ABSTRACT
Bacterial vaginosis (BV) is a common vaginal bacterial imbalance associated with risk for HIV and poor gynecologic and obstetric outcomes. Male circumcision reduces BV-associated bacteria on the penis and decreases BV in female partners, but the link between penile microbiota and female partner BV is not well understood. We tested the hypothesis that having a female partner with BV increases BV-associated bacteria in uncircumcised men. We characterized penile microbiota composition and density (i.e., the quantity of bacteria per swab) by broad-coverage 16S rRNA gene-based sequencing and quantitative PCR (qPCR) in 165 uncircumcised men from Rakai, Uganda. Associations between penile community state types (CSTs) and female partner’s Nugent score were assessed. We found seven distinct penile CSTs of increasing density (CST1 to 7). CST1 to 3 and CST4 to 7 were the two major CST groups. CST4 to 7 had higher prevalence and abundance of BV-associated bacteria, such as Mobiluncus and Dialister, than CST1 to 3. Men with CST4 to 7 were significantly more likely to have a female partner with a high Nugent score (P = 0.03). Men with two or more extramarital partners were significantly more likely to have CST4 to 7 than men with only marital partners (CST4 to 7 prevalence ratio, 1.84; 95% confidence interval [CI], 1.16 to 2.92). Female partner Nugent BV is significantly associated with penile microbiota. Our data support the exchange of BV-associated bacteria through intercourse, which may explain BV recurrence and persistence.
IMPORTANCE
Bacterial vaginosis (BV) is sexually associated but not considered a sexually transmitted disease. Our findings suggest that the uncircumcised penis is an important niche for BV-associated genital anaerobes. In addition, we found a link between extramarital sexual relationships and BV-associated bacteria in men, which parallels earlier findings of the association between sexual activity and BV in women. This suggests the sexual transmissibility of BV-associated bacteria. Reducing bacterial exchange by barrier methods and managing carriage of BV-associated bacteria in men may decrease BV persistence and recurrence in women.
INTRODUCTION
Typified by vaginal discharge, discomfort, and malodor, bacterial vaginosis (BV) results in millions of health care visits annually in the United States. The prevalence of BV varies globally (1), from 29% in the United States (2) to nearly 50% in rural Uganda (3). Key characteristics of BV include an elevated pH and vaginal microbial communities with reduced proportions of Lactobacillus spp. and increased proportions of anaerobes, including species of Mobiluncus, Atopobium, Gardnerella, Prevotella, and other taxa of the order Clostridiales (4). The same anaerobic taxa have recently been reported in the penile microbiota, particularly among uncircumcised men (5–7). However, BV is not recognized as a sexually transmitted condition (8), partly because the communicability of BV has not been established and the condition might have multiple etiologies.
The diagnosis of BV relies on Gram stain using Nugent’s criteria (i.e., a Nugent score of 7 to 10) (9) or on clinically based Amsel criteria (i.e., at least three of the four criteria, including vaginal discharge, elevated pH, clue cells, and fishy odor). While the Nugent score is less sensitive and specific for diagnosing symptomatic BV than the Amsel criteria (10), the Nugent score is commonly used in research settings because it is objective, replicable, and feasible in large-scale epidemiological studies, including those conducted in resource-limited countries (3, 11–16). Thus, Nugent-based BV diagnosis (Nugent-BV) forms the basis of most data linking BV to sexually transmitted infection (STI) and HIV susceptibility and transmission (12, 17–20) and to gynecologic and obstetric complications (21, 22). Findings based on Nugent-BV can be interpreted in the context of its epidemiological associations (9, 12, 17, 18, 21, 22).
There is growing evidence for the exchange of BV-associated bacteria through sexual intercourse. Previous studies have shown that the penis can harbor BV-associated bacteria and that these bacteria are reduced by male circumcision (5–7). Furthermore, male circumcision significantly reduces Nugent-BV in female partners (11). Persistence or recurrence of Nugent-BV after antibiotic treatment is common and may be driven by the reintroduction of BV-associated bacteria through sexual intercourse (23, 24). However, microbiological evidence establishing the exchange of BV-associated bacteria in heterosexual partners is lacking.
The primary goal of this study was to assess the relationship between penile microbiota of uncircumcised men and Nugent-BV in female partners. We characterized the penile microbiota in 165 uncircumcised men from Rakai, Uganda, and the association between the penile microbiota and female partner Nugent-BV status.
RESULTS
Study participants.
All 165 participants were HIV-negative, uncircumcised men from Rakai, Uganda. Approximately 40% of participants had a female partner with Nugent-BV, 45% had a partner with a normal Nugent score, and 15% had a partner with an intermediate Nugent score (Table 1). Most participants were in monogamous heterosexual relationships (148/165 [89.7%]), and nonmarital sexual relationships were uncommon (24/165 [14.5%]). Condom use was also uncommon, and most participants who reported using condoms used them inconsistently (58/60 [96.7%]) (Table 1).
TABLE 1 .
Study participants’ sociodemographic characteristics, sexual behaviors, and clinical history
| Parameter | No. (%) of participants (n = 165) |
|---|---|
| Age, yr | |
| 15–19 | 2 (1.2) |
| 20–24 | 30 (18.2) |
| 25–29 | 50 (30.3) |
| 30–49 | 83 (50.3) |
| Partner bacterial vaginosis status (Nugent score) | |
| Normal (0–3) | 75 (45.4) |
| Intermediate (4–6) | 30 (18.2) |
| Bacterial vaginosis (7–10) | 60 (36.4) |
| Marital status | |
| Currently married, monogomous | 148 (89.7) |
| Currently married, polygamous | 17 (10.3) |
| No. of sexual partners in past yr | |
| 1 | 93 (56.4) |
| 2 | 53 (32.1) |
| ≥3 | 19 (11.5) |
| Nonmarital sexual relationships | |
| No | 141 (85.5) |
| Yes | 24 (14.5) |
| Condom use in past yr | |
| None | 105 (63.6) |
| Inconsistent use | 58 (35.2) |
| Consistent use | 2 (1.2) |
| Current condom use | |
| No | 148 (89.7) |
| Yes | 17 (10.3) |
| Self-reported symptoms of sexually transmitted infection in past yr | |
| Genital ulcer disease | 10 (6.0) |
| Urethral discharge | 4 (2.4) |
| Dysuria | 7 (4.2) |
The seven penile CSTs.
