Abstract
Peritoneal metastasis (PM) in gastric cancer (GC) is often the cause of several complications, including ascites and bowel obstruction. The prognosis of patients with extensive PM is poor. There are only limited data available on clinical characteristics regarding the period between the initiation of chemotherapy until the death of the patient. We conducted a retrospective study to determine the frequency of major events during and after palliative chemotherapy in advanced GC patients with PM. The records of patients who received first-line palliative chemotherapy at the Tochigi Cancer Center for locally advanced or metastatic disease were reviewed. The extracted information included treatments received and emerging complications. Overall survival was compared between patients with and those without PM. A total of 97 patients were reviewed and the prevalence of complications with or without concurrent PM were as follows: bowel obstruction: PM, 37% (16/43) and non-PM, 20% (11/54) (P=0.0664); ascites: PM, 49% (21/43) and non-PM, 7% (4/54) (P<0.0001). The clinical characteristics of patients with PM from GC are unique. Therefore, it is crucial to consider PM as a predictive sign and an important factor when making clinical decisions and developing treatment strategies.
Keywords: chemotherapy, gastric cancer, peritoneal metastasis
Introduction
The prognosis of patients with unresectable or metastatic gastric cancer (GC) is poor and the median survival time ranges between 6 and 12 months (1–12). Cisplatin (CDDP) and 5-fluoropyrimidine (5-FU) are the most frequently prescribed agents. Various chemotherapeutic agents have been used in an attempt to improve patient survival. Oral fluoropyrimidines (S-1 and capecitabine) plus CDDP were found to be non-inferior to 5-FU plus CDDP (8,12). Furthermore, docetaxel and trastuzumab achieved additional results as first-line treatments in a phase III study (7,13). Although irinotecan failed to demonstrate results as a first-line treatment option in a TOP-003 trial (11), it was found to be non-inferior compared to standard treatment in a first-line setting (5,9).
Peritoneal metastasis (PM) in GC is often the cause of ascites and bowel obstruction. Chau et al (14) conducted a multivariate prognostic factor analysis and identified four independent poor prognostic factors, including PM; the patients were classified into good-, moderate- and poor-risk groups, with highly significant differences in survival among the groups.
In order to determine the association between PM and poor prognosis, it is crucial to elucidate the clinical course of the disease. It is, however, difficult to clearly define the patients' clinical characteristics from the pooled data alone. In addition, precise information associated with PM is seldom collected. One of the reasons may be the differences among individual doctors and other observers when classifying identical adverse events. Furthermore, the clinical course of PM varies widely between patients. Finally, following the termination of the protocol treatment, follow-up monitoring is also terminated, resulting in limited availability of information regarding events (i.e., whether the patient is deceased or alive). Therefore, in prospective trials, it is difficult to determine the clinical characteristics over the period between chemotherapy initiation and patient death.
We conducted a retrospective study to determine the frequency of major events during and after palliative chemotherapy in advanced GC patients with peritoneal dissemination.
Patients and methods
Inclusion criteria
The participants were recruited among patients who received first-line chemotherapy for locally advanced or metastatic disease at the Tochigi Cancer Center (TCC; Utsunomiya, Tochigi, japan) and were selected according to the following criteria: i) histologically confirmed gastric adenocarcinoma; ii) no history of prior chemotherapy; iii) adequate bone marrow, hepatic and renal function; and iv) absence of synchronous double cancer or other serious illness.
Diagnositc criteria of complications
The patient records were reviewed and the extracted information included metastatic sites, treatments received, developed complications and overall survival. The complications were determined according to the following criteria: i) Bowel obstruction, diagnostic imaging (air-fluid level formation) and/or decompression tube insertion; ii) ascitic fluid collection, a large amount of ascites and/or therapeutic drain insertion for ascites; iii) obstructive jaundice, biliary drainage and/or jaundice with imaging findings of biliary tree dilatation; iv) hydronephrosis, diagnostic imaging and/or therapeutic insertion of ureteral catheter(s); and v) thrombosis, recorded thrombotic event with abnormal blood tests.
