Abstract
Summary
Purpose:
Anterior temporal lobe resection (ATLR) controls seizures in up to 70% of patients with intractable temporal lobe epilepsy (TLE) but, in the language dominant hemisphere, may impair language function, particularly naming. Functional reorganization can occur within the ipsilateral and contralateral hemispheres. We investigated reorganization of language in left-hemisphere–dominant patients before and after ATLR; whether preoperative functional magnetic resonance imaging (fMRI) predicts postoperative naming decline; and efficiency of postoperative language networks.
Methods:
We studied 44 patients with TLE due to unilateral hippocampal sclerosis (24 left) on a 3T GE-MRI scanner. All subjects performed language fMRI and neuropsychological testing preoperatively and again 4 months after left or right ATLR.
Key Findings:
Postoperatively, individuals with left TLE had greater bilateral middle/inferior frontal fMRI activation and stronger functional connectivity from the left inferior/middle frontal gyri to the contralateral frontal lobe than preoperatively, and this was not observed in individuals with right TLE. Preoperatively, in left and right TLE, better naming correlated with greater preoperative left hippocampal and left frontal activation for verbal fluency (VF). In left TLE, stronger preoperative left middle frontal activation for VF was predictive of greater decline in naming after ATLR. Postoperatively, in left TLE with clinically significant naming decline, greater right middle frontal VF activation correlated with better postoperative naming. In patients without postoperative naming decline, better naming correlated with greater activation in the remaining left posterior hippocampus. In right TLE, naming ability correlated with left hippocampal and left and right frontal VF activation postoperatively.
Significance:
In left TLE, early postoperative reorganization to the contralateral frontal lobe suggests multiple systems support language function. Postoperatively, ipsilateral recruitment involving the posterior hippocampal remnant is important for maintaining language, and reorganization to the contralateral hemisphere is less effective. Preoperative left middle frontal activation for VF was predictive of naming decline in left TLE after ATLR.
Keywords: Anterior temporal lobe resection, Functional MRI, Naming, Temporal lobe epilepsy
Anterior temporal lobe resection (ATLR) for refractory temporal lobe epilepsy (TLE) (Wiebe et al., 2001) may be complicated by impairment of verbal memory (Hermann et al., 1995; Helmstaedter & Elger, 1996; Bonelli et al., 2010) and language, especially naming, after ATLR in the language dominant hemisphere (Davies et al., 1998).
Many functional magnetic resonance imaging (fMRI) studies have identified typical language areas in healthy controls and in patients with TLE. Verbal fluency and verb generation tasks reliably activate the left inferior frontal gyrus (IFG) (Binder et al., 1996; Woermann et al., 2003; Gaillard et al., 2004), whereas the lateral temporal neocortex is activated by sentence comprehension tasks (Gaillard et al., 2002). Few studies have reported activation in the medial temporal structures. Naming typically involves the perisylvian cortex, and basal temporal regions potentially affected in TLE (DeLeon et al., 2007; Trebuchon-Da Fonseca et al., 2009). Twenty-five percent of patients with hippocampal sclerosis (HS) experience a clinically significant naming decline after left ATLR (Saykin et al., 1995; Davies et al., 1998) and there is evidence for direct involvement of the dominant hippocampus in naming (Hamberger et al., 2007).
Few fMRI studies have systematically investigated functional reorganization of language after ATLR. Postoperatively, language activation may shift to the contralateral hemisphere in patients with seizures in the language dominant hemisphere (Hertz-Pannier et al., 2002). There is evidence for both intrahemispheric and interhemispheric reorganization of language function after ATLR (Noppeney et al., 2005). One longitudinal fMRI study showed that the language network was affected differently by left and right TLE and was reorganized after ATLR (Wong et al., 2009).
Only a few studies considered the role of fMRI to predict postoperative language outcome. Preoperative language fMRI with a semantic decision task was used to predict language decline in patients with TLE (Sabsevitz et al., 2003).
In the current longitudinal study we tested the hypotheses that:
Reorganization of language function will be different for patients undergoing left or right ATLR.
There is a relationship between preoperative language fMRI activation and language test proficiency in patients with TLE.
The relationship between language function and VF activation is affected by ATLR and differs between the dominant or nondominant hemispheres.
Preoperative language fMRI can predict postoperative language deficits, particularly naming decline, after left ATLR.
Patients and Methods
Subjects
We studied 44 patients with medically refractory TLE (24 left) due to unilateral hippocampal sclerosis (HS) (Table 1). All patients underwent ATLR for HS at the National Hospital for Neurology and Neurosurgery, London. All had structural MRI at 3T, including qualitative assessment by expert neuroradiologists and quantification of hippocampal volumes and T2 relaxation times showing unilateral HS and normal contralateral medial temporal lobe structures.
Table 1.
Demographic data and study test results
| Subjects | Gender | Median age (range), year | Median age at onset (range), year | Handedness (Oldfield, 1971) | Mean verbal IQ (SD) (WAIS-III) | ILAE postoperative seizure outcome – 1 year follow up | Mean HV cm3 (SD); paired t-test, two-tailed |
|---|---|---|---|---|---|---|---|
| 24 left TLE | 12 female | 37 (17–63) | 6 (0.25–44) | 22 right, 2 left | 93.50 (15.74) | Grade 1–2: 19 Grade 3–5: 5 | Right HV: 2.83 (0.27) Left HV: 1.87 (0.67); p < 0.0001 |
| 20 right TLE | 13 female | 35 (22–52) | 12 (0.92–55) | 18 right, 2 left | 95.60 (16.72) | Grade 1–2: 14 Grade 3–5: 6 | Right HV: 1.81 (0.44) Left HV: 2.69 (0.38); p < 0.0001 |
HV, hippocampal volume; TLE, temporal lobe epilepsy.
Prolonged interictal and ictal video–electroencephalography (EEG) confirmed seizure onset in the ipsilateral temporal lobe. All patients underwent language fMRI and standard neuropsychological assessment preoperatively and again 4 months after ATLR.
All patients’ first language was English and all were left-language dominant on fMRI (Powell et al., 2006). We calculated preoperative and postoperative lateralization indices (LIs) using the Bootstrap method of the statistical parametric mapping (SPM) toolbox (Wilke & Lidzba, 2007) for the verbal fluency (VF) contrast “VF” for each subject in the IFG and middle frontal gyrus (MFG). Left language dominance was defined by a preoperative LI of ≤−0.4 on the VF task. This threshold was chosen to ensure clear left language dominance. For patients showing a LI between −0.2 and −0.4, activation maps on a verb generation task were additionally used to decide language laterality (Sabsevitz et al., 2003; Gaillard et al., 2004; Bonelli et al., 2011). Nine patients (five left) showed atypical, bilateral language representation and were not included in this study.