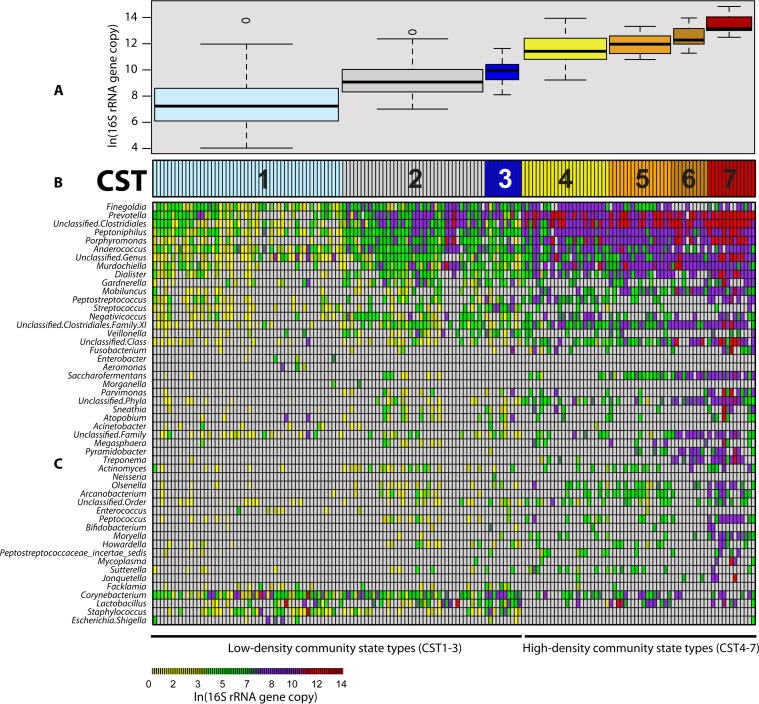
We characterized the penile microbiota using hierarchal clustering and found seven distinct community state types (CSTs) (25), which could be divided into two major groups: CST1 to 3 and CST4 to 7 (Fig. 1B; see Fig. S1 in the supplemental material). Bacterial densities differed significantly among the seven CSTs (by analysis of variance [ANOVA], P < 0.001) (Fig. 1A), indicating that the composition of penile microbiota varied along the bacterial density gradient. The lowest-density CST (CST1) had 1.0 × 106 16S rRNA gene copies per swab on average (standard deviation [SD], 8.2 × 105), and the highest-density CST (CST7) had 1.0 × 109 16S rRNA gene copies per swab on average (SD, 9.1 × 108) (Fig. 1C).
FIG 1 .
Community state types of the uncircumcised coronal sulcus microbiome. (A) Distribution of bacterial densities for each CST, shown as box plots. The box of each box plot denotes the interquartile range (IQR) (Q1 to Q3) and the corresponding median, whereas the whiskers signify the upper and lower 1.5× IQR. Across the seven CSTs, bacterial densities differed significantly (by ANOVA, P < 0.001). (B) Community state types and the BV partner status evaluated by Nugent score. CSTs were further collapsed into two major groups: the low-density community state types (CST1 to 3) and the high-density community state types (CST4 to 7). (C) Heat map representing the composition and abundance of coronal sulcus bacteria from uncircumcised men. Each column represents one man. Each row depicts the absolute abundance of a coronal sulcus bacterium (e.g., Prevotella) and can be interpreted using the annotated color-coding key (bottom, color bar), which denotes the correlation between each color with its respective ln-transformed absolute abundance.
High prevalence of BV-associated bacteria in men with CST4 to 7.
We identified the bacteria associated with female partner Nugent-BV (i.e., Nugent-BV indicators) versus a normal Nugent score (i.e., normal Nugent score indicators) among our participants using indicator analysis (see Table S1 in the supplemental material). As indicators of a normal female partner Nugent score, Corynebacterium and Staphylococcus were significantly more prevalent in CST1 to 3 than in CST4 to 7; however, even though Lactobacillus was 10% more prevalent in CST1 to 3 than in CST4 to 7, the difference was not statistically significant (Table 2; see Table S1). This discrepancy may be explained in part by the profile of Lactobacillu iners. For example, both L. iners and Lactobacillus vaginalis were associated with having a female partner with a normal Nugent score. However, L. iners was equally prevalent in CST1 to 3 and in CST4 to 7 (L. iners prevalence in CST1 to 3 of 29.7% and prevalence in CST4 to 7 of 28.1%; P = 0.97), but L. vaginalis, like Corynebacterium and Staphylococcus, was significantly more prevalent in CST1 to 3 (L. vaginalis prevalence in CST1 to 3 of 22.8% and prevalence in CST4 to 7 of 9.4%; P = 0.046). Taken together, our findings suggest that Lactobacillus associates consistently with a normal female partner Nugent score but variably with CSTs in a species-dependent manner (see Table S2 in the supplemental material).
TABLE 2 .
Prevalence and abundance of female partner Nugent score indicators in men with CST1 to 3 versus those with CST4 to 7
| Group and bacterium | Prevalence |
Proportional abundancea |
||||
|---|---|---|---|---|---|---|
| CST1 to 3 (n = 101), n (%) | CST4 to 7 (n = 64), n (%) | Chi-square P value | CST1 to 3 (n = 101), median % (IQR) | CST4 to 7 (n = 64), median % (IQR) | K-S P valueb | |
| Nugent-BV indicator | ||||||
| Dialister | 51 (50.5) | 51 (79.7) | <0.001 | 1.3 (0.5–2.9) | 1.8 (1.0–3.5) | 0.41 |
| Gardnerella | 40 (39.6) | 23 (35.9) | 0.76 | 0.7 (0.4–3.5) | 0.5 (0.2–1.2) | 0.44 |
| Mobiluncus | 22 (21.8) | 45 (70.3) | <0.001 | 0.5 (0.2–2.4) | 1.0 (0.3–2.5) | 0.22 |
| Peptostreptococcus | 38 (37.6) | 31 (48.4) | 0.23 | 2.1 (0.1–3.9) | 1.2 (0.3–2.5) | 0.15 |
| Porphyromonas | 60 (59.4) | 61 (95.3) | <0.001 | 2.7 (1.0–5.2) | 6.1 (3.0–10.3) | <0.001 |
| Prevotella | 81 (80.2) | 63 (98.4) | 0.001 | 16.0 (7.3–33.2) | 28.8 (17.2–41.9) | 0.003 |
| Saccharofermentans | 9 (8.9) | 40 (62.6) | <0.001 | 0.06 (NA)c | 0.5 (0.2–1.3) | 0.5 |
| Sneathia | 16 (15.8) | 12 (18.8) | 0.79 | 0.2 (0.1–0.7) | 0.3 (0.2–2.0) | 0.44 |
| Treponema | 1 (1.0) | 20 (31.3) | <0.001 | 0.02 (NA) | 0.1 (0.4–1.6) | 0.57 |
| Unclassified Clostridiales | 82 (81.2) | 64 (100.0) | <0.001 | 4.0 (0.8–18.7) | 22.8 (12.7–32.0) | <0.001 |
| Unclassified Clostridiales family XI | 50 (49.5) | 61 (95.3) | <0.001 | 0.6 (0.3–1.0) | 0.9 (0.5–1.6) | 0.04 |
| Unclassified phyla | 20 (19.8) | 39 (60.9) | <0.001 | 0.2 (0.1–0.4) | 0.7 (0.2–1.3) | 0.01 |
| Normal Nugent score indicators | ||||||
| Corynebacterium | 90 (89.1) | 29 (45.3) | <0.001 | 7.3 (1.4–22.9) | 0.5 (0.2–1.2) | <0.001 |
| Lactobacillus | 40 (39.6) | 19 (29.7) | 0.26 | 2.6 (0.3–28.5) | 0.9 (0.2–7.4) | 0.44 |
| Staphylococcus | 55 (54.5) | 0 (0.0) | <0.001 | 1.0 (0.1–3.5) | 0 (NA) | <0.001 |
To better delineate the difference in proportional abundance from prevalence, only participants who carried a taxon (i.e., carriers) were included in the proportional abundance comparison.
