Abstract
BACKGROUND AND OBJECTIVE
New Jersey was the first state to implement legislatively mandated newborn pulse oximetry screening (POxS) in all licensed birthing facilities to detect critical congenital heart defects (CCHDs). The objective of this report was to evaluate implementation of New Jersey’s statewide POxS mandate.
METHODS
A 2-pronged approach was used to collect data on infants screened in all New Jersey birthing facilities from August 31, 2011, through May 31, 2012. Aggregate screening results were submitted by each birthing facility. Data on failed screens and clinical characteristics of those newborns were reported to the New Jersey Birth Defects Registry (NJBDR). Three indicators were used to distinguish the added value of mandated POxS from standard clinical care: prenatal congenital heart defect diagnosis, cardiology consultation or echocardiogram indicated or performed before PoxS, or clinical findings at the time of POxS warranting a pulse oximetry measurement.
RESULTS
Of 75 324 live births in licensed New Jersey birthing facilities, 73 320 were eligible for screening, of which 99% were screened. Forty-nine infants with failed POxS were reported to the NJBDR, 30 of whom had diagnostic evaluations solely attributable to the mandated screening. Three of the 30 infants had previously unsuspected CCHDs and 17 had other diagnoses or non-CCHD echocardiogram findings.
CONCLUSIONS
In the first 9 months after implementation, New Jersey achieved a high statewide screening rate and established surveillance mechanisms to evaluate the unique contribution of POxS. The screening mandate identified 3 infants with previously unsuspected CCHDs that otherwise might have resulted in significant morbidity and mortality and also identified other significant secondary targets such as sepsis and pneumonia.
Keywords: oximetry, congenital heart disease/defects, newborn screening, surveillance and monitoring
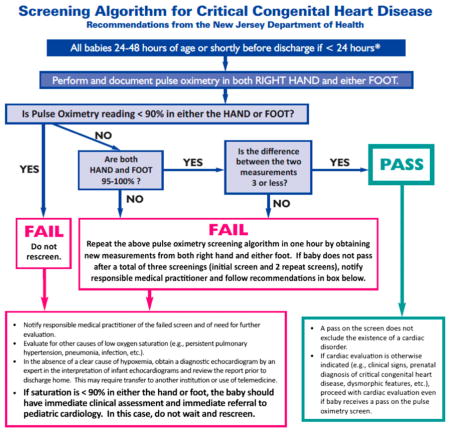
Approximately 2 per 1000 live births in the United States are infants born with critical congenital heart defects (CCHDs), of which an estimated 20% are neither diagnosed prenatally nor identified by clinical examination before hospital discharge, with wide variation by specific type of CCHD.1–3 New Jersey was the first state to implement legislatively mandated newborn pulse oximetry screening (POxS) to improve early detection of CCHDs. Legislation signed into law on June 2, 2011, and implemented 90 days later on August 31, requires all licensed birthing facilities to “perform a pulse oximetry screening a minimum of 24 hours after birth, on every newborn in its care.”4 This requirement applies to all live births regardless of health status, prenatal diagnosis of CCHDs, or location (eg, NICU/special care nursery [SCN], well-baby nursery [WBN]). To facilitate implementation of the screening requirement, the New Jersey Department of Health (NJDOH) convened a working group that developed a recommended screening algorithm that specified screening in both the right hand and either foot between 24 and 48 hours of life or when medically appropriate after 24 hours of age (Appendix 1). In all cases it is recommended for screening to be done before discharge. The NJDOH also developed an approach using surveillance mechanisms to evaluate the POxS program.
In September 2011, the US Secretary of Health and Human Services recommended that all states incorporate screening for CCHDs into their newborn screening panel.5 At least 35 states have passed or introduced legislation to mandate this screening.6 We present the first 9 months of screening experience in New Jersey, including screening rates and case detection, to highlight the benefits, challenges, and issues for consideration that might help guide implementation in other states.
METHODS
Defining CCHD
We defined CCHD to include those defects that usually cause hypoxemia (ie, hypoplastic left heart syndrome, pulmonary atresia, Tetralogy of Fallot, total anomalous pulmonary venous return, transposition of great arteries, tricuspid atresia, truncus arteriosus) and those significant defects that sometimes, but less consistently, cause hypoxemia (ie, coarctation of aorta, double outlet right ventricle, single ventricle, interrupted aortic arch, Ebstein anomaly).1,7
Data Sources
Screening Data Collection
All birthing facilities (52 facilities in operation before October 2011 and 53 after that date) submit aggregate data on a quarterly basis to the NJDOH, which includes the number of live births, the number screened, and the number of failed screening results. For purposes of this evaluation, the term “live births” refers to all infants born alive at the 53 licensed birthing facilities in New Jersey. A standard form is used to collect these data and to facilitate consistent reporting, including information about newborns who were not screened (eg, transfers, deaths, missed for unknown reasons) (Appendix 2). This report reflects the first 9 months after POxS implementation (August 31, 2011, to May 31, 2012).
