Abstract
In many human cancers including malignant glioblastoma multiforme (GBM), cancer stem cells (CSCs) are thought to be responsible for tumor initiation, metastasis and resistance to conventional anti-cancer therapies. Therefore, a CSC-targeted drug delivery strategy to eliminate CSCs is a desirable approach for developing a more effective therapeutic. Moreover, isolated CSCs will provide an invaluable tool for studying the underlying cellular mechanisms of tumor development and provide insight into therapeutic options for successful eradication of CSCs. This unit describes a method for the isolation and culture of CSCs from human GBM tumor tissue.
Keywords: glioblastoma multiforme, cancer stem cells, culture, neurosphere, tumorsphere
Introduction
The cancer stem cell (CSC) hypothesis suggests that tumors are initiated from, and maintained by, a rare fraction of cancer cells that have stem cell-like properties permitting them to evade cytotoxic therapies and continue to fuel tumor growth. CSCs have been implicated in treatment resistance and recurrence in many types of human cancer including leukemia, breast, brain, colon, lung, and prostate. A great deal of effort is currently focused on developing novel therapeutic strategies directed at eliminating CSCs for cancer treatment and prevention of recurrence. Thus, understanding the role of CSCs in tumor formation, progression, and recurrence will provide clues to developing new, effective therapeutic approaches.
In this unit, the authors detail a protocol for the tumorsphere culture method to isolate and expand CSCs from human glioblastoma multiforme (GBM) tumor tissue and a protocol for the identification of CSCs using surface markers. GBM cells are harvested from tumor specimens, mechanically dissociated and enzymatically digested. To identify CSCs, a cell suspension is labeled with fluorescence-conjugated CSC marker antibodies and sub-populations of CSC marker-positive cells are isolated using Flow cytometry. Alternatively, the cell suspension can also be cultured in a defined serum-free stem cell media for to produce three-dimensional tumorspheres.
Strategic Planning
To preserve the functional and genetic properties of the original parental tumor, the use of an appropriate method to culture CSCs is critical (Clarke et al., 2006; Chen et al., 2012). To culture GBM CSCs, culture conditions and assays were adapted from those originally established for isolation and characterization of neural stem cells (NSCs). Under these culture conditions, CSCs grow as floating multicellular spheroids, or tumorspheres, mimicking the normal NSCs and neurospheres, while hindering the growth of differentiated GBM cell populations. This tumorsphere culture is enriched for GBM cells that present stem cell-like characteristics, including expression of NSC markers, long term self-renewal, and the ability to reproduce the original tumor upon orthotopic inoculation in pre-clinical animal models. CSCs in a GBM tumor can be identified by fluorescence activated cell sorting (FACS) for NSC markers such as CD133, SSEA-1, Nestin, Nanog, and Msi1.
The use of standard serum-based adherent culture conditions to culture GBM cell lines leads to genetic and epigenetic changes that alter the cellular phenotype (Lee et al., 2006). However, when GBM CSCs are cultured using a defined serum-free stem cell media that is routinely used for NSCs, the genetic and phenotypic properties of the parental tumor are maintained. This serum-free stem cell medium allows for the maintenance of an undifferentiated stem cell state, and the addition of bFGF and EGF allows for the proliferation of multipotent, self-renewing, and expandable tumorspheres. Conversely, if serum is added to this medium the result is the differentiation of the GBM cells and their growth as an adherent monolayer.
NOTE: The following procedures are performed in a Class II biological hazard flow hood or a laminar-flow hood.
NOTE: Working conditions must ensure the highest degree of sterility. All solutions and equipment that come into contact with tissues or cells must be sterile, and proper aseptic technique should be used accordingly.
NOTE: All culture incubations are performed in a humidified 37°C, 5% CO2 incubator unless otherwise specified.
Basic Protocol 1
Isolation of CSCS from Brain Tumor
This protocol is adapted from those originally established for neurosphere culture of NSCs using a defined serum-free stem cell medium in a non-adherent manner (Reynolds and Weiss, 1992; Tropepe et al., 1999). After 7 to 10 days under neurosphere culture conditions, primary GBM tumorspheres can reach 150 to 200 μm in size and are ready for further passaging and expansion. CSCs can be identified by NSC surface markers.
