Abstract
Background
The fetal immune system is a critical window of development. The epithelial cell-derived cytokines, thymic stromal lymphopoietin (TSLP), and interleukin-33 (IL-33) have received attention for their role in allergic responses but not been studied during this critical window. The objectives were to assess correlations among IL-33, TSLP, and IgE in umbilical cord blood samples and identify prenatal predictors of these biomarkers.
Methods
This study utilized data and banked cord blood collected in the Maternal-Infant Research on Environmental Chemicals (MIREC) Study, a trans-Canada cohort study of 2001 pregnant women. Our analytic sample comprised the 1254 women with a singleton, term birth with a cord blood sample. Spearman correlation coefficients (SCC) and logistic regression models were used to examine associations between biomarkers and identify potential predictors of elevated biomarker levels.
Results
Thymic stromal lymphopoietin and IL-33 were more strongly correlated with each other (SCC = 0.75, p < 0.0001) than with IgE (IL-33 SCC = 0.14, TSLP SCC = 0.21). Maternal allergy, heavy street traffic, and elevated birth weight were significantly associated with jointly elevated TSLP and IL-33 levels, whereas maternal age and female infant sex were inversely associated with elevated IgE.
Conclusions
In this population of Canadian women and infants, TSLP and IL-33 were detectable in cord blood, more strongly correlated with each other than with IgE, and associated with maternal characteristics indicative of inflammatory responses. This study motivates investigation into the value of cord blood IL-33 and TSLP levels as childhood allergy predictors and raises interesting questions regarding in utero coordinated regulation of these cytokines.
Keywords: allergy, immunoglobulin E, interleukin-33, thymic stromal lymphopoietin, umbilical cord blood
The fetal time period is a critical window of immune system development 1–3. In utero perturbations to the regulation of immune responses and inflammatory mediators may underlie susceptibility to childhood allergic diseases. This susceptibility may manifest as alterations in fetal antibody and cytokine levels 4. Previous research into biomarkers of childhood allergic disease has primarily focused on elevated levels of cord blood immunoglobulin E (IgE) and T cell-derived cytokines 5–8. Recent research has demonstrated that the epithelial cell produced cytokines, thymic stromal lymphopoietin (TSLP), and interleukin-33 (IL-33) contribute to inflammatory-related conditions 9. TSLP has been implicated in atopic dermatitis 10, eosinophilic esophagitis 11, and allergic airway disease 12. A recent clinical trial of children treated with anti-TSLP antibodies demonstrated that, compared to controls, treated children had less bronchoconstriction and airway inflammation following an allergen challenge test 12. IL-33 has been implicated in atopic dermatitis 13, asthma 14, and inflammatory bowel disease 15. Neither TSLP nor IL-33, however, has been studied in neonates.
Although most research has examined the independent roles of these cytokines, the interaction between TSLP and IL-33 can exacerbate inflammatory responses. Specifically, IL-33 can induce TSLP and sensitize mast cells to TSLP. TSLP can, in conjunction with IL-33, induce mast cell production of Th2 cytokines 9. Despite this experimental evidence, there is a lack of knowledge regarding the correlation of these cytokines in the developing immune system. Investigation into this correlation may provide insight into early life origins of IL-33 and TSLP cross-regulation. Mechanistic interaction between these cytokines in utero could potentially promote an allergic phenotype in childhood.
In recognition of evidence regarding (i) the inflammatory roles of TSLP and IL-33 and (ii) the fetal time period as a critical window of immune system development, we sought to explore the nature of these cytokines at birth. The specific objectives of this exploratory analysis were to: (i) determine umbilical cord blood levels of and correlations among IL-33, TSLP, and IgE and (ii) identify statistical associations between maternal and infant characteristics and elevated cord blood levels of IL-33, TSLP, and IgE. Given the lack of evidence explaining the higher prevalence of allergic diseases, including asthma, among boys 16 a secondary objective was to determine whether these statistical associations differed by infant sex.
Method
Study design
Biospecimens and data for this study were from the Maternal-Infant Research on Environmental Chemicals (MIREC) Study Biobank, a trans-Canada cohort study of 2001 pregnant women from 10 Canadian cities during 2008–11, as previously described 17. Briefly, women were eligible for inclusion if they were <14 wk gestation at time of recruitment, ≥18 yr of age, able to communicate in French or in English, and planning on delivering at a local hospital 17. Participants included in the present investigation were mothers who had a singleton, live, term birth (≥37 wk) and a cord blood sample suitable for analysis. Cord blood samples (n = 5) with immunoglobulin A (IgA) levels ≥10 μg/ml were excluded to minimize the possibility of contamination from maternal blood 18. Ethical approval was obtained from Health Canada, Sainte Justine's Hospital (Montreal, QC, Canada), and the IWK Health Centre (Halifax, NS, Canada).
