Abstract
There is controversy regarding the impact of infection on long-term prognosis in osteosarcoma patients. Clinical trials and experiments relating to this field could bring reconsideration of immunotherapy for osteosarcoma. The clinical records were reviewed of 125 osteosarcoma patients with a mean follow-up of 5.1±3.9 years (range, 0.5–19.8 years), and a review of the literature was also carried out. Chronic localized infections (but not systemic infection) were determined in 6 patients (4.8%). Similar chemotherapeutic regimens (P=1.00) and histological reactions (P=0.65) were observed in patients with or without infection. Tumor location of proximal tibia (P=0.04) was more common in infected patients. More amputations (P<0.001) were necessitated in infected patients due to uncontrolled infection. The 5-year overall survival rate and event-free survival rate in infected patients were 100%, which were significantly higher than that of the non-infected patients, of whom the rates were 54 and 43% respectively (log-rank test: total survival, P=0.01; tumor-free survival, P=0.01). Distant metastasis was an independent risk factor for survival determined by Cox regression analysis (P<0.001, 95 confidence interval, 1.59–3.98). These findings suggested infection was likely to have positive effects on survival in osteosarcoma patients, however, underlying mechanisms remain to be elucidated. Reconsideration of the association of infection and survival in osteosarcoma patients will help to explore novel therapeutic routes and targets in these patients.
Keywords: oncology, osteosarcoma, surgery
Introduction
Osteosarcoma is the most frequent primary osteogenic tumor with a prevalence of 1–3 cases per million people (1). Resection of tumor (with negative surgical margin) and systemic administration of chemotherapy agents have significantly improved the long-term prognosis in osteosarcoma patients, with 5-year survival rates being 60–70% (2). Osteosarcoma patients are able to receive limb-salvage surgeries with improved postoperative function.
However, complications, including infection, local recurrence, wound dehiscence, pathological fracture and prosthetic loosening, were frequently reported in osteosarcoma patients undergoing limb-salvage surgeries (3,4). Of note, infection is deemed to be one of the most significant complications with an incidence of 5.3–13% (2,3,5,6). Despite this, severities of postoperative infections vary from mild to severe extents, and deep infection was an important reason for readmission, revision surgery and even amputation (7,8). Notably, the association of postoperative infection and improved survival in osteosarcoma patients has been observed in several preceding studies, reporting that infection could be associated with prolonged survival in canines and humans with osteosarcoma, however, the outcomes were inconsistent (2,9,10). Jeys et al (9) revealed that the 10-year survival for infected osteosarcoma patients was 84.5% compared to 62.3% in non-infected patients, and no difference was detected between the two groups in terms of histological responses to chemotherapy. However, Lee et al (2) noted no survival difference between the infected patients and non-infected group following matching for prognostic factors, which suggested that the reported positive effect on survival rate could be due to other clinical characteristics of infected patients. However, no studies have carried out further clinical observations nor laboratory experiments relating to the infection-survival association in osteosarcoma, although this association has been reported in a wide variety of cancer, showing that certain types of malignancies are possibly sensitive to immune effects associating with infection, while others are not (11–13). Sensitivity of osteosarcoma to infection could be associated with the potential efficacy of immunotherapy as a treatment for this disease, since infection involves a cascade of cellular events and inflammatory transducers, the elucidation of the association of the infection and survival in osteosarcoma patients will possibly bring reconsideration and increasing attention to immunotherapy for osteosarcoma. The aim of the present study was to determine whether inflammation has positive or negative impacts on survival in osteosarcoma patients and explore what could be obtained from this association.
Patients and methods
Inclusion and exclusion criteria
Diagnosis of Enneking IIB osteosarcoma according to the Enneking staging system (14) is determined in every patient in the present cohort between 1991 and 2012 in the Department of Orthopaedic Surgery (General Hospital of Jinan Military Region, Jinan, China). All the patients underwent limb-salvage surgeries along with neo-adjuvant chemotherapy. The mean follow-up period was 5.1 years (range 0.5–19.8 years), the minimum follow-up period was 5 years unless the patient succumbed or was lost to the cohort. Patients with the following characteristics were excluded: Infection that developed >1 year postoperatively, recurrence/metastasis and mortality that developed before infection or within 1 year postoperatively, previous surgery on the tumor location, patients who did not receive chemotherapy treatment and patients lost to follow-up. Finally, 125 patients were enrolled in the cohort (Table I).
