Abstract
The aim of this study was to compare the incidence of postoperative complications, including superficial incisional surgical site infection (SSI) following purse-string skin closure (PS) and conventional skin closure with a drainage tube (CD) following stoma closure. A total of 55 consecutive patients who underwent loop colostomy and loop ileostomy closures in our hospital between October, 2011 and September, 2014 were retrospectively assessed. The patients were divided into two groups, namely the PS group (26 patients) and the CD group (29 patients). There were no significant differences in the characteristics of the patients between the two groups. The baseline and operative characteristics also did not differ significantly between the two groups. However the incidence of superficial incisional SSI was lower in the PS group compared to that in the CD group (0 vs. 13.8%, respectively; P=0.049). The overall incidence of complications did not differ significantly between the two groups (P=0.313). The duration of postoperative hospital stay in the PS group was shorter compared to that in the CD group. In conclusion, the results of this study suggest that PS may an effective technique to reduce the incidence of superficial incisional SSI. This technique appears to be superior to the conventional technique, allowing for better cosmesis.
Keywords: stoma closure, purse-string skin closure, surgical site infection, drainage
Introduction
Stoma closure is considered a minimally invasive surgery; however, surgical site infection (SSI) is a frequent complication. Recently, the number of cases of diverting ileostomy has increased due to the increase in the number of anal-preserving surgeries, such as super-low anterior resection (sLAR) and intersphincteric resection (ISR) for rectal cancer. In this study, we focused on methods used to decrease the incidence of SSI in stoma closure.
The incidence of wound infection following stoma closure ranges between 2 and 41% across different studies (1,2). The most frequent cause of wound infection is bacterial contamination of the skin surrounding the ileostomy/colostomy due to prolonged contact with bowel contents or due to leakage of the ileostomy contents. A previous study in 1997 introduced a purse-string skin closure technique (PS) for ileostomy reversal, which reduced the risk of wound infection and resulted in smaller scars, allowing for better cosmesis (3).
The number of studies investigating the effectiveness of PS is currently limited. We previously used a Penrose drainage tube under the subcutaneous tissue to prevent SSI and have reported the efficacy of this technique in our hospital (4).
PS was reported to be better for controlling superficial incisional SSI (5,6) and we introduced this technique in June, 2013. In the present study, we examined surgical techniques by either conventional skin closure with a drainage tube (CD) or PS and compared the incidence of superficial incisional SSI between CD and PS in order to assess the efficacy of the PS in ileostomy and colostomy reversal.
Patients and methods
Patients
We retrospectively reviewed the medical records of 55 consecutive patients who underwent stoma closure. A total of 26 patients who underwent PS between June, 2013 and September, 2014 were compared to 29 patients who underwent CD between October, 2011 and May, 2013 at the Osaka Medical Center for Cancer and Cardiovascular Diseases, Japan. Medical charts were reviewed for patient demographics, including age, gender and body mass index; past medical history, such as the presence of diabetes, chronic obstructive pulmonary disease, cardiovascular disease and liver dysfunction; alcohol consumption (regular vs. moderate or non-drinker), smoking status (smoked within 1 year prior to the operation), medication records, including perioperative steroid replacement; American Society of Anesthesiology (ASA) score; and preoperative serum albumin values for the CD and PS groups.
This study was approved by the Institutional Review Board of Osaka Medical Center for Cancer and Cardiovascular Diseases and written informed consent was obtained from all the patients.
Diagnosis of SSI
Wound infection was defined as the presence of cellulitis or purulent discharge, with or without positive bacterial growth, within 30 days after the operation (adopted from the 1992 Centers for Disease Control and Prevention definition of SSI) (Table I). The surgical wounds were routinely observed and evaluated by the surgical team and surveyed until 30 days after the operation.
Table I.