P value by Kolmogorov-Smirnov test.
NA, not applicable.
In contrast, indictors of female partner Nugent-BV included Dialister, Mobiluncus, Prevotella, and Porphyromonas, which were significantly more prevalent in CST4 to 7 than in CST1 to 3 (P < 0.05) (Table 2; see Table S1 in the supplemental material). Notably, even though Gardnerella is classically associated with BV, its prevalence was not significantly higher in CST4 to 7 (Table 2).
High proportional abundance of BV-associated bacteria in men with CST4 to 7.
The proportional abundances of BV-associated bacteria were also significantly higher in CST4 to 7 than CST1 to 3, including Prevotella, Porphyromonas, and unclassified Clostridiales (P < 0.05) (Table 2); however, Lactobacillus or Gardnerella again did not differ significantly across CST group (Table 2). Further analysis of CST1 to 3 and CST4 to 7 indicator bacteria recapitulated the microbiological link between BV-associated bacteria and CST4 to 7 (see Table S3 in the supplemental material).
Men with CST4 to 7 were more likely to have a partner with Nugent-BV.
Men with CST4 to 7 were significantly more likely to have a partner with Nugent-BV than men with CST1 to 3 (CST4 to 7 versus CST1 to 3 prevalence rate ratio [PRR], 1.55; 95% confidence interval [CI], 1.07 to 2.24). Likewise, men with CST4 to 7 were less likely to have a partner with a normal Nugent score than men with CST1 to 3 (CST4 to 7 PRR, 0.68; 95% CI, 0.48 to 0.97) (Table 3). In contrast, there was no significant association between intermediate Nugent-BV and CST4 to 7 (CST4 to 7 PRR, 1.21; 95%CI, 0.65 to 2.25) (Table 3). Thus, men with CST4 to 7 had higher abundance of BV-associated bacteria and were more likely to have a female partner with Nugent-BV.
TABLE 3 .
Prevalence of female partner Nugent-BV by coronal sulcus community state type
| CST group | Female partner Nugent score |
CST4 to 7 vs CST1 to 3 PRR (95% CI) |
||||
|---|---|---|---|---|---|---|
| Nugent-BV (7–10) | Normal (0–3.0) | Intermediate (4.0–6.0) | Nugent-BV/normal | Nugent-normal/BV | Nugent-intermediate/normal | |
| CST4 to 7 | ||||||
| (n = 64) | 30 (56.6) | 23 (43.4) | 11 (17.2) | |||
| 1.55 (1.07–2.24) | 0.68 (0.48–0.97) | 1.21 (0.65–2.25) | ||||
| CST1 to 3 | ||||||
| (n = 101) | 30 (36.6) | 52 (63.4) | 19 (18.8) | |||
Link between sexual activity and CST4 to 7.
Compared to men with only marital sexual partner(s), having one nonmarital sexual partner was associated with a trend toward increased CST4 to 7 prevalence (CST4 to 7 PRR, 1.23; 95% CI, 0.79 to 1.92), and having two or more nonmarital sexual partners was associated with significantly increased CST4 to 7 prevalence (CST4 to 7 PRR, 1.84; 95% CI, 1.16 to 2.92) (see Table S4 in the supplemental material). Other sexual behaviors and urogenital symptoms were too infrequent to be investigated (Table 1). This association between CST4 to 7 and multiple nonmarital sexual partners is similar to the association between BV and the female-reported number of male nonmarital sexual partners (26).
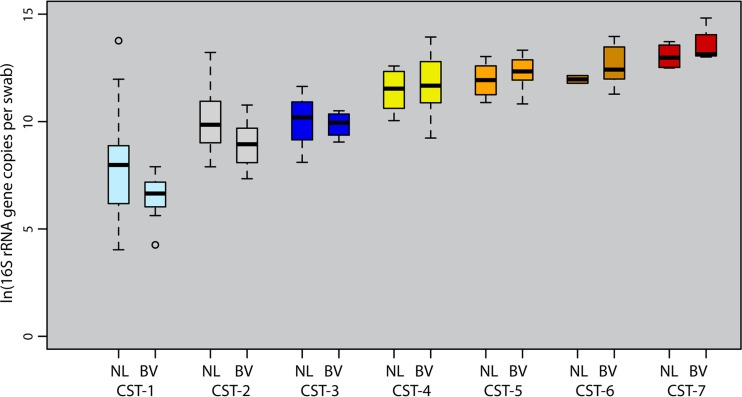
Female partner Nugent-BV was associated with increased bacterial density among men with CST4 to 7.
