Abstract
The high relapse rate of prostate cancer following radical prostatectomy is clinically problematic, and various neoadjuvant therapies aimed at reducing the rate have been examined. A previous study has shown that immune responses are increased in patients treated by adenoviral vector-mediated herpes simplex virus-thymidine kinase (HSV-tk) gene delivery followed by ganciclovir (GCV) injection. However, details of the immune responses following this form of gene therapy remain unclear. Five patients who agreed to participate in the present phase I/II trial were repeatedly administered GCV intravenously for 2 weeks following intraprostatic injection of HSV-tk. Peripheral blood samples were periodically collected following the treatments, and lymphocyte subsets were analyzed by flow-cytometry. Intracellular interferon (IFN)-γ produced by T cells was further measured in response to prostatic acid phosphatase and NY-ESO-1 overlapping peptides. Central memory (CM) cluster of differentiation 8+ (CD8+) T cells were found to increase markedly during the second round of treatment. In three patients, tumor antigen-specific T cells were clearly increased following HSV-tk + GCV treatment. An increase in prostate cancer antigen-specific T cells and CM CD8+ T cells may contribute to a reduction of relapse rates in prostate cancer patients receiving this form of gene therapy, which shows promise in a neoadjuvant setting.
Keywords: prostate cancer, gene therapy, memory T cells, antigen-specific T cells, neoadjuvant therapy
Introduction
Screening of asymptomatic men based on the serum level of prostate-specific antigen (PSA) coupled with the use of ultrasound-guided random systematic prostate biopsy has significantly improved the diagnostic accuracy and treatment of prostate cancer (1). The incidence of prostate cancer and its associated mortality rates have also increased significantly in numerous countries, including Japan (2). Despite significant progress in the detection and treatment of prostate cancer, high relapse rates (20–57%) following radical prostatectomy have been problematic, and various neoadjuvant therapies have been examined with the aim of reducing the rate of relapse (3–5). However, numerous patients have locally advanced disease at diagnosis, and therefore novel therapeutic options are required.
Treatment that involves a combination of surgery with adjuvant therapy may conceivably improve the outcome of patients who have a high risk of recurrence. Neoadjuvant therapy prior to surgery has several advantages over adjuvant therapy, as it targets presumed micro-metastases at an early stage and may reduce locally advanced tumors, thus allowing more effective surgical resection.
Gene therapy may offer a chance of surmounting the drawbacks of current therapies by inducing cytotoxicity in localized prostate cancer without serious morbidity or mortality, and stimulating immune-mediated therapeutic activities that affect not only the primary tumor but also metastases. Several protocols of gene therapy for prostate cancer have been examined in preclinical studies and clinical trials. Intraprostatic adenoviral vector transduction of the herpes simplex virus-thymidine kinase (HSV-tk) gene followed by intravenous ganciclovir (GCV) administration is a cytotoxic gene therapy approach for prostate cancer that has been tested extensively in preclinical studies and phase I/II clinical trials at Baylor College of Medicine (Houston, TX, USA) (6–8). The HSV-tk gene product, which lacks the enzyme encoded in mammalian cells, efficiently phosphorylates GCV, which is subsequently incorporated into newly synthesized DNA during cellular division, causing termination of DNA synthesis and cell death (9). This in situ gene therapy approach involves the ‘bystander effect,’ in which tumor cells close to HSV-tk-expressing cells are also killed (10–12). Baylor College of Medicine has demonstrated the safety of HSV-tk gene therapy for prostate cancer.
Previous studies have reported that activated human leukocyte antigen (HLA)-DR+ cluster of differentiation 8+ (CD8+) T cells are increased in patients treated with adenoviral vector-mediated HSV-tk gene delivery followed by GCV injection (8,13,14). However, the details of the resulting immune responses were not clear.
In the present study, the immune responses in patients who were repeatedly administered GCV intravenously following intraprostatic HSV-tk injection over a period of 2 weeks were investigated. Central memory (CM) CD8+ T cells in peripheral blood were clearly and efficiently increased during the second round of HSV-tk + GCV treatment, and prostate cancer antigen (PCa)-specific T cells were also increased.