New Jersey Birth Defects Registry
Health care professionals are required by law to report infants with CCHDs who are New Jersey residents to the New Jersey Birth Defects Registry (NJBDR) as soon as possible after diagnosis.8 As a result of the mandate, the NJBDR was expanded to monitor failed POxS. With the use of a new diagnostic code for failed POxS, all birthing facilities were requested to report individual-level data on infants’ failed POxS results, regardless of presence of CCHDs, to the NJBDR. This report includes the time of birth, screening results, findings from the diagnostic evaluation (eg, echocardiogram), any relevant prenatal diagnoses, and other clinical characteristics such as whether the infant was asymptomatic at the time of screening. For failed screens, the NJBDR staff investigated cases in which the information was unclear or if a CCHD was identified as a result of mandated POxS. The NJBDR staff also investigated registered cases of CCHDs that had no accompanying report of failed POxS to the NJBDR.
Assessment of the Impact of the Screening Mandate
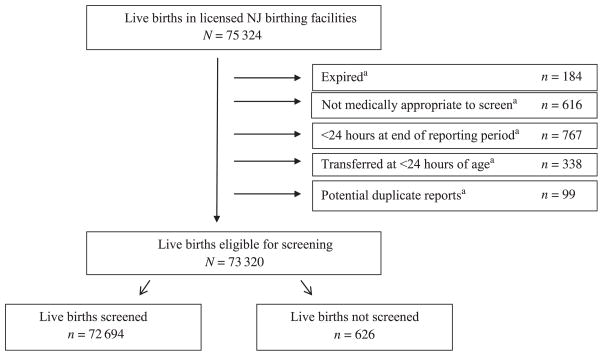
Aggregate reports were submitted every 3 months and were used to determine overall screening coverage. To determine the proportion of eligible live births who were screened, we excluded those live births who were not screened because they died before screening, were not medically appropriate to have been screened (eg, receiving oxygen in the NICU), were <24 hours old at the end of the reporting period, or were transferred to another hospital before 24 hours of age. To avoid possible double counting, we also excluded (1) live births who were not screened and transferred out of a hospital after 24 hours of age from the total live-birth count (Fig 1, Appendix 2) and (2) live births who were screened and transferred into a New Jersey birthing facility at anytime during the reporting period from the number of reported screens (Appendix 2).
FIGURE 1.
Aggregate pulse oximetry screening results from all licensed New Jersey birthing facilities for live births from August 31, 2011, to May 31, 2012. aReported reason for having not been screened. NJ, New Jersey.
The NJBDR was used to determine outcomes for those with a failed screen and to monitor CCHD cases. Three clinical indicators were used to determine whether the diagnostic evaluation was solely attributable to POxS or whether it would otherwise have occurred in the absence of the POxS mandate. These indicators included a prenatal diagnosis of congenital heart defects (CHDs), whether a cardiology consultation or echocardiogram was indicated or performed before screening on the basis of clinical factors (eg, maternal history, signs of CHD), or whether there were clinical findings at the time of the screen that would have otherwise warranted a pulse oximetry measurement.
Data Analysis
χ2 Tests of significance were used to examine differences in the proportions of missed screens over the three, 3-month aggregate data reporting periods.
This project was determined by the NJDOH Institutional Review Board to be public health practice.
RESULTS
Over the 9-month study period, 75 324 live births were reported from the licensed birthing facilities in New Jersey, of whom 97.3% (n = 73 320) were eligible for screening (Fig 1). Of those eligible to be screened, 99.1% underwent POxS. The proportion of missed screens decreased substantially over the study period from 1.8% in the first period to 0.2% in the third period (P < .001). Ninety-seven infants were reported to the NJBDR with a failed POxS, CCHD, or both.