Table I.
Comparison of the clinical patient data with and without infection.
| Characteristics | Infected | Non-infected | P-value |
|---|---|---|---|
| Age, years | 21±11 | 19±8 | 0.52 |
| Gender, n (%) | |||
| Male | 3 (50.0) | 77 (64.7) | 0.75 |
| Female | 3 (50.0) | 42 (35.3) | |
| Tumor site, n (%) | |||
| Femur | 1 (16.7) | 62 (52.6) | 0.04 |
| Tibia | 4 (66.7) | 37 (31.0) | |
| Fibula | 1 (16.6) | 11 (8.6) | |
| Other | – | 10 (7.8) | |
| Chemotherapy regimen, n (%) | |||
| DIA | 5 (83.3) | 83 (70.7) | 1.00 |
| MMIA | 1 (16.7) | 27 (22.4) | |
| Other | – | 9 (6.9) | |
| Types of surgery, n (%) | |||
| Prosthesis | 3 (50.0) | 46 (38.3) | 0.51 |
| Biological reconstruction | 3 (50.0) | 73 (61.7) | |
| Response to chemotherapy, n (%) | |||
| Good | 4 (66.7) | 70 (58.6) | 0.65 |
| Poor | 2 (33.3) | 49 (41.4) | |
| Prosthetic loosening/fracture | 1 (16.7) | 8 (6.7) | 1.00 |
| Amputation | 3 (50.0) | 2 (1.7) | 0.00 |
| Recurrence | 1 (16.7) | 28 (23.5) | 1.00 |
| Metastasis | – | 59 (49.6) | 0.03 |
| Mortality | – | 65 (54.6) | 0.00 |
| Total | 6 (4.8) | 119 (95.2) | |
DIA, cisplatin-doxorubicin-ifosfamide chemotherapy regimen; MMIA, methotrexate-doxorubicin-ifosfamide chemotherapy regimen.
Method
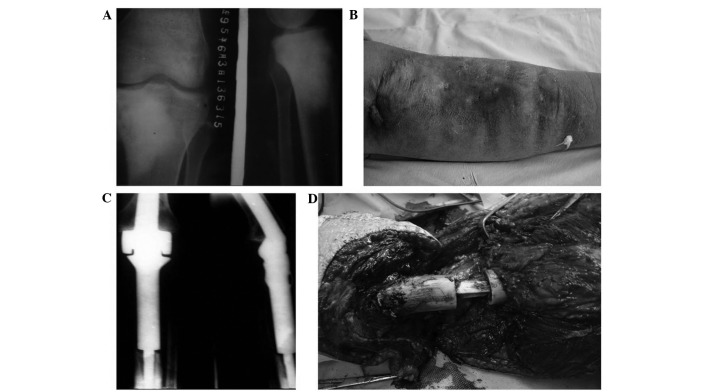
Deep infection was confirmed if the patients had clinical evidence of infection (including fever, pain, abscess, elevated white blood cell counts and elevated C-creative protein) with microbial culture within the wound location or histology compatible with infection at surgery. According to this standard, postoperative deep infection was determined in 6 patients (Fig. 1), treatment of infection included debridement and drainage (6 patients, 100%), revision surgery (1 patient, 16.7%) and amputation (4 patients, 66.7%). There was no evidence of systemic infection, and all the infections were localized. Perioperative infection (1 patient, 16.7%) was determined if it occurred within 2 months postoperatively, infections occurring >2 months postoperatively were late infections (5 patients, 83.3%). Infections of >3 months were chronic infections (6 patients, 100%).
Figure 1.