Results
Patient characteristics
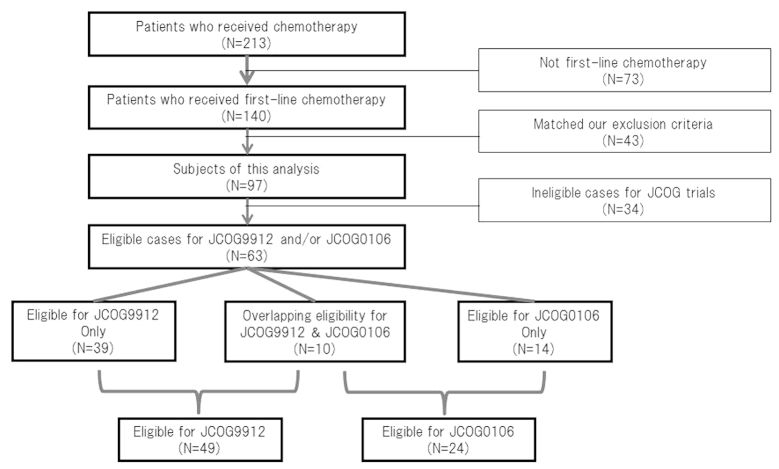
A total of 140 patients at TCC received first-line chemotherapy. Of those patients, 43 were excluded as they matched our exclusion criteria and the remaining 97 patients were reviewed and included this analysis (Fig. 2).
Figure 2.
Diagram of this analysis and patient eligibility for the JCOG9912 and JCOG0106 trials at the tochigi cancer center.
The patient characteristics are summarized in Table I. The patients included 67 men and 30 women, with a median age of 65 years (range, 33–83 years). A total of 43 patients had PM prior to chemotherapy, whereas the remaining 54 patients did not have PM (non-PM).
Table I.
Patient characteristics (n=97).
| Characteristics | Patient no. | % | |
|---|---|---|---|
| Age, years | |||
| Median (range) | 65 (33–83) | ||
| Gender | |||
| Male | 67 | 69.0 | |
| Female | 30 | 31.0 | |
| ECOG performance status | |||
| 0 | 28 | 29.0 | |
| 1 | 54 | 56.0 | |
| 2 | 12 | 12.0 | |
| 3 | 3 | 3.0 | |
| Primary tumor | |||
| Yes | 44 | 45.0 | |
| No | 53 | 55.0 | |
| Histological type | |||
| Intestinal | 37 | 38.0 | |
| Diffuse | 51 | 53.0 | |
| Mixed | 9 | 9.0 | |
| Metastatic site | |||
| Lymph nodes | 46 | 47.0 | |
| Peritoneum | 43 | 44.0 | |
| Liver | 26 | 27.0 | |
| Bone | 4 | 4.0 | |
| Lung | 3 | 3.0 | |
| Number of metastatic sites | |||
| 1 | 1 | 1.0 | |
| 2 | 66 | 68.0 | |
| 3 | 30 | 31.0 |
ECOG, eastern cooperative oncology group.
Treatment regimens
The treatment regimens are outlined in Table II. The JCOG9912 and JCOG0106 trials were conducted during the period of our analysis and 30% of the patients at TCC were also enrolled in those trials. The median survival time was 11.7 months and the median follow-up period of the survivors was 17.5 months (range, 1.4–43 months).
Table II.
Treatment regimens.
| Regimens | Patient no. | % |
|---|---|---|
| First-line (n=97) | ||
| S-1 | 30 | 31.0 |
| CPT/CDDP | 21 | 22.0 |
| MTX/5-FU | 19 | 20.0 |
| 5-FU continuous infusion | 16 | 16.0 |
| Others | 11 | 11.0 |
| Multiple-line (n=59, 120 regimens) | ||
| Paclitaxel (weekly) | 26 | 21.5 |
| Docetaxel (tri-weekly) | 20 | 17.0 |
| S-1 | 18 | 15.0 |
| CPT/CDDP | 15 | 12.5 |
| MTX/5-FU | 12 | 10.0 |
| CPT | 12 | 10.0 |
| Others | 17 | 14.0 |
CPT, irinotecan; CDDP, cisplatin; 5-FU, 5-fluorouracil; MTX, methotrexate.
Complications
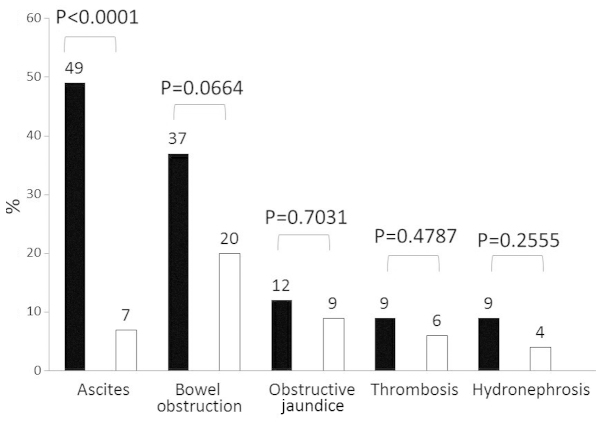
As determined by our diagnostic criteria, the following coexisting illnesses developed in our patients: bowel obstruction, 28% (27/97), ascites, 26% (25/97), obstructive jaundice, 10% (10/97), thrombosis, 7% (7/97) and hydronephrosis, 6% (6/97) (Table III).
