduced T Prod in this species since there is a discrepancy among the results of in utero studies of PEs in mice. Compared to the known male reproductive effects of the PEs in rats the FPS correctly identified all known “positives” and “negatives” tested. Seven of eight “unknowns” tested were “negatives”, they did not reduce T Prod, whereas DAP produced an “equivocal” response. Finally, a dose-response study with DPeP in CD-1 mice revealed that fetal T Prod can be inhibited by exposure to a PE in utero in this species, but at a higher dose level than required in rats.Key words. Phthalate Syndrome, Fetal endocrine biomarkers, Phthalate adverse outcome pathway, testosterone production, fetal rat testis.
Keywords: Phthalate Syndrome, Fetal endocrine biomarkers, Phthalate adverse outcome pathway, testosterone production, fetal rat testis
ABBREVIATIONS
- BPAF
bisphenol AF; hexafluorobisphenol A
- BBP
benzylbutyl phthalate
- BrDEHP
di-2-ethylhexyl tetrabromo phthalate (Uniplex FRP-45)
- DAP
diallyl phthalate
- DBP
di(n-butyl) phthalate
- DCHP
dicyclohexyl phthalate
- DEHP
di(2-ethylhexyl) phthalate
- DEP
diethyl phthalate
- DHEH
1,2-cyclohexanedicarboxylic acid, bis(2-ethylhexyl) ester
- DHeP
di(n)heptyl phthalate
- DHP
di-n-hexyl phthalate
- DiBP
diisobutyl phthalate
- DIDP
di-isodecyl phthalate
- DiHeP
di-isohepthyl phthalate
- DINCH
1,2-cyclohexane dicarboxylic acid, di-isononyl ester
- DiNP
diisononyl phthalate
- DMEP
bis(2-methoxyethyl) phthalate
- DMP
dimethyl phthalate
- DOTP
dioctyl terephthalate
- DPeP
dipentyl phthalate
- DPHCH
1,2-cyclohexanedicarboxylic acid, bis(2-propylheptyl) ester
- DPHP
bis(2-propylheptyl) phthalate (palatinol 10-P)
- DPP
dipropyl phthalate
- LIN
linuron
- PZ
prochloraz
- TOTM
palatinol TOTM (tri octyl trimellitate)
- WY-14643
pirinixic acid (PPARα agonist)
Phthalate esters (PEs) are a family of compounds used in a wide array of products including medical tubing, toys for children and adults, pharmaceuticals, personal care products, flooring, and cables, for example. There are concerns about the potential effects of PEs on human health due to widespread indirect and direct exposures (Adibi et al., 2003, 2008; Blount et al., 2000; Silva et al., 2004, 2011) and the adverse developmental and reproductive effects seen in laboratory animal studies (Gray et al., 2000; Mylchreest et al., 1998a; Saillenfait et al., 2009b). Recent trends indicate that while some human PE exposures are declining, others are increasing. For example, from 2001 to 2010 exposures to diethyl phthalate (DEP), dibutyl phthalate (DBP), benzyl butyl phthalate (BBP), and di-(2-ethylhexyl) phthalate (DEHP) have declined whereas DiBP, a reproductive toxicant in rats (Boberg et al., 2008; Hannas et al., 2011b; Saillenfait et al., 2008b), has increased by 260% (Zota et al., 2014). One would hope that as new PEs or alternatives replace older PEs in consumer products that well-studied, relatively nontoxic PEs are not replaced by ones that are less well studied, and more toxic.
At present, several regulatory bodies including the Consumer Product Safety Commission (CPSC) and U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, and Office of Chemical Safety and Pollution Prevention are developing risk assessments for individual phthalates and mixtures of phthalates. The CPSC Modernization Act of 2008 (Public Law 110–314) specifies that several phthalates, their alternatives, and mixtures of phthalates be regulated and/or evaluated “for endocrine disrupting effects.” The law bans the manufacture for sale, offer for sale, distribution in commerce, or import into the United States any children's toy or child care article that contains concentrations of >0.1% of DEHP, DBP, or BBP and the law contains a similar interim prohibition on diisononyl phthalate (DINP), diisodecyl phthalate (DIDP), and di-n-octyl phthalate (DnOP). CPSC was also required to establish a Chronic Hazard Advisory Panel (CHAP) to study the effects on children's health of all phthalates and phthalate alternatives as used in children's toys and child care articles. The CHAP was established to conduct a complete examination of the full range of phthalates that are used in products for children and to (1) examine all of the potential health effects (including endocrine disrupting effects) of the full range of phthalates; (2) consider the potential health effects of each of these phthalates both in isolation and in combination with other phthalates; (3) examine the likely levels of children's, pregnant women's, and others’ exposure to phthalates, based on a reasonable estimation of normal and foreseeable use and abuse of such products; (4) consider the cumulative effect of total exposure to phthalates, both from children's products and from other sources, such as personal care products; (5) review all relevant data, including the most recent, best-available, peer-reviewed, scientific studies of these phthalates and phthalate alternatives that employ objective data collection practices or employ other objective methods; (6) consider the health effects of phthalates not only from ingestion but also as a result of dermal, hand-to-mouth, or other exposure; (7) consider the level at which there is a reasonable certainty of no harm to children, pregnant women, or other susceptible individuals and their offspring, considering the best available science, and using sufficient safety factors to account for uncertainties regarding exposure and susceptibility of children, pregnant women, and other potentially susceptible individuals; and (8) consider possible similar health effects of phthalate alternatives used in children's toys and child care articles.
In addition, the USEPA has developed a Chemical Action Plan that includes eight phthalates: DBP, diisobutyl phthalate (DIBP), BBP, di-n-pentyl phthalate (DnPeP), DEHP, DnOP, DINP, and DIDP. EPA intends to initiate rulemaking to add these eight phthalates to the Concern List under TSCA section 5(b) (4) as chemicals that present or may present an unreasonable risk of injury to health or the environment and to add the six phthalates not already on the Toxics Release Inventory (TRI). In addition, EPA plans to consider the results of the cumulative assessment currently being developed and that was due to be completed by CPSC in 2012 pursuant to the Consumer Product Safety Improvement Act of 2008 (CPSIA), as well as the ongoing review of phthalates at FDA and the assessment for USEPA's IRIS program. When complete, these assessments would inform EPA's decision on future action to address these chemicals. EPA's potential control measures may include a ban of all or several of these chemicals, as appropriate. In 2012, EPA announced that it was proposing to regulate DPeP by issuing a significant new use rule (SNUR) under the Toxic Substances Control Act (Fed. Reg. Vol. 77, No. 66, p. 18752). This action is still pending. A SNUR requires persons who intend to manufacture, import, or process this chemical to notify EPA at least 90 days before commencing that activity.
Historically, long-term, resource-intensive multigenerational studies are required to identify the phthalates that disrupt the endocrine system and induce male reproductive tract malformations since there currently are no accepted in vitro or short term in vivo assays for this purpose. While animal studies have shown that in utero treatment with PEs such as diethylhexyl- (DEHP) (Blystone et al., 2010; Gray et al., 2000), benzyl butyl- (BBP) (Gray et al., 2000; Nagao et al., 2000; Tyl et al., 2004), dibutyl- (DBP) (Mylchreest et al., 1998b; Mylchreest and Foster 2000; Mylchreest et al., 1999), diisobutyl- (DiBP) (Saillenfait et al., 2006, 2008b), dicyclohexyl- (DCHP) (Saillenfait et al., 2009a), dipentyl- (DPeP) (Hannas et al., 2011a), dihexyl- (DHP) (Hannas et al., 2011b; Saillenfait et al., 2009a,b), diisoheptyl- (DiHeP) (Hannas et al., 2011b; McKee et al., 2006), and diisononyl- (DINP) (Borch et al., 2004; Gray et al., 2000) phthalate during the critical period of sexual differentiation cause male reproductive malformations known as the Phthalate Syndrome, many phthalates and alternatives have not been studied using such protocols. In contrast to androgen receptor antagonists like vinclozolin (Kelce et al., 1994), procymidone (Hosokawa et al., 1993), prochloraz (Noriega et al., 2005), and flutamide (McIntyre et al., 2001; Miyata et al., 2002; Wong et al., 1995; Yamasaki et al., 2005) which also cause male reproductive tract malformations, the PEs do not bind the androgen receptor, but instead disrupt Leydig cell maturation and gene expression in the fetal rat testis resulting in decreased Leydig cell androgen and insulin-like-3 (insl3) hormone production. The reduction in these hormones during sexual differentiation is causally linked to phthalate-induced malformations of several reproductive tissues in the male offspring (Hannas et al., 2011a; Howdeshell et al., 2008a; Mylchreest et al., 2002; Parks et al., 2000).
The goal of this study was to develop and validate a relatively rapid, medium-throughput in vivo screen that detects disruption of fetal testosterone synthesis and uses a minimum number of animals to identify PEs with potential to induce the Phthalate Syndrome (Foster 2006; Skakkebaek 2002). We propose that the FPS can be used to rapidly and efficiently screen phthalates to identify those with the potential to produce adverse developmental outcomes in male offspring by disrupting testosterone synthesis.
In the current study, pregnant Harlan or Charles River (CR) Sprague Dawley (SD) rats were treated by oral gavage with a single, relatively high dose of the chemical from gestational day (GD) 14 to 18, the critical period for sexual differentiation of the reproductive tract, and necropsied on GD 18. On GD 18, testis testosterone production (T Prod) was measured ex vivo from three males per litter from three litters per chemical and the remaining testes pooled by litter and used to measure mRNA levels by quantitative RT-PCR (qRT-PRC). These sample sizes were based upon power calculations from earlier work and were found to be adequate to detect reductions in T Prod greater than 50% of control (Hannas et al., 2011a,b; Howdeshell et al., 2008a,b). However, these sample sizes are not adequate to provide the statistical power needed to consistently detect anything but rather large alterations of maternal weight gain and fetal viability or the effects of chemicals that only reduce T Prod by 20–25%.
In addition to measuring fetal testis testosterone production ex vivo, we also measured mRNA expression levels in the fetal rat testis (Lambright et al., manuscript in preparation) using targeted, custom-designed qRT-PRC 96 gene arrays (arrays described in detail by (Hannas et al., 2012)) to detect phthalate-induced alterations in gene expression.
Among the PEs expected to be “positive” in the FPS, the “weak” PE used herein was DINP, and the most potent was DPeP. Other known or suspected positives were DBP, DiBP, BBP, DBP, DHP, DEHP, DiHP, DCHP, and pesticides that produce small but significant reductions in T Prod; linuron and prochloraz. The known or suspected negatives studied included dimethyl- (DMP), diethyl- (DEP), dipropyl- (DPP), dioctyl-ter- (DOTP), dipropyl heptyl (DPHP) phthalate, and diisononyl cyclohexane-1,2-dicarboxylate (DINCH) a phthalate alternative. We also included some chemicals with “unknown” activity including diheptyl- (DHeP), dimethoxy ethyl- (DMEP), tetrabromo-diethyl hexyl- (BrDEHP) phthalate, diallyl- (DAP) phthalate, 1,2-cyclohexanedicarboxylic acid, bis(2-ethylhexyl) ester (DHEH) which is a plasticizer similar in structure to DEHP but has a completely hydrogenated ring, 1,2-cyclohexanedicarboxylic acid, bis(2-propylheptyl) ester (DPHCH) which is a plasticizer similar in structure to DPHP but has a completely hydrogenated ring, Palatinol TOTM (tri octyl trimellitate), and hexafluorobisphenol A (bisphenol AF; BPAF) (Bermudez et al., 2010), an environmental estrogen in that is relatively potent in vivo with oral administration (Table 1).
TABLE 1. Observed and Expected Effects of the 27 Different Chemical Treatments on Fetal Testis Testosterone Production on Gestational Day 18. Chemicals Were Administered to the Dam on Gestational Days 14 to 18. The “Expected Outcome” Was Based Upon the Ability of the Chemical to Induce Some Component of the Phthalate Syndrome in F1 Male Rats After In Utero Exposure During Sexual Differentiation, Reduce Fetal or Neonatal AGD in the Absence of an Effect of Body Weight or Induce Reproductive Toxicity in a Transgenerational or Multigenerational Study.