Materials and methods
Patients
The study subjects were five patients with clinically localized prostate cancer with a high risk of recurrence who underwent prostate biopsy (Table I). All the patients had a Kattan preoperative nomogram score of 115, and provided study-specific informed consent prior to enrollment in the phase I/II clinical trial, which was approved by the Institutional Review Board of Kitasato University (Sagamihara, Kanagawa, Japan) and the Ministry of Health, Labour and Welfare of Japan.
Table I.
Characteristics of the patients treated with repeated HSV-tk + GCV injections.
| Patient | Age, years | PSA, ng/ml | Clinical stage | Gleason score |
|---|---|---|---|---|
| Pt1 | 70 | 13.7 | T2c | 3+3 |
| Pt2 | 63 | 6.6 | T2b | 3+5 |
| Pt3 | 60 | 44.1 | T2c | 4+3 |
| Pt4 | 68 | 7.8 | T2b | 4+3 |
| Pt5 | 71 | 28.9 | T2b | 3+3 |
HSV-tk, herpes simplex virus-thymidine kinase; GCV, ganciclovir; PSA, prostate-specific antigen.
Vector
The vector used was a serotype Ad5 adenovirus that contained the HSV-tk gene and Rous sarcoma virus long terminal repeat promoter in the region of the excised E1/E2 wild-type adenoviral genes. This replication-defective adenoviral vector was constructed, as described previously (14). A clinical-grade preparation was made by the Baylor Center for Cell and Gene Therapy, Gene Vector Laboratory under good manufacturing practice conditions. All the patients received 2×1011 viral particles of the vector into the prostate using a transrectal approach under ultrasound guidance.
Treatment course
The five patients underwent repeated intraprostatic injection of HSV-tk for 2 weeks with intravenous GCV. After a further 4 weeks, four of the patients underwent radical prostatectomy (Fig. 1). One patient was switched to radiotherapy instead of radical prostatectomy due to a prolonged activated partial thromboplastin time.
Figure 1.
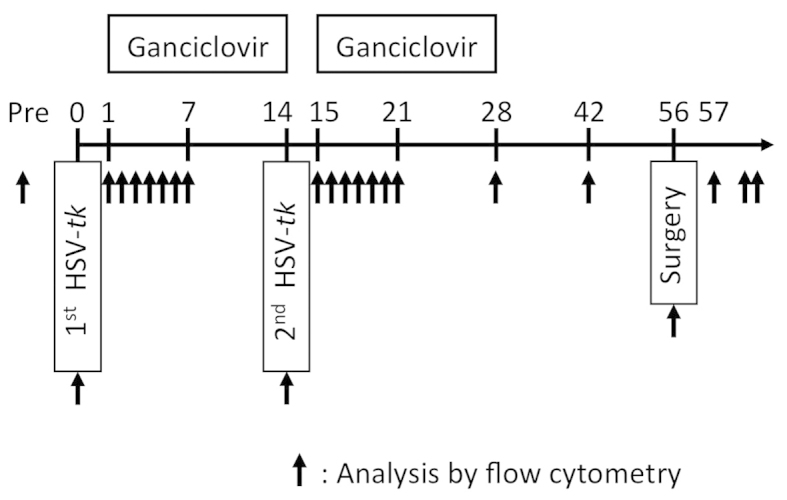
Course of repeated herpes simplex virus-thymidine kinase (HSV-tk) + ganciclovir (GCV) treatment. Five patients received repeated administration of intravenous GCV for 2 weeks following intraprostatic injection of adenovirus-mediated HSV-tk. After an additional 4 weeks, four patients underwent radical prostatectomy. Arrows indicate the timing of analyses by flow cytometry.
Lymphocyte immunophenotyping
Heparin-treated blood (100 µl) was incubated with the following fluorescence-conjugated monoclonal antibodies (mAbs): CD3/CD19 (cat. no. 349211), CD3/CD8 (cat. no. 340044), CD3/CD4 (cat. no. 340043), CD8/HLA-DR (cat. no. 349528), CD4/HLA-DR (cat. no. 340771), CD3/HLA-DR (cat. no. 340573) [all fluorescein isothiocyanate/phycoerythrin (FITC/PE); Becton, Dickinson and Co., San Jose, CA, USA], CD45RO (PE-Texas Red), CD62L (FITC) and CCR7 (PE) (Beckman Coulter, Inc., Brea, CA, USA). After incubation at room temperature for 15 min, red blood cells were lysed with BD FACS lysing solution (Becton, Dickinson and Co.). Forward light scatter and side light scatter were set to distinguish the lymphocyte, macrophage and granulocyte population from debris with an EPICS XL flow cytometer (Beckman Coulter, Inc.). Multi-color immunofluorescence analysis was performed using 10,000 lymphocytes for each analysis.