Centers for Disease Control and Prevention guidelines for the diagnosis of superfi cial SSI.
| Characteristics of superfcial SSI | |
|---|---|
| 1. | Purulent drainage, with or without laboratory confrmation, from the superfcial incision. |
| 2. | Organisms isolated from an aseptically-obtained culture of fuid or tissue from the superfcial incision. |
| 3. | At least one of the following signs or symptoms of infection: Pain or tenderness, localized swelling, redness or heat and and superfcial incision deliberately opened by surgeon, unless the incision is culture-negative. |
| 4. | Diagnosis of superfcial incisional SSI by the surgeon or attending physician. |
Superfcial SSI occurs within 30 days of an operation and involves only the skin or subcutaneous tissue surrounding the incision and at least one of the abovementioned characteristics. SSI, surgical site infection.
Surgical technique
All the patients underwent preoperative mechanical bowel preparation. After the induction of general anesthesia, prophylactic antibiosis (Cefmetazole, 2 g/day) was administered. An open-ileostomy/colostomy was sutured (Fig. 1A and B) and the wound was irrigated by povidone iodine and 500 ml of saline. The skin around the sutured stoma was resected, maintaining a ~3–5 mm margin. The intestinal tracts leading to the stoma were extracted from the abdominal cavity and dissection of the sutured stoma was performed, followed by a functional end-to-end anastomosis or Albert-Lembert anastomosis. The peritoneum and rectus fascia were closed using Vicryl 1.0; (Johnson & Johnson Co., New Brunswick, NJ, USA). Antibiotics were administered for 3 days postoperatively.
Figure 1.
Surgical procedure of the purse-string skin closure. (A and B) following irrigation with saline, an open-ileostomy/colostomy was closed by sutures. The skin was cut around the sutured stoma while maintaining a ~3–5 mm margin.
In the CD group, the skin site was closed primarily with a skin stapler. At the stoma site, a Penrose drainage tube was left subcutaneously. In the PS group, the dermis layer was sutured, drawing a purse-string form using polydioxanone 3.0 suture (Johnson & Johnson Co.). It is recommended that ~5 mm of the center of the wound are maintained open to drain any discharge (Fig. 2).
Figure 2.
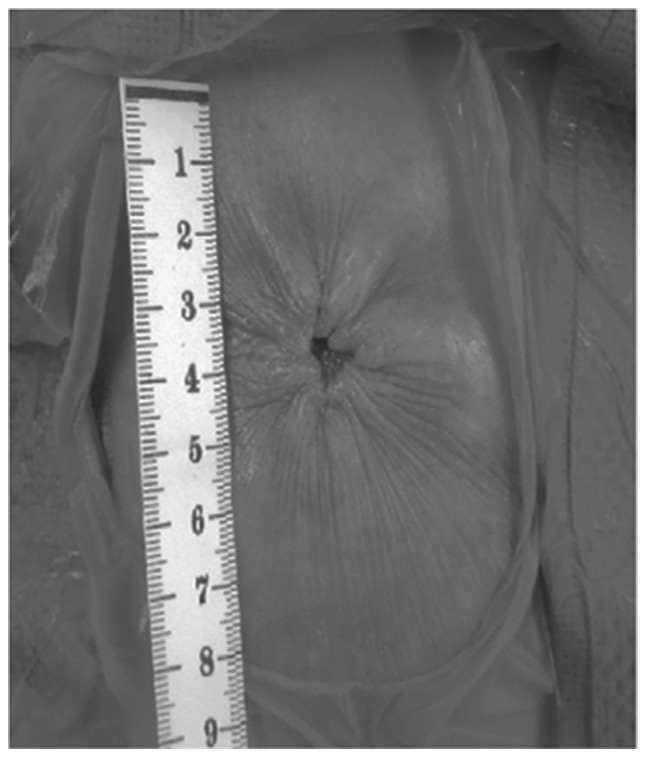
Postoperative picture of the purse-string skin closure. Approximately 5 mm of the center of the wound were mainained open to drain the discharge from the subcutaneous wound.
