Abstract
Background:
Arsenic intoxication is known to produce symptoms including diarrhea and vomiting, which are indications of gastrointestinal dysfunction.
Objective:
We investigated whether Kolaviron (KV) administration protected against sodium arsenite (NaAsO2)-induced damage to gastric and intestinal epithelium in rats.
Materials and Methods:
Control rats (Group I) were given a daily oral dose of corn oil. Rats in other groups were given a single dose of NaAsO2 (100 mg/kg; intraperitoneal) alone (Group II) or after pretreatment for 7 days with KV at 100 mg/kg (Group III) and 200 mg/kg (Group IV). Rats were sacrificed afterward and portions of the stomach, small intestine and colon were processed for histopathological examination. Hydrogen peroxide, reduced glutathione, malondialdehyde (MDA) concentrations as well as activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione S-transferase (GST) and myeloperoxidase (MPO) were measured in the remaining portions of the different gastrointestinal tract (GIT) segments.
Results:
NaAsO2 caused significant increases (P < 0.05) in MDA levels and MPO activity, with significant reductions (P < 0.05) in GST, GPX, CAT and SOD activities in the stomach and intestines. KV significantly reversed the changes (P < 0.05) in a largely dose-dependent manner. The different segments had marked inflammatory cellular infiltration, with hyperplasia of the crypts, which occurred to much lesser degrees with KV administration.
Conclusion:
The present findings showed that KV might be a potent product for mitigating NaAsO2 toxicity in the GIT.
Keywords: Chemoprevention, intestines, kolaviron, sodium arsenite, stomach
INTRODUCTION
Arsenic is a toxic metalloid (displaying some properties of both metal and a nonmetal), occurring widely in the air, water and soil, but commonly referred to as a heavy metal in toxicology.[1,2] Humans are exposed to a wide variety of inorganic forms including arsenates (AsO43-) or arsenites (AsO2-) in water, soil or food,[3] especially seafood, rice, mushrooms and poultry products[4,5] and drinking water contaminated with arsenic.[6,7]
Oral ingestion of sodium arsenite (NaAsO2) has been known to produce severe gastrointestinal symptoms, including abdominal pain, diarrhea, and nausea.[2,8] When arsenic is absorbed, it is distributed to all body tissues, after which it is reduced in vivo to trivalent arsenic which becomes methylated in the liver to methylarsonic acid and dimethylarsinic acid.[9] Inorganic arsenic is rapidly eliminated from the blood via renal elimination, although biliary excretion also does occur, creating the possibility of intestinal exposure to metabolites of arsenic.[10]
Arsenic has been primarily known to produce toxicity by interaction with enzymes and proteins containing sulfhydryl groups.[11] This interaction changes the redox status of these proteins and may cause alterations in their biological function. Arsenic also induces an increased formation of reactive oxygen (ROS) and nitrogen species, including the superoxide radical and hydroxyl radicals. An increase in lipid peroxidation as a result of the radicals causes production of several bioactive molecules of which malondialdehyde (MDA) and 4-hydroxynonenal are major examples.[2] Flavonoids are effective hydroxyl radical and peroxyl radical scavengers. They can also make complexes with metals and inhibit metal-initiating lipid peroxidation.[12] Plant polyphenols including flavonoids and carotenoids are usually not as well absorbed as low-molecular-weight antioxidants such as Vitamins C and E. Therefore, the concentrations of dietary flavonoids can be much higher in the lumen of the gastrointestinal tract (GIT) than that in the plasma or other body tissues, making their antioxidant effects more prominent in the gastrointestinal epithelium.[13] Flavonoids are also known to chelate free iron and copper which could otherwise increase ROS generation.[14] Several published reports suggest that arsenic causes damage to the body's anti-oxidative system and that this can be prevented by treatment with potential natural antioxidants.[15]
Kolaviron (KV) [Figure 1] is a fraction of the defatted ethanol extract of Garcinia kola, containing Garcinia bioflavonoids GB1, GB2, and Kolaflavanone.[16] The ability of KV to inhibit hydroxyl and superoxide radicals has been demonstrated.[17] Structurally, KV possesses many hydroxyl groups on the dual B rings [Figure 1], which confer on it the antioxidant and radical scavenging activities. KV has also been shown to offer protection against gastric ulcers[18] and ulcerative colitis.[19] The protective effects of KV against heavy metal intoxication, such as cadmium in the testes and spermatozoa has been demonstrated.[20]
Figure 1.