Intracellular interferon (IFN)-γ assay
Peripheral blood mononuclear cells (PBMC) were stimulated with prostatic acid phosphatase (PAP) or NY-ESO-1 peptides that were 15 amino acids in length, overlapping by 11 amino acids at a concentration of 1 mg/ml (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Simultaneously, monesin (200 µM) was added to block protein transport. After 16 h of incubation, the peptide-stimulated PBMC were stained with anti-CD3 (FITC), anti-CD4 (PE-Vio770) and anti-CD8 (APC-Vio770) mAbs (Miltenyi Biotec GmbH). For IFN-γ staining, the peptide-stimulated PBMCs were treated with FACS Perm2 (Becton, Dickinson and Co.) and stained with anti-IFN-γ (PE) mAb (Beckman Coulter, Inc.). IFN-γ-producing T cells were analyzed using a MACSQuant flow cytometer (Miltenyi Biotec GmbH).
Statistics
Statistical significance was analyzed using the Mann-Whitney U test. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of circulating naïve and effector T cell subsets
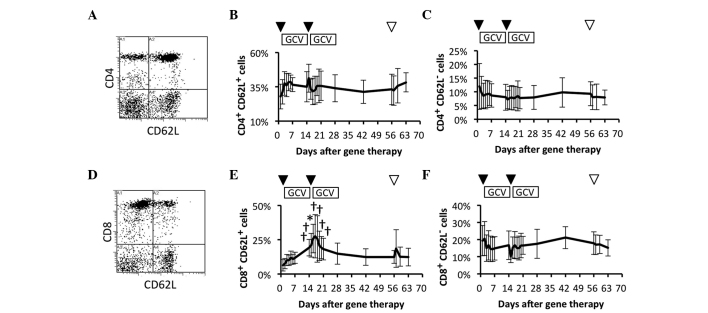
To study the efficacy of the immune system, whether the frequency of naïve and effector cells in peripheral blood was affected by the repeated HSV-tk + GCV treatment was investigated. The frequency of naïve and effector cells were compared in the circulating CD4+ T cell subsets prior and subsequent to the HSV-tk + GCV treatment (Fig. 2A). Naïve and a proportion of the memory T cell subsets (CD4+ CD62L+) were immediately and slightly increased following the 1st and 2nd HSV-tk vector injections, but there were no significant increases in comparison to the frequency of the CD4+ CD62L+ T cell subsets prior to the treatment (Fig. 2B). In contrast to the CD4+ CD62L+ T cell subsets, there was no significant difference in the effector (CD4+ CD62L–) T cell subset during the observation period (Fig. 2C). The frequency of naïve and effector cells were also compared among the circulating CD8+ T cell subsets prior and subsequent to the repeated HSV-tk + GCV treatment (Fig. 2D). Naïve and a proportion of memory T cell subsets (CD8+ CD62L+) were gradually increased following the 1st HSV-tk + GCV treatment. This was followed by a significant increase during the 2nd HSV-tk + GCV treatment, in comparison with the frequency of CD8+ CD62L+ T cell subsets prior to the treatment (Fig. 2E). In contrast to the CD8+ CD62L+ T cell subsets, the effector (CD8+CD62L–) T cell subset showed no significant change during the observation period (Fig. 2F). These results suggested that naïve, and a proportion of memory CD8+ T cells, were possibly affected by the repeated HSV-tk + GCV treatments.
Figure 2.
Circulating naïve and effector T cell subsets. Circulating T cell subsets in patients were characterized by flow-cytometric analysis using immunofluorescent antibody staining for cluster of differentiation 4 (CD4), CD8 and CD62L. (A) Representative data for naïve and effector CD4+ T cell subsets. Data for (B) naïve and a proportion of memory CD4+ T cell subsets, and (C) effector CD4+ T cell subsets. (D) Representative data for naïve and effector CD8+ T cell subsets. Data for (E) naïve and a proportion of memory CD8+ T cell subsets, and (F) effector CD8+ T cell subsets. Arrowheads indicate vector injection (▼) and radical prostatectomy (▽). †P<0.1. *P<0.05.