Data collection
Trained research staff interviewed participants during the first and third trimester to obtain data on maternal lifestyle, medical history, demographics, and ambient environment characteristics. Clinical data on the mother and infant were obtained from chart review.
Immune system biomarkers were measured in the plasma of umbilical cord blood samples in the Department of Microbiology & Immunology, Dalhousie University, Halifax, NS using specific ELISAs. TSLP concentrations were assessed using a commercial kit (Biolegend, San Diego, CA, USA). IL-33 concentrations were assessed an R&D systems duoset (Minneapolis, MN, USA). ELISA kits (EBioscience, San Diego, CA, USA) were also used to assess both total IgE and IgA concentrations. ELISA assays were performed according to manufacturer's instructions with the exception that plates were coated with sodium bicarbonate buffer (pH 8.3–8.5) and blocked with 2% BSA in PBS instead of manufacturer's coating and blocking buffers. Extended standard curves were used to improve sensitivity. The interassay and intra-assay coefficients of variation (CVs) for IL-33 were 5.9% and 11.3%, respectively; for TSLP interassay and intra-assay, CVs were 6.0% and 8.1%, respectively. The interassay and intra-assay CVs were 3.2% and 6.5%, respectively, for IgE and 5.2% and 10.4%, respectively, for IgA. Serial dilutions of both TSLP and IL-33 in spiked samples were tested and observed to be internally consistent. Both assays exhibited linearity in concentrations of serial dilutions.
Statistical analysis
Ambient maternal exposures, reproductive, and medical history characteristics as well as newborn characteristics determined a priori to be potential determinants of childhood allergic diseases were included in statistical models 19. Maternal age, household income, self-reported exposure to street traffic, parental smoking, ownership of at least one pet, pre-pregnancy body mass index (BMI) 20, maternal allergy, parity, mode of delivery, infant sex, and fetal growth were included in the analysis. The association between maternal fever and infection and elevated biomarker concentrations was examined to assess the potential influence of underlying infection-related processes.
Descriptive statistics for the immune system biomarkers were calculated using medians and interquartile ranges (IQR) due to the skewed distribution of the data. In the calculation of the median, samples below the limit of detection (LOD) were extrapolated from the ELISA curve. Spearman correlation coefficients (SCC) were calculated to determine correlation among continuous measures of TSLP, IL-33, and IgE. Due to the high percentage of samples below the (LOD), each biomarker was categorized into a binary variable. The 80th percentile was used to identify the subset of samples with elevated concentrations of TSLP (572 pg/ml) and IL-33 (942 pg/ml) due to the lack of established cutoff points. This cutoff was used instead of the detection limit because the objective was to assess prenatal predictors of elevated cytokine levels. A composite variable was developed to identify samples with elevated concentrations of both TSLP and IL-33. A sensitivity analysis was conducted to examine TSLP and IL-33 as binary variables defined at their respective LODs (TSLP = 63 pg/ml; IL-33 = 45 pg/ml). The cutoff percentile for IgE was defined at 1.2 ng/ml (0.5 kU/l) 21,22, a cutoff point previously used in studies of cord blood IgE 5,7. A bivariate analysis of all prenatal predictors and elevated IL-33, TSLP, and IgE was conducted using logistic regression. All variables with a p-value of <0.1 in this analysis were assessed for inclusion in a multivariate logistic regression model using stepwise logistic regression with a p-value criteria of 0.1 for variable inclusion and retention. Variables were entered into the model as they are depicted in Table1. To assess whether the relationship between prenatal predictors and immune system biomarkers differed by infant sex, the p-value of the product term was assessed and sex-stratified analysis were conducted. All analyses were performed using sas software v. 9.2 (SAS Institute, Cary, NC, USA).
Table 1.