A case of osteosarcoma with postoperative deep infection. A 22-year old male patient presented with swelling and pain in the left calf for 2 months, and diagnosis was osteosarcoma in the left proximal tibia by histological findings. Resection of the tumor with prosthesis was carried out. (A) X-ray indicated osteosarcoma in the left proximal tibia. (B) Two months after surgery the patient had redness, swelling of incision with fistula and purulent exudate. Debridement was carried out, however, 6 months later the was admitted again for recurrent infection. (C) Postoperative X-ray showed prosthetic loosening and bone destruction due to infection. (D) The patient underwent debridement and revision surgery, however, infection and fistula reoccurred, and amputation was applied. The patient survived until the end of follow-up (189 months fter the limb salvage surgery).
A total of 97 patients (77.6%) underwent cisplatin-doxorubicin-isofamide therapy, the remaining patients received high-dose methotrexate-doxorubicin-isofamide chemotherapy regimen (Table II). Histological responses were graded according to percentage of tumor necrosis, where good response consists of grade III and IV (necrosis of ≥90%) and grades I and II (necrosis of <90%) indicated poor responses (15). Resections of tumors were performed according to established principles of surgical margins for osteosarcoma, reconstructive methods included tumor endoprostheses, allograft-prosthetic composites and biological reconstruction. A negative tumor margin was also determined by histological findings (16). Prophylactic antibiotics (penicillin or cephalosporin) were administered within 30 min before skin incision and were discontinued within 24 h of the end of surgery recommendations by the American Academy of Othopaedic Surgeons (17). Therapeutic regimens for postoperative infections covered a variety of intravenous antibiotics, including penicillin, cephalosporin, quinolones, aminoglycosides and clindamycin. Two-agent antibiotic regimens were administered in 5 patients (83.3%), while the remaining patients received single-agent antibiotic therapy (16.7%).
Table II.
Chemotherapeutic regimen for osteosarcoma patients.
| Type of chemotherapya | Order of agents in one episode of chemotherapy | Dose and duration of agents | Total number of episodes |
|---|---|---|---|
| DDP-ADM-IFO | DDP was administered firstly, after an interval of 1 week, ADM + IFO were used | 2 preoperative + 6 postoperative | |
| DDP | 120 mg/m2, 1/day × 1, 4–6 h/time | ||
| ADM | 30 mg/m2, 1/day × 3 | ||
| IFO | 2.0 g/m2, 1/day × 5 | ||
| MTX-ADM-IFO | MTX was administered weekly for the first 2 weeks, after an interval of 1 week, ADM + IFO were used | 2 preoperative + 6 postoperative | |
| MTX | 8–12 g/m2 × 12, 4–6 h/time, at interval of 6 h | ||
| ADM | 30 mg/m2, 1/day × 3 | ||
| IFO | 2.0 g/m2, 1/day × 5 |
All agents were administered intravenously. DDP, cisplatin; ADM, doxorubicin; IFO, ifosfamide; MTX, methotrexate.
Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). χ2 test, Fisher's test and student's t-test were carried out in univariate analysis. P<0.05 was considered to indicate a statistically significant difference. End points in Kaplan-Meier analysis were recurrence/metastasis and mortality. Cox regression model was applied for multivariate analysis.
Results
A total of 6 patients had postoperative deep localized infections (4.8%, Table III). Mean postoperative time of infection was 6.8±4.0 months (1–12 months). One patient (16.7%) had perioperative infection and 5 patients (83.3%) had late infections. Bacterial culture indicated Staphylococcus aureus (4 patients, 66.7%) and Staphylococcus epidermidis (2 patients, 33.3%). Clinical characteristics of infected and non-infected patients are compared in Table I. The patients had no statistical significance in chemotherapy regimens (P=0.01) and histological response (P=0.65), infected patients were exposed to lower risks for metastasis (P=0.03) and mortality (P<0.001). There was no association of amputation and tumor recurrence (P=0.33), metastasis (P=0.06) and mortality (P=0.67). The 5-year survival rate and event-free survival rate of infected patients were 100%; 5-year survival rate of non-infected patients was 54% and event-free survival rate was 43% (Figs 2 and 3). The log-rank test indicated that the total survival rate (P=0.01) and event-free survival (P=0.01) rate of infected patients were significantly higher than those without infection.
Table III.