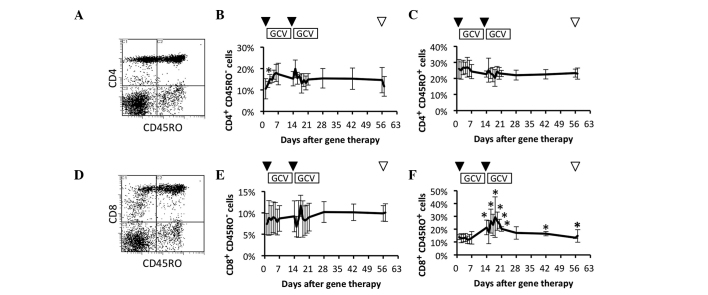
Comparison of circulating naïve and memory T cell subsets
To clarify which of the naïve and memory T cell subsets were effectively increased, these subsets were investigated in patients who had received repeated HSV-tk + GCV treatment. The frequencies of circulating naïve and memory CD4+ T cell subsets were compared prior and subsequent to HSV-tk + GCV treatment (Fig. 3A). The naïve T cell (CD4+ CD45RO–) subset was significantly increased on day 2 after the 1st HSV-tk vector injection in comparison to the frequency prior to treatment (Fig. 3B). In contrast to the naïve CD4+ T cell subset, the memory T cell subset (CD4+ CD45RO+) showed no significant change during the observation period (Fig. 3C). The frequencies of circulating naïve and memory CD8+ T cell subsets were compared prior and subsequent to the HSV-tk + GCV treatment (Fig. 3D). The naïve T cell (CD8+ CD45RO–) subset showed no significant change during the observation period (Fig. 3E). By contrast, the memory T cell (CD8+ CD45RO+) subset was significantly increased following the 2nd HSV-tk + GCV treatment in comparison to the frequency of the naïve CD8+ T cell subset prior to treatment. A significant increase of the CD8+ CD45RO+ T cell subset was further observed on days 42 and 56 after the 1st HSV-tk vector injection (Fig. 3F). These results suggested that memory CD8+ T cells were clearly and more effectively increased by repeated HSV-tk + GCV treatment.
Figure 3.
Circulating naïve and memory T cell subsets. Circulating T cell subsets in patients were characterized by flow-cytometric analysis using immunofluorescent antibody staining for cluster of differentiation 4 (CD4), CD8 and CD45RO. (A) Representative data for naïve and memory CD4+ T cell subsets. Data for (B) naïve CD4+ T cell subsets and (C) memory CD4+ T cell subsets. (D) Representative data for naïve and memory CD8+ T cell subsets. Data for (E) naïve CD8+ T cell subsets and (F) memory CD8+ T cell subsets. Arrowheads indicate vector injection (▼) and radical prostatectomy (▽). *P<0.05.
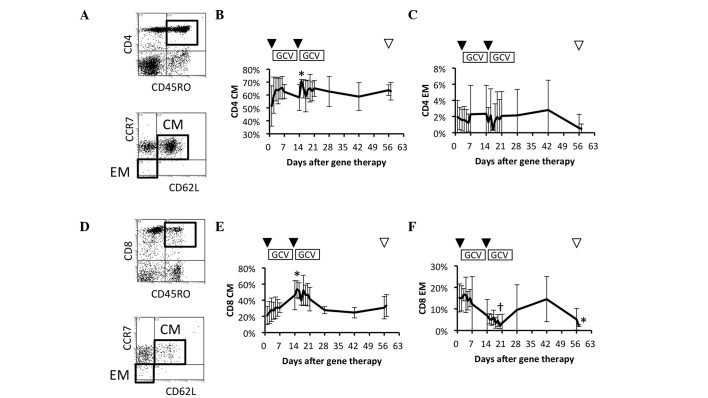
Comparison of circulating memory T cell subsets
To further study the details of memory T cell responses in patients who had received the repeated HSV-tk + GCV treatment, the frequencies of the circulating CM (CD45RO+ CD62L+ CCR7+) and effector memory (EM) (CD45RO+ CD62L– CCR7–) CD4+ T cell subsets were examined (Fig. 4A). CM CD4+ T cells increased gradually during the 1st HSV-tk + GCV treatment, and were significantly increased on day 2 after the 2nd HSV-tk vector injection (Fig. 4B). In contrast to CM CD4+ T cells, the EM CD4+ T cell subset showed no significant change during the observation period (Fig. 4C). The frequency of circulating CM (CD45RO+ CD62L+ CCR7+) and EM (CD45RO+ CD62L– CCR7–) CD8+ T cell subsets were also compared prior and subsequent to the repeated HSV-tk + GCV treatment (Fig. 4D). CM CD8+ T cells increased gradually during the 1st HSV-tk + GCV treatment, and their frequency was maximal and most significant on day 2 after the 2nd HSV-tk vector injection, in comparison with the frequency prior to treatment (Fig. 4E). By contrast, the EM CD8+ T cell subset decreased gradually during the 2nd HSV-tk + GCV treatment, and showed the lowest frequency on day 7 after the 2nd HSV-tk vector injection (Fig. 4F). These results suggested that CM CD4+ and CD8+ T cells were effectively increased following repeated HSV-tk vector injection, whereas it appeared that EM CD8+ T cells were induced to migrate from blood vessels to the local environment.