Intelligent quotient (IQ) was measured using the Wechsler Adult Intelligence Scale (WAIS-III). Verbal IQ was used as a covariate to control for the effect of ability level on fMRI activation, and performance during an out of scanner verbal fluency task was used as a proxy measure of motivation and adherence to the in scanner tasks, which could not be ascertained during scanning due to the covert nature of the responses. All patients were treated with antiepileptic medication at the time of their assessment. Medication remained unchanged in 34 patients; in 10 patients the doses were slightly reduced by the time of postoperative assessment.
All patients underwent left or right ATLR by the same neurosurgeon. The standard neurosurgical procedure was removal of the temporal pole and opening of the temporal horn, followed by en bloc resection of the hippocampus with a posterior resection margin at the midbrainstem level. The same neocortical resection was performed to 3–3.5 cm on both the left and the right sides including the superior temporal gyrus to this length on both left and right sides. The International League Against Epilepsy (ILAE) classification of postoperative seizure outcome following epilepsy surgery was used (Wieser et al., 2001).
This study was approved by the National Hospital for Neurology and Neurosurgery and the Institute of Neurology Joint Research Ethics Committee, and written informed consent was obtained from all subjects.
Neuropsychological tests
We used two language tests, phonemic verbal fluency and the McKenna Graded Naming Test (McKenna & Warrington, 1983), as patients with TLE are at particular risk of developing naming deficits after ATLR (Davies et al., 1998).
Naming
All patients completed the McKenna Graded Naming Test. This is a well-established confrontation naming test commonly used to assess expressive language functions in the United Kingdom. The subject is required to name 30 black and white line drawings of increasing difficulty. The total number of items correctly named is the performance indicator (McKenna & Warrington, 1983).
Verbal fluency (VF)
All patients completed a VF test outside the scanner. The subject is given 60 s to produce as many words starting with a given letter (“S”). The total number of words correctly produced is the performance indicator.
Neuropsychological testing occurred before and 4 months after ATLR. Postoperative – preoperative change scores were correlated with preoperative and postoperative fMRI activation patterns. Changes in language scores from baseline following left or right ATLR were correlated with preoperative fMRI activation patterns to assess language fMRI ability to predict postoperative language deficits. A clinically meaningful postoperative naming change was defined as a change of >3 points, which represented a decline of at least 15 centiles. A clinically significant VF decline was defined as change of >1 SD.
MR data acquisition
MRI studies were performed on a 3T General Electric Excite HDx scanner (General Electric, Milwaukee, WI, U.S.A.). Standard imaging gradients with a maximum strength of 40 mT/m and slew rate 150 T/m/s were used. All data were acquired using an eight-channel array head coil for reception and the body coil for transmission.
For the fMRI task, gradient-echo planar T2*-weighted images were acquired, providing blood oxygenation level–dependent (BOLD) contrast. Each volume comprised 58 contiguous 2.5 mm oblique axial slices, through the temporal and frontal lobes with a 24 cm field of view, 96 × 96 matrix, reconstructed to 128 × 128 for an in-plane resolution of 1.88 × 1.88 mm. Echo time (TE) was 30 ms and repetition time (TR) 4.5 s. The field of view was positioned to maximize coverage of the frontal and temporal lobes.
Language fMRI task and data analysis
Language paradigms
Each subject performed a VF fMRI task on a 3T GE Excite HDx scanner preoperatively and postoperatively, consisting of a blocked experimental design with 30-s activation blocks alternating with 30-s of cross-hair fixation over 5.5 min (Powell et al., 2006; Bonelli et al., 2011). Subjects were asked during the activation phase to covertly generate different words beginning with a visually presented letter (A, S, W, D, and E) contrasted by crosshair fixation as rest condition. During the verb-generation task, concrete nouns were presented visually every 3 s in blocks of 10 contrasted by 30 s of crosshair fixation as rest. Subjects were instructed to either covertly generate verbs associated with these nouns (indicated by the letter “G” preceding the noun) or silently repeat the nouns presented (indicated by the letter “R” preceding the noun) during the activation time.
Both paradigms were used to identify language regions in the inferior and middle frontal gyri; the verb generation task was additionally used to define language dominance in patients with a LI between −0.2 and −0.4 on the VF task.
Data analysis
Imaging data were analyzed using statistical parametric mapping (SPM5) (Friston et al., 1995) (Wellcome Trust Centre for Imaging Neuroscience [http://www.fil.ion.ucl.ac.uk/spm/]). The preoperative imaging time series of each subject was realigned using the mean image as a reference, spatially normalized into standard space (using a scanner specific template from 30 healthy controls, 15 patients with left HS and 15 patients with right HS), smoothed with a Gaussian kernel of 10 mm full-width half maximum (FWHM). Postoperative scans were realigned, coregistered to the preoperative mean image and spatially normalized into standard space applying each subject’s preoperative spatial normalization parameters to the subject’s postoperative realigned and coregistered scans, and again smoothed with a 10-mm FWHM Gaussian kernel. Four patients had to be excluded from further analysis because of coregistration problems.
A two-level random-effects analysis was employed for all preoperative and postoperative imaging data. At the first level, condition-specific effects were estimated according to the general linear model (Friston et al., 1995) for each subject. Regressors of interest were formed by creating boxcar functions of task against rest. Parameter estimates for regressors were calculated for each voxel. A contrast image was created for each subject preoperatively and postoperatively (“VF vs. crosshair fixation”) within the two groups. This contrast image was used for the second-level analysis.
To assess functional connectivity (FC) between activated areas, each subject’s individual peak response to the VF task was located within a region of interest (ROI) in the left IFG and MFG, defined from combined (preoperative left and right TLE) group activation maps. This peak voxel’s time series was extracted from the normalized, smoothed echo planar imaging (EPI) images and used as regressors of interest for a new general linear model fMRI analysis for each subject. Areas of functionally coupled to the left frontal seed region were compared across groups and time.
At the second level, the subjects were divided into four groups: left TLE and right TLE patients, properatively and postoperatively. Each subject’s contrast images were entered into a one sample t-test, modeling the group effect (i.e., patients preoperatively and postoperatively) on the various contrasts of interest.