Infants With Failed POxS Reported to the NJBDR
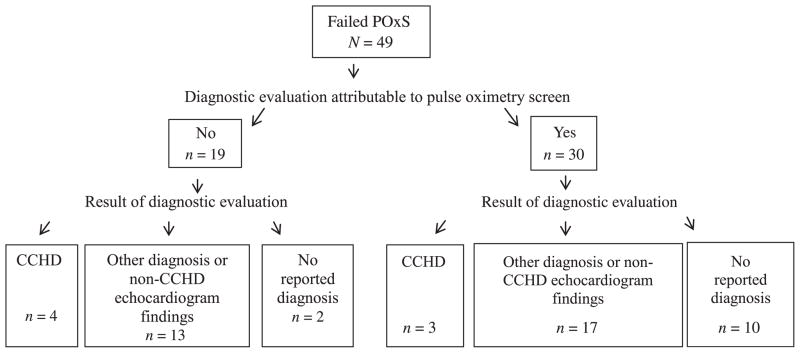
Forty-nine infants were reported to the NJBDR with a failed POxS (Fig 2). Thirty of the 49 infants (61%) with a failed POxS had none of the 3 clinical indicators and therefore their diagnostic evaluation was solely attributable to their failed screen. Of these 30 infants, 3 had CCHDs that otherwise might have resulted in serious morbidity or mortality if diagnosis had been delayed in the absence of screening. All 3 infants were in the WBN at the time of the screen, none had a prenatal diagnosis of CHD or a cardiac consult or echocardiogram indicated before the POxS, and none had clinical signs present at the time of the POxS that would have otherwise warranted a pulse oximetry measurement (Table 1). Seventeen of the 30 infants who received a diagnostic evaluation as a result of a failed POxS had other diagnoses, including culture-negative sepsis, pneumonia, pulmonary hypertension, other types of CHDs (eg, ventricular septal defect) or other echocardiogram findings (eg, patent formen ovale or patent ductus arteriosis). Ten of the 30 infants had no cardiac abnormalities found on echo-cardiogram, and no other diagnoses were made on the basis of the available follow-up information (Fig 2, Table 1).
FIGURE 2.
Failed pulse oximetry screens reported to the New Jersey Birth Defects Registry for New Jersey–resident live births screened from August 31, 2011, to May 31, 2012.
TABLE 1.
POxS Results, Echocardiogram Findings, and Other Relevant Diagnoses for Infants Whose Diagnostic Clinical Evaluation Was Attributable to Failed POxS
| Case Number | Location | Last Screen Result, %
|
Main Echocardiogram Findings | Other Relevant Diagnoses | |
|---|---|---|---|---|---|
| Preductal | Postductal | ||||
| 1a | WBN | 43 | 39 | d-TGAb | None |
| 2a | WBN | 91 | 92 | TAb | None |
| 3a | WBN | 97 | 84 | Aortic coarctation, aortic arch hypoplasia, BAVb | None |
| 4c | WBN | 88 | 90 | Echocardiogram not done | Clinical sepsis (culture negative) |
| 5c | WBN | 50 | 70 | Echocardiogram not done | Pneumonia |
| 6c | WBN | 91 | 91 | VSD (left to right shunting), PFO | None |
| 7c | WBN | 93 | 100 | PFO (left to right shunting) versus ASD | None |
| 8c | WBN | 87 | 80 | PFO (direction of flow not stated) | None |
| 9c | WBN | 94 | 100 | PPS, PFO (bidirectional shunting), mild pulmonary hypertension | None |
| 10c | WBN | 96 | 93 | Small PFO with bidirectional flow | None |
| 11c | WBN | 91 | 91 | PDA, PFO (direction of flow not stated) | TTN |
| 12c | WBN | 90 | 90 | multiple VSDs, ASD, and PPSb | None |
| 13c | WBN | 94 | 96 | Small PFO (right to left shunting) | None |
| 14c | WBN | 87 | 98 | Small PFO (left to right shunting)b | None |
| 15c | WBN | 95 | 100 | Echocardiogram not done | Poor suck-swallow coordination |
| 16 | WBN | 93 | 96 | No reported cardiac abnormalities | None |
| 17 | WBN | 94 | 99 | No reported cardiac abnormalities | None |
| 18 | WBN | 95 | 99 | No reported cardiac abnormalities | None |
| 19 | WBN | 94 | 98 | No reported cardiac abnormalities | None |
| 20 | WBN | 88 | 92 | No reported cardiac abnormalities | None |
| 21 | WBN | 96 | 100 | Echocardiogram not done | None |
| 22 | WBN | 100 | 96 | Echocardiogram not done | None |
| 23c | NICU/SCN | 97 | 91 | PFO, PDA (left to right shunting), mild pulmonary hypertensionb | History of meconium aspirationd |
| 24c | NICU/SCN | 92 | 96 | VSD (left to right shunting), PFO (left to right shunting), | None |
| 25c | NICU/SCN | 88 | 97 | No reported cardiac abnormalities | History of meconium aspirationd |
| 26c | NICU/SCN | 91 | 98 | No reported cardiac abnormalities | History of mild respiratory distressd |
| 27c | NICU/SCN | 94 | 94 | Echocardiogram not done | History of RDSd |
| 28 | NICU/SCN | 94 | 97 | Echocardiogram not done | None |
| 29 | NICU/SCN | 94 | 97 | Echocardiogram not done | None |
| 30 | NICU/SCN | 97 | 94 | Echocardiogram not done | None |
Data represent New Jersey resident live births screened from August 31, 2011, to May 31, 2012. ASD, atrial septal defect; BAV, bicuspid aortic valve; d-TGA, dextro transposition of great arteries; PDA, patent ductus arteriosus; PFO, patent foramen ovale; PPS, peripheral pulmonary stenosis; TA, tricuspid atresia; TTN, transient tachypnea of newborn; RDS, respiratory distress syndrome; VSD, ventricular septal defect.