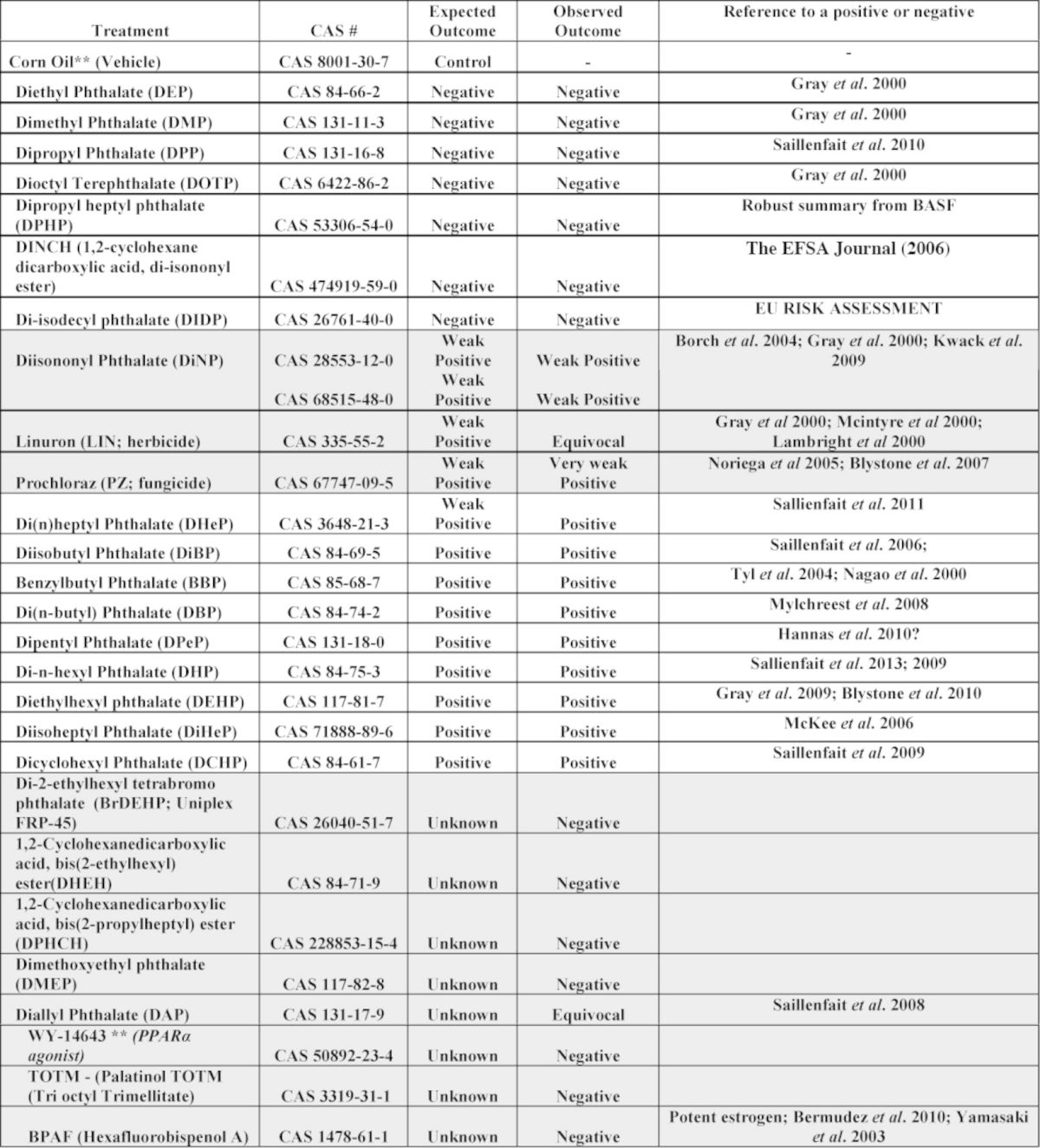
As a follow-up to these single-dose screening studies, we also have conducted dose-response studies using the FPS protocol to determine the relative potencies of the chemicals for reducing T Prod and alter gene expression (using the qRT-PCR arrays) and the results of these studies are being used to design mixture studies to determine if the chemicals in the mixture behave in a dose-additive manner. Some of the data from the dose-response studies with DPeP, DiBP, DHP, DHeP, DINP, DIDP, and the PPARα agonist Wyeth 14643 were recently published (Hannas et al., 2011b, 2012) and new dose response data from the FPS are presented herein.
We also examined the effects of DPeP, one of the more potent phthalates, in the CD-1 mouse since there is considerable uncertainty in the literature about the effects of phthalates on testosterone production in utero in this rodent species
MATERIALS AND METHODS
Animals—Rats
This project was conducted over about 2–3 years in 66 blocks. Each block consisted of about 15 pregnant rats that were typically divided into four to five different treatment groups with three to four dams per group. Block numbers that are not discussed were used for other projects and have been or will be published separately.
For the first 43 blocks, adult female Harlan SD rats (Harlan Laboratories, Inc., Indianapolis, IN) were mated by the supplier and shipped on GD 1 (Table 2). Mating was confirmed by sperm presence in vaginal smears by the supplier (day of sperm plug positive = GD 0). Following block 43, several blocks were conducted with Charles River SD rats (1) to compare effects in the CR SD rat with those seen with the Harlan SD rat and (2) to provide data on the fetal effects of in utero exposure to phthalates and mixtures in the CR SD rat to compare to the treatment-induced reductions in fetal T Prod. The reason for using the CR SD rat for postnatal studies as opposed to the Harlan SD rat is in our hands the CR SD F1 litters are more robust after birth and during lactation than are Harlan SD F1 litters.
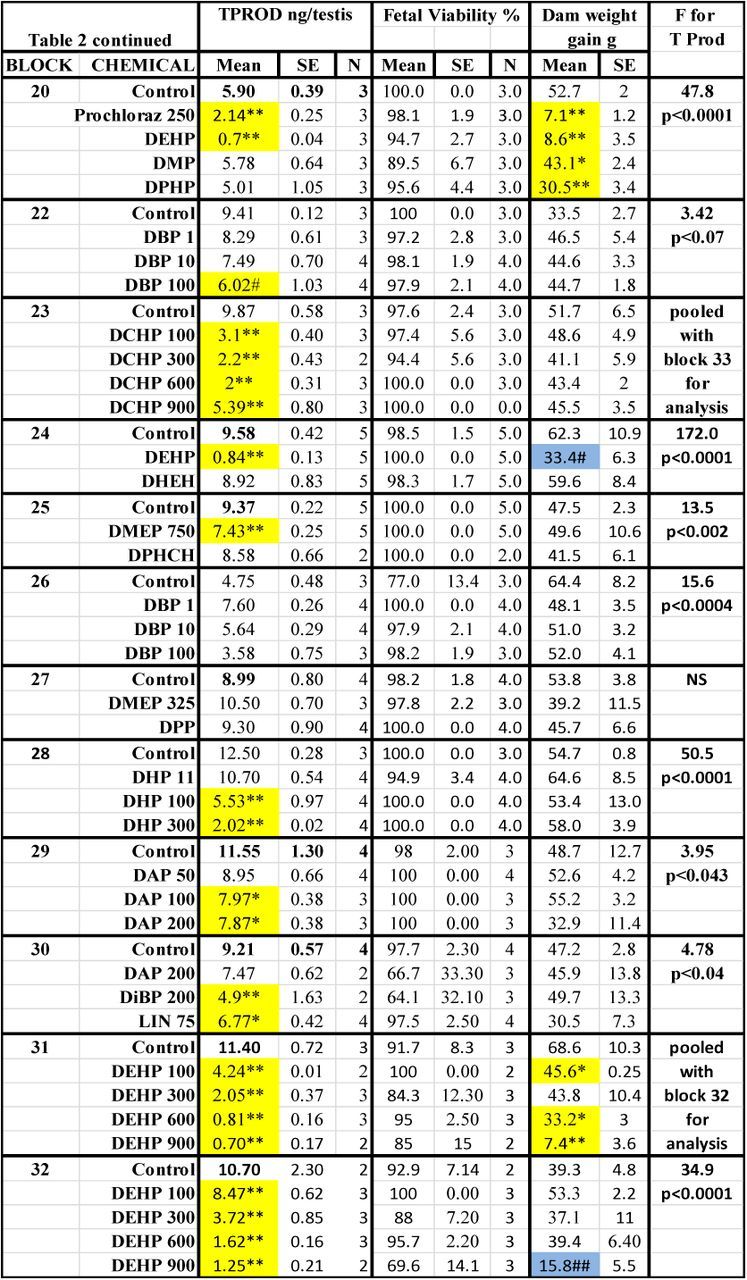
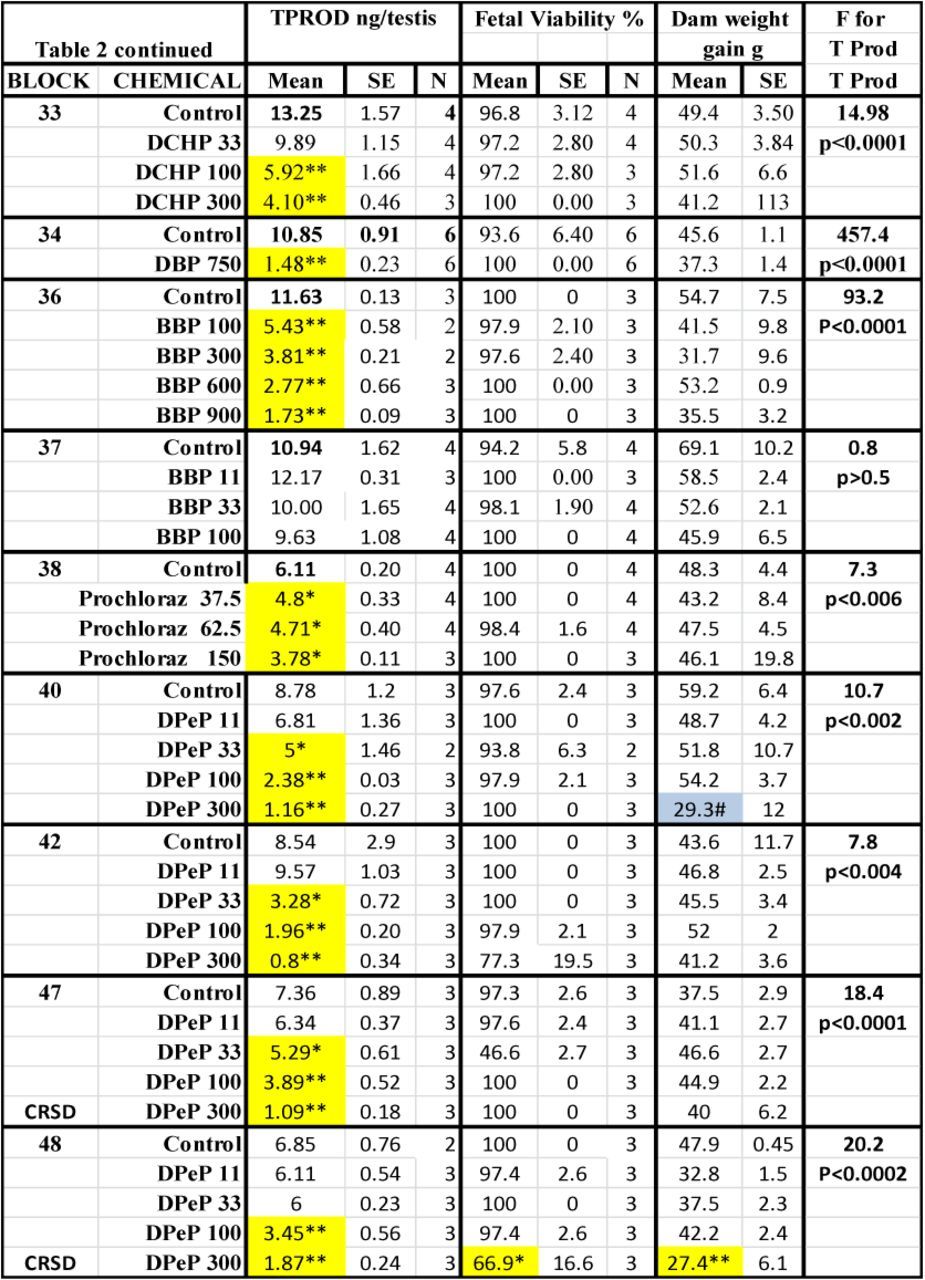
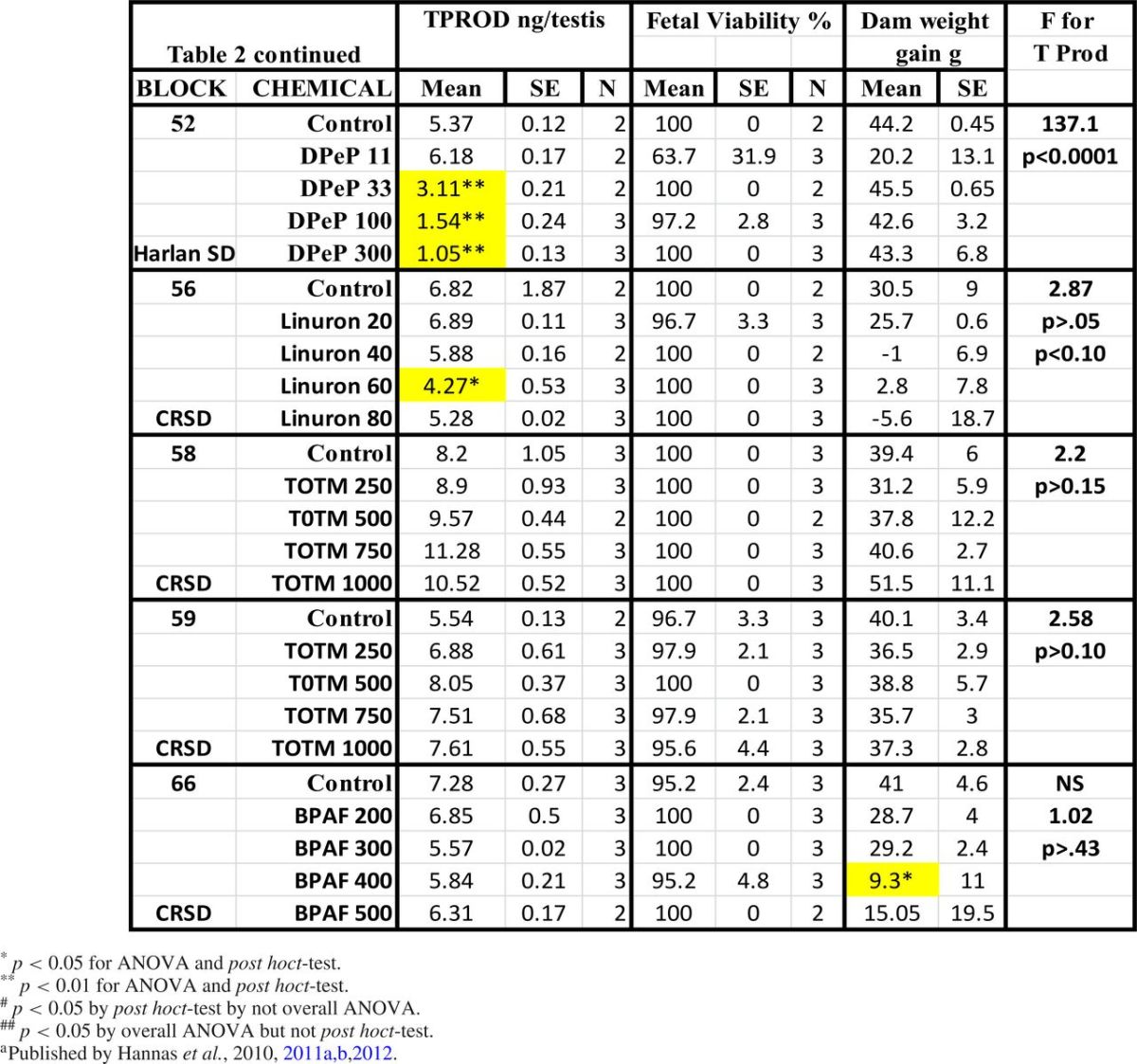
TABLE 2. Treatment Effects On Fetal Testosterone Production (T Prod) and Survival and Maternal Weight Gain. Means, Standard Errors of the Means and the Numbers of Litters Used to Measure Testosterone Production (TPROD). Also Shown are the Effects of the Chemical Treatments on Maternal Weight Gain During Dosing and Fetal Viability at GD18, and the F and p Values for the Effects of the Treatments Within Each Block on T Prod. Shaded Values Were Significant From Control by At Least p < 0.05 by a Post Hoc t-test Following a Significant F Value from the ANOVA for the Entire Block. Values Shaded in Gray Were Significant (p < 0.05) from the Control Value by a Post Hoc t-test But the Overall ANOVA Was Not Significant.
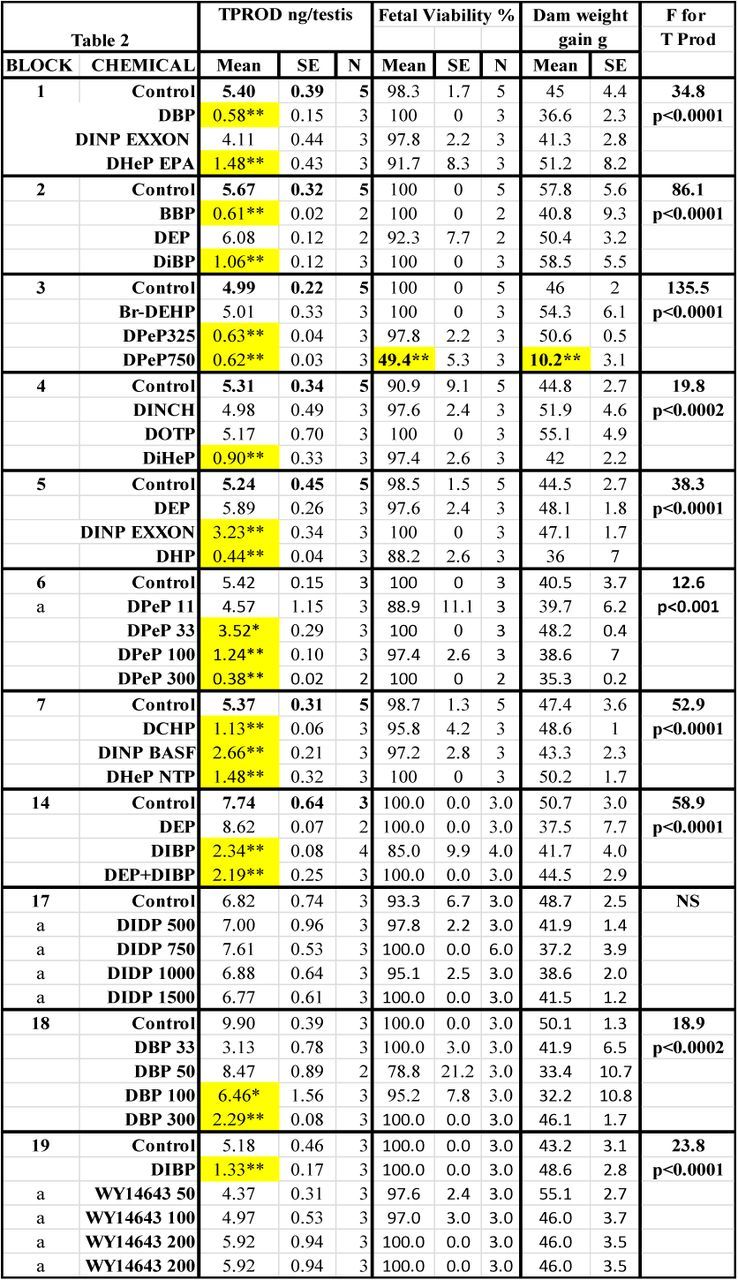
Dams were housed individually in clear polycarbonate cages (20 cm × 25 cm × 47 cm) with laboratory grade heat-treated pine shavings (Northeastern Products, Warrensburg, NY) as bedding. Pregnant dams were fed NIH07 Rat Chow and filtered (5 μm filter) municipal drinking water (Durham, NC) ad libitum.
Pregnant rats were maintained on a 12:12 h photo period (light/dark cycle, lights off at 7:00 p.m.) and 20–22º C temperature with a 45–55% relative humidity. Water was tested monthly for Pseudomonas and every 4 months for a suite of chemicals, including pesticides and heavy metals. The current study was conducted under protocols approved by the National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee and the Association for Assessment and Accreditation of Laboratory Animal Care.
Dosing and Administration of Chemicals: FPS Protocol for Single Dose Studies with 27 Chemicals in the Rat