Materials
Fresh GBM tumor tissue specimen
Enzyme digestive mix (see recipe)
Hank's balanced salt solution (HBSS, Mediatech)
Collagenase D (Roche)
DNase I (Sigma)
Bovine serum albumin (Sigma)
Trypan blue (Life Technologies)
10× Phosphate buffered saline (PBS) (Mediatech)
Dulbecco's PBS (DPBS) without Ca2+ or Mg2+ (Sigma)
Penicillin - Streptomycin – Neomycin (PSN) solution 100× stock (Sigma)
Fetal bovine serum (FBS, Sigma) - Heat inactivate at 56°C for 30 min.
Stem cell medium (see recipe)
DMEM medium (Mediatech)
F12 medium (Mediatech)
B-27 supplements (Gibco)
Recombinant human basic fibroblast growth factor (bFGF, R&D systems)
Recombinant human epidermal growth factor (EGF, R&D systems)
16% Paraformaldehyde (EMS)
FACS buffer (see recipe)
Fixing buffer (see recipe)
Human Fc block (BD Biosciences)
Anti-human SSEA-1 (Stemgent)
Anti-human Nestin (Abcam)
Anti-human Nanog (Cell Signaling Technology)
Anti-human Msi1 (BD Pharmingen)
Anti-human CD133 (Miltenyi Biotec)
Anti-human Nestin (Abcam)
Anti-human CD71 (TfR) (BioLegend)
Triton X-100 (Sigma)
Tween 20 (Sigma)
ProLong® Gold Antifade Mountant with DAPI (Life Technology)
Fine scissors and forceps (Harvard Apparatus)
No. 10 curved scapel blades (Fine Science Tools)
35 mm tissue culture dishes (Corning)
100 mm ultra-low attachment culture dishes (Corning)
LabTek 8-well chamber slide (NUNC)
15 and 50 mL conical centrifuge tubes (Corning)
5 and 10 mL disposable pipets (Denville)
Pasture pipet (Fisher Scientific)
0.22 μm syringe filter unit (Millipore)
0.22 μm cellulose-acetate disposable vacuum-filtration system (Millipore)
70 μm cell strainer (Fisher Scientific)
Polystyrene round-bottom 12 × 75 mm tubes (BD Falcon)
Glass coverslips (Fisher Scientific)
5 mL syringes (BD)
Vertical laminar flow hood certified for Level II
37°C CO2 incubator with humidity and gas control
Pipette-aid (Drummond Scientific)
Rotator multimix (VWR Scientific)
Vortex Genie (Scientific Industries)
Table top centrifuge (Eppendorf)
Hemocytometer (Hausser Scientific)
BD FACSAria flow cytometer (BD Biosciences)
IX71 inverted epifluorescent microscope (Olympus)
LSM 510 META laser scanning microscopes (Zeiss)
Dissociate GBM tumor tissue
- With written consent from patients and/or in accordance with institutional guidelines, immediately after the resection collect tumor samples (200-500 mg of tumor is recommended) into a tube containing cold sterile stem cell media without growth factors. Transport the specimen immediately to the tissue culture hood for processing.
- For surgeries at a remote site, cut the tumor sample into smaller fragments and place into a tube containing cold sterile stem cell media without growth factors (keep on ice) for transportation. The tumor can be processed within 2-3 hours after the resection. Tumor specimens from a pre-clinical animal model of human GBM tumor can also be collected and processed in the same way.
In sterile BSL II laminar flow hood, place the tumor into a 35 mm petri dish with 3 mL of HBSS. Wash tumor specimen (2 to 3 times) by transferring them sequentially to new 35 mm dishes filled with 5 mL HBSS to remove blood and debris.
- Aspirate excessive HBSS from the dish. Immediately cut the tumor into small fragments and mince with a sterile scalpel blade into approximately 1 mm3 fragments.
- The best yield can be achieved when tumors are minced to very small pieces.
- Add 3 mL of enzymatic digestion mixture to the minced tissue and collect the minced tissue with 5 mL disposable pipet, pipetting up and down a few times. Then, transfer the tumor fragments into 30 mL of pre-warmed enzymatic digestion mixture.
- The final concentration of enzymes should be 1 mg collagenase D and 0.1 mg DNase I per milliliter of HBSS. If fragments are too large to be drawn up into 5 mL pipet, further mince the tumor tissue and/or use a pipet with an opening large enough to draw up all the tumor fragments.