Statistical analysis
The data presented were analyzed using the Pearson's Chi-square and Fisher's exact tests. For continuous variables, data were expressed as median (range). The Mann-Whitney U-test was used for statistical comparison of different groups. P<0.05 was considered to indicate a statistically significant difference. All the tests were analyzed using JMP software, version 11.0 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
The patient characteristics are summarized in Table II. There were no significant differences in patient characteristics, such as age, gender, body mass index, preoperative comorbidities, ASA scores and preoperative serum albumin values between the CD and PS groups. The perioperative factors of the CD and PS groups are summarized in Table III. The median operative time of the CD group was 98 min (range, 53–213 min) and that of the PS group 106 min (range, 60–190 min); this difference was not statistically significant (P=0.377). In the CD group, the median blood loss was 15 ml (range, 0–130 ml), whereas in the PS group, it was 20 ml (range, 0–130 ml) (P=0.203). The median wound length in the PS group was 0.5 cm, which was significantly shorter compared to that in the CD group (6 cm) (P<0.001). As regards postoperative complications, superficial incisional SSI in the CD group was observed in 4 patients (13.8%); however, in the PS group, SSI was not observed. Thus, there was a significant difference in the incidence of superficial incisional SSI between the CD and PS groups (P=0.049). With regard to other complications, small bowel obstruction was observed in 2 patients (6.9%) in the CD group and 1 patient (3.8%) in the PS group. In the CD group, postoperative bleeding was observed in 1 patient (3.8%). Anastomosis leakage was not observed in any of the two groups. The overall incidence of complications in the CD and PS groups was not significantly different (P=0.313). The median postoperative hospital stay was 14 days (range, 9–50 days) in the CD group and 13 days (range, 8–29 days) in the PS group.
Table II.
Patient characteristics (N=55).
| Characteristics | CD (n=29) | PS (n=26) | P-value |
|---|---|---|---|
| Gender | 0.738 | ||
| Male | 20 | 19 | |
| Female | 9 | 7 | |
| Age, years | 58 (34–79) | 65 (27–80) | 0.255 |
| Body mass index | 22 (16–27) | 22 (14–29) | 0.831 |
| ASA score | NA | ||
| 1 | 9 | 9 | |
| 2 | 20 | 14 | |
| 3 | 0 | 3 | |
| Preoperative albumin serum value | 4.1 (3.4–5.7) | 4.1 (3.7–4.9) | 0.799 |
| Preoperative comorbidities | |||
| Diabetes | 1 | 0 | NA |
| Cardiovascular disease | 2 | 3 | NA |
| COPD | 1 | 0 | NA |
| Steroid use | 0 | 0 | NA |
| Alcohol consumption | 7 | 10 | 0.251 |
| Smoking | 7 | 6 | 0.926 |
| Liver cirrhosis | 0 | 0 | NA |
| Ileostomy/colostomy | 26/3 | 22/4 | NA |
| Anastomosis (FEEA/AL) | 27/2 | 24/2 | NA |
All the continuous variables are expressed as median (range). CD, conventional skin closure with a drainage tube; PS, purse-string skin closure; ASA, American Society of Anesthesiology; COPD, chronic obstructive pulmonary disease; FEEA, functional end-to-end anastomosis; AL, Albert-Lembert anastomosis; NA, not available.
Table III.
Perioperative factors in conventional skin closure with a drainage tube (CD) and purse-string skin closure (PS).
| Factors | CD (n=29) | PS (n=26) | P-value |
|---|---|---|---|
| Operative time, min | 98 (53–213) | 106 (60–190) | 0.377 |
| Blood loss, ml | 15 (0–130) | 20 (0–130) | 0.203 |
| Wound length, cm | 6 (4–8) | 0.5 (0.5–1) | <0.001 |
| Complications | 5 | 2 | 0.313 |
| Wound infection | 4 | 0 | 0.049 |
| Small bowel obstructiona | 2 | 1 | NA |
| Anastomosis leakagea | 0 | 0 | NA |
| Postoperative bleedinga | 0 | 1 | NA |
| Postoperative hospital stay, days | 14 (9–50) | 13 (8–29) | 0.159 |
Postoperative complications ≥grade III are listed. All the continuous variables are expressed as median (range). NA, not available.