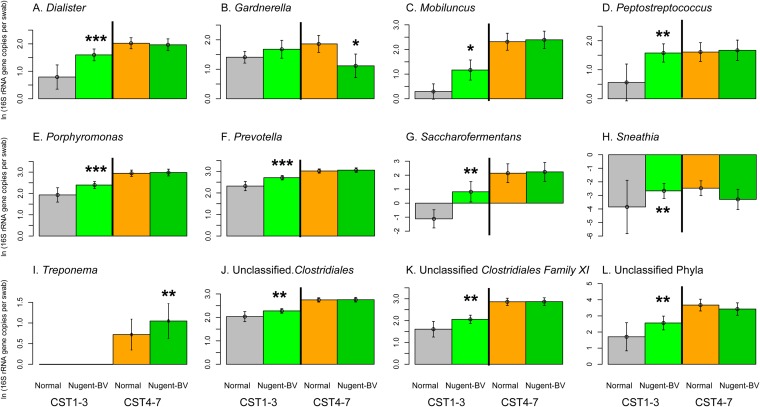
Among men with CST4 to 7, having a female partner with Nugent-BV was associated with a nearly 2-fold increase in bacterial density (men with partner Nugent-BV mean of 4.5 × 108 16S rRNA genes per swab versus men with partner normal Nugent score mean of 2.3 × 108 16S rRNA genes per swab; P = 0.06) (Fig. 2). However, the only Nugent-BV indicator that increased significantly in our quasi-Poisson models was Treponema (ΔNugent-BV − normal Nugent = +4.9 × 102 16S rRNA gene copies per swab; P = 0.01), while Gardnerella showed a borderline increase (Δ = +1.6 × 104; P = 0.09). We found no significant increases in other BV-associated bacteria (Fig. 3; see Table S5 and Fig. S2 in the supplemental material).
FIG 2 .
Coronal sulcus bacterial densities and female partner bacterial vaginosis, stratified by coronal sulcus community state type. These box plots contrast the coronal sulcus bacterial densities in men with versus men without female partner BV, stratified by CST. There was an approximately 2-fold increase in bacterial density among men with a BV partner in the high-density group (CST4 to 7) (BV group mean of 4.5 × 108 versus normal group mean of 2.3 × 108 16S rRNA gene copies per swab; P = 0.06). In contrast, there was a 5-fold bacterial density decrease in the low-density CST group (CST1 to 3) (BV group mean of 8.2 × 106 versus normal group mean of 5.3 × 107 16S rRNA gene copies per swab; P = 0.04).
FIG 3 .
Association between female partner Nugent-BV and Nugent-BV indicator in the coronal sulcus based on quasi-Poisson models, stratified by CST. The influence of partner Nugent-BV on coronal sulcus microbiome was especially visible among men with CST1 to 3. In this group, our quasi-Poisson models showed that men with female partner Nugent-BV had significantly higher absolute abundance of BV-associated bacteria than men whose female partner had a normal Nugent score. The predicted increases in BV-associated bacteria included an 18.2-fold increase in Prevotella (Δ = +4.6 × 105 16S rRNA gene copies per swab; P = 0.01), a 60.3-fold increase in Porphyromonas (P = 0.09), and a 16.4-fold increase in Dialister (Δ = +1.4 × 103; P = 0.004), while the abundance of Lactobacillus was 12.2-fold lower in men with partner Nugent-BV (Δ = −9.3 × 103; P = 0.01). Among men with CST4 to 7, Treponema (Δ = +4.9 × 102; P = 0.01) was the only BV-associated bacterium that had significantly higher absolute abundance in men with female partner Nugent-BV than men whose female partner had a normal Nugent score. Gardnerella showed a borderline increase (Δ = +1.6 × 104; P = 0.09).
Female partner Nugent-BV was linked to decreased bacterial density among men with CST1 to 3.
Some men also had CST1 to 3, despite having a female partner with Nugent-BV (n = 30/101 [29.7%]). Their penile bacterial densities were 6.5-fold lower on average than those of men whose partner had a normal Nugent score (partner Nugent-BV mean of 8.2 × 106 versus partner normal Nugent score mean of 5.3 × 107 16S rRNA gene copies per swab; P = 0.04) (Fig. 2). The decrease reflects the lower abundances of Corynebacterium (ΔNugent-BV − normal Nugent = −6.0 × 103 16S rRNA gene copies per swab; P = 0.05), Staphylococcus (Δ = −2.2 × 103; P = 0.007), and Lactobacillus (Δ = −4.5 × 101; P = 0.007), based on quasi-Poisson models (see Fig. S3 and Table S5 in the supplemental material).
We also found that female partner Nugent-BV was associated with small but significant increases in 10 of the 12 Nugent-BV indicator species. These 10 Nugent-BV indicators included Prevotella (ΔNugent-BV − normal Nugent = +4.6 × 105 16S rRNA gene copies per swab; P = 0.002), Porphyromonas (Δ = +5.9 × 104; P = 0.005), Dialister (Δ = +1.4 × 102; P < 0.001), and unclassified Clostridiales (Δ = +1.6 × 104; P = 0.02) (Fig. 3; see Table S5 and Fig. S2 in the supplemental material).
DISCUSSION
Our findings suggest that BV may be sexually transmissible, as indicated by the association between BV-associated bacteria in men and the Nugent score BV of their female partners. Sexual transmissibility of BV is also consistent with the link between multiple partners and BV-associated bacteria in men, which parallels the association between sexual behaviors and BV risk in women (23, 26–28). Sexual transmission of BV may also explain why both topical and oral antimicrobials lack long-term efficacy against BV, since BV-associated bacteria may be reintroduced from the subpreputial space to the vagina through intercourse.
In our study of 165 Ugandan men, BV-associated bacteria were prevalent and abundant in the subpreputial space, and the abundance of these bacteria was significantly associated with the Nugent scores of their female partners, except in the instance of intermediate Nugent scores. Together, these findings indicate that the subpreputial space could be an important niche for BV-associated bacteria in men. Earlier studies reported that the subpreputial space and distal urethra can harbor BV-associated bacteria (5, 6), and circumcision can reduce female partner BV (11).
The human microbiota can be highly dynamic; thus, the term “community state type” (CST) was coined to reflect the transitional nature of human microbiota (25). We showed that the penile microbiota are conserved assemblages of genital bacteria that could be represented by seven community state types. In uncircumcised men, the penile CSTs ranged from low bacterial density comprised primarily of skin-associated bacteria to high bacteria density with abundant BV-associated bacteria and other genital anaerobes.
Given the suspected sexual transmission of BV from this and earlier studies (23, 26–28), the most parsimonious explanation for the association between penile microbiota and female partner Nugent score BV is a bidirectional exchange of genital bacteria. While a simple bacterial exchange (i.e., the transfer of genital bacteria from one partner and consequent establishment in the other) may be a sufficient model to explain the meta-population dynamics of BV-associated bacteria, the finding that some men had few BV-associated bacteria (i.e., CST1 to 3) despite having a female partner with Nugent-BV suggests that bacterial interactions may shape genital microbiota composition. After exposure, CST1 to 3 may confer resistance to the establishment of BV-associated bacteria via antagonistic interactions, such as resource competition (29–32), antimicrobial production (33), stimulation of the immune system (34, 35), or predation (36). Even though little is known regarding antagonistic effects of male genital commensals, in vitro and coculture studies indicate that the vaginal commensal Lactobacillus could inhibit BV-associated bacteria (37–39). Thus, a possible explanation for the imperfect concordance between penile microbiota and female partner Nugent score is that penile microbiota composition influences the outcome from exposure to vaginal bacteria.
