Abstract
Microsatellite instability (MSI) is associated with the prognosis in several cancers and is used for determination of the chemotherapy regimen in stage II colon cancer in the National Comprehensive Cancer Network guideline. However, the association between MSI and the prognosis of gastric cancer remains unclear. PubMed database was searched until January 2014 using MeSH terms and key words to identify the studies evaluating MSI and prognosis of gastric cancer and the references were manually searched. The main outcome was the overall survival rate and the subordinate outcome was the association between high-frequency MSI (MSI-H) and clinicopathological characteristics. Eight studies met the inclusion criteria and the majority of data were collected retrospectively. There were 1,976 patients, 431 of which were MSI-H patients, with a range of 11.68–33.82%. Four studies used the National Cancer Institute panel to define MSI-H, the other four had microsatellite markers ranging 2–11. Significant associations were found in three studies and the overall summary estimate was hazard ratio, 0.63 (95% confidence interval, 0.52–0.77), with no evidence of inter-study heterogeneity (I2=0.0%). MSI-H patients were identified to have a tendency to have less lymph node (LN) metastasis, superficial tumor invasion and to be intestinal type. In conclusion, MSI-H gastric cancers have an improved prognosis, accompanied with reduced risk of LN metastasis, tumor invasion and mortality.
Keywords: microsatellite instability, gastric neoplasms, prognosis, overall survival
Introduction
Gastric cancer is one of the most common malignant tumors in the world with a poor prognosis. Although its incidence and mortality has been decreasing in recent years, it is still the second leading cause of cancer-related mortality (1,2). In order to administer and treat gastric cancer optimally, studies on the prediction for the prognosis and the treatment efficacy of gastric cancer were focused for a long time. The prognosis of gastric cancer was affected by numerous aspects, such as gastric cancer staging, location, histological type, biological behavior and therapeutic measures. However, a previous study showed that mutation or gene polymorphism in certain important molecules, such as epidermal growth factor receptor, human epidermal growth factor receptor 2 and c-Met, are also involved in the prognostic prediction (3). However, there are no commonly accepted prognostic biomarkers that are used clinically. Therefore, further investigation and analysis is required.
Microsatellite DNA are widespread, short and repetitive DNA sequences that are randomly distributed in the human genome (4). When mismatch repair genes, including hMLH1 and hMSH2, are inactivated, replication errors, such as insertions or deletions of bases within microsatellite regions, cannot be repaired. These phenomena are known as microsatellite instability (MSI). Alternatively, MSI can also be caused via epigenetic promoter methylation (5,6). For characterizing and classifying MSI, a panel of five markers for the analysis of MSI was validated and recommended by the National Cancer Institute (NCI) in 1997, including two mononucleotide repeats (BAT-25 and BAT-26) and two dinucleotide repeats (D5S346, D2S123 and D17S250) (7). If ≥2 of the five markers show instability, these genotypes are grouped into high-frequency (MSI-H). When only one marker shows instability, these genotypes are grouped into low-frequency (MSI-L), and when no marker shows instability, these are grouped into microsatellite stable (MSS). As more markers have been found, >5 markers were used in the detection of MSI, such as DP1, NM23, p53, NR-21 or DCC microsatellite loci, and the principle has been optimized. MSI-H is defined as having 30–40% instability markers, while MSI-L is defined when instability markers are <30–40% (7,8). This is not the only principle, as Bethesda Guidelines have revised that MSI-H can be defined when having instability at a mononucleotide loci and MSI-L is having limited instability at only a dinucleotide loci, and mononucleotide repeats were shown to be more sensitive compared to dinucleotide loci in detecting MSI (9–11).
MSI, since being first described in hereditary nonpolyposis colorectal cancer (CRC) (12), has been found in numerous familial and sporadic human neoplasms (5). MSI is proved to be a mostly molecular mechanism involved in carcinogenesis and development. MSI has been used in the prognosis of numerous types of cancer. For example, MSI-H is associated with a good prognosis in CRC, but no significant association was found between MSI-H and prognosis in early endometrial cancer. MSI-H forecasted a bad prognosis in breast cancer (13–15). In gastric cancers, the incidence of MSI-H varies from 8.2–37%, which was decided by the number of cases investigated. Patients with gastric cancer and MSI-H tend to be older, female, distal located, with a well-differentiated adenocarcinoma type and in lower tumor stages (6,16–22). The association between MSI-H and gastric cancer prognosis remains ambiguous. Certain studies support that MSI-H is associated with a good prognosis (6,18–21,23–25) while others are conflicting (16,26–28).