One of our objectives was to determine whether 750 mg/kg/day was sufficiently high to detect the endocrine activity of the weaker PEs like DINP, without inducing maternal or fetal toxicity with one of the most potent PEs and DPeP. We also wanted to determine if examining testosterone production from three males per litter from three to four litters per treatment group had sufficient statistical power to discriminate known “positives” from known “negatives”; the known “positives” versus “negatives” being determined by the ability of the PE to induce some aspect of the Phthalate Syndrome in male rat offspring or reduce fetal or neonatal anogenital distance in males without reducing body weight, regardless of the dosage level. PEs that induce testicular effects in pubertal male rats (Creasy et al., 1983; Foster et al., 1981, 1982, 1983; Gray et al., 1988, 1999; Gray and Butterworth 1980; Lake et al., 1984; Mangham et al., 1981; Noriega et al., 2005) were also “expected” to be positive in the FPS and reduce T Prod. This “expectation” was based upon the observation that the PEs that disrupt pubertal male testis function like DBP, DEHP, and DPeP also induce reproductive tract malformations in utero whereas PEs like DEP, DPP, and DMP do not induce reproductive effects at either life stage.
In addition, several PEs or PE alternatives with unknown activity were included in the study (Table 1). Effects on T Prod that were reduced significantly from the concurrent control by p≤ 0.01 were considered to be “positive” responses, significant effects less than p ≤ 0.05 but greater than p > 0.01 were considered “equivocal” and often repeated, and effects that did not differ from control by p ≤ 0.05 were considered to be “negatives”. Chemicals that reduced T Prod greater than 50% of control, but at relatively high dosage levels were termed as “weak positives.” Chemicals that reduced T Prod significantly (p < 0.01) were termed “very weak positives” if the reduction in T Prod did not attain a 50% reduction of control because of the chemical's maternal or fetal toxicity. This threshold was selected in order to keep sample sizes small in the “screening” protocol. In addition, the literature indicated that this threshold was attained with phthalates (Hannas et al., 2011a,b, 2012) like DBP (Struve et al., 2009) and DEHP (Parks et al., 2000) without inducing overt maternal or fetal toxicity. In addition, we are currently conducting postnatal studies to determine how much of a reduction in T Prod is necessary to produce permanent alternations later in life.
Pregnant rat dams were randomly assigned to treatment groups on GD 14 in a manner that provided each group with similar means and variances in body weight. Dams were weighed and dosed daily by oral gavage at ∼07:30 h. from GD 14 to GD 18 with 0 (vehicle control; laboratory-grade corn oil [CAS no. 8001-30-7] at 2.5 ml/kg) or with the different phthalates at 750 mg/kg/day (unless otherwise noted). Approximately 2 h after dosing on GD 18, dams were euthanized by decapitation and exsanguination, and the fetuses immediately removed and euthanized by decapitation. All fetal necropsies were conducted within a 2-h period to ensure that a similar developmental period was sampled. Generally, a block contained about 15 pregnant rats with 3–4 dams per treatment group. DMEP and DPeP also were administered at 325 mg/kg/day because the fetuses of dams treated with 750 mg DMEP /kg/day displayed anasarca and small testes (although the fetuses were viable and there was no maternal toxicity) and 750 mg DPeP/kg/day induced a high rate of fetal loss. DAP (top dose 200 mg/kg/d), and prochloraz (150 mg/kg/day) were not administered at 750 mg/kg/day because this dosage level would be toxic to the dam and/or fetus (Gray et al., 1999; Noriega et al., 2004; Saillenfait et al., 2008a) and the dose was selected from the literature.
Dosing and Administration of Chemicals: FPS Dose-Response Studies with 11 Chemicals in Rats
FPS dose-response studies were conducted using seven chemicals that we have not reported on previously including DBP (0, 1, 10, 33, 50, 100, and 300 mg/kg/day in the Harlan and CR SD rat), DAP (0, 50, 100, or 200 mg/kg/day in the Harlan SD rat), DCHP (0, 33, 100, 300, 600, or 900 mg/kg/day in the Harlan SD rat), BBP (0, 11, 33, 100, 300, 600, or 900 mg/kg/day in the Harlan SD rat), prochloraz (0, 37.5, 75, or 150 mg/kg/day in the Harlan SD rat), BPAF (0, 200, 300, 400, or 500 mg/kg/day in the CR SD rat), and TOTM (0, 250, 500, and 1000 mg/kg/day in the CR SD rat). In addition, we have new DEHP (0, 100, 300, 600, and 900 mg/kg/day in the Harlan SD rat), DHP (0, 11, 100, and 300 mg/kg/day), DiBP (0, 100, 200, 300, 500, 600, 750, and 900 mg/kg/day) and DPeP data (0, 11, 33, 100, and 300 mg/kg/day in both Harlan and CR SD rats) data that was combined with previously published data and reanalyzed (Hannas et al., 2011a,b, 2012). The sample sizes for these studies are shown in Supplemental File 2.