To test for correlations between areas of VF fMRI activation and preoperative and postoperative performance on VF and naming outside the scanner, multiple regression analyses were performed over the whole brain. Verbal IQ was entered as an additional covariate to control for performance.
Changes in VF and naming scores were used to test for correlations between preoperative fMRI activation and change in language scores from before to after ATLR.
Region of interest analysis
We defined ROIs in the left and right MFG and left and right IFG using anatomical masks (Hammers et al., 2003). In the medial temporal lobe these regions were geometric spherical ROIs (10 mm diameter) in the left and right anterior and posterior medial temporal lobe centered on the coordinates of the peak group-activation for VF. We extracted the parameter estimates in these regions and tested for correlations between subjects’ fMRI activation within these ROIs and their performance on naming and VF tests outside the scanner, preoperatively and postoperatively.
Second level of analysis
We tested for:
Evidence of reorganization of language networks after ATLR by group comparison of preoperative versus postoperative main effects;
Postoperative change in functional connectivity between typical language areas;
Efficiency of (re)organization of language function by correlating preoperative and postoperative activations for VF with preoperative and postoperative language scores;
A relationship between preoperative fMRI activation for VF and change in language scores to evaluate whether preoperative language fMRI was a useful predictor of postoperative language deficits.
Unless otherwise stated, we report activations at a threshold of p < 0.05, corrected for multiple comparisons (family-wise error rate [FWE]) across the whole brain. For correlational analyses with neuropsychological data we report all medial temporal and frontal activations at a threshold of p < 0.01, corrected for multiple comparisons in a small volume of interest (SVI).
Results
Neuropsychological test results
Naming
The left TLE group had significantly lower naming scores preoperatively (mean 14.7, SD 4.3; p = 0.03, t-test) and postoperatively (mean 11.3, SD 5.90; p 0.0001, t-test) than the right TLE group (preoperative, mean 17.9, SD 4.7; postoperative, mean 18.1, SD 4.5). There was a significant reduction of preoperative versus postoperative naming scores in the left TLE (p = 0.004, t-test).
Verbal fluency
There was no significant difference in mean VF scores between patients with left and right TLE preoperatively and postoperatively and no significant change of preoperative versus postoperative mean VF scores in both groups.
Postoperative language change. Fourteen of 24 patients with a decline in naming scores after left ATLR (12 patients classified as clinically significant, which was also reflected by clinical neuropsychological reports highlighting increased word finding difficulties elicited during the naming test and in daily life as rated by patients); mean change between preoperative and postoperative naming scores −3, ranging from −18 to +5 (5/24 patients naming scores remained unchanged, 5/24 with postoperative improvement in naming/one patient classified as clinical significant).
Nine of 24 patients with a postoperative decline in VF scores (three classified as clinical significant), 13 of 24 patients with postoperative improvement (five classified as clinical significant), and 2 of 24 patients’ scores unchanged; mean change between preoperative and postoperative VF scores +1.5, ranging from −12 to +19.
Seven of 20 patients with a decline in naming after right ATLR (not clinical significant), 5 of 20 with postoperative improvement (two classified as clinical significant), eight patients’ scores unchanged; mean change between preoperative and postoperative naming scores +0.2, ranging from −3 to +5.
Ten of 20 patients had reduced fluency scores after right ATLR, with two being clinically significant, 8 of 20 a postoperative improvement, two clinically significant, and one patient’s score unchanged. One patient did not complete postoperative VF testing. Mean change between preoperative and postoperative VF scores +0.5, ranging from −7 to +10.
Organization of language networks before and after ATLR
Preoperative main effects on fMRI activation for VF
In left and right TLE there was significant activation in the left IFG and MFG (p < 0.0001) (Fig. 1A,B, Table 2).
Figure 1.
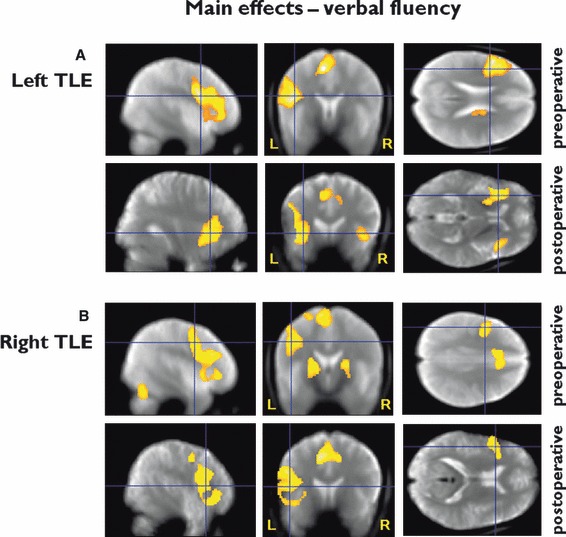
Group results in patients with left and right TLE preoperatively and postoperatively. Preoperative and postoperative main effects for verbal fluency in (A) left TLE: preoperative – left inferior and middle frontal activation; postoperative – bilateral inferior and middle frontal activation; (B) right TLE: preoperative and postoperative – left inferior and middle frontal activation. (threshold p < 0.05, FWE corrected). Preoperative: Significant regions are superimposed onto an averaged normalized mean EPI image from 30 healthy controls, 15 patients with left, and 15 patients with right hippocampal sclerosis. Postoperative: Significant regions are superimposed onto averaged normalized mean EPI images from all patients who underwent left ATLR and all patients who underwent right ATLR. The crosshair points to the peak maximum activation for the group.
Table 2.
fMRI activation peaks for preoperative and postoperative main effects of VF
| Subjects | Z-score | Corrected p-value (FWE) | Coordinates (x, y, z) in MNI space | Anatomic region |
|---|---|---|---|---|
| Left TLE preoperative | 6.96 | p < 0.0001 | −54, 10, 20 | Left IFG |
| 6.69 | p < 0.0001 | −48, 20, 26 | Left MFG | |
| 6.69 | p < 0.0001 | −32, 30, −2 | Left IFG | |
| Left TLE postoperative | 5.77 | p < 0.0001 | −42, 2, 26 | Left IFG |
| 5.74 | p < 0.0001 | −40, 18, −6 | Left IFG | |
| 4.88 | p < 0.0001 | 40, 20, −6 | Right IFG | |
| Right TLE Preoperative | 5.89 | p < 0.0001 | −46, 30, 22 | Left MFG |
| 5.81 | p < 0.0001 | −52, 8, 22 | Left IFG | |
| Right TLE Postoperative | 5.88 | p < 0.0001 | −46, 16, 10 | Left IFG |
| 5.79 | p < 0.0001 | −50, 14, 30 | Left MFG |
HC, hippocampus; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; MNI space, coordinates related to a standard brain defined by the Montreal Neurological Institute (MNI); TLE, temporal lobe epilepsy.