Unadjusted odds ratios (95% CI) of prenatal predictors and umbilical cord blood levels of high TSLP (≥80%) (pg/ml), IL-33 (≥80%) (pg/ml), and IgE (≥0.5 kU/l), MIREC Study, Canada, 2008–11
| Characteristic | N (%) | High TSLP OR (95% CI) | High IL-33 OR (95% CI) | High IL-33 and TSLP OR (95% CI) | High IgE OR (95% CI) |
|---|---|---|---|---|---|
| Maternal demographic, reproductive and medical history | |||||
| Maternal age | |||||
| ≤24 | 60 (4.8) | 1.0 | 1.0 | 1.0 | 1.0 |
| 25–29 | 270 (21.5) | 1.0 (0.5–1.9) | 0.7 (0.4–1.3) | 0.8 (0.4–1.5) | 0.8 (0.4–1.6) |
| 30–34 | 453 (36.0) | 0.7 (0.4–1.9) | 0.7 (0.4–1.2) | 0.6 (0.3–1.2) | 0.7 (0.4–1.4) |
| ≥35 | 476 (37.8) | 0.8 (0.4–1.5) | 0.7 (0.4–1.2) | 0.7 (0.3–1.3) | 0.6 (0.3–1.1)* |
| Pre-pregnancy BMI | |||||
| Underweight (<18.5) | 27 (2.3) | 1.0 | 1.0 | 1.0 | 1.0 |
| Normal (18.5–24.9) | 719 (60.5) | 1.1 (0.4–3.0) | 1.1 (0.4–2.9) | 1.1 (0.4–3.3) | 0.5 (0.2–1.2) |
| Overweight (25–29.9) | 272 (22.9) | 1.1 (0.4–3.1) | 1.1 (0.4–3.0) | 1.1 (0.4–3.3) | 0.6 (0.2–1.5) |
| Obese (≤30) | 171 (14.4) | 0.9 (0.3–2.5) | 1.0 (0.3–2.8) | 0.9 (0.3–2.8) | 0.4 (0.2–1.1) |
| Maternal allergy† | |||||
| No | 1205 (95.7) | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 54 (4.3) | 1.9 (1.1–3.4)* | 1.8 (1.0–3.2)* | 2.1 (1.1–3.9)* | 1.5 (0.8–3.0) |
| Parity | |||||
| Nulliparous | 527 (41.9) | 1.0 | 1.0 | 1.0 | 1.0 |
| Primiparous | 513 (40.8) | 1.1 (0.8–1.5) | 1.1 (0.8–1.5) | 1.3 (0.9–1.8) | 0.9 (0.7–1.3) |
| Multiparous | 217 (17.3) | 0.7 (0.4–1.0) | 0.7 (0.5–1.1) | 0.7 (0.4–1.1)* | 1.0 (0.7–1.6) |
| Mode of delivery | |||||
| Spontaneous | 900 (71.5) | 1.0 | 1.0 | 1.0 | 1.0 |
| Cesarean | 359 (25.5) | 1.0 (0.7–1.3) | 1.0 (0.7–1.4) | 0.9 (0.7–1.3) | 1.0 (0.7–1.4) |
| Household income | |||||
| ≤30,000 | 90 (7.4) | 1.0 | 1.0 | 1.0 | 1.0 |
| 30,001–50,000 | 117 (9.7) | 1.7 (0.8–3.3) | 2.8 (1.3–6.0)* | 2.6 (1.1–5.8)* | 1.2 (0.6–2.4)* |
| 50,001–100,000 | 515 (42.6) | 1.0 (0.5–1.9) | 1.5 (0.8–3.0) | 1.5 (0.7–3.1) | 0.7 (0.4–1.2) |
| >100,000 | 488 (40.3) | 1.2 (0.7–2.0) | 1.9 (0.6–3.6) | 1.8 (0.9–3.7) | 0.7 (0.4–1.2) |
| Ambient maternal exposures | |||||
| Traffic‡ | |||||
| Light | 860 (68.4) | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium | 250 (19.9) | 0.8 (0.6–1.2) | 0.9 (0.7–1.4) | 0.9 (0.6–1.3) | 1.0 (0.7–1.5) |
| Heavy | 147 (11.7) | 1.3 (0.9–2.0) | 1.5 (1.0–2.2) | 1.7 (1.1–2.6)* | 1.7 (1.1–2.6)* |
| Parental smoking§ | |||||
| No | 1009 (80.1) | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 250 (19.9) | 1.1 (0.88–1.5) | 1.2 (0.9–1.7) | 1.2 (0.8–1.7) | 1.2 (0.9–1.8) |
| Pet ownership | |||||
| No | 560 (44.5) | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 699 (55.5) | 1.1 (0.8–1.5) | 1.1 (0.8–1.4) | 1.0 (0.7–1.3) | 0.8 (0.6–1.1) |
| Infant characteristics | |||||
| Infant sex | |||||
| Male | 673 (53.5) | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 585 (46.5) | 1.2 (0.9–1.6) | 1.0 (0.8–1.3) | 1.1 (0.8–1.5) | 0.8 (0.6–1.0)* |
| Birth weight (g) | |||||
| <3000 | 138 (11.0) | 1.0 | 1.0 | 1.0 | 1.0 |
| 3000 to <3500 | 479 (38.1) | 0.9 (0.6–1.3) | 1.0 (0.6–1.8) | 1.1 (0.7–1.9) | 1.0 (0.6–1.7) |
| 3500 to <4000 | 450 (35.7) | 1.3 (0.8–2.1) | 1.2 (0.8–2.0) | 1.4 (0.8–2.4) | 1.3 (0.8–2.2) |
| ≥4000 | 192 (15.3) | 1.9 (1.1–3.2)* | 1.7 (1.0–3.0)* | 2.1 (1.2–3.9)* | 1.2 (0.6–2.2) |
BMI, body mass index; MIREC, Maternal-Infant Research on Environmental Chemicals; TSLP, thymic stromal lymphopoietin; IL-33, interleukin-33; IgE, immunoglobulin E.
