Abstract
Background:
Moringa peregrina (M. peregrina) is an important tropical tree recognized for its nutritional and medicinal properties. The objective of this study was to investigate the nutritional component in the leaves and seeds of the Persian M. peregrina (Forssk.) Fiori.
Materials and Methods:
The M. peregrina leaves and seeds of wild cultivated trees were collected from the areas of arid environment located in the South-East of the Iran. The leaves and seeds of M. peregrina were dried and grounded to a fine powder and kept in dark for the day of experiment. The acidic digested leaves and seeds were analyzed for Vitamins C and A, calcium, and potassium using atomic adsorption and flame emission spectrophotometer.
Results:
The analytical data revealed that the leaves and seeds of the Persian M. peregrina (Forssk.) Fiori contain sufficient amounts of Vitamin C: 83 ± 0.5 and 14 ± 0.6 mg/100 g/DW; and Vitamin A: 6.8 ± 0.7 and 24.8 ± 0.7 mg/100 g/DW, respectively. The elemental analysis in the leaves and seeds showed that the calcium content are 764.8 ± 1.6 and 1164.8 ± 43.4 mg/100 g/DW and for potassium content are 900.2 ± 14 and 572 ± 10 mg/100 g/DW, respectively.
Conclusions:
The nutritional characteristics of the Persian M. peregrina (Forssk.) Fiori, investigated in this study revealed that, daily use of leaves and seeds of this plant could significantly provide the recommended dietary allowance for the Vitamins C and A, and minerals, such as calcium and potassium.
Keywords: Calcium, Moringa peregrine, Potassium, Recommended dietary allowance, Vitamin A
INTRODUCTION
Moringa peregrina (M. peregrina) belongs to the family of flowering plant Moringaceae that have only one genus called Moringa, which contains 14 species from tropical and subtropical climates.[1,2] The M. peregrina and M. oleifera (M. oleifera) are the most popular between them. The M. peregrina seed oil has antimicrobial activity.[3] It has straight trunk, 3–10 m high, and white bark with high aridity adaptation. The bipinnate leaves are 30–40 cm long, the axes persistent, and deciduous leaflets. Flowers appear before leaves in May. Flowers are pentamerous, zygomorphic, hermaphrodite, and pinkish-white with white sepals. This tree bears, silique similar to pod, cylindrical fruits with 20–40 cm length. Seeds are un-winged, triquetrous, and 25 mm in length and 10–12 mm in diameter.[4] The M. peregrina flowering and fruiting time is in the months of February to April. The geographical distribution of Moringa is in tropical and non-tropical climate. Therefore, Moringa is adaptable to a wide range of environmental conditions from hot and dry to hot, humid, and wet conditions. The tree produces a tuberous tap root, which explains its tolerance to drought conditions. Originally M. peregrina is a desert species and considered a tree of hot semi-arid regions. In Iran M. peregrina is distributed in West and Central parts of Baluchestan Province. The vernacular names are Gas-e-Rooghani and Gaz Rokh.[4,5] The Moringa plant has been consumed by humans throughout the century in diverse culinary ways.[4] Almost all parts of the plant are used culturally for its nutritional value, purported medicinal properties and for taste, and flavor as a vegetable and seed. The leaves of M. oleifera can be eaten fresh and cooked.[5] The leaves contain all essential amino acids and are rich in protein and minerals.[6,7] The M. peregrina seed kernel is rich in fixed oil (42–54%) with up to 23% protein. The edible oil is used medicinally as diuretic, rubefacient, and astringent.[7] The seeds of the plants contain edible oil which is used for cooking, frying, and as a salad oil for dressing. Newer applications include the use of Moringa powder as a fish food in aqua cultural systems.[8,9] The Moringa leaves as a protein supplement are used for animals, like cows. Furthermore, Moringa seeds contain active coagulating compounds in the seeds for water treatment.[10,11] The whole seeds can also be eaten green, roasted or powdered. There is medical evidence that the M. oleifera has therapeutic and prophylactic properties. The pods and seeds, often referred to as M. kernels, have a taste that ranges from sweet to bitter and are most popularly consumed after frying to get a peanut-like taste.[12,13] M. oleifera leaf has been purported to be a good source of nutrition and a naturally organic health supplement that can be used in many therapeutic ways.