Dosing and Administration of DPeP: FPS Protocol Dose-Response Studies in CD-1 Mice
Since several investigators have claimed that PEs do not reduce fetal testosterone in the mouse, we conducted a dose-response study with DPeP in the CD-1 mouse to thoroughly examine this hypothesis over a wide dose range with a PE that is relatively potent in reducing T Prod in the rat. This PE and mouse strain were selected because the literature indicates that chronic dietary administration of DPeP produces adverse testicular effects and reduces fertility in CD-1 mice (Heindel et al., 1989).
In the dose-response study, DPeP was administered by gavage to pregnant CD-1 mice from GD 13 to 17 at 0, 50, 100, 200, 300, 400, 500, or 600 mg/kg/day. This study was conducted in several blocks with T Prod (measured as in the rat) on GD 17 from individual testes from 3 males/litter from 24, 12, 9, 14, 18, 3, 3, and 2 litters per dose group, respectively. Fewer litters were examined in the three higher dose groups because of extensive fetal loss at these dosage levels.
Dosing and Administration of Mixtures of Phthalates Using Rats
A binary mixture of phthalates
In addition to administration of individual phthalates, we also conducted a binary mixture study with DEP and DiBP using the current protocol to determine if DEP (a negative) interacted in an antagonistic or synergistic manner with DiBP (a strong positive at the dose administered in this study). Dams were dosed with the vehicle, a high level of DEP (900 mg/kg/day), an effective dose of DiBP (500 mg/kg/day) or a combination of DEP (900 mg/kg/day) and DiBP (500 mg/kg/day).
Ex Vivo Testicular Testosterone Production—Methods
The method for assessing T Prod in this study is identical to that used by Wilson et al. (2004) which is a modification of the methods used by Parks et al. (2000); methods we derived from those used in studies of fetal T Prod conducted in the 1970s (Warren et al., 1972) and 1980s (Habert and Picon 1986). Fetal testes were removed from fetal male rats and mice using a dissecting scope and three testes (one testis from three different fetuses) per litter were analyzed individually for ex vivo testosterone production as described below. Necropsies were conducted in the morning from 8:00 a.m. to 10:00 a.m, similar to previously published fetal necropsies in our laboratory. Testes were immediately transferred to a well (1 testis per well) containing 0.5 ml M199 media without phenol red for ex vivo testis hormone production (Wilson et al., 2004) and incubated with gentle rocking for 3 h at 37°C. Following incubation, the media was stored in siliconized microcentrifuge tubes and stored at −80°C until testosterone was measured by radioimmunoassay. Testosterone levels in the incubation media were measured by radioimmunoassay (RIA) using Coat-a-Count kits according to manufacturer's protocols (Siemens Corporation, Los Angeles, CA).
The testosterone intra-assay coefficient of variation was 1.25% based on variability of the standard curve and the inter-assay coefficient of variation was 9.1%. Cross-reactivity with dihydrotestosterone was 3.2%. The limit of detection of the RIA was 0.2 ng/ml testosterone for testosterone production. Data are presented and analyzed using litter mean values.
Statistics
The data from this study were analyzed by block using a one-way analysis of variance (ANOVA) using the general linear measures procedures from the Statistical Analysis Systems (SAS, Inc., Cary, NC). T Prod data were log10 transformed to correct for heterogeneity of variance for statistical analyses and percentage of control values for T Prod were generated using the control values within the same block for graphical representation of the results.
For all analyses, litter means were used as the sample size and differences were considered significant at p ≤ 0.01 (a positive response), whereas effects falling between p≤ 0.05 and p > 0.01 were considered “equivocal” effects, and responses with p > 0.05 were considered as “negatives.” If a treatment produced an “equivocal” response, the treatment was repeated in a subsequent block and the data from the blocks were pooled and the data reanalyzed to determine if the effect was statistically significant (p ≤ 0.01) or not. Post hoc treatment comparisons were made by block using the Least Squares Means procedure on SAS, which is appropriate for a priori hypotheses. Data from the dose-response studies were also analyzed using a logistic regression model with GraphPad Prism software, version 5.00 for Windows (GraphPad Software, San Diego CA, www.graphpad.com) to determine the ED50 values for each chemical.
Because DEP and DINP at 750 mg/kg/day in blocks 2 and 1, respectively and DAP at 200 mg/kg/day produced “equivocal effects” (p > 0.01 but p ≤ 0.05) on some of the endpoints (T Prod or mRNA expression), each of these was repeated at this dose level and the data pooled to determine if the PE significantly (p < 0.01) reduced T Prod or gene expression levels. The T Prod data for DINP and DEP were analyzed as log10 transformed percentage of control data for each block to adjust for heterogeneity of variance and block to block differences in the absolute T levels.
RESULTS
Rat Studies
The results of the different treatments on fetal T Prod, fetal viability and maternal weight gain are shown by block in Table 2. In utero, maternal treatment with phthalates and other chemicals during sexual differentiation produced the expected reductions in T Prod: DBP, DiBP, BBP, DPeP, DEHP, DHP, DiHeP, DCHP, DINP, and DHeP, were positive and DEP, DMP, DPP, DoTP, DPHP, DIDP, and DINCH were negative. Prochloraz was a “weak positive” and linuron produced an “equivocal” reduction in T Prod with four litters per group (p < 0.05 but p > 0.01).
The “unknowns” BPAF, BrDEHP, DPHCH, WY 14643 (Hannas et al., 2012), TOTM, and DHEH did not reduce T Prod. When blocks 29 and 30 were pooled for statistical analysis of the effects of varying doses of DAP on T Prod was considered to be an “equivocal” effect since T Prod at was reduced at 100 (p < 0.05) and 200 mg/kg/day (p < 0.02). Because the F and p values are greater than p < 0.01 for the effects of DAP, we consider this response to be “equivocal” and it is likely that the higher dose reduced fetal body weight (Saillenfait et al., 2008a). In the current study, administration of DAP did not significantly reduce maternal weight gain or induce fetal toxicity but is possible that the higher dose reduced fetal body weight (Saillenfait et al., 2008a); an endpoint we did not collect. In contrast, the effect of DMEP on T Prod at 750 mg/kg/day (block 25) was concurrent with 100% incidence of fetal anasarca, whereas treatment with 325 mg/kg/day (block 27) did not reduce T Prod or induce anasarca.
Because only 2/3 dams were pregnant in the first block that exposed pregnant rats to DEP (block 2), DEP was repeated at 750 mg/kg/day (block 5). The results of the pooled analyses indicate that DEP did not significantly reduce T Prod at 750 mg/kg/day. In addition, T Prod was not reduced by DEP administration at 900 mg/kg/day in the mixture study with DiBP (block 14).
DINP was run first in block 1 and since the effect on T Prod was equivocal (being statistically significant using untransformed T Production values (p < 0.03) but not with the log10 transformed data (p > 0.25). DINP was rerun at 750 mg/kg/day in block 5 (Fig. 1). The pooled results indicated that T Prod was significantly reduced by DINP exposure at 750 mg/kg/day.
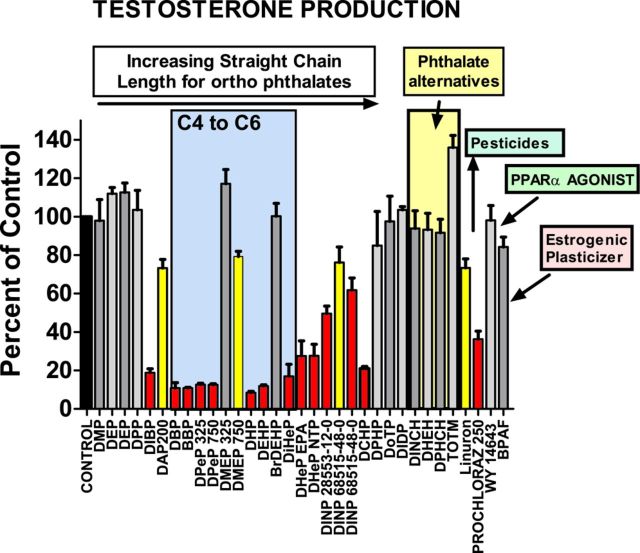
FIG. 1.
Effects of the different in utero maternal treatments on fetal testis testosterone production, collected ex vivo for 3 h incubation (one testis for each of three males per litter, with 3–4 litters per dose group in most cases). Data are expressed as percentage of control from the respective block in which the PE was tested; T Prod data were log10 transformed to correct for heterogeneity of variance. Phthalates are listed from left to right by increasing ester straight side chain length from C2 to C9. Several phthalates which do not have straight side chains from C4 to C6 disrupt fetal testis testosterone production including DIBP, DHeP, DINP, and DCHP. Gray histograms are not significantly different from control (p > 0.10), yellow were equivocal (p ≤ 0.05 to p > 0.01) and red differed significantly (p ≤ 0.01) from the concurrent control value.
The only treatments that significantly (p ≤ 0.0001) reduced maternal weight gain from GD 14 to 18 were DPeP and DEHP at 750 mg/kg/day (Table 2). DPeP at 750 mg/kg/day also significantly reduced litter sizes so there only were eight viable male fetuses (3 + 3 + 2 males from three litters) for assessment of T Prod. None of the other treatments reduced litter sizes at GD 18.
In the dose-response studies, there were dose-related reductions in T Prod (no statistically significant nonmonotonic alterations of T Prod were noted) (Fig. 2). The potency of the PEs is ranked in Table 2 and the results of the logistic regression and the relative potencies are both shown in Fig. 3).
FIG. 2.
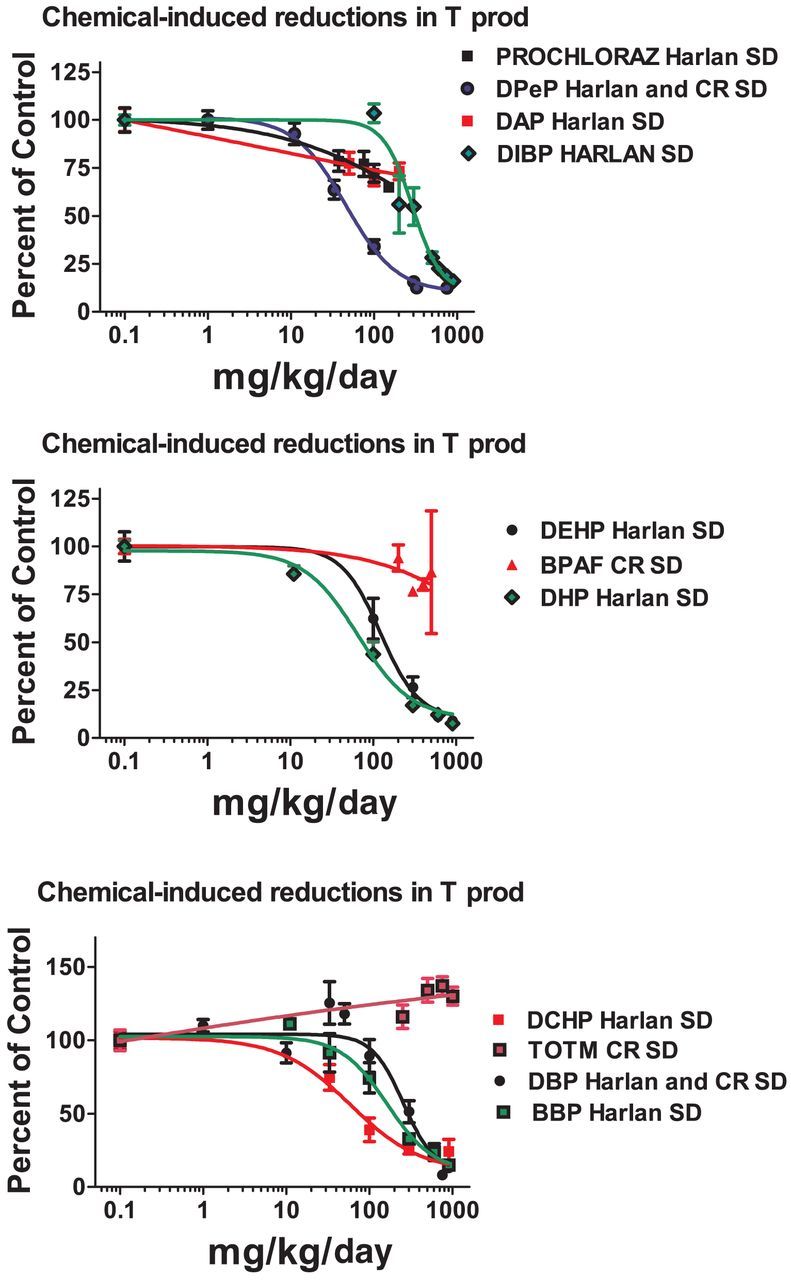
Dose-related reductions in male rat testis testosterone production on gestational day 18, expressed as percentage of control values. The graph was generated using GraphPad Prism software using the nonlinear, four parameter logistic regression model, with the bottom constrained to 10% of control testosterone production from the same block as the treatment.