p-value < 0.1.
Defined by the self-reported use an anti-allergen medication.
Defined as self-reported residential street traffic intensity.
Defined as yes if either the mother or father smoked during the pregnancy.
Results
Of the 2001 women recruited into MIREC, 18 withdrew and asked that their data and biospecimens be destroyed. Of the remaining 1983 subjects, 1363 women had provided a cord blood sample. Of these 1363 samples, 104 were excluded for a high IgA concentration, pre-term birth (<37 wk), multiple birth, or samples that were inadequate for analysis, leaving 1259 subjects for this study.
The percentages of TSLP, IL-33, and IgE samples above the LOD were 42%, 52%, and 18%, respectively (dot plots depicted in Figs S1–S3). The median and IQR of the IL-33 (pg/ml), TSLP (pg/ml), and IgE (ng/ml) were 50.7 (45.0–500.5), 63.0 (63.0–322.4), and 0.30 (0.0–2.4), respectively. The percentage of samples with IgE concentrations that exceeded 1.2 ng/ml was 16%. The correlation between TSLP and IL-33 (SCC = 0.75, p < 0.0001) was stronger than the correlations between IgE and either TSLP (SCC = 0.21) or IL-33 (SCC = 0.14). Eighty percent of samples that were categorized as having a high TSLP value had a high IL-33 value. Maternal allergy and highest birth weight were both positively associated with elevated TSLP concentrations in a bivariate analysis (p-value of <0.1) and elevated IL-33. Household income was also significantly associated with elevated IL-33 although there was no apparent dose–response relationship between these two variables. The following variables were positively associated with jointly elevated TSLP and IL-33: self-reported heavy exposure to street traffic, maternal allergy, and elevated birth weight. Household income and parity were associated with this outcome, but no clear trend of the direction of the association was observed (Table1). Maternal age, household income, and female sex were inversely associated with elevated IgE, whereas traffic exposure was positively associated with elevated IgE. There were no significant associations between either maternal fever or maternal infection and any of the tested biomarkers (data not shown).
Birth weight was the only variable that remained in the multivariate analysis of elevated IL-33, while maternal allergy and birth weight remained in the analysis of elevated TSLP (Table2). Maternal allergy (OR = 2.1; 95% CI: 1.1–3.9), self-reported exposure to traffic (heavy traffic OR = 1.8; 95% CI: 1.2–2.8), and birth weight (≥4000 g OR = 2.2; 95% CI: 1.2–4.0) were significantly and positively associated with jointly elevated TSLP and IL-33 in the multivariate analysis. A significant dose–response relationship between birth weight (test for trend p = 0.001) and jointly elevated TSLP and IL-33 was observed (Table2). The relationships between birth weight, traffic, and maternal allergy and jointly elevated TSLP and IL-33 did not differ by sex as evidenced by a non-statistically significant product term and comparable magnitudes of effects (Figs 1-3). In the sensitivity analysis using LOD rather than the 80th percentile, birth weight was the only variable significantly associated with detectable concentrations of either TSLP or IL-33. In the multivariate IgE model, maternal age (test for trend p-value = 0.02) and female sex were inversely related to elevated IgE levels. The association between maternal age and IgE did not differ by infant sex.
Table 2.