Postoperative main effects on fMRI activation for VF
Left TLE: bilateral activation in the IFG and MFG (p < 0.0001).
Right TLE: left middle and inferior frontal activation (p < 0.0001) (Fig. 1A,B, Table 2).
Group comparisons for preoperative versus postoperative main effects of VF in patients with left and right TLE
Left TLE patients demonstrated significantly less postoperative than preoperative activation in the left inferior and middle frontal gyri (p = 0.02) and in the left posterior hippocampus (p = 0.009).
There were no areas of significantly greater postoperative than preoperative activation in patients with left or right TLE.
Functional connectivity analysis
Preoperatively, left TLE had activation in the left IFG (main activation for VF) highly correlated with the response in the left MFG (p < 0.0001) and the left precentral gyrus (p = 0.016). Postoperatively, there was greater functional connectivity to the homotopic contralateral regions in the right IFG and MFG (p = 0.001) (Fig. 2A).
Figure 2.
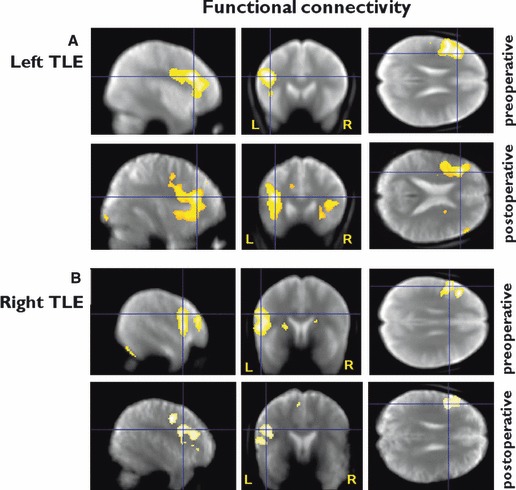
Functional connectivity: group results in patients with left and right TLE preoperatively and postoperatively, from a seed region in the left middle and inferior frontal gyri. (A) Left TLE: preoperative – functional connectivity within ipsilateral middle and inferior frontal gyri; postoperative – functional connectivity within the ipsilateral and to the contralateral middle and inferior frontal gyri; (B) Right TLE: preoperative and postoperative – functional connectivity within left inferior and middle frontal gyri; in contrast to left TLE no increased functional connectivity to the contralateral frontal lobe postoperatively (threshold p < 0.05, FWE corrected). Preoperative: Significant regions are superimposed onto an averaged normalized mean EPI image from 30 healthy controls, 15 patients with left and 15 patients with right hippocampal sclerosis. Postoperative: Significant regions are superimposed onto an averaged normalized mean EPI image from all patients who underwent left ATLR and one for all patients who underwent right ATLR. The crosshair points to the peak maximum activation for the group.
Preoperatively, the right TLE group showed findings similar to that of the left TLE group, with the left IFG being functionally connected to the left middle frontal and left precentral gyri (p = 0.001). There was no postoperative increase in functional connectivity to the contralateral hemisphere (Fig. 2B).
Efficiency of preoperative and postoperative language networks—region of interest analysis
We quantified activation in predefined ROIs in the left and right IFG, MFG, and hippocampi and correlated this with naming performance, as the most relevant clinical language parameter in TLE patients.
Preoperatively, the left TLE group showed significant correlations of VF activation in the left hippocampus with preoperative naming (p = 0.02, R2 = 0.24). After left ATLR, naming scores were correlated positively with activation in the left MFG (p = 0.02, R2 = 0.23) and in the remnant of the left posterior hippocampus (p = 0.03, R2 = 0.20).
In the right TLE there were significant correlations of VF activation with preoperative naming scores in the left MFG (p = 0.046, R2 = 0.20) and IFG (p = 0.049, R2 = 0.21), and the left hippocampus (p = 0.05, R2 = 0.19). Postoperatively, there were significant correlations between naming and VF activation in the left MFG (p = 0.039, R2 = 0.22) and IFG (p = 0.042, R2 = 0.21) and also in the right MFG (p = 0.049, R2 = 0.2). There was no significant correlation with activation in the left hippocampus after right ATLR. (Voxel by voxel analysis: Supporting Information).
Efficiency of postoperative reorganization—comparison of patients with and without clinically significant naming decline after left ATLR
Twelve of 24 patients with left TLE had clinically significant naming decline and 12 of 24 did not, or had improved naming scores after left ATLR.
Those with a clinically significant naming decline had a significant positive correlation between postoperative VF activation and postoperative naming scores in the right MFG (p = 0.02). Patients with left TLE with stable or improved naming demonstrated a significant positive correlation in the posterior remnant of the left hippocampus (p = 0.034), with greater fMRI activation correlated with better naming scores (Fig. 3, Table 3). Patients with positive naming outcomes had greater activation in the left MFG (p = 0.031), suggesting that reorganization within the ipsilateral hemisphere underpins more robust word retrieval. Patients with significant naming decline demonstrated greater activation in the right MFG (p = 0.002) than patients with no decline, from which we inferred that recruitment of contralateral frontal lobe networks does not confer proficiency.
Figure 3.
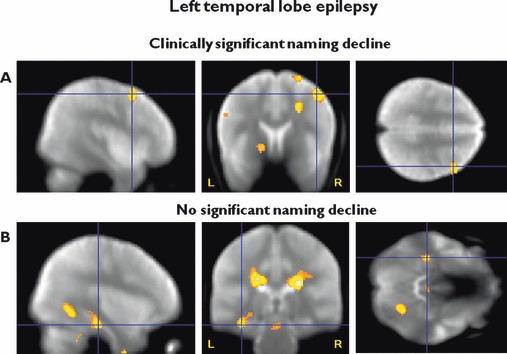
Efficiency of postoperative language networks. (A) Left TLE with clinically significant decline in naming. Greater postoperative right middle frontal gyrus fMRI activation for verbal fluency correlates with better postoperative naming scores, characterized by greater, but inefficient recruitment of the contralateral frontal lobe. (B) Left TLE without clinically significant naming decline. Greater postoperative left posterior hippocampal fMRI activation for verbal fluency correlates with better postoperative naming scores, characterized by efficient recruitment of the remaining ipsilateral posterior hippocampal structures. Threshold p < 0.01, uncorrected. Significant regions are superimposed onto an averaged normalized mean EPI image from all patients who underwent left ATLR. The crosshair points to the peak maximum activation.