Therefore, the present study is a meta-analysis to identify whether MSI is associated with the prognosis in gastric cancer by assembling the current opinions and the pooled result. The pooled analysis showed that patients with MSI-H have a reduced risk of lymph node (LN) metastasis, tumor invasion and mortality compared to those with MSI-L/MSS. Furthermore, MSI-H gastric cancers have an improved prognosis. The present analysis will provide a whole evaluation for the effect of MSI on the prognosis of gastric cancer.
Materials and methods
Search strategy
The PubMed electronic database was searched until January 2014 using MeSH terms and key words. The strategy included ‘microsatellite instability’ or ‘MSI’ or ‘replication error phenotype’ combined with ‘stomach neoplasm’ or ‘stomach cancer’ or ‘stomach carcinoma’ or ‘gastric neoplasm’ or ‘gastric cancer’ or ‘gastric carcinoma’ combined with ‘prognosis’ or ‘outcome’ or ‘survival’ or ‘DFS’ or ‘OS’. In addition, the references and review studies were manually searched to identify other relevant studies to ensure integrity. Primary authors were not contacted.
Eligibility criteria
The search was restricted to human studies that were published in peer-reviewed journals in English. Studies assessing the association between MSI and prognosis in gastric cancer with notable outcomes [overall survival (OS) in gastric cancer with MSI] were included. Reviews, single case reports, unrelated and duplicated studies were excluded. Studies that did not allow the extraction of the hazard ratio (HR) and 95% confidence interval (CI) were included in the systematic review but excluded from meta-analysis. Two investigators read the full texts of the relevant studies and applied the eligibility criteria independently. Any disagreement was assessed by a third investigator.
Quality assessment
Currently, a standard assessment for observational studies is not available. The Newcastle-Ottawa Quality Assessment Scale (NOS) for case-control studies was used for reference (29). Quality assessment was performed by the two independent investigators and any disagreement was solved by a third investigator.
Data extraction
Two investigators extracted the information from all the studies independently, including: First author, year of publication, distribution of clinical pathological factors, such as tumor stage, Lauren's classification and treatment interventions, and MSI definition.
Data analysis
Meta-analysis was performed between patients with MSI+ (MSI-H) and with MSI- (MSI-L/MSS) to explore the association between MSI and OS. Only the studies that reported HR with 95% CI were included in the meta-analysis. The HR and 95% CI were calculated for pooling if not available from the study, using the total number of events (fatalities and relapse) and numbers at risk in each group or the Kaplan-Meier survival curves provided (30). In order to explore the association between the status of MSI and OS comprehensively, the association between the MSI status and clinicopathological features in the eight studies were analyzed. Data were analyzed using Stata 12.0 software (StataCorp, College Station, TX, USA) with a random effects model. Inter-study heterogeneity was estimated using I2 statistics. Sensitivity was analyzed by removing certain studies with low quality. All the statistics were two-sided and P<0.05 was considered to indicate a statistically significant difference.
Results
Eligible studies
In total, 162 studies were searched using MeSH terms and key words from the Pubmed database until January 2014. A total of 24 review studies were removed and 12 were removed for not assessing clinical patients, while 13 did not analyze gastric cancer. The title and abstract of the remaining 113 studies were assessed; however, 80 studies were not regarding the topic between MSI and gastric cancer prognosis and four were not in English. Therefore, 29 full texts of potentially eligible studies were read independently by two investigators, and 15 studies were excluded for not providing the association between OS and MSI status (MSI-H and MSI-L/MSS), two were excluded for not reporting HR but reported the relative risk (RR) of MSI-H compared to MSI-L/MSS, while one study did not report HR but the odds ratio (OR). Two studies were excluded for offering the survival curves of MSI-H, MSI-L and MSS separately so that the HR of MSI-H could not be calculated compared to MSI-L/MSS and one study offered a rough Kaplan-Meier survival curve that a HR with an existing error was calculated from, so it was therefore removed. Finally, there were eight studies used in the meta-analysis; three of these were manually calculated (30). There was a good agreement between the two investigators (Fig. 1).
Figure 1.