Adjusted odds ratios of prenatal predictors and umbilical cord blood levels of high IL-33 (≥80%) (pg/ml), TSLP (≥80%) (pg/ml), and IgE (≥0.5 kU/l), MIREC Study, Canada, 2008–11*
| Characteristics | High IL-33 OR (95 % CI) | High TSLP OR (95 % CI) | High IL-33 and TSLP OR (95% CI) | High IgE OR (95% CI) |
|---|---|---|---|---|
| Maternal demographic, reproductive and medical history | ||||
| Maternal age | ||||
| ≤24 | – | – | – | 1.0 |
| 25–29 | 0.8 (0.4–1.6) | |||
| 30–34 | 0.7 (0.4–1.4) | |||
| ≥35 | 0.5 (0.3–1.0) | |||
| Test for trend | 0.02 | |||
| Maternal allergy | ||||
| No | – | 1.0 | 1.0 | – |
| Yes | 1.8 (1.0–3.3) | 2.1 (1.1–3.9) | ||
| Ambient maternal exposures | ||||
| Traffic | ||||
| Light | – | – | 1.0 | – |
| Medium | 0.9 (0.6–1.3) | |||
| Heavy | 1.8 (1.2–2.8) | |||
| Test for trend | 0.04 | |||
| Infant characteristics | ||||
| Sex | ||||
| Male | – | – | – | 1.0 |
| Female | 0.8 (0.6–1.0) | |||
| Birth weight (g) | ||||
| <3000 | 1.0 | 1.0 | 1.0 | – |
| 3000 to <3500 | 1.0 (0.6–1.5) | 0.9 (0.6–1.3) | 1.1 (0.6–1.9) | |
| 3500 to <4000 | 1.2 (0.8–2.0) | 1.3 (0.8–2.1) | 1.5 (0.8–2.6) | |
| ≥4000 g | 1.7 (1.0–3.0) | 1.9 (1.1–3.2) | 2.2 (1.2–4.0) | |
| Test for trend | 0.006 | 0.001 | 0.001 | |
MIREC, Maternal-Infant Research on Environmental Chemicals; TSLP, thymic stromal lymphopoietin; IL-33, interleukin-33; IgE, immunoglobulin E.
Models adjusted for those variables identified as having p < 0.1 based on findings in Table 2. IL-33 model adjusted for birth weight, TSLP model adjusted for allergy and birth weight, IL-33/TSLP model adjusted for allergy, traffic, and birth weight, IgE model adjusted for maternal age and sex.
Figure 1.
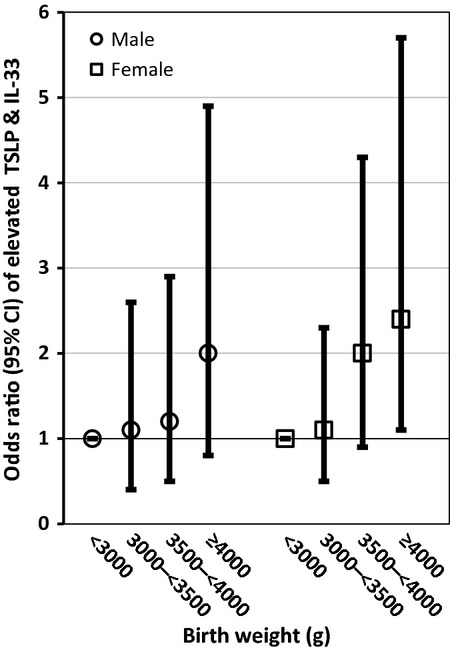
Adjusted odds ratios (95% CI) of birth weight and elevated thymic stromal lymphopoietin (TSLP) and IL-33 stratified by infant sex.
Figure 3.
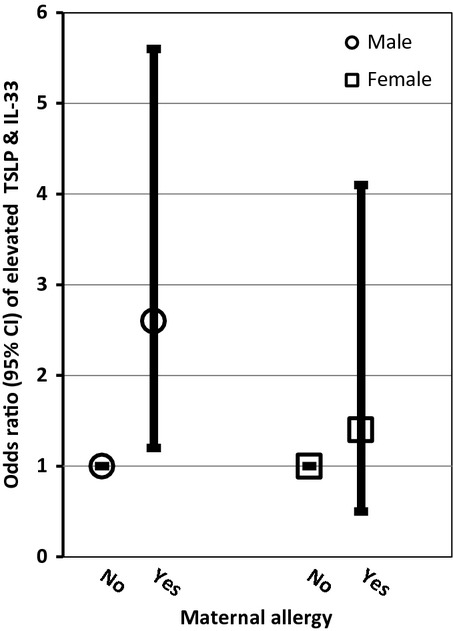
Adjusted odds ratios (95% CI) of maternal allergy and elevated thymic stromal lymphopoietin (TSLP) and IL-33 stratified by infant sex.