[14,15] Identification of these vitamins would be a great advantage to the nutritional attributes of the M. peregrina. It was reported that M. peregrina showed anti-hyperglycemic effect of the ethanolic extract from which quercetin, quercetin-3-O-rutinoside, chryseriol-7-O-rhamnoside, and 6, 8, 3′, 5′-tetramethoxy apigenin were isolated.[16] The aerial parts of M. peregrina yielded lupeol acetate, β-amyrin, α-amyrin, β-sitosterol, β-sitosterol-3-O-glucoside, apigenin, rhamnetin, neochlorogenic acid, rhamnetin-3-O-rutinoside, and 6-methoxy-acacetin-8-C-β-glucoside.[17] Occurrence of isothiocyanates in seed extracts of M. peregrina growing in Iran and elsewhere was reported.[7,18] Even though various studies have been done on the M. oleifera, there are few experimental studies regarding the potential nutritional value of the M. peregrina. These include the total vitamins, mineral, protein, and phenolic content.[14] The ethanolic M. oleifera leaf extract could prevent testicular toxicity of rats exposure to high dose of chromium.[19] Although must people in some countries have used the M. oleifera plant as food, there is a little evidence about its chemical contents and nutritional characteristics. In a study revealed that M. oleifera leaves contain considerable amount of crude protein, whereas nearly 30% of total proteins are digestible.[20] The research conducted in this study seeks to assess the nutritional and medicinal values of M. peregrina leaves and seeds collected from the province of Bluchestan/Iran. Our objectives are to quantitatively analyze the Vitamin C, Vitamin A, calcium, and potassium in the leaves and seeds samples to get an overarching idea of the nutritional value of this species located in Iran.
MATERIALS AND METHODS
Sample preparations
The M. peregrina leaves and seeds of wild cultivated trees were collected from the city of Nikshahr/Kerman under authorization of the Jahad Keshavarzi and Agriculture Organization of Sistan and Bluchestan Province/Iran from the areas of arid environment. The leaves and seeds of M. peregrina (No. 2025) were kept in the Herbarium of Pharmacy Faculty/Isfahan University of Medicine. The leaves and seeds were dried in clean, cool, and dark area at room temperature for 20 days. The dry leaves and seeds of M. peregrina were grounded to a fine powder and kept in dark at room temperature for the day of analysis.
Phytochemical tests
Tests for alkaloids
Most alkaloids are precipitated from neutral or slightly acid solution by Mayer's reagent (potassium-mercuric iodide solution), by Wagner's reagent (solution of iodine in potassium iodide). The Mayer's and Wagner's tests were performed to detect the total alkaloids content of M. peregrina and compared with Datura stramonium alkaloid content.[21,22,23,24]
Tests for tannins
The tannin compounds are widely distributed in plants. It plays a role in protection from predation and growth regulation. The ferric chloride (FeCl3) and lead acetate tests were applied to measure the total tannin content of M. peregrina and compared with tannins content from the leaves of Punica.
Test for cardenolide
Many plants contain sugar-steroid derivatives known as cardenolides. Cardenolide glycosides are often toxic with heart-arresting. The Baljet reaction and the 3,5-dinitrobenzoic acid color reaction with cardenolides were used to clarify the total cardenolides content of M. peregrina and compared with the digitalis nervosa L as control.[25,26,27,28,29,30] The Kodde-reaction was performed in alkaline pH with 2 mL KOH (1N) and 3,5-dinitrobenzoic acid.
Tests for steroids and terpenoids
The Liebermann–Burchard or acetic anhydride test was used for the estimation of cholesterol contents in M. peregrina. The formation of a reddish ring at the junction of two layers after a few minutes was taken as positive. The Salkowski's test was also used for semi-quantification of terpenoids content. When concentrated sulfuric acid was added to the chloroform solution of M. peregrina, at the lower of the chloroform layer a yellow with deep red color at the junction of two layers were taken as positive terpenoids content and compared with Glycyrrhiza glabra L. as control.[31]
Tests for saponins
The powdered sample of leaves and seeds (5 g) were boiled with 20 mL of distilled water and the filtered. The filtered of each sample (10 mL) was mixed with distilled water (5 mL). The frothing was then mixed with three drops of olive oil for the formation of emulsion that was taken positive, if saponins present comparing with Zizyphus spina-christi (sedr.).