Discussion
Wound infection (particularly, superficial incisional SSI) is the most common complication following stoma closure, reportedly occurring in one of every three cases (7,8). A prospective review of the complications of ileostomy closure reported an SSI rate of 18.3% (9). The high incidence rate of SSI was considered to be associated with the primary closure of these wounds. Generally, superficial incisional SSI is not a fatal complication; however, there is a risk of severe infection and abdominal wall scar hernia. Therefore, we reported the utility of the Penrose drain placement under the subcutaneous tissue for reducing the incidence of SSI (4). However, with this technique, wound infection may not be sufficiently controlled; thus, we introduced PS for stoma closure in order to reduce the incidence of SSI.
In the present study, the SSI rate in the CD group was 8.3%, which is consistent with previous reports (10,11). However, no patients developed SSI in the PS group. This finding is in accordance with a previous report (12). PS of stoma reversal is a cosmetically superior approach (3). A recent study compared patients treated using PS to those treated using primary closure (12) and found that no SSI occurred in the PS group compared to the CD group, in which 40% of patients developed SSI (P=0.001). These results suggest a lower risk of SSI when PS is used. To effectively prevent SSI, it is important to maintain the drainage hole until the discharge from the wound is reduced (Fig. 2).
Recently, PS has been a focus of investigation with regard to better cosmetic appearance (Fig. 3) (13,14). In the present study, the wound length was evaluated in both groups. PS was performed and ~0.5 cm of the wound was maintained open in order to drain the discharge. By contrast, the patients in the CD group required a ~6-cm linear suture. Taken together, the results suggest that PS allows for better cosmesis in addition to the lower risk of SSI development.
Figure 3.
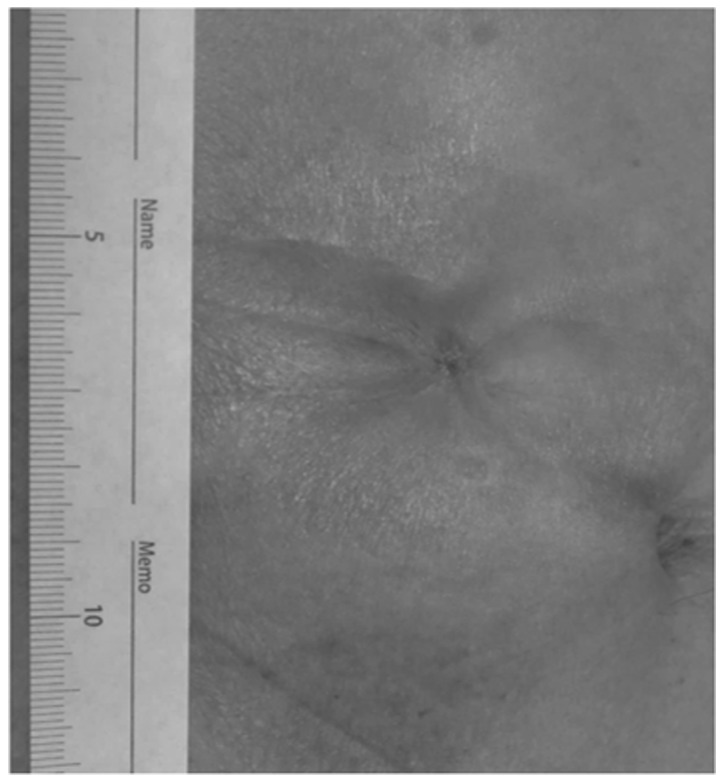
Postoperative picture 1 month following purse-string skin closure. The scar became smaller, allowing for better cosmesis.
With the improvement of surgical techniques for anal preservation by sLAR and ISR in patients with rectal cancer, the opportunities for primary artificial anus construction have increased. Stoma closure is associated with a high risk of wound infection; therefore, it is important to evaluate the usefulness of PS and open drainage system to prevent severe SSI. We are currently conducting a randomized trial to confirm our findings and assess additional end points, including cost-effectiveness and patient satisfaction.