Using 16S rRNA gene absolute abundance as a surrogate of taxon absolute abundance in this study was key to elucidating the associations between Nugent score and penile microbiota, likely because taxon absolute abundance is the most relevant metric for examining ecological interactions (40). However, to better evaluate our surrogate metric’s clinical interpretation and to more fully assess the influence of penile bacteria on female partner vaginal microbiota and vice versa, longitudinal partner studies are needed to assess treatments that eradicate BV-associated bacteria from men and the temporal effects of sexual intercourse on genital microbiota. It will also be important to determine if our findings could be generalized to non-Ugandan populations. Previous studies suggest that antimicrobial resistance and poor drug penetration can challenge decolonization efforts (41–45). However, study design limitations—including, suboptimal treatment regimen, insufficient randomization methods, limited power, and unknown adherence—may have contributed to previous failed attempts to decrease BV by decolonizing male sexual partners (46).
If BV is redefined as a sexually transmitted condition, it has the potential to expand the infectious disease framework from transmission of single pathogens to encompass transmission of bacterial communities. This could affect clinical care of BV and justify new preventative and treatment strategies, such as prebiotic, probiotics, or narrow-spectrum antimicrobials to modify the penile microbiota or microenvironments aimed at reducing male carriage, which may ameliorate the persistence and recurrence of BV.
MATERIALS AND METHODS
Study design and subjects.
Uncircumcised HIV-negative men, 15 to 49 years of age, were enrolled in a randomized trial of male circumcision (MC) for HIV prevention in Rakai, Uganda (47). At enrollment, coronal sulcus samples were collected by clinicians using sterile cotton-tipped applicators premoistened with sterile saline, which were rolled over the coronal sulcus twice and immediately placed in 1 ml of Amplicor transport medium (Roche Diagnostics, Indianapolis, IN) and stored at −80°C until analysis. Among the enrollment samples from married men who remained HIV negative together with their partner(s) during the trial, 165 samples were selected at random for this study.
The female partners were enrolled into a parallel study (48) and provided self-collected vaginal swabs, which were evaluated by Nugent’s criteria and scored as normal (Nugent score of 0 to 3), intermediate (Nugent score of 4 to 6), or BV (Nugent score of 7 to 11). Among the 17 polygamous men, 15 only had one female partner enrolled. The female partners for the two other polygamous men had concordant BV assignments. Male herpes simplex virus 2 and syphilis serology were assessed as previously described (49). Extensive demographic and sexual activity data were collected by interview (47).
Human subject research.
This study was approved by four institutional review boards: the Science and Ethics Committee of the Uganda Virus Research Institute (Entebbe, Uganda), the National Council for Science and Technology (Kampala, Uganda), the Committee for Human Research at Johns Hopkins University’s Bloomberg School of Public Health (Baltimore, MD), and the Western Institutional Review Board (Olympia, WA).
Sample processing.
From each sample, 100 µl of the swab eluent was lysed using pressure-cycling technology (Pressure Biosciences, South Easton, MA), purified using the Qiagen AllPrep DNA/RNA minikit (Qiagen, Valencia, CA), and eluted using 100 µl of buffer EB as previously described (5).
Penile microbiota bacterial density characterization.
Using the purified DNA, we quantified penile microbiota bacterial density, measured as 16S rRNA gene copies per microliter of swab eluent using a broad-coverage quantitative PCR (qPCR) targeting the 16S rRNA gene (V3-V4) (50). Results were reported as 16S rRNA gene copies per swab (based on 1000 µl of eluent from each swab). A detailed description of laboratory analyses can be found in Text S1 in the supplemental material.
Penile bacterial prevalence, proportional abundance, and absolute abundance calculation.
We characterize the penile microbiota by sequencing the bacterial 16S rRNA gene (V3 to V6) using the same qPCR V3F primer on GS FLX (454 Life Sciences, Branford, CT) as previously described (5). The resultant pyrosequences were chimera checked, demultiplexed, quality checked, and classified taxonomically as previously described (5) and specifically for Lactobacillus species as previously described (25). We obtained a total of 202,241 reads, with a mean of 1,210 reads per sample (SD, 973; range, 124 to 5,143; median, 930). Taxonomic groups with a single sequence were excluded. Clostridiales and Clostridiales family XI sequences classified at a <80% bootstrap confidence level were reported as unclassified Clostridiales and unclassified Clostridiales family XI, respectively. A detailed description of bioinformatics analysis can be found in Text S1 in the supplemental material.
Bacterial prevalence was calculated for CST1 to 3 and CST4 to 7 as the no. of participants with CST who had taxon/total no. of participants with each CST stratum. Bacterial proportional abundance was calculated for each participant as the no. of sequences of taxon/total no. of sequences, and bacterial absolute abundance was calculated as the proportional abundance of taxon × total microbiota bacterial density, as in a previous study (50). Neither proportional nor absolute abundance estimates based on 16S rRNA gene copies are identical to estimates of absolute population sizes or their relative abundances because of variation in 16S rRNA gene copy numbers per cell (51). Still, 16S rRNA gene copies provide a useful proxy for abundance; thus, we use the 16S rRNA gene copy number as a proxy for absolute abundance, allowing comparisons of bacterial communities across human subjects. The 50 most prevalent coronal sulcus bacteria, comprising 99.7% of total sequences, were included in the analysis.
Microbiota CST assignment by hierarchal clustering.
To identify community state types (CSTs), we used hierarchal clustering by Ward linkage in Euclidean distance using the cutree algorithm through an iterative process as previously described (25). Comparisons of the 6-, 7-, and 8-CST solutions revealed the 7-CST solution to be the most parsimonious and effective. We further divided the seven CSTs into two major strata—CST1 to 3 and CST4 to 7—based on the first bifurcation of the clustering dendrogram. Bacterial densities among CSTs were compared using analysis of variance.
Identification of indicator taxa for CST, major CST strata, and female partner Nugent score.