Figure 2.
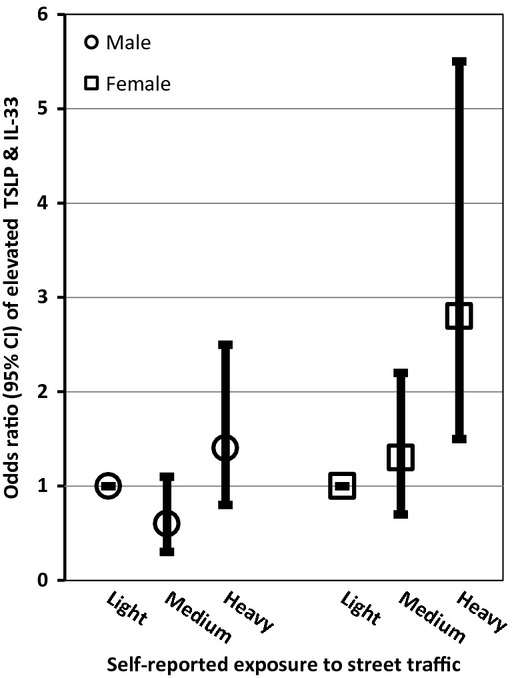
Adjusted odds ratios (95% CI) of traffic exposure and elevated thymic stromal lymphopoietin (TSLP) and IL-33 stratified by infant sex.
Discussion
In this prospective cohort study of Canadian women, we observed several novel findings. First, IL-33 and TSLP levels were detectable at birth and highly correlated with each other. Second, elevated levels of the composite TSLP and IL-33 variable were significantly and positively associated with maternal allergy, birth weight, and self-reported exposure to traffic. The magnitudes of these associations were greater in the joint IL-33 TSLP model than in the independent cytokine models. Further, cord blood IgE levels were inversely associated with maternal age and female sex, but not the presence of maternal allergy, in a multivariate model.
The observed strong correlation between TSLP and IL-33 provides preliminary evidence that TSLP and IL-33 may be operating in a dependent manner in the developing immune system. These speculations require further investigation to clarify whether the observed levels of TSLP and IL-33 persist into childhood and promote inflammatory responses that manifest as allergic disease. The link with higher birth weight might also suggest an early inflammatory component involving TSLP and IL-33 that is linked to adiposity. Although birth weight is an imperfect surrogate for adiposity, high birth weight has been consistently associated with an increased risk of childhood obesity independent of infant sex 23. The observed dose–response relationship between birth weight and elevated cytokine levels in the primary and sensitivity analysis is consistent with evidence of common phenotypes among both childhood asthma and obesity 24. The observed association raises the question of whether a childhood asthma–obesity association is rooted in fetal development.
Although, to our knowledge, there are no published investigations of TSLP or IL-33 levels in cord blood, the present findings are consistent with evidence regarding atopic disease determinants. The observed statistically significant association between maternal allergy and joint elevation of levels of TSLP and IL-33 suggests that maternal allergy has an influence on these biomarkers that is detectable at birth. This finding builds upon evidence of a genetic component to childhood allergic disease 25. The lack of significant association in the sensitivity analysis may indicate that the subset of infants with elevated cytokine levels have a different risk profile that than those with detectable concentrations.
The finding between self-reported street traffic exposure and jointly elevated TSLP and IL-33 levels, although lacking a strong dose–response relationship, is supported by toxicological evidence that air pollution, specifically diesel exhaust and particulate matter, can induce inflammatory processes including epithelial cell production of TSLP 26. The lack of dose–response relationship may be explained by the potential challenge in differentiating between ‘low’ and ‘medium’ levels of traffic exposure and resulting exposure misclassification 27. Given the coherence between the present results and the literature on allergic disease determinants, further investigation is warranted to elucidate: (i) the mechanisms underlying the observed statistical associations; (ii) the long-term implications of elevated cord blood levels of TSLP and IL-33, and (iii) whether immune system sequelae are enhanced when TSLP and IL-33 are elevated.
The present findings of an inverse relation between elevated cord blood IgE and both maternal age and female sex have also been reported in a US-based study 28. This study reported that residence in low-income areas, Hispanic ethnicity, and maternal total IgE were associated with elevated cord blood IgE levels 28. The MIREC study did not measure maternal total IgE as it was not part of the original cohort analyses. Moreover, the MIREC study population is primarily urban, Caucasian and of higher household income levels than the general population 17. A biologic mechanism for the findings in regard to maternal age remains to be identified. The inverse relationship between females and IgE motivates investigation into how early life development of allergic disease differs between girls and boys.