Test for anthraquinones
Anthraquinones may be detected by the Borntraeger's reactions. 0.2 g of leaves and seeds were added to sulfuric acid solution (5 mL, 2N) and boiled for 2 min. After cooling, 10 mL toluene solvent was added and decanted to stay for 10 min. The yellow color of the toluene layer turns to red in alkaline pH if the anthraquinones present. The Cassia angustifolia L. was applied as a positive control.
Test for flavonoids
A few drop of 1% NH3 or NaOH solution was added to the aqueous extract of each plant sample in a test tube. A yellow coloration was observed if flavonoids compound are present. It should be colorless with a few drop of diluted acid.[32,33]
Chemical analysis
Atomic adsorption/flame emission spectroscopy
Atomic adsorption/flame emission spectroscopy (AA/FES, Shimadzu AA-670) were used for determination of elements in samples after digestion of dry sediment in 7 M HNO3 .
Quantitative analysis of calcium and potassium
The Moringa sample (5 g) with concentrated nitric acid (20 mL, 7N) was placed in a small dish and heated slowly to boil for 2 h. After cooling, 10 mL deionized water was carefully added and the mixed boiled for 3 min. After cooling, the samples were filtered with filter paper in volumetric (1000 mL) flasks and then the volume made to 1 L with de-ionized water.[19] The pure potassium chloride and calcium carbonate were incubated in oven (110°C) to dehydrate for 1 h and then transferred to a desiccator. The compounds were dissolved in 1000 mL double distilled water to make a serial concentration of standards, 1 ppm, 2 ppm, 3 ppm, 4 ppm, 5 ppm, and 10 ppm. The M. peregrina leaves and seeds were dissolved in HNO3 (10 mL, 7N) and then diluted 10 times with double distilled water. FES was used for determination of calcium and potassium by standard curves.
Quantitative analysis of Vitamin C
To measure the Vitamin C contents of the samples a standard method was used with a little modification.[25] All analysis was performed in triplicates. Ascorbic acid is highly sensitive to various modes of processing. There are factors that can influence the degradation of Vitamin C.[34] This water soluble compound is extracted from the M. peregrina (powdered seeds and leaves 1 g) with acidic solution (water 75 mL and phosphoric acid 25 mL) over a shaker for 1 h. Ascorbic Acid was purchased from Merck (Germany). Ascorbic acid (50 mg) was dissolved in 100 mL of metaphosphoric acid to make a stock solution. 2,4-dinitro hydrazin (10 g) was added to 500 mL of sulfuric acid (4.5 M). Tioreh (5 g) was added to 100 mL of water and anhydrous copper sulfate; (0.6 g) was added to 100 mL of water and stirred to dissolve. Tiore solution (5 mL) and CuSO4 solution (5 mL) were added to 2,4-dinitro hydrazin solution (100 mL) while stirring to get a reagent. All the solutions were sealed and refrigerated before use. A blank, containing only meta-phosphoric acid and the reagent of 2,4-dinitro hydrazin, were similarly prepared. The absorbance of each sample was read at 520 nm. Using the standard stock solution of ascorbic acid, a calibration curve was established of three dilutions across a range of concentrations 0.1–2 g/mL. The stand curve equation was, y = 0.02356 × −0.009 (r2 = 0.9988).
Quantitative analysis of Vitamin A
The experimental methods described by Sebrell, et al. were used to measure the Vitamin A contents of the sample.[35] All analysis was done in triplicates. Standard compounds, Vitamin A vial (Darupakhsh, Iran). Vitamin A (50,000 IU/mL, Batch No. 12 OSVE) were dissolved in 330 mL of a mixture of Et-OH: KOH (300–30) to obtain the standard stock solution which was then diluted to make work solutions of five concentration ranging from 500 IU/mL to 5 IU/mL for the standard curve with an equation, y = 105 × +0.0123, r2 = 0.991. After saponification by heating the solution (30 min) and extraction with diethyl ether (3X), the reagent (Antimony tri chloride 10 mL) was added to make solution 100 mL with CHCL3. A blank containing only solutions and the reagent was similarly prepared. The absorbance of each sample was read at 620 nm.