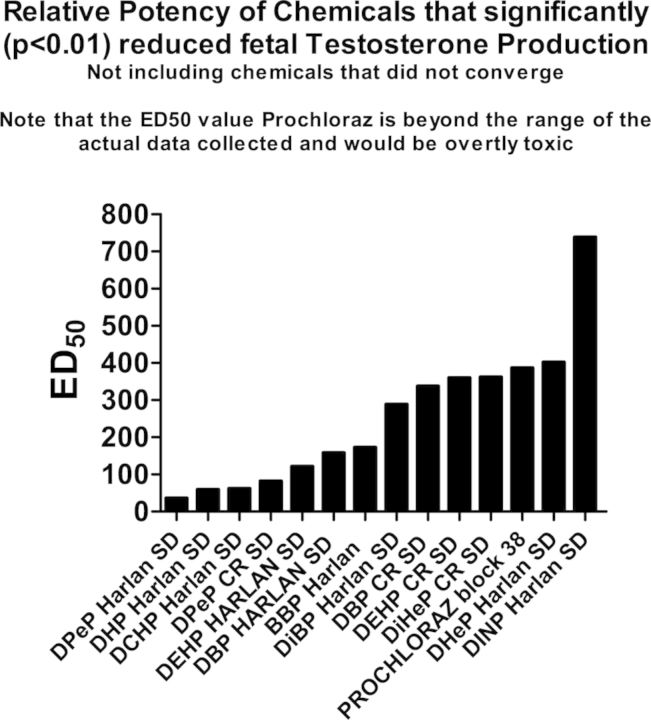
FIG. 3.
The ED50 values from the logistic regression analyses of the dose-response data were ranked from left to right by decreasing potency to reduce testosterone production with the most potent chemical with the lowest ED50 value on the left and the weakest chemical. Chemicals that did not significantly reduce testosterone production are not included in the figure.
When the dose-response data for DPeP, DEHP, and DBP were analyzed by strain, rather than with the data from the Harlan and CR SD rats pooled, we found that T Prod in the Harlan SD was significantly more sensitive to disruption than in the CR SD rat for each PE (Fig. 4)
FIG. 4.
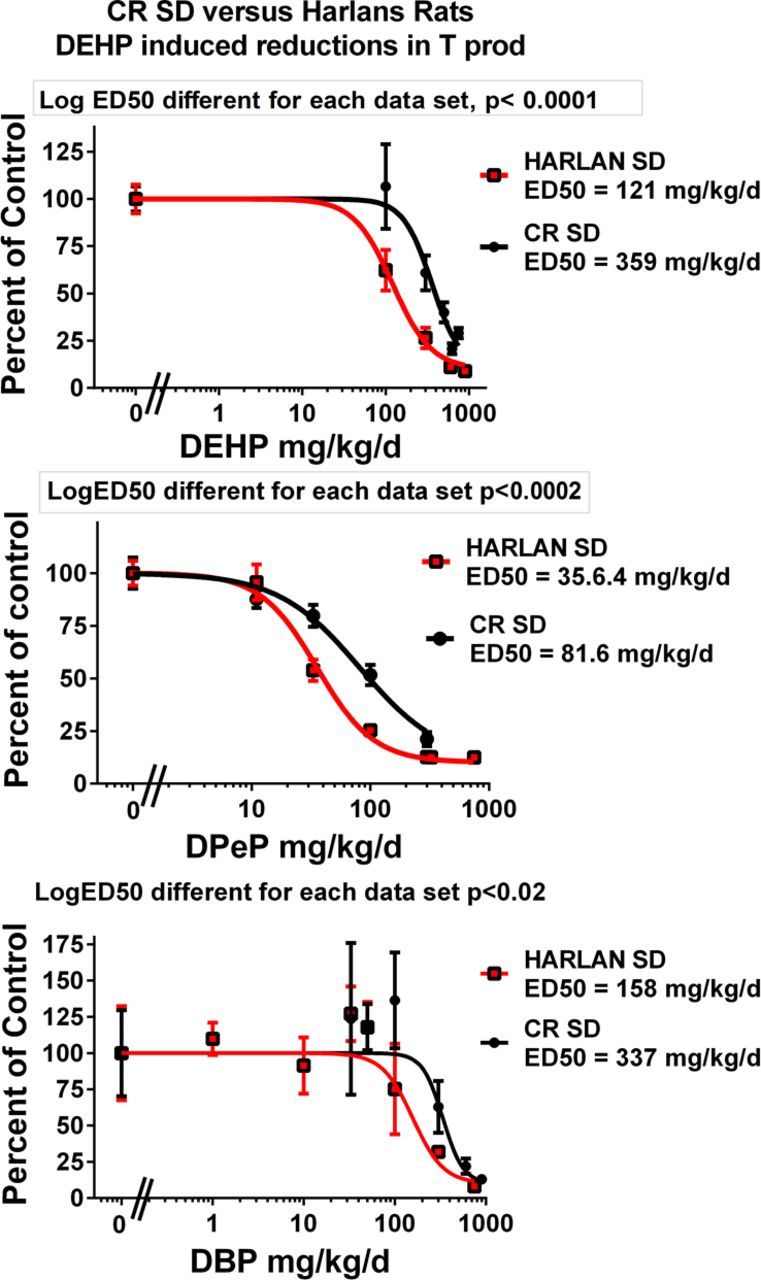
Dipentyl, dibutyl, and diethylhexyl phthalate were run in several blocks in both Harlan SD and Charles Rivers SD (CR SD) rats in order to compare the sensitivity of these SD rats from different suppliers to phthalate-induced reduction of fetal testosterone production on GD 18. The results of the statistical comparison of the two logistic regression models with GraphPad Prism software indicate that the Harlan SD was slightly more sensitive than is the CR SD.
In the binary mixture study with DEP and DiBP, DiBP alone significantly reduced T Prod from 7.74 (±0.64) ng/testis in controls to 2.34 (±0.37) whereas DEP was without effect (8.62 ± 0.07). DEP did not interact with DiBP; the combination of DEP plus DiBP (2.19 ± 0.24) did not differ from the effect of DiBP alone (Table 2, block 14).
Mice
In the dose-response study, oral DPeP administration to pregnant CD-1 mice from GD 13 to 17 significantly reduced fetal testis T Prod at 100 mg/kg/day and above in a dose-related manner (F(7, 77) = 5.9; p < 0.0001). However, the ED50 was about four fold higher than in the rat (ED50 = 193 mg/kg/day vs. 48 mg/kg/day) and the dose-related decline in T Prod reached a plateau at about 50% of control, whereas T Prod in the rat reached a plateau at about 10–15% of control. In contrast to T Prod, DPeP induced fetal loss and reduced maternal weight gain during dosing at lower doses in the mouse than in the rat (Fig. 5).
FIG. 5.
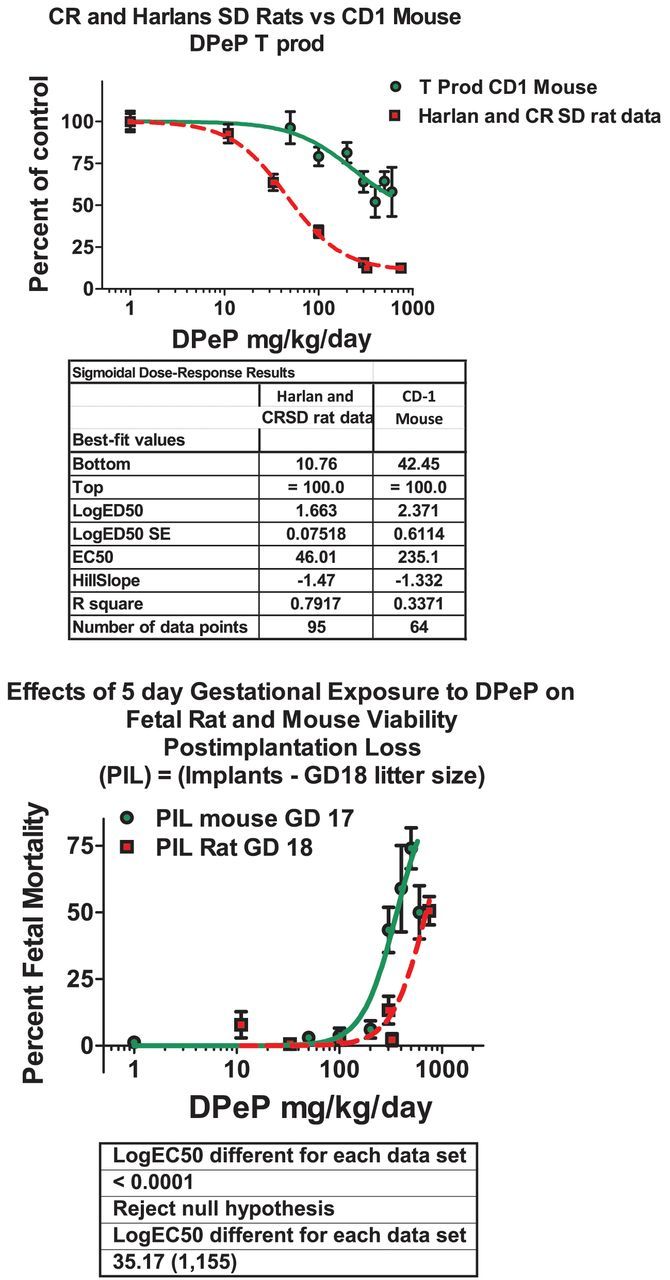
Comparison of the dose-related effects of dipentyl phthalate (DPeP) on testosterone production (T Prod) on gestational day 18 and fetal mortality as measured by postimplantation loss (PIL = 100 × (number of implantation sites – number of live fetuses)) in the fetal male rat and mouse. The results of the statistical comparison of the two logistic regression models with GraphPad Prism software indicate that T Prod was more sensitive to DPeP in the rat versus the mouse, whereas, fetal mortality was more affected in the mouse than the rat.
DISCUSSION
Concordance of Fetal Phthalate Screening Results on Individual Chemicals with “Expected” Outcomes
A major objective of this study was to develop a screening protocol to identify and characterize PEs that disrupt fetal rat testis endocrine function and potential disruptions of sexual differentiation. Although there are literally thousands of publications on the reproductive toxicity of the PEs, the vast majority of these have studied two active PEs, DBP, and DEHP. Beyond these two well-studied PEs only a handful of other PEs or alternatives have been studied in utero for reproductive toxicity out of the several hundred extant PEs and alternatives. The data from the published studies (listed in Table 1) were used to predict expected “positives” and “negatives.” Chemicals reported to produce the Phthalate Syndrome in F1 male rats after in utero exposure, or reduce AGD in male rats during neonatal or late fetal life without affecting body weight were expected to be “positive” in the FPS and reduce T Prod. PEs that induce testicular effects in pubertal male rats (Creasy et al., 1983; Foster et al., 1981, 1982, 1988; Gray et al., 1988, 1999; Gray and Butterworth, 1980; Lake et al., 1984; Mangham et al., 1981; Noriega et al., 2005) were also expected to be positive in the FPS and reduce T Prod. We also expected the pesticides prochloraz (Blystone et al., 2007) and linuron (Wilson et al., 2009) to reduce T Prod, as reported in the literature. However, we would not necessarily expect chemicals like vinclozolin (Kelce et al., 1994) or procymidone (Hosokawa et al., 1993; Ostby et al., 1999) that disrupt male rat testis function and pubertal development via other adverse outcome pathways (e.g., androgen receptor antagonism) to reduce T Prod in the FPS.
In the screening protocol termed the FPS, pregnant rats and rat fetuses were exposed to one of 27 chemicals, including PEs, several PE alternatives, two pesticides and a potent PPARα agonist (Hannas et al., 2011b, 2012) to determine which chemicals suppressed fetal rat testis testosterone production during the “masculinizing window” of fetal development (Carruthers and Foster 2005; Scott et al., 2008; Wolf et al., 2000) (Tables 1 and 2). A companion paper (in preparation) describes a highly reproducible genomic “signature” (mRNA expression levels) for the effects of these chemicals on testis gene expression using a custom-designed 96 gene qRT-PCR array containing mRNA for key genes involved in androgen synthesis, gonadal and sexual differentiation and PPAR function; a genomic signature identical that reported by Hannas et al. (2012) and it compares the ED50 values for the reductions in mRNA levels with the ED50 values for T Prod.