Table 3.
Association of postoperative naming and postoperative VF fMRI activation after left ATLR in patients with and without a clinically significant naming decline
| Subjects | Postoperative fMRI activation – neuropsychology score | Z-score | Corrected p-value (FWE) | Coordinates (x, y, z) in MNI space | Anatomic region |
|---|---|---|---|---|---|
| Left TLE with clinically significant decline | Postoperative VF activation – naming | 3.27 | p = 0.022 | 24, 6, 38 | Right MFG |
| Left TLE with no significant decline | Postoperative VF activation – naming | 2.58 | p = 0.034 | −36, −20, −30 | Left HC/PHG |
| Left TLE with decline > left TLE without decline | Postoperative VF activation – naming | 2.86 | p = 0.002 | 44, 12, 52 | Right MFG |
| Left TLE without decline > left TLE with decline | Postoperative VF activation – naming | 3.14 | p = 0.031 | −36, 16, 6 | Left MFG |
HC, hippocampus; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; MNI space, coordinates related to a standard brain defined by the Montreal Neurological Institute (MNI); PHG, parahippocampal gyrus; TLE, temporal lobe epilepsy; VF, verbal fluency.
There were no significant correlations between preoperative hippocampal volumes or postoperative volumes of the residual hippocampi and preoperative or postoperative naming scores in patients with left or right HS.
Prediction of naming decline
Preoperative language fMRI and change in naming scores—voxel by voxel analysis
Twelve of 24 patients with left TLE had a clinically significant naming decline after left ATLR and no patient had a clinically significant naming decline after right ATLR.
In left TLE there was a significant correlation between preoperative fMRI activation in the left MFG and postoperative decline in naming scores (p = 0.003), characterized by greater preoperative fMRI activation being correlated with greater postoperative decline (Table 4, Fig. 4A).
Table 4.
Association of change of naming scores with preoperative VF fMRI activation in patients with left temporal lobe epilepsy
| Subjects | fMRI contrast – change in neuropsychology task | Z-score | Corrected p-value (FWE) | Coordinates (x, y, z) in MNI space | Anatomic region |
|---|---|---|---|---|---|
| Left TLE | VF fMRI – 1/naming | 3.95 | p = 0.003 | −42, 6, 56 | Left MFG |
| 2.34 | p = 0.064 | −20, −8, −18 | Left HC |
HC, hippocampus; MFG, middle frontal gyrus; MNI space, coordinates related to a standard brain defined by the Montreal Neurological Institute (MNI); TLE, temporal lobe epilepsy; VF, verbal fluency.
Figure 4.
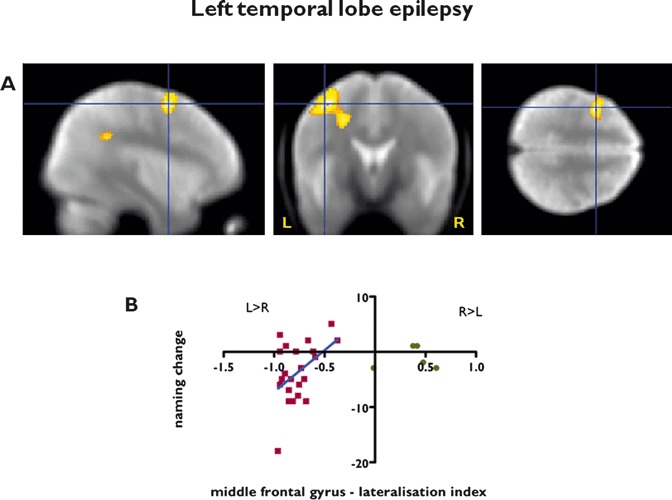
Prediction of naming decline using preoperative verbal fluency fMRI in patients with left TLE. (A) Whole brain voxel by voxel analysis. Left middle frontal gyrus activation for verbal fluency correlates with change in naming scores after left ATLR, characterized by greater naming decline in subjects with greater preoperative fMRI activation. Threshold p < 0.01, uncorrected. Significant regions are superimposed onto an averaged normalized mean EPI image from 30 healthy controls, 15 patients with left, and 15 patients with right hippocampal sclerosis. The crosshair points to the peak maximum activation. (B) Prediction of naming decline in individual subjects—language lateralization index for verbal fluency in the middle frontal gyrus. Strongly left lateralized middle frontal gyrus activation for verbal fluency correlates with clinical significant naming decline after left ATLR (r2 = 0.21, p = 0.03). For comparison, five patients with left TLE and atypical language representation are indicated by green circles; these patients were not included in this study.
There was a significant correlation between preoperative fMRI activation for VF and change in VF scores outside the scanner in the left MFG (p = 0.001), characterized by greater fMRI activation being associated with greater decline in VF. From a clinical perspective a decline in VF scores was less relevant than a decline in naming scores and therefore VF was not considered further.
Prediction of naming decline in individual subjects—positive predictive value (PPV), sensitivity, and specificity of language fMRI and preoperative naming performance
A clinically significant naming decline was defined as a decline of >3 on the McKenna Graded Naming Test. Two of our 24 patients with left TLE performed in the impaired range (i.e., <1st centile) preoperatively and therefore were excluded from further analysis, as floor effects prevented identification of a postoperative decline.
Having identified that fMRI activation in the left MFG was significantly related to a decline in naming scores, we quantified this activation and calculated a preoperative lateralization index in the MFG for each individual subject using an anatomic mask (Hammers et al., 2003) and correlated this with each patient’s change in naming scores. There was a significant correlation between the lateralization index for VF in the left MFG and change in naming scores (p = 0.03, R2 = 0.21), characterized by patients with greater left lateralized language in this area being at higher risk for postoperative naming decline after left ATLR (Fig. 4B). A lateralization index of less than −0.65 in the MFG was defined as a cutoff—representing strongly left lateralized language representation for VF, which identified all patients with a clinically significant naming decline with a positive predictive value (PPV) of 60% with 100% sensitivity and 33.33% specificity because of a relatively high number of false positives. Preoperative naming scores alone identified all but two patients with a clinical significant naming decline (PPV of 54.54%, 100% sensitivity, 16.67% specificity). By considering both independent predictors to calculate the risk for the individual patients, all left TLE patients with a clinically significant naming decline were identified with a PPV of 66.67%, 100% sensitivity and 40% specificity.