Infants with CCHD.
Transferred out of birthing facility.
Infants with other relevant diagnoses or non-CCHD echocardiogram findings.
Infant appeared to have clinical resolution of this condition at time of screen.
The remaining 19 infants with a failed POxS had 1 of the 3 clinical indicators, suggesting that the mandated POxS would not have changed the already indicated evaluation and subsequent clinical management of these infants (Fig 2). Therefore, their diagnostic evaluation was not solely attributable to the mandated POxS. Four of these infants had CCHDs (transposition of the great arteries, hypoplastic left heart syndrome, total anomalous pulmonary venous return, interrupted aortic arch). Of the remaining 15, 13 had other forms of CHDs or echocardiogram findings (eg, bicuspid aortic valve, patent formen ovale, patent ductus arteriosis) or other noncardiac diagnoses (eg, pneumothorax), and 2 reportedly had no diagnosis made.
Infants With CCHDs but No Failed POxS Reported to the NJBDR
Forty-eight infants were reported to the NJBDR with CCHDs without an accompanying failed POxS reported to the registry (Table 2). These infants had 3 identified reasons for not having an accompanying failed POxS registered. The infants could have (1) passed the POxS, (2) failed the POxS without being reported, or (3) might not have been screened. Based on available clinical information, at least 98% of these infants with CCHDs but no accompanying failed POxS registered, experienced ≥1 of the following: a prenatal CHD diagnosis, transfer out of the birthing hospital within 1 day of life, clinical monitoring with pulse oximetry in the NICU/SCN, or passing POxS results.
TABLE 2.
Number of Children With CCHDs Reported to the New Jersey Birth Defects Registry Without Failed POxS
| Type of CCHD | n (Total N = 48) |
|---|---|
| Tetralogy of Fallot | 15 |
| Coarctation of aorta | 13 |
| Transposition of great arteries | 4 |
| Double outlet right ventricle | 2 |
| Hypoplastic left heart syndrome and coarctation of aorta | 2 |
| Interrupted aortic arch | 2 |
| TAPVR | 2 |
| Truncus arteriosus | 2 |
| Ebstein anomaly | 1 |
| Hypoplastic left heart syndrome and single ventricle | 1 |
| Tetralogy of Fallot and pulmonary atresia | 1 |
| Transposition of great arteries and single ventricle | 1 |
| Tricuspid atresia | 1 |
| Tricuspid atresia, pulmonary atresia, and TAPVR | 1 |
Data represent New Jersey resident live births from August 31, 2011 to May 31, 2012. TAPVR, total anomalous pulmonary venous return.