In conclusion, the present study suggests that PS is associated with a lower risk of postoperative SSI compared to primary skin closure, even with the placement of a drainage tube; it also allows for better cosmesis, including a more favorable wound length, indicating that PS may be a favorable alternative to primary skin closure.
References
- 1.Hackam DJ, Rotstein OD. Stoma closure and wound infection: an evaluation of risk factors. Can J Surg. 1995;38:144–148. [PubMed] [Google Scholar]
- 2.Wong KS, Remzi FH, Gorgun E, et al. Loop ileostomy closure after restorative proctocolectomy: outcome in 1,504 patients. Dis Colon Rectum. 2005;48:243–250. doi: 10.1007/s10350-004-0771-0. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee A. Pursestring skin closure after stoma reversal. Dis Colon Rectum. 1997;40:993–994. doi: 10.1007/BF02051210. [DOI] [PubMed] [Google Scholar]
- 4.Imada S, Noura S, Ohue M, et al. Efficacy of subcutaneous penrose drains for surgical site infections in colorectal surgery. World J Gastrointest Surg. 2013;5:110–114. doi: 10.4240/wjgs.v5.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirbagheri N, Dark J, Skinner S. Factors predicting stomal wound closure infection rates. Tech Coloproctol. 2013;17:215–220. doi: 10.1007/s10151-012-0908-4. [DOI] [PubMed] [Google Scholar]
- 6.Chow A, Tilney HS, Paraskeva P, Jeyarajah S, Zacharakis E, Purkayastha S. The morbidity surrounding reversal of defunctioning ileostomies: a systematic review of 48 studies including 6,107 cases. Int J Colorectal Dis. 2009;24:711–723. doi: 10.1007/s00384-009-0660-z. [DOI] [PubMed] [Google Scholar]
- 7.Vermulst N, Vermeulen J, Hazebroek EJ, Coene PP, van der Harst E. Primary closure of the skin after stoma closure. Management of wound infections is easy without (long-term) complications. Dig Surg. 2006;23:255–258. doi: 10.1159/000095399. [DOI] [PubMed] [Google Scholar]
- 8.Williams LA, Sagar PM, Finan PJ, Burke D. The outcome of loop ileostomy closure: a prospective study. Colorectal Dis. 2008;10:460–464. doi: 10.1111/j.1463-1318.2007.01385.x. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Botello SA, Garcia-Armengol J, Garcia-Granero E, et al. A prospective audit of the complications of loop ileostomy construction and takedown. Dig Surg. 2004;21:440–446. doi: 10.1159/000083471. [DOI] [PubMed] [Google Scholar]
- 10.Parks SE, Hastings PR. Complications of colostomy closure. Am J Surg. 1985;149:672–675. doi: 10.1016/S0002-9610(85)80153-7. [DOI] [PubMed] [Google Scholar]
- 11.Pittman DM, Smith LE. Complications of colostomy closure. Dis Colon Rectum. 1985;28:836–843. doi: 10.1007/BF02555488. [DOI] [PubMed] [Google Scholar]
- 12.Milanchi S, Nasseri Y, Kidner T, Fleshner P. Wound infection after ileostomy closure can be eliminated by circumferential subcuticular wound approximation. Dis Colon Rectum. 2009;52:469–474. doi: 10.1007/DCR.0b013e31819acc90. [DOI] [PubMed] [Google Scholar]
- 13.Marquez TT, Christoforidis D, Abraham A, Madoff RD, Rothenberger DA. Wound infection following stoma takedown: primary skin closure versus subcuticular purse-string suture. World J Surg. 2010;34:2877–2882. doi: 10.1007/s00268-010-0753-4. [DOI] [PubMed] [Google Scholar]
- 14.Lee JR, Kim YW, Sung JJ, et al. Conventional linear versus purse-string skin closure after loop ileostomy reversal: comparison of wound infection rates and operative outcomes. J Korean Soc Coloproctol. 2011;27:58–63. doi: 10.3393/jksc.2011.27.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]