Figure 4.
Circulating central memory (CM) and effector memory (EM) T cell subsets. Circulating T cell subsets in patients were characterized by flow-cytometric analysis using immunofluorescent antibody staining for cluster of differentiation 4 (CD4), CD8, CD45RO, CD62L and CCR7 (CD197). (A) Representative data for naïve and memory CD4+ T cell subsets (upper panel). CM and EM T cell subsets gated on CD4+ CD45RO+ T cells (lower panel). Data for (B) CM CD4+ T cell subsets and (C) EM CD4+ T cell subsets. (D) Representative data for naïve and memory CD8+ T cell subsets (upper panel). CM and EM T cell subsets gated on CD8+ CD45RO+ T cells (lower panel). Data for (E) CM CD8+ T cell subsets and (F) EM CD8+ T cell subsets. Arrowheads indicate vector injection (▼) and radical prostatectomy (▽). †P<0.1. *P<0.05.
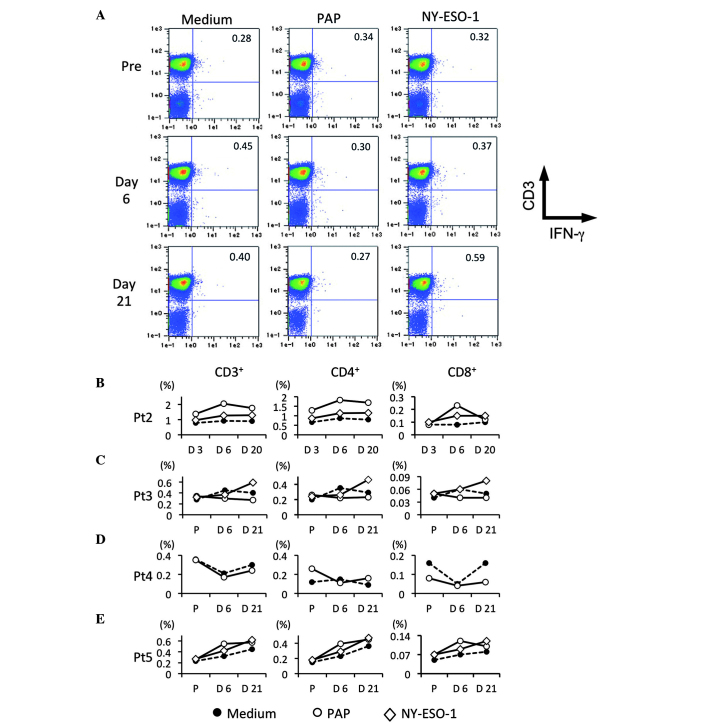
Detection of PCa-specific T cells
To study whether PCa-specific T cells were also increased by repeated HSV-tk + GCV treatment, the IFN-γ-producing T cells in patients following stimulation with PAP and NY-ESO-1-overlapping peptides were detected. Fig. 5A shows representative flow-cytometric data for tumor antigen-specific IFN-γ producing T cells prior and subsequent to HSV-tk vector injection (Fig. 5A). In patient 2, PAP-specific T cells were maximally increased on day 6 after the 1st HSV-tk vector injection, whereas NY-ESO-1-specific T cells were slightly increased (Fig. 5B). In patient 3, NY-ESO-1-specific T cells were clearly increased following repeated HSV-tk + GCV treatment, whereas PAP-specific T cells were slightly increased on day 6 after the 1st HSV-tk vector injection in comparison to PCa-specific T cells prior to treatment (Fig. 5C). In patient 4, PAP-specific T cells were only examined, and no difference was observed prior and subsequent to the treatment (Fig. 5D). In patient 5, an increase of PAP-specific T cells on day 6 after the 1st HSV-tk vector injection was observed, whereas NY-ESO-1-specific T cells increased after the 2nd injection of HSV-tk vector, in comparison to PCa-specific T cells prior to treatment (Fig. 5E). Although PAP-specific T cells were increased following the 1st injection of HSV-tk vector, NY-ESO-1-specific T cells appeared to be increased following the 2nd injection. These results suggested that PCa antigen-specific T cells may be efficiently induced by repeated treatment with HSV-tk + GCV.