RESULTS
The M. peregrina leaves and seeds were collected from the city of Nikshahr/Iran and the plant originality and the specification were confirmed by botanic specialist from the Jahad-e-Keshavarzi and Agriculture Organization of Sistan and Bluchestan Province and by an Isfahan University Pharmacognosy Professor. The vernacular names were Gas-e-Rooghani and Gaz Rokh. The leaves and seeds were dried in clean, cool, and dark area at room temperature to protect vitamins. The dry leaves and seeds of M. peregrina were ground to a fine powder and kept in dark at room temperature for the day of analysis. Semi-quantitative analysis of phytochemicals of M. peregrina leaves and seeds are shown in Figure 1. As shown in Figures 2 and 3, the Vitamin C as ascorbic acid in the leaves and seeds are 83 ± 0.5 and 14 ± 0.6 mg/100 g/DW and Vitamin A 6.8 ± 0.7 and 24.8 ± 0.7 mg/100 g/DW, respectively. The compounds of leaves and seeds measured, and the nutritional standards were compared with the Vitamin A and Vitamin C content of carrot, liver, orange, and kiwi per serve. Figures 4 and 5 show the calcium and potassium content of the leaves and seeds which are 764.8 ± 1.6 and 1164.8 ± 43.4 mg/100 g/DW and for potassium content are 900.2 ± 14 and 572 ± 10 mg/100 g/DW, respectively. The nutritional standards as shown in Figure 6 were compared with the calcium and potassium in whole milk and banana per serve.
Figure 1.
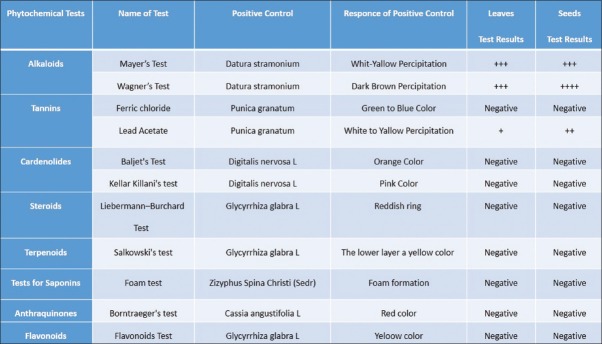
Semi-quantitative analysis of phytochemicals of Moringa peregrina leaves and seeds
Figure 2.
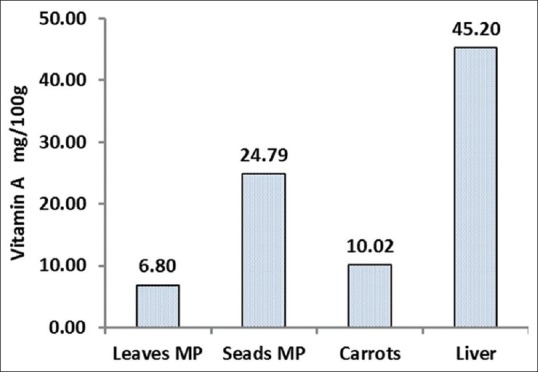
The Vitamin A content in 100 g dry weight of Moringa peregrina leaves and seeds. The figure is comparing the Vitamin A content of 100 g of carrot and liver per serve (data taken from FDA and published reports for Vitamin A source reference) with the vitamin contents of M. peregrina leaves and seeds
Figure 3.
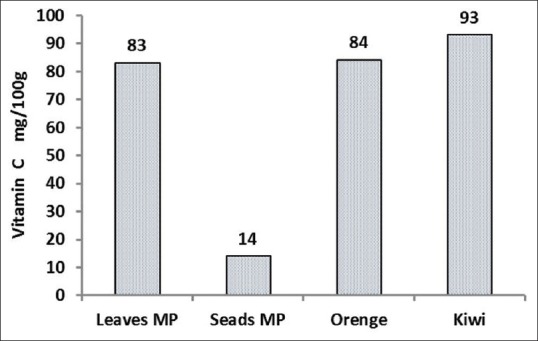
The Vitamin C content in 100 g dry weight of Moringa peregrina leaves and seeds. The figure is comparing the Vitamin C content of 100 g of Orange and Kiwi per serve (data taken from FDA and published reports for vitamin C reference) with the vitamin contents of M. peregrina leaves and seeds
Figure 4.