We used indicator analysis to identify penile bacteria uniquely associated with CSTs and female partner Nugent score (52). The indicator species analysis is an objective assessment of a particular genus’ representation in an environment or a study group. A genus’ indicator value (IV) for a study group is determined based on its proportional abundance and prevalence in the given study group. The IV can range from 0 to 1, with 0 as no indication to 1 as perfect indication. To test the null hypothesis of no difference between our observation and what might be observed by chance, we built IV null distributions by the Monte Carlo procedure using 1,000 resampled data sets with randomized study group assignments. We determined the P value for each observed IV based on its location within the null distribution and adjusted for false discovery. A significance level of α = 0.10 was used.
Assessment of relationship between female partner Nugent-BV and penile microbiota across CST strata.
We compared the prevalence of female partner Nugent-BV versus normal Nugent score in CST1 to 3 versus CST4 to 7 based on prevalence rate ratio (PRR) and its 95% confidence interval (CI) by the Breslow test of heterogeneity in EpiR (version 0.9 to 48) (53). We then compared partner Nugent-BV indicator prevalence and proportional abundance in CST1 to 3 versus CST4 to 7. We compared female partner Nugent score indicator and Lactobacillus species prevalence by chi-square test. Due to the large differences in prevalence, we only included participants with the taxon in the proportional abundance comparisons, which we performed using the Kolmogorov-Smirnov test. A significance level of α = 0.05 was used.
Assessment of relationship between female partner Nugent-BV and penile microbiota within CST stratum.
We examined the association between penile microbiota and female partner Nugent-BV (normal and Nugent-BV) within each CST stratum. We compared the bacterial density using a two-tailed t test with unequal variance, after excluding outliers based on the interquartile range rule. We compared Nugent-BV indicator absolute abundance using a quasi-Poisson model to predict each indicator’s absolute abundance by female partner Nugent-BV status for each CST stratum. Polygamy and extramarital relationship were included in the starting model. The predicted absolute abundances were presented as circle plots, where the area of the circle is proportional to mean absolute abundance. Detailed description of the statistical analyses can be found in Text S1 in the supplemental material.
Nucleotide sequence accession number.
Sequence data have been deposited in GenBank under accession no. SRP058681.
SUPPLEMENTAL MATERIAL
Supplementary materials, methods, and results. Download
Community state types of the uncircumcised coronal sulcus microbiome, identified by hierarchal clustering. This heat map visualization illustrates how we clustered microbiota profiles by hierarchal clustering, which produced the resultant dendrogram (top). The seven community state types (CSTs) were identified by cutting the dendrogram using the cutree algorithm in R. As shown, collapsing the dendrogram to its first bifurcation produced two major CST groups: the low-density community state types (CST1 to 3) and the high-density community state types (CST4 to 7). In a heat map, each column represents one male participant, and each row depicts the absolute abundance (16S rRNA gene copies per swab) of bacterial genus-level taxa in the coronal sulcus microbiota (e.g., Prevotella). The abundance is interpreted using the annotated color-coding key (bottom, color bar), which denotes the correlation between each color with its respective ln-transformed absolute abundance. Download
Absolute abundances of Prevotella, Porphyromonas, Dialister, Mobiluncus, and Gardnerella by CST group and partner BV status, shown in box plots. Download
Absolute abundances of Lactobacillus, Staphylococcus, Finegoldia, and Corynebacterium for CST1 to 3 by partner BV status, shown in box plots. Download
Indicator genera associated with female partner Nugent score in full cohort and in monogamous men only, based on absolute abundance.
Lactobacillus species prevalence by penile CSTs, female partner Nugent score, and penile CSTs.
Indicator genera for CST1 to 3 versus CST4 to 7, as determined based on absolute abundance.
Association between host sexual activity and prevalence of CST1 to 3 versus CST4 to 7.
Absolute abundance of indicator genera associated with female partner normal and Nugent-BV scores in men with CST1 to 3 and men with CST4 to 7 by the quasi-Poisson model.
ACKNOWLEDGMENTS
This work was provided by R01AI087409-01A1 and U01AI51171 from the National Institutes of Health, the Bill and Melinda Gates Foundation (22006.02), and the Doris Duke Charitable Foundation (no. 2011036). C.M.L. was supported by the Northern Arizona University Technology and Research Initiative Fund (TRIF) and the Cowden Endowment in Microbiology at Northern Arizona University. A.A.R.T. was supported by NIH 1K23AI093152-01A1 and the Doris Duke Charitable Foundation Clinician Scientist Development Award.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
We thank Allison Abraham for assistance with epidemiological methods.
D.S, G.K, R.M.G., F.N., M.J.W., and R.H.G. conducted the field study. F.N. and J.L.P. contributed to metadata collection. C.M.L. contributed to the laboratory analysis. C.M.L., B.A.H., P.K., and L.B.P. contributed to data analysis. C.M.L. drafted the manuscript. B.A.H., A.A.T., J.R., J.L.P., P.K., M.J.W., R.H.G., and L.B.P. contributed to revisions of the manuscript. All authors have reviewed the manuscript.
Footnotes
Citation Liu CM, Hungate BA, Tobian AAR, Ravel J, Prodger JL, Serwadda D, Kigozi G, Galiwango RM, Nalugoda F, Keim P, Wawer MJ, Price LB, Gray RH. 2015. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. mBio 6(3):e00589-15. doi:10.1128/mBio.00589-15.