We found that “expected positive” PEs could be correctly identified with the screen and in most cases evaluating T Prod from only 3 litters per chemical (one testis each from 3 males per litter) provided an adequate sample size. However, a sample size of four litters per dose group was not sufficient to label the 26% reduction in T Prod as statistically significant at a p < 0.01 level by 75 mg linuron/kg/day.
We also determined that administering a PE at a dose of 750 mg/kg/day for 5 days during sexual differentiation was appropriate for most, but not all, of the PEs. This dose was high enough to detect the reduction in T Prod induced by the weaker PEs like DINP, without inducing maternal or fetal toxicity, whereas PEs like DAP, DMEP ,and DPeP had to be administered at lower dosage levels. Our use of a single, relative high dosage level to screen for reductions in T Prod is based on the assumption that few if any chemicals reduce fetal testis T Prod at a low dose level but have no effect at high dosage levels. This assumption is supported by the PE dose response data on fetal rat Prod from our laboratory and other laboratories including one study that administered DBP in utero with a low dose of 0.1 mg/kg/day which they reported as equivalent to high dose human exposures (Lehmann et al., 2004). The use of dosage levels lower than 0.1 mg/kg/day is problematic because PEs are found in rodent diets and beddings within this dose range (Kondo et al., 2010).
FPS Dose-Response Studies
In addition to executing single dose level studies to identify “positives” and “negatives” we also conducted dose-response studies on 11 of the chemicals in order to determine the ED50 values for reduced T Prod (Fig. 3 and Table 3). Some of these data were presented previously by Hannas et al. (2011a,b, 2012) whereas others are presented here for the first time. We found that the ED50 dose of the chemicals that significantly reduced T Prod varied by about 25-fold from 45 to 1100 mg/kg/day. These potency values are currently being used to design a fixed-ratio mixture study with nine active phthalates to determine if the mixture reduces T Prod and testis mRNA expression in a dose-additive manner in the CR SD rat as was seem in the Harlan SD rat (Hannas et al., 2011b) and to determine how much of a reduction in each fetal endocrine endpoint is required to induce permanent effects later in life. For the three PEs (DPeP, DEHP, and DBP) administered in the FPS to both Harlan and CR SD rats, we found that T Prod in Harlan SD was reduced at a significantly lower ED50 values than in the CR SD (Fig. 4).
TABLE 3. Logistic Regression Analyses of the Effects of Chemicals Testosterone Production.
| Chemical and strain | ED50 | ED50 95% CI | log ED50 | log ED50 SE | Hill slope | No. of litters | R2 | Rank |
|---|---|---|---|---|---|---|---|---|
| DPeP Harlan SD | 35.59 | 28.26 to 44.82 | 1.551 | 0.05011 | −1.816 | 66 | 0.819 | 1 |
| DHP Harlan SD | 59.21 | 42.85 to 81.83 | 1.772 | 0.06845 | −1.255 | 29 | 0.93 | 2 |
| DCHP Harlan SD | 61.62 | 39.56 to 95.97 | 1.79 | 0.09279 | −1.035 | 24 | 0.7683 | 3 |
| DPeP CR SD | 81.62 | 62.96 to 105.8 | 1.912 | 0.05494 | −1.259 | 29 | 0.8534 | 4 |
| DEHP HARLAN SD | 121.2 | 92.02 to 159.5 | 2.083 | 0.05789 | −1.87 | 26 | 0.8675 | 5 |
| DBP HARLAN SD | 157.9 | 100.5 to 248.0 | 2.198 | 0.09746 | −2.576 | 50 | 0.5364 | 6 |
| BBP Harlan | 172.4 | 115.7 to 256.6 | 2.236 | 0.0841 | −1.63 | 28 | 0.7949 | 7 |
| DiBP Harlan SD | 288.2 | 247.9 to 335.2 | 2.46 | 0.03273 | −2.508 | 60 | 0.8778 | 8 |
| DBP CR SD | 337.1 | 250.2 to 454.2 | 2.528 | 0.06286 | −3.906 | 27 | 0.6911 | 9 |
| DEHP CR SD | 359.8 | 281.3 to 460.3 | 2.556 | 0.05183 | −2.517 | 26 | 0.8059 | 10 |
| DiHeP CR SD | 361.6 | 290.0 to 450.8 | 2.558 | 0.0454 | −2.396 | 19 | 0.8639 | 11 |
| DHeP Harlan SD | 401.7 | 310.3 to 520.0 | 2.604 | 0.05146 | −2.539 | 14 | 0.8383 | 12 |
| DINP Harlan SD | 738.3 | 616.7 to 883.9 | 2.868 | 0.03853 | −1.681 | 38 | 0.7691 | 13 |
| PROCHLORAZ | 386.5a | 50.32 to 2968 | 2.587 | 0.4257 | −0.565 | 23 | 0.5336 | 14 |
| DAP CR SD | BAD FIT | None | None | None | None | 20 | None | |
| BPAF CR SD | BAD FIT | None | None | None | None | 14 | None | |
| TOTM CR SD | BAD FIT | None | None | None | None | 28 | None | |
| DIDP Harlan SD | Not converged | None | None | None | None | 15 | None | |
| WYTHE 14643 Harlan SD | Not converged | None | None | None | None | 12 | None |
Note. Chemicals in the table are ranked from the lowest to highest ED50 value in mg/kg/day.
aIndicates that the ED50 value would be toxic to the dam and was above the dose range tested in the current study.
As discussed by Hannas et al. (2011a), the relative potencies of the different PEs in the FPS also are well correlated with the relative potencies for induction of the postnatal Phthalate Syndrome and other reproductive effects as well. For example, among the positive chemicals DPeP was one of the most potent PEs in reducing fetal T Prod in the FPS and also in producing Phthalate Syndrome malformations, while DINP is one of the weakest PEs examined in the FPS and it produced a low incidence of Phthalate Syndrome malformations and only at very high dosage levels. With DINP, we found that 7.7% of the F1 males were affected at 750 mg/kg/day whereas 100% of the F1 males were severely malformed following exposure to DPeP at 300 mg/kg/day. Although DPeP and DINP represent the extremes among the active PEs in regards to their potencies, others like DiBP, DBP, and BBP for example, are intermediary in their potencies between these two in the FPS and in postnatal assessments. Furthermore, we have shown (Hannas et al., 2011b) that the relative potencies obtained in the FPS can be used to accurately predict the effects of mixtures of PEs on fetal T Prod and testis gene expression.
The potency estimates from the FPS are likely to be less useful in predicting the postnatal effects of in utero exposure to chemicals like prochloraz (p <0.01 reduction in T Prod) (Blystone et al., 2007b; Noriega et al., 2005; Vinggaard et al., 2006) and linuron (Lambright et al., 2000; Wilson et al., 2009) because these chemicals disrupt fetal endocrine pathways by at least two mechanisms of toxicity; by weakly inhibiting testis T Prod, and by acting as an androgen receptor antagonist, and for chemicals like vinclozolin and procymidone that disrupt male reproductive tract differentiation as androgen receptor antagonists.
PE SAR and In Utero Effects on T Prod
In the current project, we examined the ability of ortho PEs and PE alternatives with ortho ester groups varying in from C1 (one carbon side chain; DMP) to as many as 10 carbons (DIDP). As noted by other authors, although most if not all alkyl PEs with side-chain lengths of C4 to C6 are reproductive toxicants (Fabjan et al., 2006), some C3 and C7 PEs reduce fetal T Prod and alter male rat reproductive development. It would seem that the claim “…that molecules with linear alkyl chains of 4–6 carbons profoundly affect fertility in rodents, with DEHP being the most active. Molecules with longer or shorter side chains are essentially inactive in these assays.” (report from Exxon/Mobil to USEPA, 2001) is not consistent with more recent observations. DPeP is clearly more potent than DEHP as a reproductive toxicant and PEs like DIBP (straight chain length of C3) (Saillenfait et al., 2006, 2008b) and DnHeP (C7 straight chain) (Saillenfait et al., 2011) also can reduce fetal T Prod in the FPS and induce reproductive toxicity.
Screening PEs In Vivo Versus In Vitro
Although our protocol does not eliminate animal use, it reduces the numbers of animals needed to detect PEs that induce the Phthalate Syndrome in rats by altering testis endocrine function during sexual differentiation and also allows us to characterize the relative potency of the “positive” PEs. An in vivo screening protocol is necessary for chemical classes like the phthalates since tests conducted in vitro do not accurately reflect the effects that phthalates have in the more complex and complete environment of the developing whole animal (in vitro limitations discussed by McPartland, 2011; http://blogs.edf.org/nanotechnology/2011/06/14/chemical-safety-evaluation-limitations-of-emerging-test-methods/). Some of the major limitations of the current batteries of in vitro assays that are relevant to the phthalates are the lack of metabolic activity (activation and detoxification), the absence of important biological pathways and key molecular events, the inability to integrate all of the in vitro effects across all the biological systems in a whole animal and the absence of assay validation. For example, the reported reproductive toxicity “signature” for phthalates, focused on PPAR activation (Knudsen et al., 2013; Martin et al., 2011), is incongruous with the endocrine pathways actually disrupted in the developing testis in utero by PEs (Hannas et al., 2011b, 2012). In fact, in the FPS protocol the potent PPARα agonist WY 14643 (Pirinixic Acid), had no effect on testis T Prod or expression of the mRNA for any of the genes disrupted by PEs (Hannas et al., 2011b, 2012). Additionally, the PPARγ agonist rosiglitazone also did not affect these fetal endocrine measures or induce any aspect of the Phthalate Syndrome in F1 animals (Boberg et al., 2008). The lack of effect of a potent PPARα or PPARγ agonist on testis endocrine function indicates that activation of either PPAR pathway is unlikely to be a key event in the adverse outcome pathway for the effects of PEs on fetal testis endocrine function (Boberg et al., 2008; Hannas et al., 2012). Furthermore, the structure activity relationship (SAR) for the reproductive toxicity of the PEs is very different than the SAR for activation of PPARα pathways (Bility et al., 2004). In the current study, we stated that we “expected” PEs that induce testicular effects in pubertal male rats (Creasy et al., 1983; Foster et al., 1981, 1982, 1983; Gray et al., 1988, 1999; Gray and Butterworth 1980; Lake et al., 1984; Mangham et al., 1981; Noriega et al., 2005) would be positive in the FPS and reduce T Prod. This hypothesis was based upon the observation that the PEs that induce testicular lesions in the pubertal male testis also induce reproductive tract malformations in utero whereas PEs like DEP, DPP and DMP do not alter testis function at either life stage. Since the phenotypic effects of PEs on testis function during these two stages of development are so different, one would not necessarily expect this to be the case. The fact that the SAR for testicular toxicity appears to be similar during these two stages of development may indicate that the same molecular initiating event (MIE) is disrupted by PEs in utero and during puberty but the downstream events regulated by this MIE differ considerably; plausible outcomes given the major differences between the signaling pathways in the fetal and pubertal rat testis (Scott et al., 2008).
In Utero Dipentyl Phthalate Exposure Reduces Fetal Testosterone in the CD-1 Mouse
In the current study, we also conducted a dose-response study of DPeP in the pregnant CD-1 mouse and found that this relatively potent PE did significantly reduced T Prod (Fig. 5). This mouse strain and PE were selected because DPeP has been shown to produce testicular effects in this strain in a high dose Reproductive Assessment by Continuous Breeding (RACB) study with effects sufficient to render the F1 males and females infertile (Heindel et al., 1989). In addition, DEHP induces testicular lesions in this mouse strain, but not in the ICR mouse strain (Oishi 1993). This study was conducted to begin to clarify some of the discrepancies on effects of PEs in utero on fetal mouse T Prod.