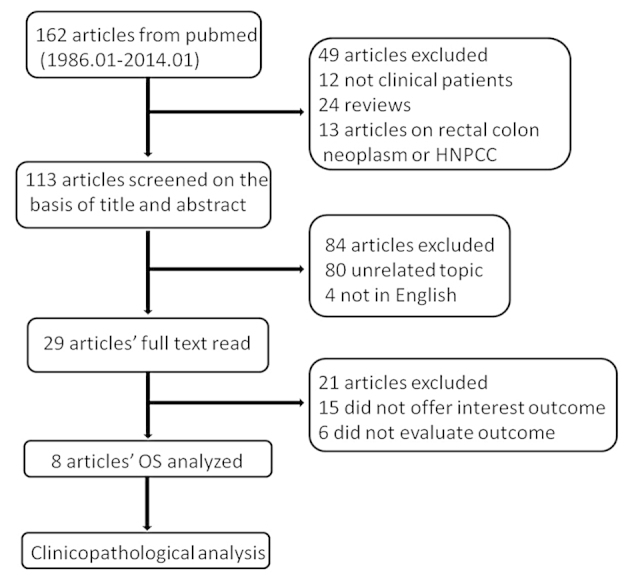
Search strategy process. HNPCC, hereditary nonpolyposis colorectal cancer; OS, overall survival.
Characteristics of the studies
The characteristics of the studies are summarized in the Table I. In total, 1,976 patients were analyzed, of which there were 431 MSI-H patients, range 11.68–33.82%. Two studies collected the data prospectively. The follow-up period ranged 1–260.9 months. The majority of the patients had adenocarcinoma and advanced gastric cancer. More than half the patients had intestinal type according to the Lauren classification. Almost all the patients received surgery (Table I).
Table I.
Characteristics of each study in the meta-analysis.
| Study (year) | No. of patients | No. of MSI-H, % | Period of follow-up, month | Stage distribution, n | No. of intestinal type | Treatment strategy | No. of MSI marker | MSI-H defination | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|
| Fang et al (2012) | 214 | 11.7 | 1–243 | Early, 44 Advanced, 170 | 134 | Surgery II/III;got adjuvant CT | 5 | ≥2 | (6) |
| Corso et al (2009) | 250 | 25.2 | 36–260.9 | Early, 183 Advanced, 67 | 163 | Surgery; no neoadjuvant CT | 5 | ≥2 | (18) |
| Chiaravalli et al (2001) | 185 | 19.5 | NA | All advanced | 108 | Surgery; no neoadjuvant CT | 3, all mono-neoadjuvant | ≥1 | (31) |
| Beghelli et al (2006) | 510 | 16.3 | Until July 2004 | Early, 64 Advanced, 446 | 286 | Surgery; no neoadjuvant CT | 2, all mono-nucleotide | ≥1 | (21) |
| Kim et al (2011) | 476 | 33.8 | 1–57 | Early, 166 Advanced, 310 | 289 | Surgery | 5 | ≥2 | (20) |
| Falchetti et al (2008) | 159 | 17.0 | 8.8–20.4 | Early, 15 Advanced, 144 | 77 | Surgery | 8 | ≥1 mono-nucleotide | (19) |
| Hayden et al (1997) | 101 | 20.8 | NA | Early, 15 Advanced, 66 | 75 | Surgery | 11 | ≥1 | (24) |
| An et al (2005) | 81 | 18.5 | 47–57.7 | Early, 14 Advanced, 67 | 53 | Surgery | 5 | ≥2 | (28) |
MSI-H, high-frequency microsatellite instability; CT, computed tomography; NA, not available.
MSI analysis
A variety of MSI markers were used in the assessment of the MSI status within the meta-analysis. Four studies analyzed the MSI-H status using ≥2 loci of five markers showing instability (6,9,18,20). Two studies used only mononucleotide markers (21,31); in which ≥1 loci showed instability, and was defined as MSI-H. The other two studies used 8 and 11 markers, respectively (19,24). Tumors with MSI-L and MSS showed similar clinicopathological characteristics, so therefore, these two types were classified together to compare to the tumors with MSI-H.