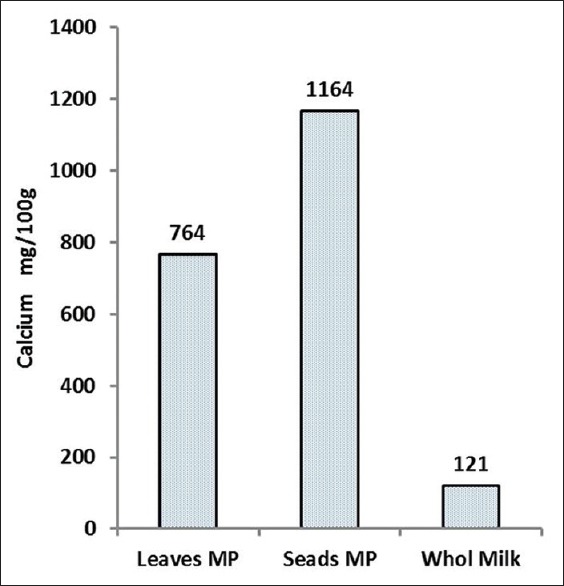
The calcium content in 100 g dry weight of Moringa peregrina leaves and seeds. The figure is comparing the calcium content of 100 g of whole milk per serve (data taken from FDA and published reports for calcium source reference) with the calcium contents of M. peregrina leaves and seeds
Figure 5.
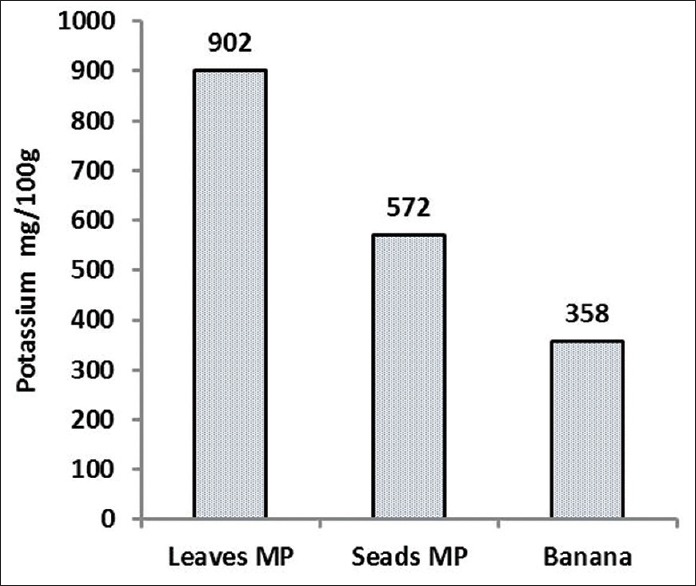
The potassium content in 100 g dry weight of Moringa peregrina leaves and seeds. The figure is comparing the potassium content of 100 g of banana per serve (data taken from FDA and published reports for potassium source reference) with the potassium content of M. peregrina leaves and seeds
Figure 6.
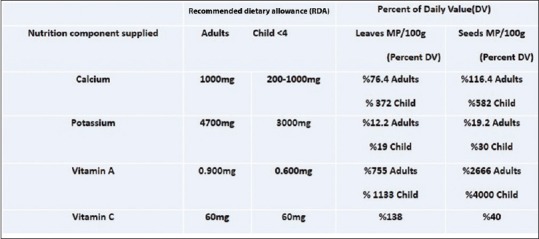
The Recommended dietary allowance of various nutrients
DISCUSSIONS
The results were expressed for the macronutrients as mg of element per 100 g on a dry weight bases (% g DW). The Moringa leaves and seeds samples contained relatively high amounts of examined minerals. As the data shows for these samples, the highest value of calcium was obtained from the seeds in which it was found to be a good source of calcium (1.16 g/100 g DW). It was reported M. oleifera leaves contain calcium (2003 mg/DW) and potassium (1324 mg/g/DW).[35] This research suggests that seeds of M. peregrina like leaves of M. oleifera were excellent sources for the recommended daily requirements of calcium as shown in Figure 6. Our findings also confirm and support the traditional idea that the use of Moringa could be a good source for our diet to provide vitamins and minerals. This plant is safe for human consumption even in a concentrated formula, as traditionally seeds of the plant are eaten in the way peas and seeds are fried or roasted like groundnuts.[7,8] The results from the analysis of compounds showed that the leaves and seeds of M. peregrina contain Vitamin C and Vitamin A, with higher amounts of Vitamin C than A. The significant difference in Vitamin C content was observed among plant parts. There was no direct relationship between the values obtained for Vitamin C and Vitamin A in the leaves and seeds. That is clear that, leaves that exhibited high concentrations of Vitamin C did not necessarily have high concentrations of Vitamin A. Although Vitamin A content in M. peregrina leaves (6.8 mg/100 g DW) is less than Vitamin A content of M. oleifera leaves but M. peregrina seeds showed higher concentrations of Vitamin A (24.8 mg/100 g DW) than seeds of M. oleifera (18.9 mg/100 g DW).[35,36] The seed kernel is rich in fixed oil and Vitamin A is a soluble in oil. The oil is edible therefore, it is important to note that the M. peregrina seeds can serve as a valuable source of Vitamin A.[37] Similarly, M. oleifera leaves are a very rich source of nutrients and contain the essential Vitamins A and C. Leaves are rich in biologically active carotenoids and Vitamin C have health-promoting potential in maintaining a balanced diet and preventing free-radical damage that can initiate many illnesses.