Although some authors have stated emphatically that, unlike the rat, T levels in the mouse do not respond to PEs at this stage of development (Gaido et al., 2007; Johnson et al., 2012), other authors have reported reductions in fetal and neonatal (Moody et al., 2013) testosterone and related gene expression levels and increases in malformations of androgen- and insl3-dependent tissues in the male mouse reproductive tract. For example, Johnson et al. (2012) stated in their review article “Of mice and men (and rats): phthalate-induced fetal testis endocrine disruption is species-dependent” that inhibition of fetal Leydig cell hormone synthesis is not observed in the mouse following in utero PE administration. These authors conclude that T Prod in mice is not affected by PE exposures in utero and, for this reason; mice are a better animal model for humans than is the fetal rat. However, our results demonstrate that T prod in fetal mice is significantly reduced at 100 mg DPeP/kg/day and above. In the current study with CD-1 mice, we found that DPeP significantly reduced T Prod by 25–30% at a dose level that did not induce any maternal or fetal toxicity. The ED50 for this effect was about fourfold higher than in the rat and the effect reached a plateau at about 50% of control, whereas the T Prod in the rat can be reduced to ∼10–15% of control before reaching a plateau. Although the biological basis for the species difference in the level of T Prod at the plateau is not known, this could result if the MIE disrupted by the PEs only inhibited one of multiple potential pathways regulating T Prod and the affected pathway was more important in the rat than in the mouse.
In contrast to being less sensitive to the effects of DPeP on T Prod, pregnancy maintenance and fetal viability in the mouse were affected at lower dosage levels than in the rat (Fig. 5). In addition to the current investigation, several other studies have reported that PEs reduce fetal mouse T Prod, testis genes related to T Prod and insl3 synthesis, and increase the incidence of malformations in androgen- and insl3-dependent tissues in postnatal life. For example, Song et al. (2006,2008) and Wang et al. (2004) reported that in utero DEHP reduced fetal testis insl3 levels, induced abnormal development of the gubernaculum, induced cryptorchidism, and caused testis histopathology, dysplasia and dysfunction of Sertoli cells, Leydig cells, and spermatogenic cells in fetal KM mice. Wu et al. (2010) reported that in utero DEHP reduced fetal and postnatal testosterone and fetal insl3 levels and Liu et al. (2009) found that DEHP induced hypospadias and altered TGFβ1 levels in the genital tubercle. One study even reported a nonmonotonic effect on fetal mouse T Prod, which increased and then decreased with increasing maternal dosages of DEHP (Do et al., 2012)). However, the latter study (Do et al., 2012) reported nonmonotonic effects at several dose levels that are well below those that have been reported in rodent diets and beddings (Kondo et al., 2010).
It is possible that the discrepancies in the literature on the effects of PEs in the mouse can be attributed to strain differences, as it is known that the pubertal effects of PEs on the mouse testis vary greatly from strain to strain (Oishi 1993).
CONCLUSIONS
In summary, in the current project we developed and validated a short-term in vivo protocol, termed the FPS, to screen phthalates, phthalate alternatives and other chemicals for their ability to disrupt testis endocrine function in utero; an effect causally related to the development of male rat reproductive tract lesions and reproductive problems after birth in adulthood. The FPS protocol also can be used to determine the relative potency of the PEs that reduce fetal T Prod and a comparison of these results with those seen in multigenerational or one-generation studies reveals that the FPS accurately predicts PEs that do, or do not, induce the Phthalate Syndrome in F1 male rats after in utero exposure.
Although the current screening protocol for PE-induced reproductive toxicity does not eliminate animal use, as would an in vitro study, this protocol significantly reduces the numbers of animals, as well as the amount of other resources (labor, time, etc.) required to predict whether PEs will or will not induce the Phthalate Syndrome and other reproductive effects. Until all the key events in this adverse outcome pathway can be identified and quantified, validated in vitro assays developed and the results integrated, it will remain necessary to conduct animal studies for human health assessments with this class of chemicals.
FUNDING
Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (in part); National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH); NTP/NIEHS IA (RW7592285501-1).
Supplementary Material
Acknowledgments
We would like to thank the following scientists for their assistance with the execution of this study: Nicola Evans, Bethany Hannas, Mary Cardon, Phillip Hartig, Hunter Sampson, and Brandy Beverly.
Disclaimer: The research described in this article has been reviewed by the U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The statements, opinions, or conclusions contained therein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, or the United States government.
Footnotes
These authors contributed equally to this study.
REFERENCES
- Adibi J. J., Perera F. P., Jedrychowski W., Camann D. E., Barr D., Jacek R., Whyatt R. M. Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environ. Health Perspect. 2003;111:1719–1722. doi: 10.1289/ehp.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi J. J., Whyatt R. M., Williams P. L., Calafat A. M., Camann D., Herrick R., Nelson H., Bhat H. K., Perera F. P., Silva M. J., Hauser R. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect. 2008;116:467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez D. S., Gray L. E., Jr, Wilson V. S. Modeling the interaction of binary and ternary mixtures of estradiol with bisphenol A and bisphenol AF in an in vitro estrogen-mediated transcriptional activation assay (T47D-KBluc) Toxicol. Sci. 2010;116:477–487. doi: 10.1093/toxsci/kfq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bility M. T., Thompson J. T., McKee R. H., David R. M., Butala J. H., Vanden Heuvel J. P., Peters J. M. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol. Sci. 2004;82:170–182. doi: 10.1093/toxsci/kfh253. [DOI] [PubMed] [Google Scholar]
- Blount B. C., Silva M. J., Caudill S. P., Needham L. L., Pirkle J. L., Sampson E. J., Lucier G. W., Jackson R. J., Brock J. W. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. 2000;108:979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blystone C. R., Furr J., Lambright C. S., Howdeshell K. L., Ryan B. C., Wilson V. S., Leblanc G. A., Gray L. E., Jr Prochloraz inhibits testosterone production at dosages below those that affect androgen-dependent organ weights or the onset of puberty in the male Sprague Dawley rat. Toxicol. Sci. 2007;97:65–74. doi: 10.1093/toxsci/kfm004. [DOI] [PubMed] [Google Scholar]
- Blystone C. R., Kissling G. E., Bishop J. B., Chapin R. E., Wolfe G. W., Foster P. M. Determination of the di-(2-ethylhexyl) phthalate NOAEL for reproductive development in the rat: Importance of the retention of extra animals to adulthood. Toxicol. Sci. 2010;116:640–646. doi: 10.1093/toxsci/kfq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg J., Metzdorff S., Wortziger R., Axelstad M., Brokken L., Vinggaard A. M., Dalgaard M., Nellemann C. Impact of diisobutyl phthalate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats. Toxicology. 2008;250:75–81. doi: 10.1016/j.tox.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Borch J., Ladefoged O., Hass U., Vinggaard A. M. Steroidogenesis in fetal male rats is reduced by DEHP and DINP, but endocrine effects of DEHP are not modulated by DEHA in fetal, prepubertal and adult male rats. Reprod. Toxicol. 2004;18:53–61. doi: 10.1016/j.reprotox.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Carruthers C. M., Foster P. M. Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate. Birth Defects Res. 2005;74:277–285. doi: 10.1002/bdrb.20050. [DOI] [PubMed] [Google Scholar]
- Creasy D. M., Foster J. R., Foster P. M. The morphological development of di-N-pentyl phthalate induced testicular atrophy in the rat. J. Pathol. 1983;139:309–321. doi: 10.1002/path.1711390307. [DOI] [PubMed] [Google Scholar]
- Do R. P., Stahlhut R. W., Ponzi D., Vom Saal F. S., Taylor J. A. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod. Toxicol. 2012;34:614–621. doi: 10.1016/j.reprotox.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exxon Mobil Biomedical Sciences, Inc. 553 pages. High production volume (HPV) chemical challenge program, Test Plan, For the Phthalate Esters Category. 2001 submitted to EPA 2001. EPA document AR 201-13646A. [Google Scholar]
- Fabjan E., Hulzebos E., Mennes W., Piersma A. H. Acategory approach for reproductive effects of phthalates. Crit Rev Toxicol. 2006;36:695–726. doi: 10.1080/10408440600894914. [DOI] [PubMed] [Google Scholar]
- Foster P. M. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181–185. [DOI] [PubMed] [Google Scholar]
- Foster P. M., Cook M. W., Thomas L. V., Walters D. G., Gangolli S. D. Differences in urinary metabolic profile from di-n-butyl phthalate-treated rats and hamsters. A possible explanation for species differences in susceptibility to testicular atrophy. Drug Metab. Dispos. 1983;11:59–61. [PubMed] [Google Scholar]
- Foster P. M., Foster J. R., Cook M. W., Thomas L. V., Gangolli S. D. Changes in ultrastructure and cytochemical localization of zinc in rat testis following the administration of di-n-pentyl phthalate. Toxicol. Appl. Pharmacol. 1982;63:120–132. doi: 10.1016/0041-008x(82)90031-x. [DOI] [PubMed] [Google Scholar]
- Foster P. M., Lake B. G., Cook M. W., Thomas L. V., Gangolli S. D. Structure-activity requirements for the induction of testicular atrophy by butyl phthalates in immature rats: Effect on testicular zinc content. Adv. Exp. Med. Biol. 1981;136:445–452. doi: 10.1007/978-1-4757-0674-1_33. [DOI] [PubMed] [Google Scholar]
- Gaido K. W., Hensley J. B., Liu D., Wallace D. G., Borghoff S., Johnson K. J., Hall S. J., Boekelheide K. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol. Sci. 2007;97:491–503. doi: 10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- Gray T. J., Butterworth K. R. Testicular atrophy produced by phthalate esters. Arch. Toxicol. Suppl. 1980;4:452–455. doi: 10.1007/978-3-642-67729-8_106. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Ostby J., Furr J., Wolf C. J., Lambright C., Parks L., Veeramachaneni D. N., Wilson V., Price M., Hotchkiss A., et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum. Reprod. Update. 1999;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J., Furr J., Price M., Veeramachaneni D. N., Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J., Sigmon R., Ferrell J., Rehnberg G., Linder R., Cooper R., Goldman J., Laskey J. The development of a protocol to assess reproductive effects of toxicants in the rat. Reprod. Toxicol. 1988;2:281–287. doi: 10.1016/0890-6238(88)90032-9. [DOI] [PubMed] [Google Scholar]
- Habert R., Picon R. Relationships between plasma progesterone levels and testicular testosterone production in the rat fetus: Effects of maternal ovariectomy. J. Endocrinol. 1986;108:361–367. doi: 10.1677/joe.0.1080361. [DOI] [PubMed] [Google Scholar]
- Hannas B. R., Furr J., Lambright C. S., Wilson V. S., Foster P. M., Gray L. E., Jr Dipentyl phthalate dosing during sexual differentiation disrupts fetal testis function and postnatal development of the male Sprague-Dawley rat with greater relative potency than other phthalates. Toxicol. Sci. 2011a;120:184–193. doi: 10.1093/toxsci/kfq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B. R., Lambright C. S., Furr J., Evans N., Foster P. M., Gray E. L., Wilson V. S. Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: A targeted RT-PCR array approach for defining relative potency. Toxicol. Sci. 2012;125:544–557. doi: 10.1093/toxsci/kfr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B. R., Lambright C. S., Furr J., Howdeshell K. L., Wilson V. S., Gray L. E., Jr Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol. Sci. 2011b;123:206–216. doi: 10.1093/toxsci/kfr146. [DOI] [PubMed] [Google Scholar]
- Heindel J. J., Gulati D. K., Mounce R. C., Russell S. R., Lamb J. C. t. Reproductive toxicity of three phthalic acid esters in a continuous breeding protocol. Fundam. Appl. Toxicol. 1989;12:508–518. doi: 10.1016/0272-0590(89)90024-9. [DOI] [PubMed] [Google Scholar]