Survival analysis
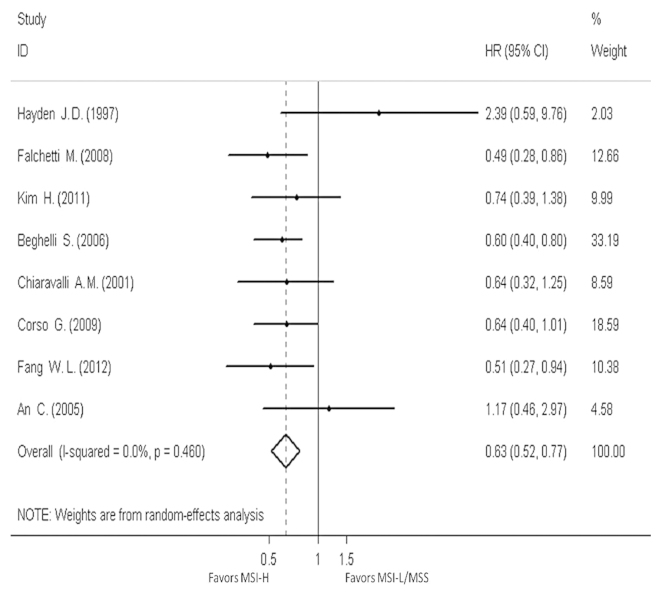
Eight studies provided the OS data for pooling. The HR and 95% CI for each study and the summarized HR are shown in Fig. 2. The estimate pooled was HR, 0.63 (95% CI, 0.52–0.77), with no evidence of heterogeneity (I2=0.0%). The funnel plot is shown in Fig. 3, and a little asymmetry can be observed. This result was verified by the quantified evaluation using Begg's test (P=0.063), therefore no evident publication bias was discovered in these studies. The studies by Hayden et al (24) and Beghelli et al (21) had clear differences in the definition of MSI-H, so therefore these were removed when evaluating sensitivity; the outcome changed respectively into HR, 0.62 (95% CI, 0.50–0.75) and HR, 0.66 (95% CI, 0.51–0.85), with no evidence of heterogeneity (I2=0.0 and 8.6%, respectively) and the funnel plot showed a little more symmetry than previously.
Figure 2.
Forest plots evaluating hazard ratio (HR) of the high-frequency microsatellite instability (MSI-H) compared to microsatellite stable (MSS), based on overall survival (OS). CI, confidence interval; MSI-L, low-frequency microsatellite instability.
Figure 3.
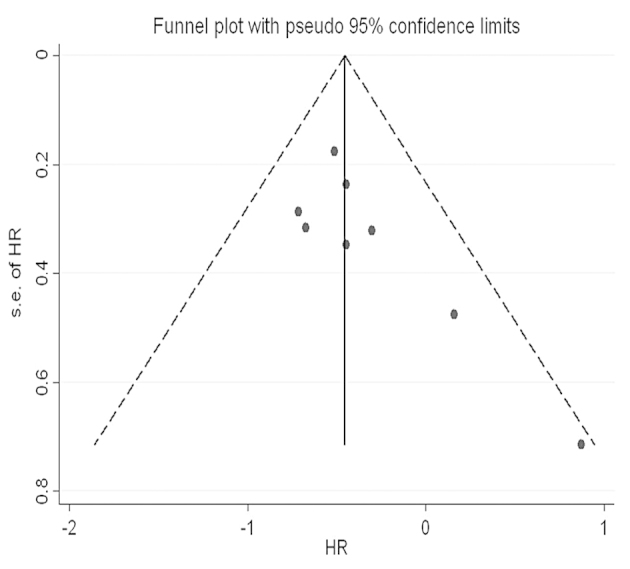
Funnel plot of eight studies. s.e., standard error; HR, hazard ratio.