[37] Other reports show fresh M. oleifera leaves as having high concentrations of ascorbic acid.[38,39] Researchers used fresh leaves. In this research, the dried leaves gave lower concentration of ascorbic acid (83 mg/100 g DW) compared to values as higher as 5500 mg/100 g DW determined from fresh materials.[6] In addition, other reports indicated that the Vitamin C content ranges around 204 mg/100 g of fresh weight.[39,40] Previous research on M. oleifera showed, with one fresh Moringa leaves contain approximately 220 mg/100 g of ascorbic in the leaves.[14] Studies have shown that ascorbic acid is highly sensitive to a number of factors, including temperature and light. The lack of ascorbic acid in processed food has been explained mostly by heat, oxygen, and light samples.[6,39] Therefore, as ascorbic acid has a very short half-life and the leaves collected in this analysis were dried, the ascorbic acid should be far lower than that which would be found in fresh tissue. Another reason comes from the variation of species in the Moringa plant. To the best of our knowledge, there is no report on the vitamins of M. peregrina.
The M. oleifera leaves are rich of Vitamin C and phenolic compounds which are responsible for its antioxidant activity.[40,41] Antioxidant systems have evolved to help defend the body against free-radicals, which are commonly produced within the body under normal circumstances. Vitamin C or ascorbic acid acts as an antioxidant that, along with other vitamins, protects the body from oxidative stress, maintains the immune system. In addition, ascorbic acid neutralizes any radicals, damaged lipids, and DNA in the blood that may cause illnesses to emerge.[41] These findings demonstrate that Moringa is a potential plant candidate to be used to improve the health and nutrition. Together, the macronutrients and micronutrients provide energy, structure, and regulation which are needed for growth, maintenance, repair, and reproduction.
The results of the present study are consistent with previous finding that the M. peregrina (Forssk.) Fiori has valuable nutritional components and could be a complementary food for individuals with vitamins and mineral deficiencies. The bioavailability of the nutrients demands more studies such as digestibility of the proteins and fats and existence of anti-nutrients, like metal chelators (e.g., phytates oxalates), and protease inhibitors. Exploration of these issues could provide a more complete picture of the nutritional significances of the seeds and leaves of M. peregrina (Forssk.) Fiori.
ACKNOWLEDGMENTS
The authors would like express special thanks to the Isfahan University of Medical Sciences/Iran for financial support (grant number 387055).
Footnotes
Source of Support: Isfahan University of Medical Sciences, Isfahan, IRAN
Conflict of Interest: None declared.