- Incubate the minced tumor at 37°C for 30 to 90 min with gentle mixing in a rotator multimix.
- Incubation time depends on tumor size. The rotator mltimix provides excellent digestion and yield of cells.
- Triturate the fragment mixture 3 to 5 times with a 10 mL pipette.
- Avoid the formation of air bubbles. Excessive air bubbles will lower the cell viability and enhance the possibility of contamination.
- Place a 70 μm sterile mesh filter into a clean 50 mL conical tube and carefully pass the solution through the filter to remove any large, undigested tumor pieces.
- Pass the solution through the center of the filter with a steady, semi-forceful pipet stream.
Using a plunger from a 5 mL syringe, gently mash the remaining tumor pieces against mesh filter to enable more tumor cells to pass through.
Wash the filter twice with 4 to 5 mL of HBSS and pellet the filtered cells by centrifugation at 200 ×g for 10 min at 4°C.
- Discard the supernatant and resuspend the cell pellet in 10 mL HBSS.
- If the solution appears clumpy, pass it through a second 70 μm mesh filter.
- Determine the cell number and viability by counting an aliquot of the cells using a hemocytometer and trypan blue.
- At this point, isolated GBM cells can be maintained either in the defined serum-free stem cell medium for primary tumorsphere culture or in serum-containing medium as adherent monolayer culture. Isolated GBM cells can also be subjected to fluorescence activated cell sorting (FACS) to fractionate the CSC subsets.
Identification of CSCs using FACS Analysis
12. To identify CSC population, the cell suspension produced as described above is stained with antibodies against CSC markers.
13. Adjust the cell number to a concentration of 1-5×106 cells/mL in ice cold FACS buffer. Aliquot 100 μL of cell suspension into separate 5 mL polystyrene round-bottom tubes for each antibody to be used.
14. (Optional) if cells express high levels of Fc receptors which will contribute to non-specific binding and background fluorescence, add 100 μL of Fc block to each sample (Fc block diluted in FACS buffer at 1:50 ratio). Incubate on ice for 20 min.
- 15. Centrifuge at 200 ×g for 5 min at 4°C. Discard supernatant. Resuspend cell pellet in a volume of 100 μL FACS buffer containing fluorochrome-labelled primary antibody (0.1-10 μg/mL) for 30 min on ice in the dark.
- The optimal dilution of antibody, which gives the best staining with minimum background and non-specific staining, must be determined experimentally for each antibody. All dilutions should be made in FACS buffer.
16. Wash cells in 1 mL of FACS buffer and pellet by centrifugation at 200 ×g for 5 min at 4°C.
17. If necessary, perform the secondary antibody staining (if using unlabelled primary antibody). Dilute the fluorochrome-labelled secondary antibody in FACS buffer at the optimal dilution, resuspend cells in this solution and incubate for 30 min on ice in the dark. Wash the cells with 1 mL of FACS buffer and pellet by centrifugation at 200 ×g for 5 min at 4°C. Repeat the washing procedure once.
- 18. Resuspend the pellet in 100 μL of fixing buffer. Analyze the cells on the flow cytometer as soon as possible for best results.
- Cells may be stored in this buffer for up to 3 days at 4°C in the dark.
Culturing GBM cells as tumorspheres
- 19. Plate isolated GBM cells isolated as described above at a density of 50,000 cells/mL in stem cell medium using 100 mm ultra-low attachment culture dishes. Incubate in a humidified 37°C, 5% CO2 incubator.
- The biological activity of the growth factors in the stem cell medium decreases with time. For optimal results, supplement the medium with the growth factors just before use. For best results the spheres should be grown in ultra-low attachment surface dishes.
- 20. Once spheres have formed, replace medium every other day by letting them settle to the bottom by gravity at 37°C. After the spheres have settled, carefully remove the medium and replace with fresh stem cell medium.
- Centrifugation is not recommended because this will alter the shape of the viable neurospheres.
- 21. Passage the cells approximately every 7 to 10 days or when primary tumorspheres reach 150 to 200 μm in size to prevent the cell clusters from growing too large, which can lead to necrosis as a result of a lack of oxygen and nutrient exchange at the center of the neurospheres.
- There is variability between tumors and each tumor must be evaluated daily to follow sphere formation.