DISCUSSION
This article represents the first published report in the United States from a legislatively mandated, statewide POxS program for all live hospital births. In the first 9 months after the New Jersey mandate, ~73 000 infants were screened, leading to the identification of 3 infants with previously unsuspected CCHDs. Consistent with findings from other studies, New Jersey’s POxS program identified several newborns with other potentially important and unsuspected conditions or CHDs.2,9–13 Similar to other time-sensitive biochemical newborn screening tests for related metabolic and endocrine disorders, our findings support that POxS can improve the timing of diagnosis of and intervention for CCHDs. Although our findings address the clinical utility of POxS, information on related costs and resource use for statewide POxS implementation is limited. Recent data from a random sample of New Jersey hospitals indicate that the estimated costs of POxS per newborn ($14) is lower than that for laboratory metabolic screening ($20) and hospital-based hearing screening ($36–$39, adjusted to 2011 dollars).14–16 However, these estimates do not include costs incurred from follow-up diagnostic evaluations and/or medical intervention as a result of the failed POxS. Our data indicated that the number of echocardiograms conducted as a result of failed mandatory POxS for infants with no subsequent potentially significant findings was only 7 over this 9-month period. In addition, it does not appear that resources for transferring infants who failed the POxS were used unnecessarily because at least 5 of the 6 infants whose transfers were triggered solely by the failed POxS had potentially significant echocardiogram findings. Importantly, many of the infants who failed the mandatory screen had previous clinical history or signs that would have triggered a subsequent diagnostic evaluation regardless of the statewide mandate, hence requiring no additional resources. Although we do not know how many of the 48 infants with CCHDs without a registered failed screen would have actually failed the POxS, for most of these infants, the mandated POxS likely would not have caused additional resource use beyond that which was already clinically indicated.
Implementing and evaluating statewide POxS necessitated a bridging of traditional newborn screening and birth defects surveillance perspectives. Because most previous studies assessing POxS excluded symptomatic and/or prenatally diagnosed infants,2,9–11,13,17–19 there was no existing approach for implementing and/or evaluating the POxS for all live births, including infants in the NICU and those prenatally diagnosed with CCHDs. Within 90 days of the signing of the legislative mandate, the NJDOH developed a recommended screening algorithm, and the POxS mandate was implemented for all live births in all New Jersey birthing hospitals with 94% of WBNs and 88% of NICU/SCNs reporting to have adopted the algorithm specifically recommended by the NJDOH. Data from a recent investigation in New Jersey revealed that the addition of the new mandated screening processes posed minimal burden on hospital nursing staff.20
From the New Jersey experience, it is clear that to evaluate the added value of a statewide POxS mandate, establishing mechanisms or using existing systems for surveillance is necessary. In New Jersey, as is possible in other states, there was no feasible, immediate mechanism (eg, bloodspot card or electronic birth certificate) to quickly incorporate individual-level screening results and details on infants’ clinical characteristics into a statewide, systematic reporting system. Results from New Jersey’s universal newborn hearing screening program, another statewide point-of-care screening program, are captured within NJDOH’s electronic birth certificate. At the time of implementation of POxS, the electronic birth certificate was being reengineered and was not modifiable statewide in all birthing facilities. The introduction of aggregate screening data reports and individual-level reporting of failed screens to the NJBDR enabled us to quickly capture data to evaluate our statewide mandate. We found that providing a prescriptive template for the aggregate data reporting request was quite useful in obtaining consistent reports across all 53 birthing facilities. Understanding the discrepancies between the number of live births and number of infants screened enabled us to adjust the proportion of live births screened to more accurately reflect infants eligible to be screened. Therefore, we believe the aggregate-level data provide us with a close approximation of implementation coverage. The planned implementation of a new statewide electronic birth reporting system will enable us to collect individual-level data and therefore provide a more accurate reconciliation of infants transferred. Use of the NJBDR to collect detailed relevant clinical characteristics (eg, whether CHD was prenatally detected, a cardiac consult was indicated before the POxS, and/or clinical signs were present at the time of the POxS) of infants who failed the POxS was crucial to evaluate the unique contribution of POxS. As such, we anticipate that the NJBDR will continue to be a necessary tool for collection of detailed information on infants with failed POxS.
Our data collection efforts suggested that some degree of underreporting may have occurred. In particular, the identification of 48 infants with CCHDs without a registered failed POxS revealed that pulse oximetry was not consistently used or reported as a screening tool. With high relevance to implementation of a statewide mandate, these findings underscore that training and education are needed to emphasize that all infants are required to receive the mandated POxS regardless of their location in the hospital or prenatal diagnosis and any infant that fails the screening protocol should be reported to the NJBDR. Our findings also highlight the need to clarify and emphasize the distinction between the use of pulse oximetry as part of a diagnostic assessment and its use as a universal screening tool. These potential sources of underreporting of failed screens appear to be most relevant to NICU/SCN infants or those with preexisting diagnoses or symptoms rather than those in the WBN. In addition, the NJDOH received reports of several infants whose failed screens were erroneously deemed a “pass” by hospital staff. This further highlights the need for significant and ongoing education, outreach, and technical support for health care providers and nursery staff. Performing audits to determine the extent of underreporting at the hospital level is an essential part of future quality assurance activities as is encouraging hospitals to establish their own internal audit protocols. Quality assurance and training efforts in New Jersey need to also focus on POxS techniques (eg, sensor placement) and interpretation of results to assess and ensure the validity of POxS implementation.