Table III.
Developed complications.
| Complications | Patient no. (n=97) | % |
|---|---|---|
| Bowel obstruction | 27 | 28 |
| Ascites | 25 | 26 |
| Obstructive jaundice | 10 | 10 |
| Thrombosis | 7 | 7 |
| Hydronephrosis | 6 | 6 |
In total, 43 (44%) of our patients had GC with PM (Fig. 1). Of these patients, 16 (37%) developed bowel obstruction, whereas the prevalence of this condition in non-PM patients was only 20% (P=0.0664). There was a statistically significant difference between the two groups regarding the development of ascites, with 21 (49%) of the PM patients compared to only 4 (7%) of the non-PM patients (P=0.0001). Obstructive jaundice (n=5, 12%), thrombosis (n=4, 9%) and hydronephrosis (n=4, 9%) were also encountered more frequently among patients with PM compared to those without PM.
Figure 1.
Developed complications in relation to peritoneal metastasis. Black bar, patients with peritoneal metastasis. White bar, patients without peritoneal metastasis.
Discussion
The findings of this retrospective study demonstrated that <1/3 of the patients with advanced or recurrent GC developed ascites or bowel obstruction, with a higher frequency among patients with PM. However, development of obstructive jaundice, thrombosis and hydronephrosis was observed in <10% of the patients. There were no differences with regards to metastatic sites.
In phase III trials of metastatic GC, the frequency of patients with PM is ~20–30%. Although the rate of PM is very similar between Western countries and Japan, there is a difference in the frequency of non-target lesions according to the Response Evaluation Criteria In Solid Tumors (RECIST) criteria (15). It is generally considered that the majority of the patients with non-target lesions are patients with PM. The rates of non-target lesions in Japanese trials are higher compared to those in Western trials. One of the reasons for this difference is the eligibility criteria; Japanese trials tend to allow enrollment of patients with vey small metastases that cannot be detected by imaging techniques, but are detectable only by laparotomy. This may provide an explanation as to why patients with PM in the JCOG9912 trial had a better outcome compared to the patients enrolled in Western trials (1–16).
Although several other studies have described PM as a prognostic factor for survival (16–18), the Japanese Clinical Oncology Group (JCOG) indicated in the JCOG9912 trial that PM was not a significant factor (19). The JCOG0106 clinical trial, a phase III, non-platinum comparison trial with eligibility limited only to patients with PM (20), was conducted during the same period by JCOG; patients with extensive PM were not enrolled in this trial due to poor prognosis and the severe toxicity. It is possible that certain investigators preferred to enroll patients with moderate PM in the less toxic JCOG0106 trial.
The JCOG trials assigned patients with PM into two different studies. However, 16% (10/63) of the eligible cases included in the two JCOG trials overlapped (Fig. 2). Selection bias is one reason why the prognostic index of JCOG differed from those of other analyses and any evaluation of their results of a prognostic index should be performed with caution.
Our data suggest unique trends of PM and the present treatment strategy for PM appears to be reasonable. However, there is currently no widely accepted specific treatment for PM; JCOG failed to demonstrate a clear strategy in two trials limited only to patients with PM (JCOG0106 and JCOG0407). Japanese investigators are attempting to establish a PM limited strategy in one of two manners: one group of investigators intends to achieve a survival benefit by intraperitoneal direct infusional chemotherapy (21), similar to the treatment of ovarian cancer, whereas another group is investigating non-platinum systemic treatment with a paclitaxel-based regimen for PM-limited disease (22).
An ongoing trial in cooperation with the West Japan Oncology Group (WJOG) has already completed enrollment and is partly considering the unique clinical course of PM. The WJOG4007 trial, comparing second-line treatment with irinotecan or paclitaxel, is not limited to PM patients. Even if the prevalence of PM is not extensive at the time of enrollment, it is expected that ~1/3 of the patients will develop PM during cancer progression. A proportion of the PM patients are likely to develop bowel obstruction and would in turn lose their opportunity to receive irinotecan, due to its poor elimination from the intestine and liver. The majority of oncologists tend to avoid selecting irinotecan as a first-line treatment for patients with PM due to its characteristic excretion mechanism. However, certain experienced gastrointestinal oncologists predict disease progression and the development of PM and, therefore, prefer to use irinotecan as early as possible, suggesting that, by using all active chemotherapeutic agents, including irinotecan, patients may achieve a survival benefit.