- 22. To passage the tumorspheres, centrifuge for 5 min at 200 ×g. Remove the supernatant and resuspend the tumorspheres in 3 mL of stem cell medium. Triturate with a pasteur pipette 60-70 times.
- There should no longer be any visible spheres. If visible spheres remain, continue to triturate another 30-60 times. Avoid excessive trituration to minimize damaging the cells. Avoid the formation of air bubbles. Excessive air bubbles will lower the cell viability and enhance the possibility of contamination.
- 23. Determine the cell density and resuspend in the desired volume of stem cell medium for replating.
- Best results are obtained when cells are plated at a density of about 50,000 cells/mL of stem cell medium.
Immunofluorescence staining of GBM tumorspheres
- 24. Transfer tumorspheres from a culture dish to one well of 8-well chamber slide using 1 mL pipette. Leave spheres undisturbed for 5 min to allow the tumorspheres to settle and carefully remove the culture medium from each well using a 1 mL pipette.
- After aspiration, verify the spheres are at the bottom of the well by visualization with the naked eye or by microscopy. Centrifugation of the viable tumorspheres will alter the shape of the tumorspheres.
25. Wash the spheres with 400 μL of PBS three times by adding the PBS to the well swirl gently and let settle for 5 min prior to carefully removing the PBS with a 1ml pipette.
26. After the 3 washes add 200 μL of the 4% paraformaldehyde solution directly into the well. Incubate for 30 min at room temperature.
27. Aspirate the paraformaldehyde solution carefully. Add 400 μL of PBS and incubate for 5 min. Repeat this washing procedure three times.
28. Permeabilize cells by adding 200 μL of PBS containing 0.3% Triton X-100 to each well and incubate for 5 min at room temperature.
29. Remove PBS/Triton-X 100 by aspiration. Add 400 μL of PBS and incubate for 5 min. Repeat this washing procedure twice.
30. Block the spheres by incubation in 200 μL of 1% BSA in PBST for 30 min.
31. Incubate the spheres in 200 μL of the diluted primary antibody (e.g. anti-human CD133, nestin, and TfR) in 1% BSA in PBST in a humidified chamber overnight at 4°C.
32. Wash spheres with 400 μL of PBS three times, 5 min each wash as above.
33. Dilute secondary antibodies 1/100 in 1% BSA in PBST and incubate secondary antibodies for 30 min at room temperature in the dark.
34. Wash spheres with 400 μL of PBS three times, 5 min each wash as above.
- 35. Remove the chambers from the glass slides and mount coverslip with a drop of mounting medium containing DAPI for counter staining.
- Avoid trapping any air bubbles.
36. Seal coverslip with nail polish to prevent drying and movement under microscope.
37. Visualize immunostaining under a fluorescent microscope using the appropriate filters for each fluorochrome.
Reagents and Solutions
Use deionized, distilled water or equivalent for all recipes and protocol steps.
Enzymatic digestion mixture
100 mg Collagenase D
10 mg DNase I
Dissolve above components in 100 mL of HBSS and filter through a 0.22 μm disposable vacuum-filtration system. This enzyme digestive mix must be made up fresh prior to each use.
Stem cell medium (Tumor sphere medium)
50 mL of DMEM medium
50 mL of F12 medium
1 mL Penicillin-Streptomycin-Neomycin (100× stock)
2 mL B27 supplement (50× stock)
Store up to 1 week at 4°C. Supplement the medium just before use with:
100 μL recombinant human basic fibroblast growth factor (bFGF, 10 ng/mL final) (1000× stock)
100 μL recombinant human epidermal growth factor (EGF, 20 ng/mL final) (1000× stock)
The stock solution of penicillin/streptomycin/neomycin contains 5,000 U/ml penicillin, 5 mg/ml streptomycin and 10 mg/ml neomycin. Store this stock solution up to reported expiration date in 5 ml aliquots at -20°C.
Recombinant human bFGF stock solution, 10 ng/μL (1000×)
Resuspend lyophilized bFGF to a final concentration of 10 ng/μL in 1× PBS containing 0.1% (w/v) BSA. Store at -70°C.
Recombinant human EGF stock solution, 20 ng/μL (1000×)
Resuspend lyophilized EGF to a final concentration of 20 ng/μL in 1× PBS containing 0.1% (w/v) BSA. Store at -70°C.