Clinical characteristics and prognosis of infected patients.
| No. | Gender | Age at surgery, years | Tumor site | Types of surgery | Management of infection | Prognosis | Time of infection | Event-free survival, months | Survival, months |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 33 | Distal femur | Prosthesis | Debridement | Survival | Late | 115 | 115 |
| 2 | Female | 18 | Proximal tibia | Inactivation and re-implantation | Amputation | Survival | Late | 171 | 171 |
| 3 | Female | 17 | Proximal tibia | Inactivation and re-implantation | Debridement | Survival | Perioperative | 62 | 62 |
| 4 | Female | 10 | Proximal tibia | Inactivation and re-implantation | Amputation | Survival | Late | 22 | 22 |
| 5 | Male | 16 | Proximal tibia | Inactivation and re-implantation | Amputation | Survival | Late | 189 | 189 |
| 6 | Male | 22 | Proximal tibia | Resection of tumor | Amputation | Survival | Late | 24 | 237 |
Figure 3.
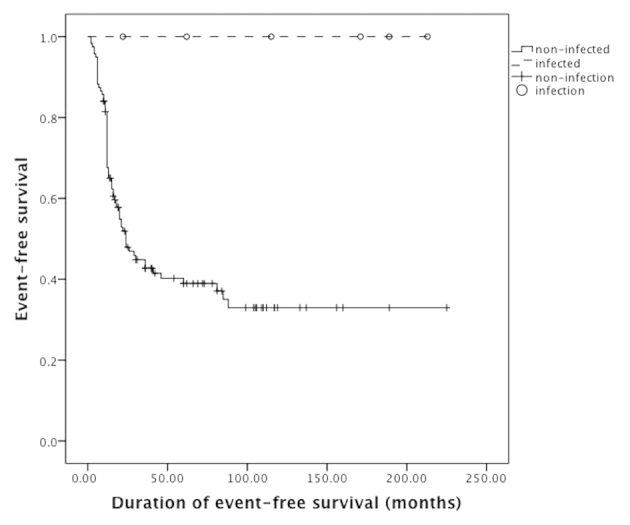
Kaplan-Meier analysis suggested that the event-free survival rate of infected patients was higher compared to the non-infected patients (P=0.01).
A variety of factors indicated by single-variate analysis and clinical observation were included in COX regression analysis. These factors comprised amputation, postoperative infection, chemotherapeutic regimen, response to chemotherapy, tumor recurrence and metastasis. Results showed that tumor metastasis was an independent risk factor for survival (P<0.001, 95 % confidence interval, 1.59–3.98). Patients with evidence of metastasis were exposed to a risk of mortality that was 15.6 times as high as those without metastasis.
Discussion
In 1891, William B. Coley injected streptococcal organisms into a patient with unresectable sarcoma and sequential infection in this patient resulted in shrinking of the tumor. Since then, Coley used a series of heat-inactivated streptococci and Bacillus prodigiosus (known as Coley's toxins) in the treatment for cancer (18). However, Coley's toxins did not significantly improve long-term survival in patients with cancer, and development of chemotherapy and radiation therapy caused Coley's toxins to gradually disappear from use (19). In recent decades, immunotherapy has drawn increasing attention as an adjuvant therapy for human malignancies, including osteosarcoma, showing that Coley's principles of treatment for cancer is correct and that certain malignant tumors are sensitive to an enhanced immune system while others are not. However, it remains unclear whether infection is associated with improved survival in osteosarcoma patients and what role the immune system could play in this process. These results have shown that patients with postoperative deep infections had improved survival compared to the uninfected patients, despite that the majority of the postoperative infections resulted in amputation and/or postponed chemotherapy.
The importance of the present clinical observation consists in that osteosarcoma is possibly sensitive to an enhanced immune system associated with infection, indicating that immunotherapy could be a valuable therapeutic route for osteosarcoma. However, the underlying mechanisms remain unclear. Preceding studies showed that infection possibly plays antitumor roles by enhancing cellular immune system, as elevated levels of tumor necrosis factor-α concomitant with infection stimulated the innate immune system in patients, resulting in enhanced antitumor effects (18). Buddingh et al (20) reported that chemoresistant osteosarcoma cells are susceptible to lysis of interleukin-15-induced natural killing (NK) cells, and the results indicated the potential antitumor effects of NK cells or NK cell-activating agents in high-grade osteosarcoma patients. In addition to these findings, infection may play antitumor roles through other routes; in vivo studies have shown that infection is associated with angio-suppressive effects in tumors, yet further explorations remain to be carried out to elucidate this connection (21).