The present study benefited from the relatively large sample size and comparatively rich covariate data. The sample size exceeds any previous analysis of either TSLP or IL-33 in humans. The MIREC study population represents multiple geographic regions within the country. We were able to restrict the analysis to cord blood samples not contaminated by maternal blood by excluding samples with elevated IgA concentrations. We were also able to rule out the role of underlying inflammatory responses in promoting high biomarker concentrations. This study was, however, subject to at least four limitations common to observational studies. First, as data on childhood allergic diagnoses were not available in the MIREC study, we were not able to examine these outcomes. Second, both maternal allergy and traffic were self-reported. Given that the estimated allergy prevalence in the MIREC study (4.3%) is lower than other national estimates of allergy medication use (4–9%) 29, it is unlikely that any resulting bias from the self-reported nature of the variable overestimated allergy prevalence. Furthermore, the available data on traffic exposure in the MIREC study does not capture time or duration of exposure. Third, the study sample included higher income, more educated women, who were less likely to smoke than the general population. Although this potential selection bias is unlikely to have influenced the observed associations, which operate independently of socioeconomic status, it does restrict examination of certain prenatal characteristics. Fourth, the influence of residual confounding on the observed associations from unmeasured variables such as maternal stress or vitamin D cannot be ruled out. There are also unmeasured factors, such as endogenous corticosteroids and other inflammatory cytokines, which could modify IL-33 and TSLP simultaneously. For example, the epithelial-derived cytokine IL-25 (IL-17E) and IL-17 family member has received attention for interacting with IL-33 and TSLP to enhance inflammatory responses 9.
In conclusion, the present findings suggest that neonates with elevated cord blood levels of TSLP and IL-33 may represent an immunologically distinct subset. These cytokines were associated with maternal and infant characteristics that reflect underlying inflammation and/or increased risk of allergic disease development. Thus, cord blood levels of TSLP and IL-33 warrant further investigation as potential predictive factors for inflammatory and allergic disease. Given what is known regarding the integral role of TSLP and IL-33 in allergic disease and inflammatory processes, the present findings also raise interesting questions regarding in utero coordinated regulation of these cytokines and motivate further research on this topic.
Acknowledgments
We would like to recognize the valuable contributions of Yisong Wei, Nong Xu (and other members of the Marshall laboratory) for their assistance in the analysis of the cord blood samples. We would also like to acknowledge the MIREC Study Group and the MIREC Biobank for access to the cord blood samples as well as the MIREC study participants for their dedication. This study was funded by a Category A grant from the IWK Health Centre (Halifax, NS).
Supporting Information
Figure S1. Dot plot of log10 IgE concentrations in cord blood plasma samples (n = 1254).
Figure S2. Dot plot of log10 TSLP concentrations in cord blood plasma samples (n = 1254).
Figure S3. Dot plot of log10 IL-33 concentrations in cord blood plasma samples (n = 1254).