REFERENCES
- 1.Al Khateeb W, Bahar E, Lahham J, Schroeder D, Hussein E. Regeneration and assessment of genetic fidelity of the endangered tree Moringa peregrina (Forsk.) Fiori using Inter Simple Sequence Repeat (ISSR) Physiol Mol Biol Plants. 2013;19:157–64. doi: 10.1007/s12298-012-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal S, Bhanger MI. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J Food Compost Anal. 2006;19:544–51. [Google Scholar]
- 3.Lalas S, Gortzi O, Athanasiadis V, Tsaknis J, Chinou I. Determination of antimicrobial activity and resistance to oxidation of Moringa peregrina seed oil. Molecules. 2012;17:2330–4. doi: 10.3390/molecules17032330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghahreman A. Flora of Iran. Tehran: Institute of Forests and Rangelands Publications; 2001. p. 2578. [Google Scholar]
- 5.Gopalan C, Rama Sastri BV, Balasubramanian SC. Nutritive Value of Indian Foods. India: National Institute of Nutrition, Indian Council of Medical Research; 1989. [Google Scholar]
- 6.Janick J, Paull RE. The Encyclopedia of Fruit and Nuts. Wallingford, United Kingdom: Cabi Publishing; 2008. pp. 509–10. [Google Scholar]
- 7.Afsharypuor S, Asghari G, Mohagheghzadeh A, Dehshahri S. Volatile constituents of the seed kernel and leaf of Moringa peregrina (Forssk.) Fiori, Agricolt. Cultivated in Chabahar (Iran) Iran J Pharm Sci. 2010;6:141–4. [Google Scholar]
- 8.Al-kahtani HA. Moringa peregrina (Al-Yassar or Al-Ban) seeds oil from Northwest Saudi Arabia. J King Saud Univ. 1995;1:31–45. [Google Scholar]
- 9.Dongmeza E, Siddhuraju P, Francis G, Becker K. Effects of dehydrated methanol extracted Moringa (Moringa oleifera Lam.) leaves and three of its fractions on growth performance and feed nutrient assimilation in Nile tilapia (Oreochromis niloticus (L.)) Aquaculture. 2006;261:407–22. [Google Scholar]
- 10.Bina B, Shahsavani A, Asghari G, Hasanzadeh A. Comparison of water turbidity removal efficiencies of Moringa oleifera seed extract and poly aluminum chloride. Water Wastewater. 2007;61:24–34. [Google Scholar]
- 11.Fahey J. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005;1:5–20. [Google Scholar]
- 12.Makkar HP, Becker K. Nutritional value and antinutritional components of whole and ethanol extracted Moringa oleifera leaves. Anim Feed Sci Technol. 1996;63:211–28. [Google Scholar]
- 13.McBurney RP, Griffin C, Paul AA, Greenberg DC. The nutritional composition of African wild food plants: From compilation to utilization. J Food Compost Anal. 2004;17:277–89. [Google Scholar]
- 14.DanMalam HU, Abubakar Z, Katsayal UA. Pharmacognostic studies on the leaves of Moringa oleifera. Niger J Nat Prod Med. 2001;5:45–9. [Google Scholar]
- 15.El-Batran SA, Abdel-Salam OM, Abdelshfeek KA, Nazif NM, Ismail SI, Hammouda FM. Phytochemical and pharmacological investigation on Moringa peregrina (Forssk) Fiori. Nat Prod Sci. 2005;11:199–206. [Google Scholar]
- 16.El-Alfy TS, Ezzat SM, Hegazy AK, Amer AM, Kamel GM. Isolation of biologically active constituents from Moringa peregrina (Forssk.) Fiori. (family: Moringaceae) growing in Egypt. Pharmacogn Mag. 2011;7:109–15. doi: 10.4103/0973-1296.80667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjaer A, Malver O, El-Menshawi B, Reisch J. Isothiocyanates in myrosinase-treated seed extracts of Moringa peregrina. Phytochemistry. 1979;18:1485–7. [Google Scholar]
- 18.Afsharypuor S, Asghari G, Mohagheghzadeh A, Dehshahri S. Volatile Constituents of the Seed Kernel and Leaf of Moringa peregrina (Forssk.) Fiori, Agricolt. Cultivated in Chabahar (Iran) Iran J Pharm Sci. 2010;6:141–4. [Google Scholar]
- 19.Marczenko Z. Ellis Horwood Series in Analytical Chemistry. 2 Sub Edition. West Sussex, UK: John Wiley and Sons; 1986. Separation and Spectrophotometric Determination of Elements. [Google Scholar]