When the advances in immunotherapy of osteosarcoma are reviewed, interferon (IFN)-α has obtained considerable attention as an adjuvant treatment of osteosarcoma. Evidence from fundamental research has indicated that IFN played direct antitumor and/or indirect immune roles, particularly in osteosarcoma. IFN signaling was found to be intact in the periphery blood of osteosarcoma patients, while in other malignancies (such as melanoma), the signaling is impaired suggesting that osteosarcoma patients could be sensitive to treatment of IFN (22). A cooperative clinical trial being conducted by European and American Osteosarcoma Study Group-1 is currently the largest prospective study associated with immunotherapy of osteosarcoma patients. Patients with good responses to chemotherapy (tumor necrosis rate ≥90% by histological findings at surgery) underwent maintenance treatment of IFN-α as an adjuvant treatment to chemotherapy (methotrexate, cisplatin and doxorubicin). However, no evidence has shown that IFN-α had significant roles in improving the survival rate of osteosarcoma patients (22,23). The discrepancy between clinical trials and laboratory findings may be associated with various confounding factors. Additionally, an enhanced immune system is associated with a cascade of immune cells and cytokines, which could be more complicated than the effects induced by one type of inflammatory cytokine. Therefore, further research with a wider spectrum of immune cells and cytokines is required.
In recent years, antitumor effects relating to tumor associated macrophages (TAM) and neutrophils (TAN) are gaining increasing attention. Preceding studies suggested that TAM and TAN are integrated in the regulation of innate and adaptive immune responses. Strong evidence showed protumoral macrophages could be stimulated by IFN and become antitumoral cells attracting T helper 1 lymphocytes to the microenvironment of cancer (24,25). Of note, neutrophils have long been considered to be terminal effector cells playing a major role in inflammation and resistance against microbes. Various studies have shown that neutrophils could play an important role in tumor growth and progression. However, in the analogy with macrophages, neutrophils have double-edged roles in tumor progression. Neutrophils could be driven by transforming growth factor-β to acquire a protumoral phenotype, and by contrast, it could play antitumoral roles through cytotoxic and anti-angiogenic effects (26,27). Based on the outcomes of the present study, osteosarcoma could be sensitive to an immune system enhanced by infection. In addition, macrophages and neutrophils are important effector cells in infection, and studies in this area could offer valuable information in the treatment of osteosarcoma. However, current studies associated with TAM and TAN in osteosarcoma are limited.
Another important aspect associated with postoperative infection is local high temperature within the wound. Over the past decades, therapy applying thermal effects has been widely utilized as an adjuvant treatment route of malignant tumor, and the thermoablative technique is a typical example in this field. In clinical practice, thermal ablation comprises of radiofrequency ablation, microwave ablation, high-intensity focused ultrasound and laser-induced thermotherapy (28). The strengths of thermal ablation are not only its advantages as a surgical technique, but also the immunomodulation by thermotherapy in cancers. Fundamental studies and clinical observations have shown that thermal ablation plays important roles in modulating immune system in cancer patients. In addition, different thermoablative techniques have shown immunostimulating effects with similar immune cells and transducers profiles, however, studies associated with the immune effects aroused by thermal therapy for osteosarcoma patients are extremely scarce (28). In clinical practice, microwave ablation is currently used in the treatment of osteosarcoma, and in certain cases, inactivated bone tumor remained in situ instead of being entirely resected. As a result, postoperative exudation, redness and swelling of incisions were frequently observed. Microbiological culture excluded localized infection in all the patients, and the prognosis of these patients is favorable thus far. We believe the patients who underwent thermotherapy in the Department of Orthopaedic Surgery experienced a situation imitating postoperative wound infection (with similar clinical presentations). Thermoablative techniques and inactivated tumor tissues in vivo could possibly cause a wide variety of immune response, and this will have impacts on the long-term survival of osteosarcoma patients, however, further studies are required to explore the immune effects by thermotherapy in osteosarcoma and to determine its efficacy in the treatment of osteosarcoma patients. Currently the related studies are scarce.