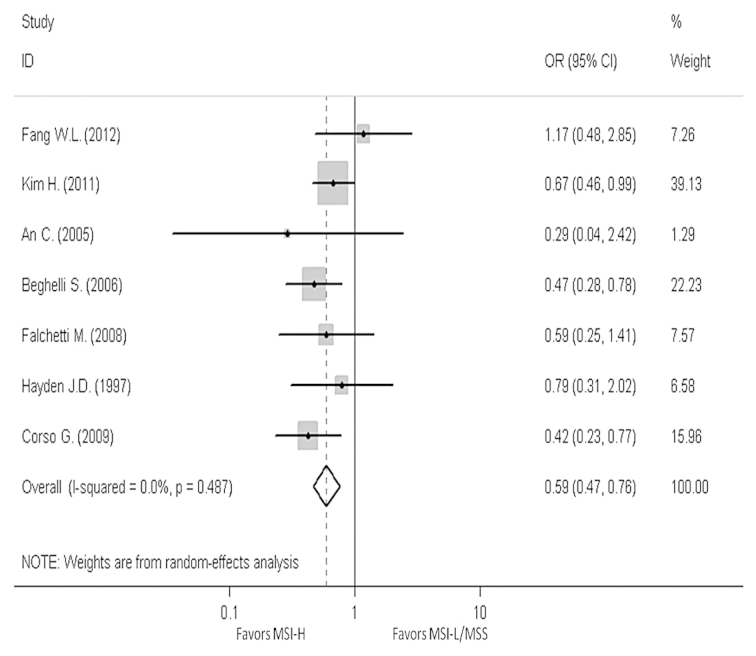
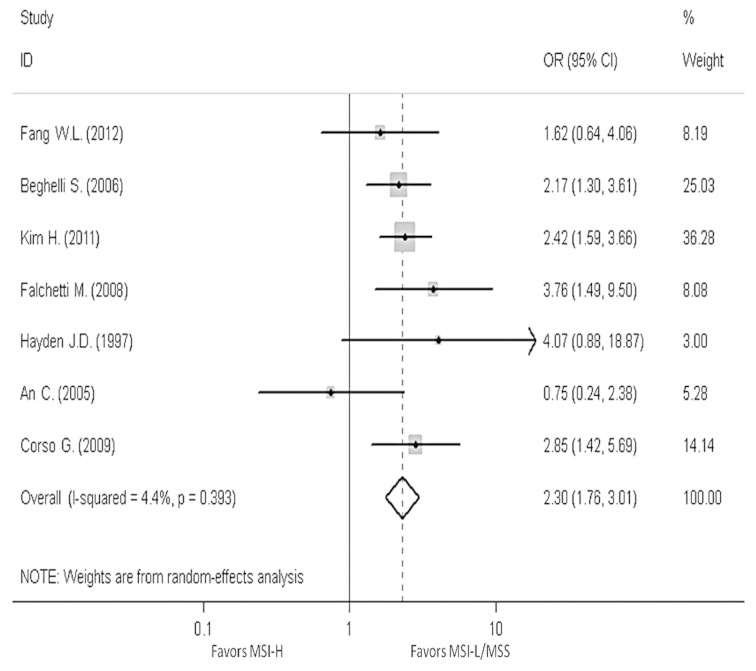
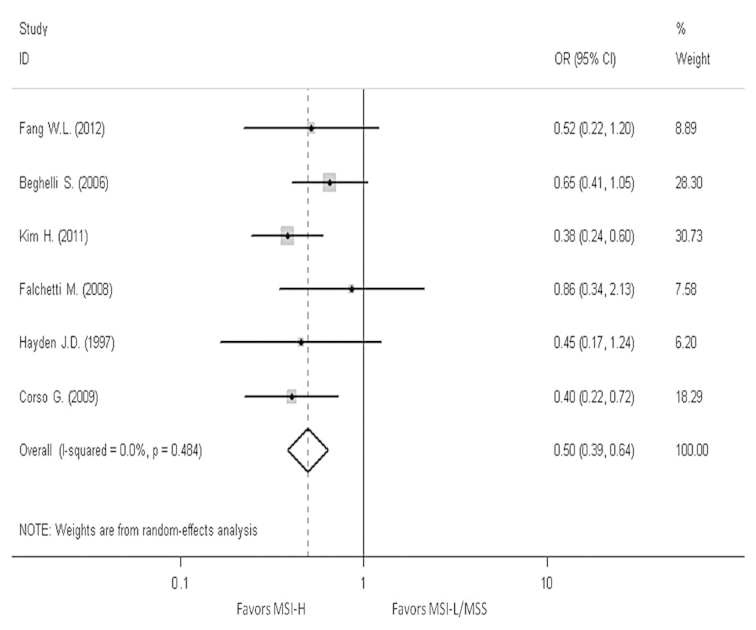
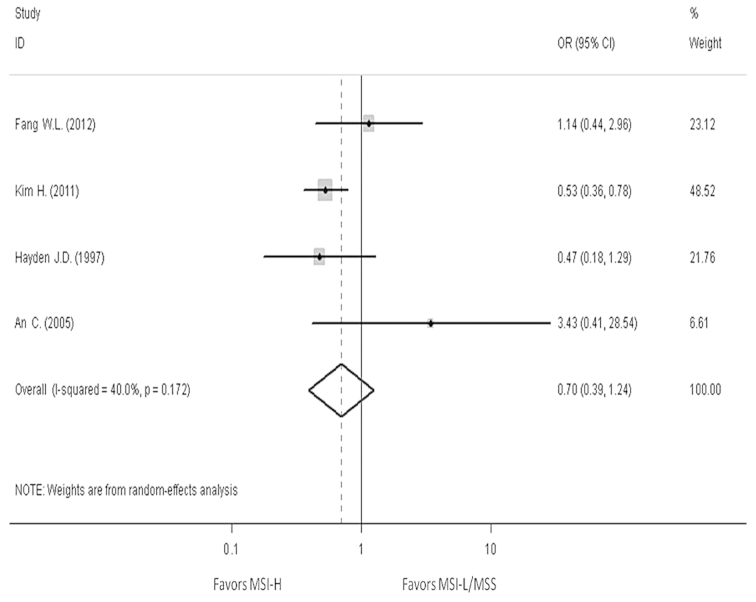
In order to reveal the association, the clinicopathological characters, such as LN metastasis, tumor invasion, tumor-node-metastasis (TNM) stage and Lauren classification, have been analyzed. The pooled OR in LN metastasis that was positive for MSI-H compared to MSI-L/MSS is 0.59 (95% CI, 0.47–0.76), the pooled OR in T3-T4 invasion is 0.5 (95% CI, 0.39–0.64) and the pooled OR in II–IV stage in TNM phase is 0.7 (95% CI, 0.39–1.24). The pooled OR in intestinal type is 2.3 (95% CI, 1.76–3.01). All the results have no evident heterogeneity (Figs. 4–7).
Figure 4.
Forest plots evaluating odds ratio (OR) of the high-frequency microsatellite instability (MSI-H) compared to microsatellite stable (MSS), based on lymph node metastasis. CI, confidence interval; MSI-L, low-frequency microsatellite instability.
Figure 7.
Forest plots evaluating odds ratio (OR) of the high-frequency microsatellite instability (MSI-H) compared to microsatellite stable (MSS), based on the Lauren classification. CI, confidence interval; MSI-L, low-frequency microsatellite instability.
Discussion
Mismatch repair deficiency is an important molecular mechanism in carcinogenesis and has a clear effect on prognosis. In CRC patients, cancers with MSI-H have an improved prognosis and a worse sensitiveness to 5-fluorouracil (5-FU)-based chemotherapy compared to MSI-L/MSS cancer (32). While in gastric cancer, the clinical significance remains controversial. All the studies regarding the effect of MSI status on prognosis in PubMed were collected and the information was obtained from 1,976 patients enrolled. The result showed that patients with MSI-H had a reduced 37% mortality risk compared to those with MSI-L/MSS, indicating that MSI-H patients have an improved OS compared to MSI-L/MSS patients, in accordance with CRC. In addition, the RR of another two studies excluded in the present meta-analysis were 0.47 (1.23–0.18) and 1.94 (MSI-L vs. MSI-H) (18,33), while one OR was 0.50 (0.27–0.93) (34); these studies also supported an improved OS outcome for MSI-H cancer.