There was no significant correlation between preoperative naming scores or left hippocampal volume and naming change in patients with left TLE.
Discussion
Summary of main findings
In this longitudinal study we performed language fMRI in patients with TLE preoperatively and 4 months after left/right ATLR to assess reorganization and efficiency of preoperative and postoperative language networks.
We demonstrated reorganization to the contralateral hemisphere within 4 months of left ATLR, which was not observed after right ATLR, suggesting that multiple systems support language. These findings were corroborated by functional connectivity analysis showing greater postoperative than preoperative connectivity to the contralateral frontal lobe in patients with left TLE.
Preoperatively, a significant correlation between naming function and fMRI VF activation in the left hippocampus and the left frontal lobe in left and right TLE highlighted the role of the dominant hippocampus for word retrieval. After left ATLR, the posterior remnant of the left hippocampus and the left frontal system had a continued role in successful naming, and reorganization to the contralateral frontal lobe network was less productive.
Preoperatively, greater left middle frontal activation was predictive of greater postoperative naming decline after left ATLR.
Language fMRI in temporal lobe epilepsy—reorganization of language function after ATLR
Several small studies have investigated postoperative reorganization and plasticity of language function (Hertz-Pannier et al., 2002; Backes et al., 2005; Pataraia et al., 2005; Helmstaedter et al., 2006; Wong et al., 2009). A postoperative fMRI study showed more bilateral language representation after left ATLR than right ATLR, and healthy controls (Backes et al., 2005). Without preoperative data, no conclusion on the effects of surgery can be drawn.
One fMRI study found evidence for reallocation of language processing to other regions than typical language areas in left TLE, compared to patients with right TLE whose activation patterns remained unchanged postoperatively (Wong et al., 2009). This study was limited by small numbers, heterogeneous pathologies, and ROI analysis restricted to typical language areas, so that possible compensatory mechanisms in other brain areas were not assessed.
In our longitudinal study we used VF and neuropsychological assessment preoperatively and postoperatively to show reorganization/recruitment of the contralateral frontal lobe network within 4 months of left ATLR in a large cohort of patients with HS, who were all left dominant for language. Most likely these findings reflect multiple systems supporting language, which come into action once the main system in the speech dominant hemisphere is disrupted. A similar dynamic was described in stroke patients (Saur et al., 2006). We are carrying out long-term follow-up studies at 1 year to investigate subsequent reorganization after ATLR.
Earlier age of epilepsy onset is associated with a higher incidence for atypical language representation (Gaillard et al., 2007) but also early intrahemispheric reorganization (Bell et al., 2002). Postoperative interhemispheric reorganization of language function is more likely in patients with preexisting atypical language representation (Pataraia et al., 2005). We did not observe an effect of age of epilepsy onset, but our sample was purposefully restricted to patients with preoperative left language dominance in order to study interhemispheric and intrahemispheric postoperative reorganization and its functional capacity. Within our sample there was no relationship between age of onset and naming performance.
Neurobiologic implications
Naming deficits have been reported after language dominant hemisphere ATLR (Davies et al., 1998, 2005,; Seidenberg et al., 1998; Hermann et al., 1999). The underlying mechanisms are not fully understood.
The hippocampus has a key role in verbal learning and memory (Squire, 1992) and there is evidence that the hippocampus is essential for naming. Several studies reported increased hippocampal activity during naming tasks (Tomaszewki Farias et al., 2005; Bonelli et al., 2011). Further, prior to surgery, naming was poorer in patients with HS and the risk of naming decline after ATLR was lower in patients with HS than in those without HS (Davies et al., 1998; Hamberger et al., 2007) suggesting a key role for the hippocampus. A pediatric study concluded that reorganization of language function occurred when there was a hippocampal lesion (Liegeois et al., 2004). Patients with left HS had a higher chance of atypical language representation than TLE patients with other pathologies (Weber et al., 2006), suggesting that the speech-dominant hemisphere’s hippocampus plays an important role in language function, particularly naming, and that this function is impaired in HS, and if it is resected. Language lateralization in patients with TLE is not straightforward, and there may be complex findings such as crossed lateralization for anterior and posterior language areas (Gaillard et al., 2004), which could partly explain preoperative naming deficits in some patients with right TLE.
Functional MRI activates a network of cerebral regions. A requirement for naming and VF tasks is the retrieval of semantically or lexically associated words from long-term memory. Given the role of the dominant hippocampus in verbal learning and memory, it most likely has an essential role in the acquisition of phonemic, lexical and conceptual information, which are crucial for naming and word retrieval (Bonelli et al., 2011).
Language functions, particularly naming and reading, can be transferred to the contralateral hemisphere after injuries to the language dominant hemisphere in early life, or the development of TLE (Devinsky et al., 1993; Liegeois et al., 2004). Preservation of naming in patients with HS after ATLR has been attributed to intrahemispheric reorganization, in particular to the posterior and inferior temporal regions (Hamberger et al., 2007). Our recent memory fMRI study also suggested that it was the capacity of the remaining posterior ipsilateral hippocampus that preserved verbal and visual memory encoding function after ATLR (Bonelli et al., 2010).
In the current study we showed that, preoperatively, in left TLE with left-hemisphere speech dominance, the left hippocampus and frontal lobe supported efficient naming function. Postoperatively, patients with left TLE with no significant naming decline relied on the recruitment of the residual left posterior hippocampus for word retrieval, whereas patients demonstrating a decline showed greater reliance on the contralateral frontal lobe. The implication is that reorganization within 4 months of speech-dominant ATLR to the contralateral hemisphere is less effective, whereas reorganization involving the ipsilateral posterior hippocampus underpins good naming functions postoperatively. The circumstances that determine whether language function is reorganized to the contralateral hemisphere and/or the ipsilateral hemisphere and temporal lobe still need to be established. Possible factors are age of onset, preoperative lateralization index for language, genetic factors, severity of sclerosis, electroclinical findings (Helmstaedter et al., 1997; Janszky et al., 2003), and extent of hippocampal resection.