Although the present study is inherent to several limitations of observational research, including that this is a single-institute retrospective study with a relatively small sample size (particularly the infected patients), the outcome has shown that postoperative infection was likely to improve the survival rate of osteosarcoma patients. However, the association between infection and survival rate of osteosarcoma patients remains to be elucidated. A wide spectrum of immune cells and transducers has shown potential in the treatment of osteosarcoma. Immune effects induced by thermal therapy also showed a possibility of exploring new treatment modalities. However, studies associated with these fields are limited and therefore, further studies are required to elucidate the association of the immune system and survival rate in osteosarcoma patients to improve the prognosis of these patients.
Figure 2.
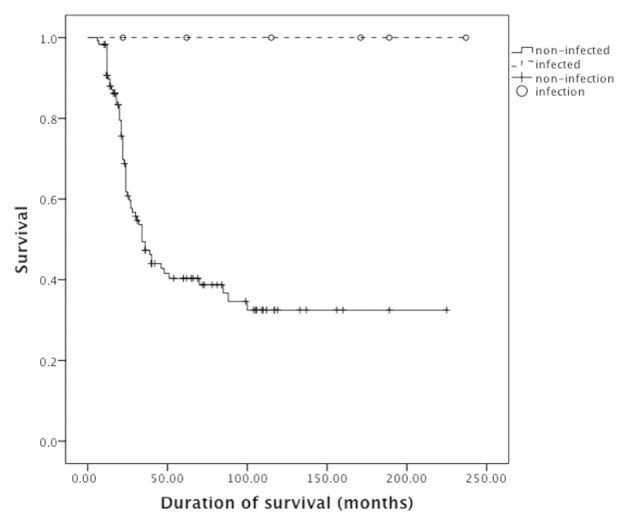
Kaplan-Meier analysis indicates that the survival rate in infected patients was higher compared to the non-infected controls (P=0.01).
Glossary
Abbreviations
- DIA
cisplatin-doxorubicin-ifosfamide
- MMIA
methotrexate-doxorubicin-ifosfamide
- IFN
interferon
- IL
interleukin
- TNF
tumour necrosis factor
- TGF
transforming growth factor
- NK
natural killing
References
- 1.Agarwal M, Anchan C, Shah M, Puri A, Pai S. Limb salvage surgery for osteosarcoma: Effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82–91. doi: 10.1097/BLO.0b013e31805d85c4. [DOI] [PubMed] [Google Scholar]
- 2.Lee JA, Kim MS, Kim DH, Lim JS, Park KD, Cho WH, Song WS, Lee SY, Jeon DG. Postoperative infection and survival in osteosarcoma patients. Ann Surg Oncol. 2009;16:147–151. doi: 10.1245/s10434-008-0184-8. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Moretti VM, Ashana AO, Lackman RD. Perioperative infection rate in patients with osteosarcomas treated with resection and prosthetic reconstruction. Clin Orthop Relat Res. 2011;469:2889–2894. doi: 10.1007/s11999-011-1877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin DS, Weber KL, Chao EY, An KN, Sim FH. Reoperation for failed prosthetic replacement used for limb salvage. Clin Orthop Relat Res. 1999;358:53–63. doi: 10.1097/00003086-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick MH, Owen WC, Seibel NL, Reaman GH. Lack of association between neutropenia and the incidence of bacteremia associated with indwelling central venous catheters in febrile pediatric cancer patients. Pediatr Infect Dis J. 1991;10:506–510. doi: 10.1097/00006454-199107000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Hingorani P, Seidel K, Krailo M, Mascarenhas L, Meyers P, Marina N, Conrad EU, Hawkins DS. Body mass index (BMI) at diagnosis is associated with surgical wound complications in patients with localized osteosarcoma: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2011;57:939–942. doi: 10.1002/pbc.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirganowicz PZ, Eckardt JJ, Dorey FJ, Eilber FR, Kabo JM. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clin Orthop Relat Res. 1999;358:64–74. doi: 10.1097/00003086-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Pan KL, Chan WH, Ong GB, Premsenthil S, Zulkarnaen M, Norlida D, Abidin Z. Limb salvage in osteosarcoma using autoclaved tumor-bearing bone. World J Surg Oncol. 2012;10:105. doi: 10.1186/1477-7819-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeys LM, Grimer RJ, Carter SR, Tillman RM, Abudu A. Post operative infection and increased survival in osteosarcoma patients: Are they associated? Ann Surg Oncol. 2007;14:2887–2895. doi: 10.1245/s10434-007-9483-8. [DOI] [PubMed] [Google Scholar]