Our data did not elucidate at which timepoint the complications occur. It is considered that disease progression in cases with PM is closely associated with bowel obstruction and ascites. In general, bowel obstruction and ascites represent typical symptoms and clinical evidence of disease progression in GC patients with PM. It is difficult to assess disease progression in patients with small PM. Japanese investigators recently reported that progression-free survival does not directly reflect on overall survival in GC (23). There may be several reasons for this and we hypothesized that one of the possible explanations is the unique clinical characteristics of PM in GC. The majority of the patients with PM do not have RECIST target lesions; thus, it is very difficult to define a distinct point of disease progression. When conducting control trials by using progression-free survival as a primary endpoint in GC, we must consider enrolling only patients who have target lesions, while indirectly considering bowel obstruction and ascites as progression events.
Our data also suggests that we should consider a change of active regimens in patients with PM when there are clinical symptoms of bowel obstruction and ascites without any definitive progression of metastases on imaging. In the presence of bowel obstruction and massive ascites, it is impossible to continue with anticancer treatment, unless the patient's clinical condition is reversed. The clinical characteristics of patients with PM of GC are unique and it is crucial to consider PM as a predictive sign and an important factor when making clinical decisions and developing treatment strategies.
References
- 1.Wils JA, Klein HO, Wagener DJT, et al. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin – a step ahead in the treatment of advanced gastric cancer: a trial of the european organization for research and treatment of cancer gastrointestinal tract cooperative group. J Clin Oncol. 1991;9:827–831. doi: 10.1200/JCO.1991.9.5.827. [DOI] [PubMed] [Google Scholar]
- 2.Vanhoefer U, Rougier P, Wilke H, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil and doxorubicin versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the european organization for research and treatment of cancer gastrointestinal tract cancer cooperative group. J Clin Oncol. 2000;18:2648–2657. doi: 10.1200/JCO.2000.18.14.2648. [DOI] [PubMed] [Google Scholar]
- 3.Ohtsu A, Shimada Y, Shirao K, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with advanced gastric cancer: JCOG study 9205. J Clin Oncol. 2003;21:54–59. doi: 10.1200/JCO.2003.04.130. [DOI] [PubMed] [Google Scholar]
- 4.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin and fluorouracil versus fluorouracil, doxorubicin and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1016/S0959-8049(97)86090-X. [DOI] [PubMed] [Google Scholar]
- 5.Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. V325 Study Group: Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 8.Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–1553. doi: 10.1200/JCO.2009.25.4706. [DOI] [PubMed] [Google Scholar]
- 9.Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomized phase 3 study. Lancet Oncol. 2009;10:1063–1069. doi: 10.1016/S1470-2045(09)70259-1. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 11.Narahara H, Iishi H, Imamura H, et al. Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002) Gastric Cancer. 2011;14:72–80. doi: 10.1007/s10120-011-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 13.Bang YJ, Cutsem E, Feyereislova A, et al. ToGA Trial Investigators: Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 14.Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer. Pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Kim JG, Ryoo BY, Park YH, et al. Prognostic factors for survival of patients with advanced gastric cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2008;61:301–307. doi: 10.1007/s00280-007-0476-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee SS, Lee JL, Ryu MH, et al. Combination chemotherapy with capecitabine (X) and cisplatin (P) as first line treatment in advanced gastric cancer: experience of 223 patients with prognostic factor analysis. Jpn J Clin Oncol. 2007;37:30–37. doi: 10.1093/jjco/hyl134. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Lim T, Uhm JE, et al. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18:886–891. doi: 10.1093/annonc/mdl501. [DOI] [PubMed] [Google Scholar]
- 19.Takahari D, Takashima A, Mizusawa J, et al. Prognostic factors in Japanese patients with advanced gastric cancer using the data from JCOG9912 study. Proc ASCO. 2011 abstract no. 4059. [Google Scholar]
- 20.Shirao K, Boku N, Yamada Y, Yamaguchi K, Doi T, Takiuchi H, et al. Randomized phase III study of 5-fluorouracil continuous infusion (5FUci) versus methotrexate and 5-FU sequential therapy (MF) in gastric cancer with peritoneal metastasis (JCOG0106) Proc ASCO. 2009 doi: 10.1093/jjco/hyt114. abstract no. 4545. [DOI] [PubMed] [Google Scholar]
- 21.Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70. doi: 10.1093/annonc/mdp260. [DOI] [PubMed] [Google Scholar]
- 22.Matsubara J, Shimada Y, Takashima A, et al. A phase i study of bolus 5-fluorouracil and leucovorin combined with weekly paclitaxel (FLTAX) as first-line therapy for advanced gastric cancer. Jpn J Clin Oncol. 2008;38:540–546. doi: 10.1093/jjco/hyn062. [DOI] [PubMed] [Google Scholar]
- 23.Fuse N. Progression-free survival as surrogate endpoint of overall survival in patients with advanced/recurrent gastric cancer: individual patient data analysis on 4,102 patients from 20 randomized trials. Proc JSCO: Plenaly. 2011;1 [Google Scholar]