REFERENCES
- 1.Kenyon C, Colebunders R, Crucitti T. 2013. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol 209:505–523. doi: 10.1016/j.ajog.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, Markowitz LE. 2007. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 34:864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 3.Thoma ME, Gray RH, Kiwanuka N, Wang MC, Sewankambo N, Wawer MJ. 2011. The natural history of bacterial vaginosis diagnosed by Gram stain among women in Rakai, Uganda. Sex Transm Dis 38:1040–1045. doi: 10.1097/OLQ.0b013e3182275499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredricks DN, Fiedler TL, Marrazzo JM. 2005. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 5.Liu CM, Hungate BA, Tobian AA, Serwadda D, Ravel J, Lester R, Kigozi G, Aziz M, Galiwango RM, Nalugoda F, Contente-Cuomo TL, Wawer MJ, Keim P, Gray RH, Price LB. 2013. Male circumcision significantly reduces prevalence and load of genital anaerobic bacteria. mBio 4(2):e00076. doi: 10.1128/mBio.00076-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson DE, Dong Q, Van der Pol B, Toh E, Fan B, Katz BP, Mi D, Rong R, Weinstock GM, Sodergren E, Fortenberry JD. 2012. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PLoS One 7:e36298. doi: 10.1371/journal.pone.0036298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eren AM, Zozaya M, Taylor CM, Dowd SE, Martin DH, Ferris MJ. 2011. Exploring the diversity of Gardnerella vaginalis in the genitourinary tract microbiota of monogamous couples through subtle nucleotide variation. PLoS One 6:e26732. doi: 10.1371/journal.pone.0026732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Workowski KA, Berman S, Centers for Disease Control and Prevention . 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 59:1–110. [PubMed] [Google Scholar]
- 9.Nugent RP, Krohn MA, Hillier SL. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram-stain interpretation. J Clin Microbiol 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. 1996. Validity of the vaginal Gram-stain for the diagnosis of bacterial vaginosis. Obstet Gynecol 88:573–576. doi: 10.1016/0029-7844(96)00233-5. [DOI] [PubMed] [Google Scholar]
- 11.Gray RH, Kigozi G, Serwadda D, Makumbi F, Nalugoda F, Watya S, Moulton L, Chen MZ, Sewankambo NK, Kiwanuka N, Sempijja V, Lutalo T, Kagayii J, Wabwire-Mangen F, Ridzon R, Bacon M, Wawer MJ. 2009. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 20042e1–42.e7. doi: 10.1016/j.ajog.2008.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier SL, Rabe L, Gaydos CA, Quinn TC, Konde-Lule J. 1997. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 350:546–550. doi: 10.1016/S0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 13.Thoma ME, Gray RH, Kiwanuka N, Aluma S, Wang MC, Sewankambo N, Wawer MJ. 2011. The short-term variability of bacterial vaginosis diagnosed by Nugent Gram stain criteria among sexually active women in Rakai, Uganda. Sex Transm Dis 38:111–116. doi: 10.1097/OLQ.0b013e3181f0bdd0. [DOI] [PubMed] [Google Scholar]
- 14.Kaul R, Nagelkerke NJ, Kimani J, Ngugi E, Bwayo JJ, Macdonald KS, Rebbaprgada A, Fonck K, Temmerman M, Ronald AR, Moses S, Kibera HIV Study Group . 2007. Prevalent herpes simplex virus type 2 infection is associated with altered vaginal flora and an increased susceptibility to multiple sexually transmitted infections. J Infect Dis 196:1692–1697. doi: 10.1086/522006. [DOI] [PubMed] [Google Scholar]
- 15.Schellenberg JJ, Card CM, Ball TB, Mungai JN, Irungu E, Kimani J, Jaoko W, Wachihi C, Fowke KR, Plummer FA. 2012. Bacterial vaginosis, HIV serostatus and T-cell subset distribution in a cohort of East African commercial sex workers: retrospective analysis. AIDS 26:387–393. doi: 10.1097/QAD.0b013e32834ed7f0. [DOI] [PubMed] [Google Scholar]
- 16.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG. 1998. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. 2003. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis 37:319–325. doi: 10.1086/375819. [DOI] [PubMed] [Google Scholar]
- 18.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 19.Warren D, Klein RS, Sobel J, Kieke B Jr, Brown W, Schuman P, Anderson J, Cu-Uvin S, Mayer K, Jamieson DJ, Holmberg S, Duerr A, HIV Epidemiology Research Study Group . 2001. A multicenter study of bacterial vaginosis in women with or at risk for human immunodeficiency virus infection. Infect Dis Obstet Gynecol 9:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, Donnell D, Celum C, Kapiga S, Delany S, Bukusi EA. 2012. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med 9:e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillier SL, Krohn MA, Cassen E, Easterling TR, Rabe LK, Eschenbach DA. 1995. The role of bacterial vaginosis and vaginal bacteria in amniotic fluid infection in women in preterm labor with intact fetal membranes. Clin Infect Dis 20(Suppl 2):S276–S278. doi: 10.1093/clinids/20.Supplement_2.S276. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad AH, Copper RL, Das A, Thom E, Johnson F, McNellis D, Miodovnik M, Van Dorsten JP, Caritis SN, Thurnau GR, Bottoms SF. 1998. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU Network. Am J Public Health 88:233–238. doi: 10.2105/AJPH.88.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. 2006. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 193:1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 24.Schwebke JR, Desmond RA. 2007. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin Infect Dis 44:213–219. doi: 10.1086/509577. [DOI] [PubMed] [Google Scholar]
- 25.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, Abdo Z, Forney LJ, Ravel J. 2012. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4:132ra152. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fethers KA, Fairley CK, Hocking JS, Gurrin LC, Bradshaw CS. 2008. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis 47:1426–1435. doi: 10.1086/592974. [DOI] [PubMed] [Google Scholar]
- 27.Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, De Guingand D, Morton AN, Fairley CK. 2013. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis 56:777–786. doi: 10.1093/cid/cis1030. [DOI] [PubMed] [Google Scholar]
- 28.Larsson PG, Brandsborg E, Forsum U, Pendharkar S, Andersen KK, Nasic S, Hammarström L, Marcotte H. 2011. Extended antimicrobial treatment of bacterial vaginosis combined with human lactobacilli to find the best treatment and minimize the risk of relapses. BMC Infect Dis 11:223. doi: 10.1186/1471-2334-11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fooks LJ, Gibson GR. 2002. Probiotics as modulators of the gut flora. Br J Nutr 88(Suppl 1):S39–S49. doi: 10.1079/BJN2002628. [DOI] [PubMed] [Google Scholar]
- 30.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O’Neill SL. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 31.Lina G, Boutite F, Tristan A, Bes M, Etienne J, Vandenesch F. 2003. Bacterial competition for human nasal cavity colonization: role of staphylococcal agr alleles. Appl Environ Microbiol 69:18–23. doi: 10.1128/AEM.69.1.18-23.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uehara Y, Nakama H, Agematsu K, Uchida M, Kawakami Y, Abdul Fattah AS, Maruchi N. 2000. Bacterial interference among nasal inhabitants: eradication of Staphylococcus aureus from nasal cavities by artificial implantation of Corynebacterium sp. J Hosp Infect 44:127–133. doi: 10.1053/jhin.1999.0680. [DOI] [PubMed] [Google Scholar]