References
- Holsapple MP, Paustenbach DJ, Charnley G, et al. Symposium summary: children's health risk–what's so special about the developing immune system? Toxicol Appl Pharmacol. 2004;199:61–70. doi: 10.1016/j.taap.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Etzel R, Chen D, Halonen M, Holladay S, Jarabek A. Workshop to identify critical windows of exposure for children's health: immune and respiratory system work group summary. Environ Health Perspect. 2000;108(Suppl 3):483–90. doi: 10.1289/ehp.00108s3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selgrade MK. Immunotoxicity: the risk is real. Toxicol Sci. 2007;100:328–32. doi: 10.1093/toxsci/kfm244. [DOI] [PubMed] [Google Scholar]
- Warner JO. The early life origins of asthma and related allergic disorders. Arch Dis Child. 2004;89:97–102. doi: 10.1136/adc.2002.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen M, Kallio MJT, Siimes MA, Elg P, Björksten F, Ranki A. Cord serum immunoglobulin E as a risk factor for allergic symptoms and sensitization in children and young adults. Pediatr Allergy Immunol. 2009;20:12–8. doi: 10.1111/j.1399-3038.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- Ferguson A, Dimich-Ward H, Becker A, et al. Elevated cord blood IgE is associated with recurrent wheeze and atopy at 7 yrs in a high risk cohort. Pediatr Allergy Immunol. 2009;20:710–3. doi: 10.1111/j.1399-3038.2009.00869.x. [DOI] [PubMed] [Google Scholar]
- Edenharter G, Bergmann R, Bergmann K, et al. Cord blood-IgE as risk factor and predictor for atopic diseases. Clin Exp Allergy. 1998;28:671–8. doi: 10.1046/j.1365-2222.1998.00241.x. [DOI] [PubMed] [Google Scholar]
- Fu Y, Lou H, Wang C, et al. T cell subsets in cord blood are influenced by maternal allergy and associated with atopic dermatitis. Pediatr Allergy Immunol. 2013;24:178–86. doi: 10.1111/pai.12050. [DOI] [PubMed] [Google Scholar]
- Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2009;226:172–90. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J, Omori M, Gyarmati D, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–9. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noti M, Wojno EDT, Kim BS, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005–13. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvreau GM, O'Byrne PM, Boulet L-P, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–10. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- Cevikbas F, Steinhoff M. IL-33: a novel danger signal system in atopic dermatitis. J Invest Dermatol. 2012;132:1326–9. doi: 10.1038/jid.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Stolarski B, Kewin P, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–77. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- Pastorelli L, De Salvo C, Cominelli MA, Vecchi M, Pizarro TT. Novel cytokine signaling pathways in inflammatory bowel disease: insight into the dichotomous functions of IL-33 during chronic intestinal inflammation. Therap Adv Gastroenterol. 2011;4:311–23. doi: 10.1177/1756283X11410770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kynyk JA, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med. 2011;17:6–11. doi: 10.1097/MCP.0b013e3283410038. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Fraser WD, Fisher M, et al. Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr Perinat Epidemiol. 2013;27:415–25. doi: 10.1111/ppe.12061. [DOI] [PubMed] [Google Scholar]
- Ownby DR, McCullough J, Johnson CC, Peterson EL. Evaluation of IgA measurements as a method for detecting maternal blood contamination of cord blood samples. Pediatr Allergy Immunol. 1996;7:125–9. doi: 10.1111/j.1399-3038.1996.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ. 2009;181:E181–90. doi: 10.1503/cmaj.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2000. Obesity:preventing and managing the global epidemic Report of a WHO Consulation (WHO Technical Report Series 894). Available at: http://www.who.int.nutrition/publications/obesity/WHO_TRS_894/en. Accessed February 4, 2015.
- Seagroatt V, Anderson S. The second international reference preparation for human serum immunoglobulin E and the first British standard for human serum immunoglobulin E. J Biol Stand. 1981;4:431–7. doi: 10.1016/s0092-1157(81)80034-0. [DOI] [PubMed] [Google Scholar]
- Amarasekera M. Immunoglobulin E in health and disease. Asia Pac Allergy. 2011;1:12–5. doi: 10.5415/apallergy.2011.1.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes Relat Metab Disord. 2006;30:610–7. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- Rasmussen F, Hancox RJ. Mechanisms of obesity in asthma. Curr Opin Allergy Clin Immunol. 2014;14:35–43. doi: 10.1097/ACI.0000000000000024. [DOI] [PubMed] [Google Scholar]
- Westman M, Kull I, Lind T, et al. The link between parental allergy and offspring allergic and nonallergic rhinitis. Allergy. 2013;68:1571–8. doi: 10.1111/all.12267. [DOI] [PubMed] [Google Scholar]
- Bleck B, Grunig G, Chiu A, et al. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J Immunol. 2013;190:3757–63. doi: 10.4049/jimmunol.1201165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni G, Badaloni C, Porta D, Forastiere F, Perucci CA. Comparison between various indices of exposure to traffic-related air pollution and their impact on respiratory health in adults. Occup Environ Med. 2008;65:683–90. doi: 10.1136/oem.2007.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scirica CV, Gold DR, Ryan L, et al. Predictors of cord blood IgE levels in children at risk for asthma and atopy. J Allergy Clin Immunol. 2007;119:81–8. doi: 10.1016/j.jaci.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Manfreda J, Becklake MR, Sears MR, et al. Prevalence of asthma symptoms among adults aged 20–44 years in Canada. CMAJ. 2001;164:995–1001. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Dot plot of log10 IgE concentrations in cord blood plasma samples (n = 1254).
Figure S2. Dot plot of log10 TSLP concentrations in cord blood plasma samples (n = 1254).
Figure S3. Dot plot of log10 IL-33 concentrations in cord blood plasma samples (n = 1254).