FACS buffer
2% (v/v) heat-inactivated FBS in 1× DPBS without calcium and magnesium. Add 10 mL FBS to 500 mL DPBS. Store buffer up to 1 month at 4°C.
Fixing buffer
2% (v/v) paraformaldehyde in FACS buffer. Add 2 mL FBS to 100 mL FACS buffer. Store buffer up to 1 month at 4°C.
PBST
0.1% (v/v) Tween 20 in 1× PBS. Add 1 mL Tween 20 to 1 L 1× PBS. Store buffer up to 1 month at 4°C.
Commentary
Background Information
The development of the neurosphere culture method to isolate and expand stem cells from neural tissue (Reynolds and Weiss 1992) has greatly advanced our understanding of NSCs. In the presence of EGF and bFGF, these cell populations can be reliably expanded and maintained in the form of neurospheres and can efficiently differentiate into the major CNS cell types upon removal of growth factors.
This culture method was also successfully applied to isolation and expansion of CSCs from GBM tumor tissue allowing CSCs to grow as floating tumorspheres, mimicking the normal NSCs. GBM CSCs presents stem cell-like characteristics, including expression of NSC markers, long term self-renewal. Propagated by this method, these CSCs also have the ability to reproduce the original tumor upon orthotopic inoculation in pre-clinical animal models. Tumor spheres from adult GBM have the potential to express glial and/or neuronal markers and form highly infiltrating gliomas in the brain of immunedeficient mice (Tunici et al. 2004).
Various surface markers such as CD133, SSEA-1, L1CAM, or integrin α6 were suggested to identify CSCs in GBM tumors. However, significant inconsistency of stem cell marker expression was observed among the GBM tumors. Given the complex genetic and epigenetic heterogeneity of GBMs, it is unlikely that the expression of a single marker will define CSCs in every GBM tumors (Brescia et al. 2012).
Critical Parameters and Troubleshooting
When isolating GBM tumor cells, optimization of enzymatic digestion is critical. Both the duration of digestion and the choice of enzyme will influence the yield and viability of tumor cells. For example, it is reported that the often-used trypsin-based enzymatic dissociation can be deleterious to GBM cells, resulting in a high percentage of cell death after isolation (Hutton and Pevny 2008). Moreover, trypsin digestion might cleave and modify the surface expression of proteins such as CD133, thereby affecting how the cells are sorted and the ability of these molecules to play a role in tumor progression (Sakariassen et al. 2007). In the current protocol, we use collagenase D as an alternative for the enzymatic digestion which provides a high yield of viable stem cells.
In addition, it should be noted that optimization of culture conditions and plating densities will significantly affect the yield and growth of tumor spheres with good cell viability across different GBM tumors. We recommend plating the cells at a high density (e.g., 50,000 cells/mL), which may need to be adjusted on an individual basis.
For sorting with FACS, it is critical to obtain a single cell suspension not to compromise the staining and sorting procedure. Thorough but gentle mechanical trituration and filtering through cell-strainer caps into FACS tubes as described above is highly recommended as it prevents clumping during the sort.
Anticipated Results
This isolation protocol should yield 1 to 50 × 106 viable GBM cells depending on the size of the tumor specimen. After plating the isolated single cells using tumorsphere culture condition, GBM cells initiate to form small clumps of few cells in around 3-4 days. As these clumps grow, they change into a more spherical shape. By 7-10 days, primary tumorspheres of 150 to 200 μm in average diameter can be observed and are ready for further passaging and expansion.
Time Considerations
Tumor dissociation and plating of single cells for tumorsphere culture usually takes approximately 2 to 3 h. The initial sphere formation of GBM CSCs from a primary tumor specimen may take about 3 to 4 days. FACS staining can be done within 2 h. Immunofluorescence staining of GBM tumorspheres may take about 2 days.
Figure 1.