Clinical implications—prediction of postoperative naming decline
Functional MRI is useful in predicting decline of memory (Rabin et al., 2004; Richardson et al., 2004; Janszky et al., 2005; Powell et al., 2008; Bonelli et al., 2010) and naming (Sabsevitz et al., 2003) after ATLR. Identifying who is at risk for language impairment postoperatively improves the advice that is given to patients considering surgery. Sabsevitz et al. ()2003 previously highlighted the role of temporal lobe regions compared to frontal regions in predicting postoperative naming deficits after temporal lobe surgery as assessed by a semantic decision fMRI task. In contrast to these findings, using the verbal fluency paradigm in left dominant for language left TLE patients (therefore at risk of a postoperative naming decline), those with greater VF activation in the left MFG were at greater risk of a postoperative naming decline implying an important interaction between MFG and anterior temporal lobe. As shown previously, in patients with left TLE due to hippocampal sclerosis, left hippocampal disengagement during language tasks was paralleled by greater frontal activation, suggesting compensatory strategies in less functionally developed regions in the frontal lobe (Bonelli et al., 2011). A similar process has been described previously for episodic memory in left TLE (Dupont et al., 2000).
To establish a robust method to predict postoperative decline in individual subjects we calculated a lateralization index in the anatomically defined MFG, which would be easy to apply in a clinical setting. Patients with strongly left-lateralized VF in this area (LI < −0.65) were at risk for clinically significant naming decline after left ATLR. Combining preoperative performance on the naming test and language lateralization indices we were able to predict a clinically significant naming decline in all our patients with a PPV of 67%. Furthermore, none of the five patients with left TLE with preoperative atypical language representation (who were not included in this study) had a clinically significant naming decline after ATLR (Fig. 4B).
Methodologic strengths and limitations
Strengths
This study has the advantage of comparing fMRI data and neuropsychological assessment before and 4 months after left or right ATLR in a large cohort of patients with TLE, all left language dominant with the same pathology. This allowed explicit study of the effects of surgery with respect to cortical reorganization of language function.
We confined this study to a homogenous group of left hemisphere dominant patients, to determine the effects of left ATLR.
Limitations
The VF task had to be carried out covertly, so that performance was not directly measured in the scanner.
Our results may be influenced by the effect of volume averaging on the extent and magnitude of hippocampal signal, given that all patients with TLE had hippocampal sclerosis.
Postoperatively, language was assessed 4 months after ATLR, and reorganization may continue over a longer time. In addition, we did not include healthy controls for repeated-measures effects in this study. At present we are undertaking longitudinal follow-up studies of language organization over 12 months after ATLR and in healthy controls to address these issues.
We examined expressive language skills by combining a VF fMRI task with out of scanner VF and naming performance, which is of great clinical concern in TLE patients and following ATLR. The VF task does not primarily elicit temporal activation but requires word retrieval and was associated with hippocampal activation. Future studies will benefit from fMRI paradigms that evaluate receptive language functions and explore the network-sustaining naming processes, particularly posterior and basal temporal areas. We are currently implementing and validating a library of such tasks.
Early age of onset is associated with a higher incidence of atypical language dominance. We considered a selected population of left language dominant patients with HS, so the effects of age of onset were not investigated. It is necessary to establish patterns of change in typical language dominance before assessing the input of preoperative atypical language representation.
Conclusion
In left TLE we demonstrated early postoperative activation in the contralateral frontal lobe for basic language function. The capacity of the remaining ipsilateral posterior hippocampus was important to maintain naming function postoperatively, while involvement of the contralateral frontal lobe was less proficient.
VF-fMRI was predictive of postoperative naming decline in individual patients.
Acknowledgments
The Wellcome Trust [programme grant numbers 067176, 083148]; The Big Lottery Fund; The Wolfson Trust and Epilepsy Society support the Epilepsy Society MRI scanner; the Austrian Section of the ILAE supported S.B. by a fellowship, Vienna 2006. This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. We are grateful to the radiographers at the Epilepsy Society MRI Unit, Philippa Bartlett, Jane Burdett and Elaine Williams, who scanned the subjects, to all our subjects and our colleagues for their enthusiastic cooperation, particularly to Dr. Sallie Baxendale for her neuropsychological expertise, and for devising the epilepsy surgery neuropsychology database.
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
Table S1. Association of pre- and postoperative VF/naming scores with pre- and postoperative VF fMRI activation.
Supporting info item
References
- Backes WH, Deblaere K, Vonck K, Kessels AG, Boon P, Hofman P, Wilmink JT, Vingerhoets G, Boon PA, Achten R, Vermeulen J, Aldenkamp AP. Language activation distributions revealed by fMRI in post-operative epilepsy patients: differences between left- and right-sided resections. Epilepsy Res. 2005;66:1–12. doi: 10.1016/j.eplepsyres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bell B, Hermann B, Seidenberg M, Davies K, Cariski D, Rosenbek J, Woodard A, Rutecki P, Bishop M. Ipsilateral reorganization of language in early-onset left temporal lobe epilepsy. Epilepsy Behav. 2002;3:158–164. doi: 10.1006/ebeh.2002.0322. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, Benbadis S, Frost JA, Rao SM, Haughton VM. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Bonelli SB, Powell RH, Yogarajah M, Samson RS, Symms MR, Thompson PJ, Koepp MJ, Duncan JS. Imaging memory in temporal lobe epilepsy: predicting the effects of temporal lobe resection. Brain. 2010;133:1186–1199. doi: 10.1093/brain/awq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli SB, Powell R, Thompson PJ, Yogarajah M, Focke NK, Stretton J, Vollmar C, Symms MR, Price CJ, Duncan JS, Koepp MJ. Hippocampal activation correlates with visual confrontation naming: fMRI findings in controls and patients with temporal lobe epilepsy. Epilepsy Res. 2011;95:246–254. doi: 10.1016/j.eplepsyres.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KG, Bell BD, Bush AJ, Hermann BP, Dohan FC, Jr, Jaap AS. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia. 1998;39:407–419. doi: 10.1111/j.1528-1157.1998.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Davies KG, Risse GL, Gates JR. Naming ability after tailored left temporal resection with extraoperative language mapping: increased risk of decline with later epilepsy onset age. Epilepsy Behav. 2005;7:273–278. doi: 10.1016/j.yebeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, Lee A, Hillis AE. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Perrine K, Llinas R, Luciano DJ, Dogali M. Anterior temporal language areas in patients with early onset of temporal lobe epilepsy. Ann Neurol. 1993;34:727–732. doi: 10.1002/ana.410340517. [DOI] [PubMed] [Google Scholar]