Figure 5.
Detection of tumor antigen-specific T cells. Circulating tumor antigen-specific T cell subsets in patients were characterized by flow-cytometric analysis using immunofluorescent antibody staining for cluster of differentiation 3 (CD3), CD4, CD8 and interferon-γ (IFN-γ). (A) Representative data for CD3 and IFN-γ production in peripheral blood lymphocytes (PBL). PBL were stimulated with prostatic acid phosphatase (PAP) and NY-ESO-1 overlapping peptides. CD3+ IFN-γ+, CD4+ IFN-γ+ and CD8+ IFN-γ+ T cells of (B) patients 2, (C) 3, (D) 4 and (E) 5 are shown, respectively. Each symbol indicated medium (●), PAP (○) and NY-ESO-1 (◇).
Discussion
Gene therapy is a promising experimental approach for prostate cancer. Numerous in vitro and animal studies have shown that HSV-tk is effective against prostate cancer cell lines and prostate tumors grown in animals (15–18). However, its efficacy and immune responses in human prostate cancer patients are not well defined. In the present study, the dynamics of systemic T cell subsets and prostate cancer-derived antigen-specific T cells were investigated, and it was identified that they were efficiently increased in patients who received repeated treatment with HSV-tk + GCV.
Several preclinical studies have shown that tumors treated with HSV-tk + GCV contain areas of necrosis highlighted by infiltration of macrophages, CD4+ and CD8+ T cells (11,12,19,20). Additionally, interleukin (IL)-1, IL-2, IL-6, IL-12, IFN-γ, tumor necrosis factor-α and granulocyte macrophage colony-stimulating factor are detectable in tumors following HSV-tk + GCV treatment (19,21). Furthermore, previous studies have indicated that circulating total CD8+ and activated CD8+ T cells are significantly increased 2 weeks after injection of HSV-tk vector (8,13,14). Our previous study reported the dynamics of circulating lymphocyte subsets in patients who had received repeated HSV-tk + GCV treatment (22). Total CD8+ and activated CD8+ T cells were significantly and efficiently increased by this type of treatment, particularly following the 2nd round of treatment. However, it was not clear whether the increased CD8+ T cells were specifically active against prostate cancer antigens. Previous studies have utilized adenoviral vectors for suicide gene delivery to prostate tumors as a form of neoadjuvant therapy (14,23,24). These increased CD8+ T cells possibly represent an immune response to adenovirus. van der Linden et al (24) reported that adenovirus-specific neutralizing antibody responses are clearly enhanced in patients who received adenovirus-mediated suicide gene therapy. The study further demonstrated that adenovirus-specific PBMC proliferation and IFN-γ production occurred in vitro. By contrast, van der Linden et al (24) also found no correlation between anti-adenovirus antibody titers (neutralizing antibody) and the PSA response. To the best of our knowledge, the present study is the first to indicate that repeated HSV-tk + GCV treatment can efficiently induce circulating CM CD8+ T cells and PCa-specific T cell responses in human patients. The high rate of relapse of prostate cancer following surgery is clinically problematic. Although radical prostatectomy is intended to be curative, the high recurrence rates of prostate cancer may be due to minimal residual disease (MRD) following surgery. To eliminate MRD, induction of memory T cells, which are also involved in immunological surveillance for adaptive immunity, may be important.