- 20.Sadek KM. Chemotherapeutic efficacy of an ethanolic Moringa oleifera leaf extract against chromium-induced testicular toxicity in rats. Andrologia. 2014;46:1047–54. doi: 10.1111/and.12196. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira EM, Carvalho MR, Neves VA, Silva MA, Arantes-Pereira L. Chemical characteristics and fractionation of proteins from Moringa oleifera Lam. leaves. Food Chem. 2014;147:51–4. doi: 10.1016/j.foodchem.2013.09.135. [DOI] [PubMed] [Google Scholar]
- 22.Cordell GA. Chemistry and pharmacology. Vol. 43. London: Academic Press; 1993. The alkaloids; pp. 23–43. [Google Scholar]
- 23.Roberts MR, Wink M. Biochemistry, Ecology and Medical Applications. New York: Plenum; 1998. Alkaloids. [Google Scholar]
- 24.Southon IW, Buckingham J. Dictionary of Alkaloids. London: Chapman and Hall; 1989. [Google Scholar]
- 25.Hussein SA, Barakat HH, Merfort I, Nawwar MA. Tannins from the leaves of Punica granatum. Phytochemistry. 1997;45:819–23. [Google Scholar]
- 26.Carl AB, Ashwood ER, Burns DE. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th ed. Philadelphia: Elsevier Health Sciences; 2012. Clinical Toxicology. [Google Scholar]
- 27.Bell FK, Krantz JC., Jr Digitalis; the Baljet reaction, digitoxin and digitoxigenin. J Am Pharm Assoc Am Pharm Assoc. 1949;38:107–9. doi: 10.1002/jps.3030380214. [DOI] [PubMed] [Google Scholar]
- 28.Neuwald F. The photometric assay of digitoxin by the Baljet reaction. J Am Pharm Assoc Am Pharm Assoc. 1950;39:172–4. doi: 10.1002/jps.3030390314. [DOI] [PubMed] [Google Scholar]
- 29.Silberman H, Thorp RH. The estimation of the component cardiac glycosides in Digitalis plant samples. III. The separation and estimation of the genins and anhydrogenins. J Pharm Pharmacol. 1957;9:401–5. doi: 10.1111/j.2042-7158.1957.tb12291.x. [DOI] [PubMed] [Google Scholar]
- 30.Yisa J. Phytochemical analysis and antimicrobial activity of Scoparia dulcis and Nymphaea lotus. Aust J Basic Appl Sci. 2009;3:3975–9. [Google Scholar]
- 31.Smithuis AL. The 3,5-dinitrobenzoic acid color reaction with cardenolides. Pharm Weekbl. 1956;91:253–72. [PubMed] [Google Scholar]
- 32.Burke RW, Diamondstone BI, Velapoldi RA, Menis O. Mechanisms of the Liebermann-Burchard and Zak color reactions for cholesterol. Clin Chem. 1974;20:794–81. [PubMed] [Google Scholar]
- 33.Hossain MA, AL-Raqmi KA, AL-Mijizy ZH, Weli AM, Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3:705–10. doi: 10.1016/S2221-1691(13)60142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bineesh NP, Singhal RS, Pandit AB. A study on degradation kinetics of ascorbic acid in drumstick (Moringa oleifera) leaves during cooking. J Sci Food Agric. 2005;85:1953–8. [Google Scholar]
- 35.Sebrell WH, Harris RS, editors. The Vitamins. 2nd ed. Vol. 1. New York: Academic Press; 1967. Descriptive information on the chemistry, physicochemical properties and physiology of Vitamin A; p. 140. [Google Scholar]
- 36.Vanisha SN, Shilpa P. Standardization and organoleptic evaluation of drumstick (Moringa oleifera) leaves incorporated in to traditional Indian recipes. JTFL. 2008;3:2–7. [Google Scholar]
- 37.Somali MA, Bajneid MA, Al-Fhaimani SS. Chemical composition and characteristics of Moringa peregrina seeds and seeds oil. J Oil Fat Ind (J Am Oil Chem Soc) 1984;61:85–6. [Google Scholar]
- 38.Smolin LA, Grosvenor MB. Nutrition: Science and Application. Hoboken, NJ: John Wiley and Sons, Inc; 2007. [Google Scholar]
- 39.Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–55. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 40.Koheil MA, Hussein MA, Othman AM, EL-Haddad A. Anti-inflammatory and antioxidant activities of Moringa peregrina seeds. FRA. 2011;1:49–61. [Google Scholar]
- 41.Wilson T. Antioxidants, Human Health and Health Diseases. Wallingford, United Kingdom: Cabi Publishing; 1999. Whole foods, antioxidants and health; pp. 141–50. [Google Scholar]