Compared to the previous research, MSI-H patients have a long survival in gastric cancer. For investigating the association between MSI-H and a superior prognosis, the information was repeatedly analyzed and identified that one study (24) used only the English population; this study was carried out in 1997, which is much earlier than the other studies. Furthermore, the markers in this study are extremely different to the other studies (11 markers in which ≥1 was showing instability and can be classified as MSI+), therefore the study was excluded. When the study was excluded, the conclusion did not change and the funnel plot became a little more symmetrical than previously. The reduced publication bias was also confirmed by Begg's test (P=0.23). The sample in this study is small (101 patients) and the weight is 2.03, which leads to the meta-analysis having little sensitivity to the study. Subsequently, another study was removed that had a different MSI-H definition and the weight was a little heavier (21); the conclusion and the publication bias did not change. Therefore, MSI-H patients have an improved prognosis in gastric cancer. This conclusion can possibly indicate that MSI-H patients may have an improved prognosis compared to MSI-L. With this conclusion, the concrete reflection of the different prognoses is required. Subsequently, the clinicopathological features of the different patients were analyzed and it was identified that those with MSI-H appeared to have a smaller risk in LN metastasis and T invasion. These patients were also inclined to have the intestinal type. Less LN metastasis, shallower T invasion and intestinal type are known to predict a good prognosis, so this is possibly the reason why patients of MSI-H have a long OS. As a result, increasing attention should be paid to the MSI-L gastric cancer patient by administering a stronger treatment.