In the present study, circulating memory CD8+ T cells were more effectively increased compared to memory CD4+ T cells, whereas IFN-γ-producing PCa-specific CD4+ T cells were more efficiently induced compared to IFN-γ-producing PCa-specific CD8+ T cells. PAP and NY-ESO-1 overlapping peptides were utilized to stimulate PCa-specific T cells. These peptides were 15 amino acids in length, overlapping by 11 amino acids. These overlapping peptides may be presented more easily by the major histocompatibility complex (MHC)-II on antigen-presenting cells compared to MHC-I. Cytopathic and bystander effects appeared to be efficiently induced by this repeated HSV-tk + GCV treatment. As a result, prostate cancer-associated antigens may be released from cytopathic areas, thus initiating PCa-specific T cell responses in patients.
In conclusion, the present study reported evidence of systemic T cell responses following repeated HSV-tk + GCV treatment. CM CD8+ T cells and PCa-specific T cells in peripheral blood were enhanced following injection of the vector, suggesting the potential for activation of components of the cell-mediated immune response in the clinical trial. This cell-mediated immune response may contribute to a reduction of relapse rates in patients with prostate cancer. This repeated treatment with HSV-tk + GCV is expected to be promising for neoadjuvant therapy.
Acknowledgements
The present study was supported by a grant from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Grant-In-Aid for Scientific Research C21592060), Japan Science and Technology Agency and Asahi Kasei Pharma Urological Academy.
Glossary
Abbreviations
- HSV-tk
herpes simplex virus-thymidine kinase
- GCV
ganciclovir
- CM
central memory
- EM
effector memory
- PAP
prostatic acid phosphatase
- PCa
prostate cancer
References
- 1.Jones GW, Mettlin C, Murphy GP, et al. Patterns of care for carcinoma of the prostate gland: Results of a national survey of 1984 and 1990. J Am Coll Surg. 1995;180:545–554. [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Ohori M, Wheeler TM, Kattan MW, Goto Y, Scardino PT. Prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 1995;154:1818–1824. doi: 10.1016/S0022-5347(01)66792-2. [DOI] [PubMed] [Google Scholar]
- 4.Zietman AL, Edelstein RA, Coen JJ, Babayan RK, Krane RJ. Radical prostatectomy for adenocarcinoma of the prostate: The influence of preoperative and pathologic findings on biochemical disease-free outcome. Urology. 1994;43:828–833. doi: 10.1016/0090-4295(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 5.Namiki K, Rosser CJ. Neoadjuvant therapy and prostate cancer: What a urologist should know. Curr Opin Urol. 2007;17:188–193. doi: 10.1097/MOU.0b013e3280e08802. [DOI] [PubMed] [Google Scholar]
- 6.Herman JR, Adler HL, Aguilar-Cordova E, Rojas-Martinez A, Woo S, Timme TL, Wheeler TM, Thompson TC, Scardino PT. In situ gene therapy for adenocarcinoma of the prostate: A phase I clinical trial. Hum Gene Ther. 1999;10:1239–1249. doi: 10.1089/10430349950018229. [DOI] [PubMed] [Google Scholar]
- 7.Shalev M, Kadmon D, Teh BS, Butler EB, Aguilar-Cordova E, Thompson TC, Herman JR, Adler HL, Scardino PT, Miles BJ. Suicide gene therapy toxicity after multiple and repeat injections in patients with localized prostate cancer. J Urol. 2000;163:1747–1750. doi: 10.1016/S0022-5347(05)67534-9. [DOI] [PubMed] [Google Scholar]
- 8.Miles BJ, Shalev M, Aguilar-Cordova E, et al. Prostate-specific antigen response and systemic T cell activation after in situ gene therapy in prostate cancer patients failing radiotherapy. Hum Gene Ther. 2001;12:1955–1967. doi: 10.1089/104303401753204535. [DOI] [PubMed] [Google Scholar]
- 9.Thompson TC. In situ gene therapy for prostate cancer. Oncol Res. 1999;11:1–8. [PubMed] [Google Scholar]
- 10.Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, Abraham GN. The ‘bystander effect’: Tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53:5274–5283. [PubMed] [Google Scholar]