The New Jersey experience produced meaningful qualitative and quantitative information for evaluating and improving our program as well as for assisting other states as they begin implementation and evaluation of POxS. Nevertheless, generalization of our observed number of infants with failed POxS and/or CCHDs to what other states might expect should be done with consideration of a few limitations. The lower number of infants with CCHDs and/or failed POxS in New Jersey, compared with other studies,10,11 is likely attributable to several factors, with prenatal detection being a major consideration.21 Given the close geographic proximity to major out-of-state pediatric cardiac surgical centers, New Jersey resident mothers of infants with prenatally diagnosed CCHDs may choose to deliver in a neighboring state. In addition, infants receiving care exclusively out of state are not routinely reported to the NJBDR. Although the overall proportion of New Jersey resident infants with CCHDs prenatally diagnosed is not known, the estimate may be higher than the 36% to 50% previously reported1,11 due to the increased use of ultrasounds in routine prenatal care22 as well as the significant training efforts23 in parts of New Jersey. A higher prenatal detection rate coupled with the possibility of infants with prenatally detected CCHDs being born and solely receiving care out of state could result in an underestimation of CCHD cases and infants with reported failed POxS in the NJBDR. Although this report is well beyond the average time from birth to the age of registration in the NJBDR (~50 days), it is still possible that additional infants with CCHDs may be reported to the NJBDR due to late diagnosis or late reporting.24 Other potential sources of underascertainment include providers not screening infants, providers not reporting infants with a failed POxS, and exclusion of infants born in New Jersey to mothers who were residents of another state. Historically, the NJBDR has only included infants born to New Jersey residents, although it has recently been modified to accommodate registration of failed POxS for out-of-state residents born in New Jersey.
The NJDOH’s approach and data collection mechanisms used to evaluate the statewide POxS mandate may provide insight and guidance to other states implementing or considering similar legislation. Despite the challenges of a short implementation period and being first in the nation to implement statewide POxS, the NJDOH was able to implement a statewide screening effort with a reported high coverage rate, a means to track the proportion of the population screened, and a mechanism by which to obtain relevant clinical information on those infants with a failed POxS. As other states plan and evaluate their POxS programs, we encourage consideration of a number of factors including obtaining information on clinical indicators, exploring the role of prenatal diagnosis, and assessing the ability of surveillance systems to ascertain all failed screens and CCHD cases born in and/or to residents of the state.25 National efforts are currently underway to develop standardized protocols and minimal core data sets and to facilitate sharing of information between states with the ultimate goal of creating more efficient and effective programs to improve the health of infants. From a statewide experience, the results from the first 9 months in New Jersey revealed that mandated POxS successfully identified 3 infants with previously undetected CCHDs who likely would have been discharged from the hospital in the absence of a failed POxS, potentially resulting in significant morbidity or death.
WHAT’S KNOWN ON THIS SUBJECT
Prenatal diagnosis and clinical examination do not identify all infants with critical congenital heart defects before hospital discharge. To improve early critical congenital heart defect detection, New Jersey was the first state to implement legislatively mandated newborn pulse oximetry screening.
WHAT THIS STUDY ADDS
This report is the first to evaluate statewide pulse oximetry screening implementation. New Jersey had a high statewide screening rate and identified 3 infants with previously unsuspected critical congenital heart defects that otherwise might have resulted in significant morbidity and mortality.
Acknowledgments
We thank Dr Margaret Honein for her scientific review of the manuscript, Drs Matt Oster and Tiffany Riehle-Colarusso for their clinical insight on CHDs, Drs Suzanne Gilboa and Elizabeth Ailes for their input regarding prenatal identification of CHDs, and the staff at all 53 New Jersey birthing facilities for their participation in all aspects of the implementation of pulse oximetry screening.
FUNDING: No external funding.
ABBREVIATIONS
- CCHD
critical congenital heart defect
- CHD
congenital heart defect
- NICU/SCN
neonatal intensive care unit/special care nursery
- NJBDR
New Jersey Birth Defects Registry
- NJDOH
New Jersey Department of Health
- POxS
pulse oximetry screening
- WBN
well-baby nursery
APPENDIX 1. New Jersey Department of Health’s recommended pulse oximetry screening algorithm used during the study period. Subsequent to the study period, the initial algorithm shown above was updated.26
APPENDIX 2. Aggregate Data–Reporting Template
| Date of reporting period: | |
|---|---|
| Hospital name: | |
| Total | |
| Explanation of live births screened and not screened in current reporting period | |
Live births who were screened at your birthing facility in current reporting period
|
|
Live births born at your birthing facility during current reporting period who were not screened in current reporting period
|
Definitions for data items above:
(1) Number of infants in the WBN and NICU/SCN who were born at your birthing facility during the current reporting period. This number does not represent all admissions (ie, excludes those transferred into your facility).