- 33.Cash HL, Whitham CV, Behrendt CL, Hooper LV. 2006. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belkaid Y, Naik S. 2013. Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol 14:646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose WA II, McGowin CL, Spagnuolo RA, Eaves-Pyles TD, Popov VL, Pyles RB. 2012. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS One 7:e32728. doi: 10.1371/journal.pone.0032728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dwidar M, Monnappa AK, Mitchell RJ. 2012. The dual probiotic and antibiotic nature of Bdellovibrio bacteriovorus. BMB Rep 45:71–78. doi: 10.5483/BMBRep.2012.45.2.71. [DOI] [PubMed] [Google Scholar]
- 37.Teixeira GS, Carvalho FP, Arantes RM, Nunes AC, Moreira JL, Mendonça M, Almeida RB, Farias LM, Carvalho MA, Nicoli JR. 2012. Characteristics of Lactobacillus and Gardnerella vaginalis from women with or without bacterial vaginosis and their relationships in gnotobiotic mice. J Med Microbiol 61:1074–1081. doi: 10.1099/jmm.0.041962-0. [DOI] [PubMed] [Google Scholar]
- 38.Matu MN, Orinda GO, Njagi EN, Cohen CR, Bukusi EA. 2010. In vitro inhibitory activity of human vaginal lactobacilli against pathogenic bacteria associated with bacterial vaginosis in Kenyan women. Anaerobe 16:210–215. doi: 10.1016/j.anaerobe.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Atassi F, Brassart D, Grob P, Graf F, Servin AL. 2006. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol Med Microbiol 48:424–432. doi: 10.1111/j.1574-695X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 40.Leibold MA. 1995. The niche concept revisited: mechanistic models and community context. Ecology 76:1371. doi: 10.2307/1938141. [DOI] [Google Scholar]
- 41.Bukusi E, Thomas KK, Nguti R, Cohen CR, Weiss N, Coombs RW, Holmes KK. 2011. Topical penile microbicide use by men to prevent recurrent bacterial vaginosis in sex partners: a randomized clinical trial. Sex Transm Dis 38:483–489. doi: 10.1097/OLQ.0b013e318214b82d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colli E, Landoni M, Parazzini F. 1997. Treatment of male partners and recurrence of bacterial vaginosis: a randomised trial. Genitourin Med 73:267–270. doi: 10.1136/sti.73.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moi H, Erkkola R, Jerve F, Nelleman G, Bymose B, Alaksen K, Tornqvist E. 1989. Should male consorts of women with bacterial vaginosis be treated? Genitourin Med 65:263–268. doi: 10.1136/sti.65.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vejtorp M, Bollerup AC, Vejtorp L, Fanøe E, Nathan E, Reiter A, Andersen ME, Strømsholt B, Schrøder SS. 1988. Bacterial vaginosis: a double-blind randomized trial of the effect of treatment of the sexual partner. Br J Obstet Gynaecol 95:920–926. doi: 10.1111/j.1471-0528.1988.tb06581.x. [DOI] [PubMed] [Google Scholar]
- 45.Vutyavanich T, Pongsuthirak P, Vannareumol P, Ruangsri RA, Luangsook P. 1993. A randomized double-blind trial of tinidazole treatment of the sexual partners of females with bacterial vaginosis. Obstet Gynecol 82:550–554. [PubMed] [Google Scholar]
- 46.Mehta SD. 2012. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex Transm Dis 39:822–830. doi: 10.1097/OLQ.0b013e3182631d89. [DOI] [PubMed] [Google Scholar]
- 47.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, Kiwanuka N, Moulton LH, Chaudhary MA, Chen MZ, Sewankambo NK, Wabwire-Mangen F, Bacon MC, Williams CF, Opendi P, Reynolds SJ, Laeyendecker O, Quinn TC, Wawer MJ. 2007. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 48.Wawer MJ, Tobian AA, Kigozi G, Kong X, Gravitt PE, Serwadda D, Nalugoda F, Makumbi F, Ssempiija V, Sewankambo N, Watya S, Eaton KP, Oliver AE, Chen MZ, Reynolds SJ, Quinn TC, Gray RH. 2011. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet 377:209–218. doi: 10.1016/S0140-6736(10)61967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tobian AA, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, Laeyendecker O, Charvat B, Ssempijja V, Riedesel M, Oliver AE, Nowak RG, Moulton LH, Chen MZ, Reynolds SJ, Wawer MJ, Gray RH. 2009. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 360:1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu CM, Aziz M, Kachur S, Hsueh P-R, Huang Y-T, Keim P, Price LB. 2012. BactQuant: an enhanced broad-coverage bacterial quantitative real-time PCR assay. BMC Microbiol 12:56. doi: 10.1186/1471-2180-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoddard SF, Smith BJ, Hein R, Roller BR, Schmidt TM. 2015. rrnDB: improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res 43:D593–D598. doi: 10.1093/nar/gku1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roberts DW. 2010. labdsv: ordination and multivariate analysis for ecology. R package version 1.4-1. R Development Core Team. [Google Scholar]
- 53.Stevenson M, Nunes T, Sanchez J, Thornton R, Reiczigel J, Robison-Cox J, Sebastiani P, Solymos P. 2013. epiR: an R package for the analysis of epidemiological data, version 0.9-48. R Development Core Team. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials, methods, and results. Download
Community state types of the uncircumcised coronal sulcus microbiome, identified by hierarchal clustering. This heat map visualization illustrates how we clustered microbiota profiles by hierarchal clustering, which produced the resultant dendrogram (top). The seven community state types (CSTs) were identified by cutting the dendrogram using the cutree algorithm in R. As shown, collapsing the dendrogram to its first bifurcation produced two major CST groups: the low-density community state types (CST1 to 3) and the high-density community state types (CST4 to 7). In a heat map, each column represents one male participant, and each row depicts the absolute abundance (16S rRNA gene copies per swab) of bacterial genus-level taxa in the coronal sulcus microbiota (e.g., Prevotella). The abundance is interpreted using the annotated color-coding key (bottom, color bar), which denotes the correlation between each color with its respective ln-transformed absolute abundance. Download
Absolute abundances of Prevotella, Porphyromonas, Dialister, Mobiluncus, and Gardnerella by CST group and partner BV status, shown in box plots. Download
Absolute abundances of Lactobacillus, Staphylococcus, Finegoldia, and Corynebacterium for CST1 to 3 by partner BV status, shown in box plots. Download
Indicator genera associated with female partner Nugent score in full cohort and in monogamous men only, based on absolute abundance.
Lactobacillus species prevalence by penile CSTs, female partner Nugent score, and penile CSTs.
Indicator genera for CST1 to 3 versus CST4 to 7, as determined based on absolute abundance.
Association between host sexual activity and prevalence of CST1 to 3 versus CST4 to 7.
Absolute abundance of indicator genera associated with female partner normal and Nugent-BV scores in men with CST1 to 3 and men with CST4 to 7 by the quasi-Poisson model.