- 11.Barba D, Hardin J, Sadelain M, Gage FH. Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci USA. 1994;91:4348–4352. doi: 10.1073/pnas.91.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagandeep S, Brew R, Green B, Christmas SE, Klatzmann D, Poston GJ, Kinsella AR. Prodrug-activated gene therapy: Involvement of an immunological component in the ‘bystander effect’. Cancer Gene Ther. 1996;3:83–88. [PubMed] [Google Scholar]
- 13.Ayala G, Wheeler TM, Shalev M, Thompson TC, Miles B, Aguilar-Cordova E, Chakraborty S, Kadmon D. Cytopathic effect of in situ gene therapy in prostate cancer. Hum Pathol. 2000;31:866–870. doi: 10.1053/hupa.2000.8453. [DOI] [PubMed] [Google Scholar]
- 14.Satoh T, Teh BS, Timme TL, et al. Enhanced systemic T-cell activation after in situ gene therapy with radiotherapy in prostate cancer patients. Int J Radiat Oncol Biol Phys. 2004;59:562–571. doi: 10.1016/j.ijrobp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Hall SJ, Mutchnik SE, Chen SH, Woo SL, Thompson TC. Adenovirus-mediated herpes simplex virus thymidine kinase gene and ganciclovir therapy leads to systemic activity against spontaneous and induced metastasis in an orthotopic mouse model of prostate cancer. Int J Cancer. 1997;70:183–187. doi: 10.1002/(SICI)1097-0215(19970117)70:2<183::AID-IJC8>3.3.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 16.Hall SJ, Sanford MA, Atkinson G, Chen SH. Induction of potent antitumor natural killer cell activity by herpes simplex virus-thymidine kinase and ganciclovir therapy in an orthotopic mouse model of prostate cancer. Cancer Res. 1998;58:3221–3225. [PubMed] [Google Scholar]
- 17.Hassan W, Sanford MA, Woo SL, Chen SH, Hall SJ. Prospects for herpes-simplex-virus thymidine-kinase and cytokine gene transduction as immunomodulatory gene therapy for prostate cancer. World J Urol. 2000;18:130–135. doi: 10.1007/s003450050185. [DOI] [PubMed] [Google Scholar]
- 18.Loimas S, Toppinen MR, Visakorpi T, Jänne J, Wahlfors J. Human prostate carcinoma cells as targets for herpes simplex virus thymidine kinase-mediated suicide gene therapy. Cancer Gene Ther. 2001;8:137–144. doi: 10.1038/sj.cgt.7700286. [DOI] [PubMed] [Google Scholar]
- 19.Vile RG, Castleden S, Marshall J, Camplejohn R, Upton C, Chong H. Generation of an anti-tumour immune response in a non-immunogenic tumour: HSVtk killing in vivo stimulates a mononuclear cell infiltrate and a Th1-like profile of intratumoural cytokine expression. Int J Cancer. 1997;71:267–274. doi: 10.1002/(SICI)1097-0215(19970410)71:2<267::AID-IJC23>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 20.Kianmanesh AR, Perrin H, Panis Y, Fabre M, Nagy HJ, Houssin D, Klatzmann D. A ‘distant’ bystander effect of suicide gene therapy: Regression of nontransduced tumors together with a distant transduced tumor. Hum Gene Ther. 1997;8:1807–1814. doi: 10.1089/hum.1997.8.15-1807. [DOI] [PubMed] [Google Scholar]
- 21.Whartenby KA, Abboud CN, Marrogi AJ, Ramesh R, Freeman SM. The biology of cancer gene therapy. Lab Invest. 1995;72:131–145. [PubMed] [Google Scholar]
- 22.Satoh T, Kubo M, Tabata K, et al. Systemic T-cell activation following neoadjuvant in situ gene therapy in high-risk prostate cancer patients. J Urol. 2012;187:e323. doi: 10.1016/j.juro.2012.02.878. [DOI] [Google Scholar]
- 23.Ayala G, Satoh T, Li R, Shalev M, Gdor Y, Aguilarcordova E, Frolov A, Wheeler T, Miles B, Rauen K. Biological response determinants in HSV-tk + ganciclovir gene therapy for prostate cancer. Mol Ther. 2006;13:716–728. doi: 10.1016/j.ymthe.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 24.van der Linden RR, Haagmans BL, Mongiat-Artus P, van Doornum GJ, Kraaij R, Kadmon D, Aguilar-Cordova E, Osterhaus AD, van der Kwast TH, Bangma CH. Virus specific immune responses after human neoadjuvant adenovirus-mediated suicide gene therapy for prostate cancer. Eur Urol. 2005;48:153–161. doi: 10.1016/j.eururo.2005.02.013. [DOI] [PubMed] [Google Scholar]