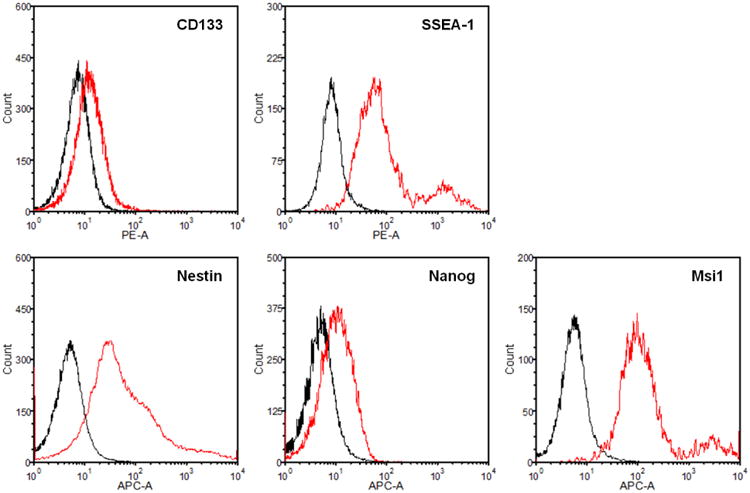
Identification of CSCs in GBM tumor using FACS Analysis.
Figure 2.

Photographs of CSCs forming tumorsphere in vitro. (A) Tumor spheres of human GBM after 7 day in culture (magnification 4×). (B) One GBM tumor sphere (magnification 20×).
Figure 3.

Enrichment of CSCs by tumorsphere culture.
Figure 4.
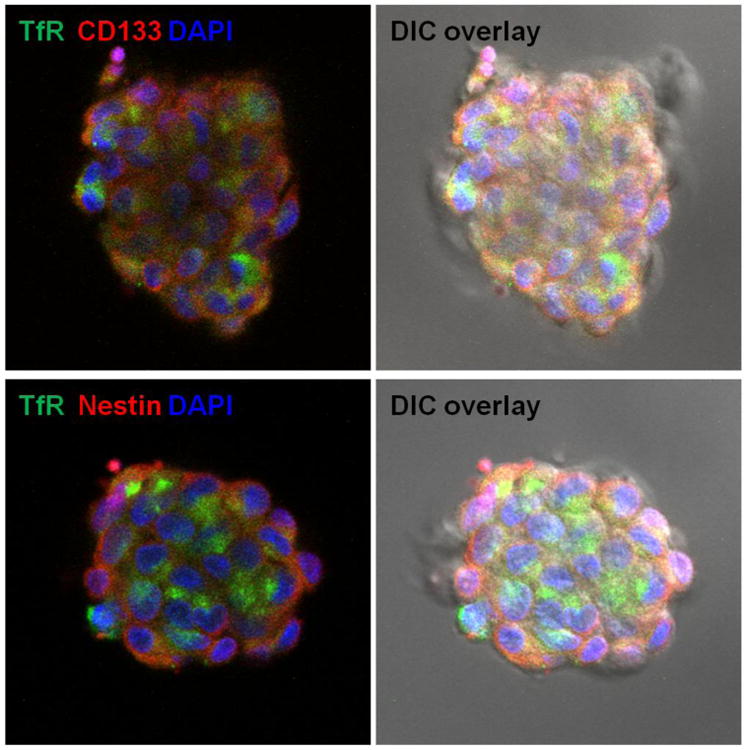
Immunofluorescence staining of GBM tumor spheres. GBM tumor spheres were stained for transferrin receptor (TfR) and CSC markers (CD133 or Nestin).
Acknowledgments
This work was funded by NIH grant HHSN261201400038C.
Literature Cited
- Brescia P, Richichi C, Pelicci G. Current strategies for identification of glioma stem cells: adequate or unsatisfactory? J Oncol. 2012;2012:376894. doi: 10.1155/2012/376894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, McKay RM, Parada LF. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 2012;149:36–47. doi: 10.1016/j.cell.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells - perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Hutton SR, Pevny LH. Isolation, culture, and differentiation of progenitor cells from the central nervous system. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot5077. pdb.prot5077. [DOI] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Sakariassen PØ, Immervoll H, Chekenya M. Cancer Stem Cells as Mediators of Treatment Resistance in Brain Tumors: Status and Controversies. Neoplasia. 2007;9:882–892. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Tunici P, Bissola L, Lualdi E, Pollo B, Cajola L, Broggi G, Sozzi G, Finocchiaro G. Genetic alterations and in vivo tumorigenicity of neurospheres derived from an adult glioblastoma. Mol Cancer. 2004;3:25. doi: 10.1186/1476-4598-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]