- Hosokawa S., Murakami M., Ineyama M., Yamada T., Yoshitake A., Yamada H., Miyamoto J. The affinity of procymidone to androgen receptor in rats and mice. J. Toxicol. Sci. 1993;18:83–93. doi: 10.2131/jts.18.83. [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L., Rider C. V., Wilson V. S., Gray L. E., Jr Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ. Res. 2008a;108:168–176. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L., Wilson V. S., Furr J., Lambright C. R., Rider C. V., Blystone C. R., Hotchkiss A. K., Gray L. E., Jr A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague-Dawley rat in a cumulative, dose-additive manner. Toxicol. Sci. 2008b;105:153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Heger N. E., Boekelheide K. Of mice and men (and rats): Phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicol. Sci. 2012;129:235–248. doi: 10.1093/toxsci/kfs206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelce W. R., Monosson E., Gamcsik M. P., Laws S. C., Gray L. E. Environmental hormone disruptors: Evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol. Appl. Pharmacol. 1994;126:276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- Knudsen T., Martin M., Chandler K., Kleinstreuer N., Judson R., Sipes N. Predictive models and computational toxicology. Methods Mol. Biol. 2013;947:343–374. doi: 10.1007/978-1-62703-131-8_26. [DOI] [PubMed] [Google Scholar]
- Kondo F., Ikai Y., Hayashi R., Okumura M., Takatori S., Nakazawa H., Izumi S., Makino T. Determination of five phthalate monoesters in human urine using gas chromatography-mass spectrometry. Bull. Environ. Contam. Toxicol. 2010;85:92–96. doi: 10.1007/s00128-010-0051-8. [DOI] [PubMed] [Google Scholar]
- Lake B. G., Gray T. J., Foster J. R., Stubberfield C. R., Gangolli S. D. Comparative studies on di-(2-ethylhexyl) phthalate-induced hepatic peroxisome proliferation in the rat and hamster. Toxicol. Appl. Pharmacol. 1984;72:46–60. doi: 10.1016/0041-008x(84)90248-5. [DOI] [PubMed] [Google Scholar]
- Lambright C., Ostby J., Bobseine K., Wilson V., Hotchkiss A. K., Mann P. C., Gray L. E., Jr Cellular and molecular mechanisms of action of linuron: An antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicol. Sci. 2000;56:389–399. doi: 10.1093/toxsci/56.2.389. [DOI] [PubMed] [Google Scholar]
- Lehmann K. P., Phillips S., Sar M., Foster P. M., Gaido K. W. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol. Sci. 2004;81:60–68. doi: 10.1093/toxsci/kfh169. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang D. Y., Li Y. S., Xiong J., He D. W., Lin T., Li X. L., Wei G. H. Di-(2-ethylhexyl) phthalate upregulates ATF3 expression and suppresses apoptosis in mouse genital tubercle. J. Occup. Health. 2009;51:57–63. doi: 10.1539/joh.l8091. [DOI] [PubMed] [Google Scholar]
- Mangham B. A., Foster J. R., Lake B. G. Comparison of the hepatic and testicular effects of orally administered di(2-ethylhexyl) phthalate and dialkyl 79 phthalate in the rat. Toxicol. Appl. Pharmacol. 1981;61:205–214. doi: 10.1016/0041-008x(81)90410-5. [DOI] [PubMed] [Google Scholar]
- Martin M. T., Knudsen T. B., Reif D. M., Houck K. A., Judson R. S., Kavlock R. J., Dix D. J. Predictive model of rat reproductive toxicity from ToxCast high throughput screening. Biol. Reprod. 2011;85:327–339. doi: 10.1095/biolreprod.111.090977. [DOI] [PubMed] [Google Scholar]
- McIntyre B. S., Barlow N. J., Foster P. M. Androgen-mediated development in male rat offspring exposed to flutamide in utero: Permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicol. Sci. 2001;62:236–249. doi: 10.1093/toxsci/62.2.236. [DOI] [PubMed] [Google Scholar]
- McKee R. H., Pavkov K. L., Trimmer G. W., Keller L. H., Stump D. G. An assessment of the potential developmental and reproductive toxicity of di-isoheptyl phthalate in rodents. Reprod. Toxicol. 2006;21:241–252. doi: 10.1016/j.reprotox.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Miyata K., Yabushita S., Sukata T., Sano M., Yoshino H., Nakanishi T., Okuno Y., Matsuo M. Effects of perinatal exposure to flutamide on sex hormones and androgen-dependent organs in F1 male rats. J. Toxicol. Sci. 2002;27:19–33. doi: 10.2131/jts.27.19. [DOI] [PubMed] [Google Scholar]
- Moody S., Goh H., Bielanowicz A., Rippon P., Loveland K. L., Itman C. Prepubertal mouse testis growth and maturation and androgen production are acutely sensitive to di-n-butyl phthalate. Endocrinology. 2013;154:3460–3475. doi: 10.1210/en.2012-2227. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Cattley R. C., Foster P. M. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: An antiandrogenic mechanism. Toxicol. Sci. 1998a;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Cattley R. C., Foster P. M. D. Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: An antiandrogenic mechanism. Toxicol. Sci. 1998b;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Foster P. M. DBP exerts its antiandrogenic activity by indirectly interfering with androgen signaling pathways. Toxicol. Appl. Pharmacol. 2000;168:174–175. doi: 10.1006/taap.2000.9031. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Sar M., Cattley R. C., Foster P. M. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol. Appl. Pharmacol. 1999;156:81–95. doi: 10.1006/taap.1999.8643. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Sar M., Wallace D. G., Foster P. M. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod. Toxicol. 2002;16:19–28. doi: 10.1016/s0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Nagao T., Ohta R., Marumo H., Shindo T., Yoshimura S., Ono H. Effect of butyl benzyl phthalate in Sprague-Dawley rats after gavage administration: A two-generation reproductive study. Reprod. Toxicol. 2000;14:513–532. doi: 10.1016/s0890-6238(00)00105-2. [DOI] [PubMed] [Google Scholar]
- Noriega N., Ostby J., Lambright C., Wilson V., Gray L. Prenatal exposure to the fungicide prochloraz alters the onset of parturition in the dam and sexual differentiation in male rat offspring. Toxicologist. 2004;73 [Google Scholar]
- Noriega N. C., Ostby J., Lambright C., Wilson V. S., Gray L. E. Late gestational exposure to the fungicide prochloraz delays the onset of parturition and causes reproductive malformations in male but not female rat offspring. Biol. Reprod. 2005;72:1324–1335. doi: 10.1095/biolreprod.104.031385. [DOI] [PubMed] [Google Scholar]
- Oishi S. Strain differences in susceptibility to di-2-ethylhexyl phthalate-induced testicular atrophy in mice. Toxicol. Lett. 1993;66:47–52. doi: 10.1016/0378-4274(93)90078-c. [DOI] [PubMed] [Google Scholar]
- Ostby J., Kelce W. R., Lambright C., Wolf C. J., Mann P., Gray L. E., Jr The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicol. Ind. Health. 1999;15:80–93. doi: 10.1177/074823379901500108. [DOI] [PubMed] [Google Scholar]
- Parks L. G., Ostby J. S., Lambright C. R., Abbott B. D., Klinefelter G. R., Barlow N. J., Gray L. E. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Saillenfait A. M., Gallissot F., Sabate J. P. Evaluation of the developmental toxicity of diallyl phthalate administered orally to rats. Food Chem. Toxicol. 2008a;46:2150–2156. doi: 10.1016/j.fct.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Saillenfait A. M., Gallissot F., Sabate J. P. Differential developmental toxicities of di-n-hexyl phthalate and dicyclohexyl phthalate administered orally to rats. J. Appl. Toxicol. 2009a;29:510–521. doi: 10.1002/jat.1436. [DOI] [PubMed] [Google Scholar]
- Saillenfait A. M., Roudot A. C., Gallissot F., Sabate J. P. Prenatal developmental toxicity studies on di-n-heptyl and di-n-octyl phthalates in Sprague-Dawley rats. Reprod. Toxicol. 2011;32:268–276. doi: 10.1016/j.reprotox.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Saillenfait A. M., Sabate J. P., Gallissot F. Developmental toxic effects of diisobutyl phthalate, the methyl-branched analogue of di-n-butyl phthalate, administered by gavage to rats. Toxicol. Lett. 2006;165:39–46. doi: 10.1016/j.toxlet.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Saillenfait A. M., Sabate J. P., Gallissot F. Diisobutyl phthalate impairs the androgen-dependent reproductive development of the male rat. Reprod. Toxicol. 2008b;26:107–115. doi: 10.1016/j.reprotox.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Saillenfait A. M., Sabate J. P., Gallissot F. Effects of in utero exposure to di-n-hexyl phthalate on the reproductive development of the male rat. Reprod. Toxicol. 2009b;28:468–476. doi: 10.1016/j.reprotox.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Scott H. M., Hutchison G. R., Jobling M. S., McKinnell C., Drake A. J., Sharpe R. M. Relationship between androgen action in the “male programming window,” fetal sertoli cell number, and adult testis size in the rat. Endocrinology. 2008;149:5280–5287. doi: 10.1210/en.2008-0413. [DOI] [PubMed] [Google Scholar]
- Silva M. J., Barr D. B., Reidy J. A., Malek N. A., Hodge C. C., Caudill S. P., Brock J. W., Needham L. L., Calafat A. M. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. J., Furr J., Samandar E., Preau J. L., Jr, Gray L. E., Needham L. L., Calafat A. M. Urinary and serum metabolites of di-n-pentyl phthalate in rats. Chemosphere. 2011;82:431–436. doi: 10.1016/j.chemosphere.2010.09.052. [DOI] [PubMed] [Google Scholar]
- Skakkebaek N. E. Endocrine disrupters and testicular dysgenesis syndrome. Horm. Res. 2002;57:43. doi: 10.1159/000058100. [DOI] [PubMed] [Google Scholar]
- Song X. F., Wei G. H., Deng Y. J., Chen X., Liu X., Zhang D. Y. [Di(2-ethylhexyl) phthalate affects the testes and leydig cells of neonatal KM mice] Zhonghua Nan Ke Xue. 2006;12:775–779. [PubMed] [Google Scholar]
- Song X. F., Wei G. H., Liu X., Zhang D. Y., Chen X., Deng Y. J. Effects of diethylhexyl phthalate (DEHP) on INSL3 mRNA expression by Leydig cells derived from mouse embryos and in newborn mice. J. Int. Med. Res. 2008;36:512–521. doi: 10.1177/147323000803600316. [DOI] [PubMed] [Google Scholar]
- Struve M. F., Gaido K. W., Hensley J. B., Lehmann K. P., Ross S. M., Sochaski M. A., Willson G. A., Dorman D. C. Reproductive toxicity and pharmacokinetics of di-n-butyl phthalate (DBP) following dietary exposure of pregnant rats. Birth Defects Res. 2009;86:345–354. doi: 10.1002/bdrb.20199. [DOI] [PubMed] [Google Scholar]
- The EFSA Journal. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 12th list of substances for food contact materials. 2006;395–401:1–21. [Google Scholar]
- Tyl R. W., Myers C. B., Marr M. C., Fail P. A., Seely J. C., Brine D. R., Barter R. A., Butala J. H. Reproductive toxicity evaluation of dietary butyl benzyl phthalate (BBP) in rats. Reprod. Toxicol. 2004;18:241–264. doi: 10.1016/j.reprotox.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Vinggaard A. M., Hass U., Dalgaard M., Andersen H. R., Bonefeld-Jorgensen E., Christiansen S., Laier P., Poulsen M. E. Prochloraz: an imidazole fungicide with multiple mechanisms of action. Int J Androl. 2006;29:186–192. doi: 10.1111/j.1365-2605.2005.00604.x. [DOI] [PubMed] [Google Scholar]
- Wang W., Wei G., Deng Y., Zhang X. Histopathological changes of the cryptorchid testis and epididymis of mice exposed to DEHP. Zhonghua Nan Ke Xue. 2004;10:807–810. 814. [PubMed] [Google Scholar]
- Warren D. W., Haltmeyer G. C., Eik-Nes K. B. Synthesis and metabolism of testosterone in the fetal rat testis. Biol. Reprod. 1972;7:94–99. doi: 10.1093/biolreprod/7.1.94. [DOI] [PubMed] [Google Scholar]
- Wilson V. S., Lambright C. R., Furr J. R., Howdeshell K. L., Earl Gray L., Jr The herbicide linuron reduces testosterone production from the fetal rat testis during both in utero and in vitro exposures. Toxicol. Lett. 2009;186:73–77. doi: 10.1016/j.toxlet.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Wilson V. S., Lambright C., Furr J., Ostby J., Wood C., Held G., Gray L. E. Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicol. Lett. 2004;146:207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Wolf C. J., LeBlanc G. A., Ostby J. S., Gray L. E., Jr Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicol. Sci. 2000;55:152–161. doi: 10.1093/toxsci/55.1.152. [DOI] [PubMed] [Google Scholar]
- Wong C., Kelce W. R., Sar M., Wilson E. M. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J. Biol. Chem. 1995;270:19998–20003. doi: 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]
- Wu S., Zhu J., Li Y., Lin T., Gan L., Yuan X., Xu M., Wei G. Dynamic effect of di-2-(ethylhexyl) phthalate on testicular toxicity: Epigenetic changes and their impact on gene expression. Int. J. Toxicol. 2010;29:193–200. doi: 10.1177/1091581809355488. [DOI] [PubMed] [Google Scholar]
- Yamasaki K., Noda S., Muroi T., Mitoma H., Takakura S., Sakamoto S. Effects of in utero and lactational exposure to flutamide in SD rats: Comparison of the effects of administration periods. Toxicology. 2005;209:47–54. doi: 10.1016/j.tox.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Zota A. R., Calafat A. M., Woodruff T. J. Temporal trends in phthalate exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect. 2014 doi: 10.1289/ehp.1306681. http://dx.doi.org/10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.