(2) Number of infants screened at your birthing facility during the current reporting period. This number includes infants born at your facility and those transferred into your facility who were screened.
(3) Number of infants who failed the pulse oximetry screening at your birthing facility in the current reporting period.
(a) Number of infants both born and screened at your birthing facility during the current reporting period.
(b) Number of infants born at your birthing facility during the previous reporting period, but who were screened in the current reporting period. This number is likely to represent infants who were previously medically unstable or too young to be screened in the previous reporting period.
(c) Number of infants not born at your birthing facility but who were transferred into your birthing facility and screened during the current reporting period. Below list the name of the hospital the infant was transferred from. If transfers were received from multiple hospitals, create a row for each hospital. Include the number of infants who were admitted.
(d) Explanation of why an infant was screened in the current reporting period, if not listed above (eg, home births).
(e) Number of infants born at your birthing facility during the current reporting period that expired before conducting the pulse oximetry screening.
(f) Number of infants born at your birthing facility during the current reporting period who were not medically appropriate for pulse oximetry screening. This number is primarily applicable to NICU infants.
(g) Number of infants born at your birthing facility during the current reporting period who were <24 hours of age at the end of the current reporting period and were therefore were not screened during the current reporting period.
(h) Number of infants born at your birthing facility during the current reporting period who were not screened and were transferred out at <24 hours. Below list the name of the hospital the infant was transferred to. If infants were transferred to different hospitals, create a row for each hospital. Include the number of infants who were transferred.
(i) Number of infants born at your birthing facility during the current reporting period who were not screened and were transferred out of your birthing facility at ≥24 hours. Below list the name of the hospital the infant was transferred to. If infants were transferred to different hospitals, create a row for each hospital. Include the number of infants who were transferred.
(j) Explanation of why an infant was not screened, if not listed (eg, discharged, 24 hours, parents refused screen, etc).
Important: Infants who were in both the WBN and the NICU or SCN during the current reporting period should only be counted once for all reporting items above.
Footnotes
Dr Garg conceptualized and designed all aspects of the study, assisted in data acquisition, analyzed and interpreted study data, drafted the initial manuscript, revised it critically for important intellectual content, and approved the final version for submission; Dr Van Naarden Braun conceptualized and designed all aspects of the study, acquired study data, analyzed and interpreted data, drafted the initial manuscript, revised it critically for important intellectual content, and approved the final version for submission; Ms Knapp assisted in study design and conceptualization, acquired study data, analyzed and interpreted data, reviewed and revised the manuscript critically for important intellectual content, and approved the final version for submission; Drs Anderson, Koppel, Hirsch, Olney, Hinton, and Kemper assisted in interpretation of study data, reviewed and revised the manuscript critically for important intellectual content, and approved the final version for submission; Ms Beres assisted in study design and conceptualization, analyzed and interpreted data, reviewed and revised the manuscript critically for important intellectual content, and approved the final version for submission; Dr Sweatlock analyzed and interpreted study data, reviewed and revised the manuscript critically for important intellectual content, and approved the final version for submission; and Ms Glidewell assisted in study conceptualization and interpretation of study data, reviewed and revised the manuscript critically for important intellectual content, and approved the final version for submission.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the New Jersey Department of Health.
FINANCIAL DISCLOSURE: The authors indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Mahle WT, Newburger JW, Matherne GP, et al. American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research; American Academy of Pediatrics Section on Cardiology and Cardiac Surgery, and Committee on Fetus and Newborn. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120(5):447–458. doi: 10.1161/CIRCULATIONAHA.109.192576. [DOI] [PubMed] [Google Scholar]
- 2.Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine—results from a prospective multicenter study. Eur J Pediatr. 2010;169(8):975–981. doi: 10.1007/s00431-010-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F33–F35. doi: 10.1136/adc.2007.119032. [DOI] [PubMed] [Google Scholar]
- 4.N.J.S.A. 26:2–111.3 et seq.
- 5.Recommended Uniform Screening Panel of the Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. [Accessed July 23, 2012]; Available at: www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendedpanel/index.html.