In contrast to the patients with MSI-L/MSS cancer, MSI-H patients in CRC had an improved prognosis, as well as a worse sensitivity to 5-FU-based chemotherapy. This phenomenon indicates that the MSI-H patients with gastric cancer may also have the same characteristic. Therefore, the research regarding the medicinal sensitiveness between MSI-H and MSI-L is extremely necessary, which may guide the physician to improve the choice of the chemotherapy treatment. However, there is little research regarding the medicinal sensitiveness of MSI-H patients in gastric cancer.
The present meta-analysis has certain limitations. First, the majority of studies are retrospective and cannot be controlled manually leading to certain selective bias. Therefore, the random effects model was used. Second, there is not a unified standard for the evaluation of observational study, so NOS was used for reference. In addition, the patients analyzed have a variety of different parameters, including pathology, stage, location, treatment strategy and MSI-H definition. Four studies used the NCI panel and three studies used ≥1 mononucleotide loci, while the remaining study used ≥1 loci that did not limit the type of loci; this study was removed to conduct a sensitivity analysis and the result did not show a significant difference. Eight studies were pooled, only two of which provided the association between the MSI status and OS information in the tumors of different stage. Therefore, no pooled conclusion regarding the MSI status and OS in stages I–IV was achieved.
The pooling of studies is not sufficient, so a large sample clinical trial focusing on prognosis and prediction is required, in which the independent outcome in different stages or different treatment strategies should be stated. A convenient and economical MSI detection method and evaluation standard of observational study should be unified.
Figure 5.
Forest plots evaluating odds ratio (OR) of the high-frequency microsatellite instability (MSI-H) compared to microsatellite stable (MSS), based on T invasion. CI, confidence interval; MSI-L, low-frequency microsatellite instability.
Figure 6.
Forest plots evaluating odds ratio (OR) of the high-frequency microsatellite instability (MSI-H) compared to microsatellite stable (MSS), based on TNM. CI, confidence interval; MSI-L, low-frequency microsatellite instability; TNM, tumor-node-metastasis.
Acknowledgements
This study was supported by National Science and Technology Major Project (grant no. 2013ZX09303002) and the Science and Technology Plan Project of Liaoning Province (grant nos. 2011404013-1 and 2012225001).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer - pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida K, Yasui W, Yokozaki H, et al. New molecular prognostic markers in gastric carcinoma. Gan To Kagaku Ryoho. 1998;25:2021–2027. (In Japanese) [PubMed] [Google Scholar]
- 4.Leung WK, Kim JJ, Kim JG, Graham DY, Sepulveda AR. Microsatellite instability in gastric intestinal metaplasia in patients with and without gastric cancer. Am J Pathol. 2000;156:537–543. doi: 10.1016/S0002-9440(10)64758-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arzimanoglou II, Gilbert F, Barber HR. Microsatellite instability in human solid tumors. Cancer. 1998;82:1808–1820. doi: 10.1002/(SICI)1097-0142(19980515)82:10<1808::AID-CNCR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Fang WL, Chang SC, Lan YT, et al. Microsatellite instability is associated with a better prognosis for gastric cancer patients after curative surgery. World J Surg. 2012;36:2131–2138. doi: 10.1007/s00268-012-1652-7. [DOI] [PubMed] [Google Scholar]
- 7.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 8.Shemirani AI, Haghighi MM, Zadeh SM, et al. Simplified MSI marker panel for diagnosis of colorectal cancer. Asian Pac J Cancer Prev. 2011;12:2101–2104. [PubMed] [Google Scholar]
- 9.Brennetot C, Buhard O, Jourdan F, Flejou JF, Duval A, Hamelin R. Mononucleotide repeats BAT-26 and BAT-25 accurately detect MSI-H tumors and predict tumor content: implications for population screening. Int J Cancer. 2005;113:446–450. doi: 10.1002/ijc.20586. [DOI] [PubMed] [Google Scholar]
- 10.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baudhuin LM, Burgart LJ, Leontovich O, Thibodeau SN. Use of microsatellite instability and immunohistochemistry testing for the identification of individuals at risk for Lynch syndrome. Fam Cancer. 2005;4:255–265. doi: 10.1007/s10689-004-1447-6. [DOI] [PubMed] [Google Scholar]