- Dupont S, Van de Moortele PF, Samson S, Hasboun D, Poline JB, Adam C, Lehericy S, Le Bihan D, Samson Y, Baulac M. Episodic memory in left temporal lobe epilepsy: a functional MRI study. Brain. 2000;123:1722–1732. doi: 10.1093/brain/123.8.1722. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Jabbari B, Vezina LG, Frattali C, Theodore WH. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256–265. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Vezina LG, Frattali C, Theodore WH. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–1408. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, Conry JA, Pearl PL, Ritter FF, Sato S, Vezina LG, Vaidya CJ, Wiggs E, Fratalli C, Risse G, Ratner NB, Gioia G, Theodore WH. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Goodman RR, Williams A, Perrine K, Devinsky O, McKhann GM., 2nd Evidence for cortical reorganization of language in patients with hippocampal sclerosis. Brain. 2007;130:2942–2950. doi: 10.1093/brain/awm187. [DOI] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C, Elger CE. Cognitive consequences of two-thirds anterior temporal lobectomy on verbal memory in 144 patients: a three-month follow-up study. Epilepsia. 1996;37:171–180. doi: 10.1111/j.1528-1157.1996.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Linke DB, Elger CE. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings, EEG, sex, and age at onset of epilepsy. Brain Cogn. 1997;33:135–150. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Fritz NE, Gonzalez Perez PA, Elger CE, Weber B. Shift-back of right into left hemisphere language dominance after control of epileptic seizures: evidence for epilepsy driven functional cerebral organization. Epilepsy Res. 2006;70:257–262. doi: 10.1016/j.eplepsyres.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Haltiner A, Wyler AR. Relationship of age at onset, chronologic age, and adequacy of preoperative performance to verbal memory change after anterior temporal lobectomy. Epilepsia. 1995;36:137–145. doi: 10.1111/j.1528-1157.1995.tb00972.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Perrine K, Chelune GJ, Barr W, Loring DW, Strauss E, Trenerry MR, Westerveld M. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999;13:3–9. doi: 10.1037//0894-4105.13.1.3. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Chiron C, Jambaque I, Renaux-Kieffer V, Van de Moortele PF, Delalande O, Fohlen M, Brunelle F, Le Bihan D. Late plasticity for language in a child’s non-dominant hemisphere: a pre- and post-surgery fMRI study. Brain. 2002;125:361–372. doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- Janszky J, Jokeit H, Heinemann D, Schulz R, Woermann FG, Ebner A. Epileptic activity influences the speech organization in medial temporal lobe epilepsy. Brain. 2003;126:2043–2051. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- Janszky J, Jokeit H, Kontopoulou K, Mertens M, Ebner A, Pohlmann-Eden B, Woermann FG. Functional MRI predicts memory performance after right mesiotemporal epilepsy surgery. Epilepsia. 2005;46:244–250. doi: 10.1111/j.0013-9580.2005.10804.x. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha-Khadem F, Baldeweg T. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127:1229–1236. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- McKenna P, Warrington EK. The graded naming test. England NFER Nelson; 1983. , Windsor, UK. [Google Scholar]
- Noppeney U, Price CJ, Duncan JS, Koepp MJ. Reading skills after left anterior temporal lobe resection: an fMRI study. Brain. 2005;128:1377–1385. doi: 10.1093/brain/awh414. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pataraia E, Billingsley-Marshall RL, Castillo EM, Breier JI, Simos PG, Sarkari S, Fitzgerald M, Clear T, Papanicolaou AC. Organization of receptive language-specific cortex before and after left temporal lobectomy. Neurology. 2005;64:481–487. doi: 10.1212/01.WNL.0000150900.71773.E6. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Powell HW, Richardson MP, Symms MR, Boulby PA, Thompson PJ, Duncan JS, Koepp MJ. Preoperative fMRI predicts memory decline following anterior temporal lobe resection. J Neurol Neurosurg Psychiatry. 2008;79:686–693. doi: 10.1136/jnnp.2007.115139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin ML, Narayan VM, Kimberg DY, Casasanto DJ, Glosser G, Tracy JI, French JA, Sperling MR, Detre JA. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain. 2004;127:2286–2298. doi: 10.1093/brain/awh281. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Thompson PJ, Baxendale SA, Duncan JS, Dolan RJ. Pre-operative verbal memory fMRI predicts post-operative memory decline after left temporal lobe resection. Brain. 2004;127:2419–2426. doi: 10.1093/brain/awh293. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL, 3rd, Mueller WM, Binder JR. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Stafiniak P, Robinson LJ, Flannery KA, Gur RC, O’Connor MJ, Sperling MR. Language before and after temporal lobectomy: specificity of acute changes and relation to early risk factors. Epilepsia. 1995;36:1071–1077. doi: 10.1111/j.1528-1157.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Hermann B, Wyler AR, Davies K, Dohan FC, Jr, Leveroni C. Neuropsychological outcome following anterior temporal lobectomy in patients with and without the syndrome of mesial temporal lobe epilepsy. Neuropsychology. 1998;12:303–316. doi: 10.1037//0894-4105.12.2.303. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Tomaszewki Farias S, Harrington G, Broomand C, Seyal M. Differences in functional MR imaging activation patterns associated with confrontation naming and responsive naming. AJNR Am J Neuroradiol. 2005;26:2492–2499. [PMC free article] [PubMed] [Google Scholar]
- Trebuchon-Da Fonseca A, Guedj E, Alario FX, Laguitton V, Mundler O, Chauvel P, Liegeois-Chauvel C. Brain regions underlying word finding difficulties in temporal lobe epilepsy. Brain. 2009;132:2772–2784. doi: 10.1093/brain/awp083. [DOI] [PubMed] [Google Scholar]
- Weber B, Wellmer J, Reuber M, Mormann F, Weis S, Urbach H, Ruhlmann J, Elger CE, Fernandez G. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;129:346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, Sperling MR, Luders H, Pedley TA. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]
- Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007;163:128–136. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, Okujava M, Wolf P, Tuxhorn I, Ebner A. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- Wong SW, Jong L, Bandur D, Bihari F, Yen YF, Takahashi AM, Lee DH, Steven DA, Parrent AG, Pigott SE, Mirsattari SM. Cortical reorganization following anterior temporal lobectomy in patients with temporal lobe epilepsy. Neurology. 2009;73:518–525. doi: 10.1212/WNL.0b013e3181b2a48e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