- 6. [Accessed June 10, 2013];Screening map for CCHD. Available at: www.cchdscreeningmap.com/
- 7.Oster ME, Lee KA, Honein MA, et al. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131(5) doi: 10.1542/peds.2012-3435. Available at: www.pediatrics.org/cgi/content/full/131/5/e1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.N.J.S.A. 26:8–40.20 et seq.
- 9.Bradshaw EA, Cuzzi S, Kiernan SC, Nagel N, Becker JA, Martin GR. Feasibility of implementing pulse oximetry screening for congenital heart disease in a community hospital. J Perinatol. 2012;32(9):710–715. doi: 10.1038/jp.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de-Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338:a3037. doi: 10.1136/bmj.a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewer AK, Middleton LJ, Furmston AT, et al. PulseOx Study Group. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet. 2011;378(9793):785–794. doi: 10.1016/S0140-6736(11)60753-8. [DOI] [PubMed] [Google Scholar]
- 12.Kemper AR, Mahle WT, Martin GR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128(5) doi: 10.1542/peds.2011-1317. Available at: www.pediatrics.org/cgi/content/full/128/5/e1259. [DOI] [PubMed] [Google Scholar]
- 13.Koppel RI, Druschel CM, Carter T, et al. Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics. 2003;111(3):451–455. doi: 10.1542/peds.111.3.451. [DOI] [PubMed] [Google Scholar]
- 14.Peterson C, Grosse SD, Glidewell J, et al. A public health economic assessment of hospitals’ cost to screen newborns for critical congenital heart disease. Public Health Reports. doi: 10.1177/003335491412900113. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll AE, Downs SM. Comprehensive cost-utility analysis of newborn screening strategies. Pediatrics. 2006;117(5 pt 2):S287–S295. doi: 10.1542/peds.2005-2633H. [DOI] [PubMed] [Google Scholar]
- 16.Vohr BR, Oh W, Stewart EJ, et al. Comparison of costs and referral rates of 3 universal newborn hearing screening protocols. J Pediatr. 2001;139(2):238–244. doi: 10.1067/mpd.2001.115971. [DOI] [PubMed] [Google Scholar]
- 17.Meberg A, Brügmann-Pieper S, Due R, Jr, et al. First day of life pulse oximetry screening to detect congenital heart defects. J Pediatr. 2008;152(6):761–765. doi: 10.1016/j.jpeds.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 18.Sendelbach DM, Jackson GL, Lai SS, Fixler DE, Stehel EK, Engle WD. Pulse oximetry screening at 4 hours of age to detect critical congenital heart defects. Pediatrics. 2008;122(4) doi: 10.1542/peds.2008-0781. Available at: www.pediatrics.org/cgi/content/full/122/4/e815. [DOI] [PubMed] [Google Scholar]
- 19.Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379(9835):2459–2464. doi: 10.1016/S0140-6736(12)60107-X. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Rapid implementation of pulse oximetry newborn screening to detect critical congenital heart defects—New Jersey, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(15):292–294. [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh W. Evaluation of pulse oximetry screening in Middle Tennessee: cases for consideration before universal screening. J Perinatol. 2011;31(2):125–129. doi: 10.1038/jp.2010.70. [DOI] [PubMed] [Google Scholar]
- 22.Friedberg MK, Silverman NH, Moon-Grady AJ, et al. Prenatal detection of congenital heart disease. J Pediatr. 2009;155(1):26–31. e1. doi: 10.1016/j.jpeds.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 23.Levy DJ, Pretorius DH, Rothman A, et al. Improved prenatal detection of congenital heart disease in an integrated health care system. Pediatr Cardiol. 2013;34(3):670–679. doi: 10.1007/s00246-012-0526-y. [DOI] [PubMed] [Google Scholar]
- 24.Aamir T, Kruse L, Ezeakudo O. Delayed diagnosis of critical congenital cardiovascular malformations (CCVM) and pulse oximetry screening of newborns. Acta Paediatr. 2007;96(8):1146–1149. doi: 10.1111/j.1651-2227.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 25.Olney RS, Botto LD. Newborn screening for critical congenital heart disease: essential public health roles for birth defects monitoring programs. Birth Defects Res A Clin Mol Teratol. 2012;94(12):965–969. doi: 10.1002/bdra.23103. [DOI] [PubMed] [Google Scholar]
- 26.State of New Jersey Department of Health. [Accessed June 9, 2013];Critical Congenital Heart Defects (CCHD) Screening Resources. Available at: www.nj.gov/health/fhs/nbs/cchd_resources.shtml.