- 12.Aaltonen LA, Peltomaki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 13.Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46:2788–2798. doi: 10.1016/j.ejca.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Paulson TG, Wright FA, Parker BA, Russack V, Wahl GM. Microsatellite instability correlates with reduced survival and poor disease prognosis in breast cancer. Cancer Res. 1996;56:4021–4026. [PubMed] [Google Scholar]
- 15.Diaz-Padilla I, Romero N, Amir E, et al. Mismatch repair status and clinical outcome in endometrial cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2013;88:154–167. doi: 10.1016/j.critrevonc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Oki E, Kakeji Y, Zhao Y, et al. Chemosensitivity and survival in gastric cancer patients with microsatellite instability. Ann Surg Oncol. 2009;16:2510–2515. doi: 10.1245/s10434-009-0580-8. [DOI] [PubMed] [Google Scholar]
- 17.Seo HM, Chang YS, Joo SH, et al. Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J Surg Oncol. 2009;99:143–147. doi: 10.1002/jso.21220. [DOI] [PubMed] [Google Scholar]
- 18.Corso G, Pedrazzani C, Marrelli D, Pascale V, Pinto E, Roviello F. Correlation of microsatellite instability at multiple loci with long-term survival in advanced gastric carcinoma. Arch Surg. 2009;144:722–727. doi: 10.1001/archsurg.2009.42. [DOI] [PubMed] [Google Scholar]
- 19.Falchetti M, Saieva C, Lupi R, et al. Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Hum Pathol. 2008;39:925–932. doi: 10.1016/j.humpath.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, An JY, Noh SH, Shin SK, Lee YC, Kim H. High microsatellite instability predicts good prognosis in intestinal-type gastric cancers. J Gastroenterol Hepatol. 2011;26:585–592. doi: 10.1111/j.1440-1746.2010.06487.x. [DOI] [PubMed] [Google Scholar]
- 21.Beghelli S, de Manzoni G, Barbi S, et al. Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery. 2006;139:347–356. doi: 10.1016/j.surg.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 22.An JY, Kim H, Cheong JH, Hyung WJ, Kim H, Noh SH. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer. 2012;131:505–511. doi: 10.1002/ijc.26399. [DOI] [PubMed] [Google Scholar]
- 23.dos Santos NR, Seruca R, Constancia M, Seixas M, Sobrinho-Simoes M. Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology. 1996;110:38–44. doi: 10.1053/gast.1996.v110.pm8536886. [DOI] [PubMed] [Google Scholar]
- 24.Hayden JD, Cawkwell L, Quirke P, et al. Prognostic significance of microsatellite instability in patients with gastric carcinoma. Eur J Cancer. 1997;33:2342–2346. doi: 10.1016/S0959-8049(97)00343-2. [DOI] [PubMed] [Google Scholar]
- 25.Chiaravalli AM, Feltri M, Bertolini V, et al. Intratumour T cells, their activation status and survival in gastric carcinomas characterised for microsatellite instability and Epstein-Barr virus infection. Virchows Arch. 2006;448:344–353. doi: 10.1007/s00428-005-0066-4. [DOI] [PubMed] [Google Scholar]
- 26.Perez RO, Jacob CE, D'Ottaviano FL, et al. Microsatellite instability in solitary and sporadic gastric cancer. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:279–285. doi: 10.1590/S0041-87812004000500010. [DOI] [PubMed] [Google Scholar]
- 27.Wirtz HC, Muller W, Noguchi T, et al. Prognostic value and clinicopathological profile of microsatellite instability in gastric cancer. Clin Cancer Res. 1998;4:1749–1754. [PubMed] [Google Scholar]
- 28.An C, Choi IS, Yao JC, et al. Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin Cancer Res. 2005;11:656–663. [PubMed] [Google Scholar]
- 29.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 30.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Chiaravalli AM, Cornaggia M, Furlan D, et al. The role of histological investigation in prognostic evaluation of advanced gastric cancer. Analysis of histological structure and molecular changes compared with invasive pattern and stage. Virchows Arch. 2001;439:158–169. doi: 10.1007/s004280100441. [DOI] [PubMed] [Google Scholar]
- 32.Carethers JM, Smith EJ, Behling CA, et al. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology. 2004;126:394–401. doi: 10.1053/j.gastro.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol. 2003;16:641–651. doi: 10.1097/01.MP.0000076980.73826.C0. [DOI] [PubMed] [Google Scholar]
- 34.Fang WL, Chang SC, Lan YT, et al. Molecular and survival differences between familial and sporadic gastric cancers. Biomed Res Int. 2013;2013:396272. doi: 10.1155/2013/396272. [DOI] [PMC free article] [PubMed] [Google Scholar]