- 10.Lascelles BD, Dernell WS, Correa MT, Lafferty M, Devitt CM, Kuntz CA, Straw RC, Withrow SJ. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol. 2005;12:1073–1083. doi: 10.1245/ASO.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 13.Sottnik JL, U'Ren LW, Thamm DH, Withrow SJ, Dow SW. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother. 2010;59:367–378. doi: 10.1007/s00262-009-0755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noor S, Thornormoethsson HS, Zervas CT, et al. Limb versus life-the outcomes of osteosarcoma in Cambodia. Int Orthop. 2014;38:579–585. doi: 10.1007/s00264-013-2173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando K, Heymann MF, Stresing V, Mori K, Rédini F, Heymann D. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers (Basel) 2013;5:591–616. doi: 10.3390/cancers5020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon DG, Song WS, Kong CB, Cho WH, Cho SH, Lee JD, Lee SY. Role of surgical margin on local recurrence in high risk extremity osteosarcoma: A case-controlled study. Clin Orthop Surg. 2013;5:216–224. doi: 10.4055/cios.2013.5.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prokuski L. Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg. 2008;16:283–293. doi: 10.5435/00124635-200805000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: A historical perspective. Pharmacol Ther. 1994;64:529–564. doi: 10.1016/0163-7258(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 19.Werneke U. A guide to using complementary alternative medicines in cancer. Nurs Times. 2005;101:32–35. [PubMed] [Google Scholar]
- 20.Buddingh EP, Schilham MW, Ruslan SE, Berghuis D, Szuhai K, Suurmond J, Taminiau AH, Gelderblom H, Egeler RM, Serra M, et al. Chemotherapy-resistant osteosarcoma is highly susceptible to IL-15-activated allogeneic and autologous NK cells. Cancer Immunol Immunother. 2011;60:575–586. doi: 10.1007/s00262-010-0965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas-Tikhonenko A, Hunter CA. Infection and cancer: The common vein. Cytokine Growth Factor Rev. 2003;14:67–77. doi: 10.1016/S1359-6101(02)00071-0. [DOI] [PubMed] [Google Scholar]
- 22.Buddingh EP, Ruslan SE, Berghuis D, Gelderblom H, Anninga JK, Hogendoorn PC, Egeler RM, Schilham MW, Lankester AC. Intact interferon signaling in peripheral blood leukocytes of high-grade osteosarcoma patients. Cancer Immunol Immunother. 2012;61:941–947. doi: 10.1007/s00262-012-1232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: Results from pre-randomisation treatment. Ann Oncol. 2015;26:407–414. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, Mazzoleni S, Moi D, Venneri MA, Indraccolo S, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008;14:299–311. doi: 10.1016/j.ccr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Matsushima H, Geng S, Lu R, Okamoto T, Yao Y, Mayuzumi N, Kotol PF, Chojnacki BJ, Miyazaki T, Gallo RL, et al. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood. 2013;121:1677–1689. doi: 10.1182/blood-2012-07-445189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng S, Matsushima H, Okamoto T, Yao Y, Lu R, Page K, Blumenthal RM, Ward NL, Miyazaki T, Takashima A. Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood. 2013;121:1690–1700. doi: 10.1182/blood-2012-07-445197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C. More than just tumor destruction: Immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011;2011:160250. doi: 10.1155/2011/160250. [DOI] [PMC free article] [PubMed] [Google Scholar]