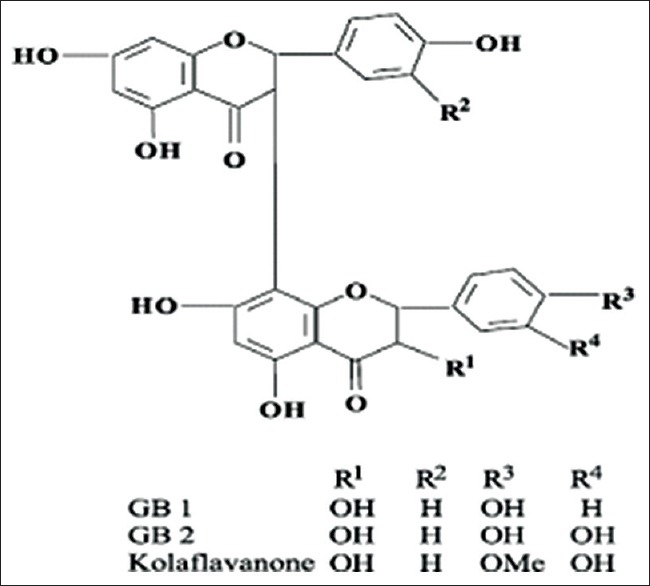
Structure of Kolaviron
Arsenic administered orally via drinking water or by oral gavage has been shown to induce oxidative stress and structural impairment in GIT and other tissues.[21,22] The oral route is also the major means of natural exposure to arsenic compounds. However, the intraperitoneal route is the most frequently used route of administration in toxicity studies involving arsenic in laboratory animals.[23]
This protocol will also enable the comparison of parenteral arsenic effects on the GIT with previous reports involving oral exposures in the literature.[22,24] To our knowledge, no study has been carried out to explore the protective role of KV against arsenic gastrointestinal toxicity. We therefore sought to investigate whether NaAsO2 administered intraperitoneally induces significant gastrointestinal injury and whether KV could offer protection to gastrointestinal tissues in arsenic-induced toxicity.
MATERIALS AND METHODS
Chemicals
NaAsO2, Epinephrine, glutathione, 5,5'- dithiobis-2-nitrobenzoic acid, hydrogen peroxide (H2 O2), thiobarbituric acid, and trichloroacetic acid, were purchased from Sigma Chemical (St. Louis, MO). All other reagents used were of analytical grade and were obtained from British Drug houses.
Animal protocol and experimental design
A total of 24 adult male Wistar rats weighing 100–120 g were obtained from the Experimental Animal Unit of Faculty of Veterinary Medicine, University of Ibadan, Nigeria. The animals were kept in plastic cages in a well-ventilated animal house under controlled light cycle (12 h light/12 h dark) and fed with commercial rat chow and water ad libitum. All of the animals received humane care according to the criteria outlined in the Guide for the Care and the Use of Laboratory Animals prepared by the National Academy of Science and published by the National Institute of Health. The ethics regulations were followed in accordance with national and institutional guidelines for the protection of the animals' welfare during experiments.[25] They were randomly divided into four groups of six animals each as follows:
Group I: Control rats receiving corn oil for 8 days
Group II: Rats treated with NaAsO2 at day 8
Group III: Rats pretreated with KV (100 mg/kg; per oral) for 8 days, followed by single administration of NaAsO2 (100 mg/kg; intraperitoneal) on the 8th day
Group IV: Rats pretreated with KV (200 mg/kg; per oral) for 8 days, followed by a single administration of NaAsO2 (100 mg/kg; intraperitoneal) on the 8th day.
Rats were sacrificed 24 h after the last administration by cervical dislocation, followed by removal of the gastrointestinal segments, stomach, small intestine and colon. The stomach was cut open along the greater curvature while the intestines were opened up along their entire lengths. Small portions of the different segments were cut for histopathological examination. The remaining portions were rinsed and homogenized using 50 mM Tris-HCl buffer (pH 7.4) containing 1.15% KCl. The homogenate was subjected to cold centrifugation at 4°C at a speed of 10,000x g for 15 min. The supernatant which was taken as the postmitochondrial fraction was used for the estimation of different biochemical parameters.
Biochemical assays
Protein concentration was determined by the Biuret method of Gornal et al.[26] H2 O2 generation was assessed by the method of Wolff.[27] MDA concentration as an index of lipid peroxidation was quantified according to the method described by Farombi et al.[28] Reduced glutathione (GSH) concentration was determined using the method of Jollow et al.[29] Catalase (CAT) activity using H2 O2 as substrate was measured by the method of Claiborne.[30] Superoxide dismutase (SOD) assay was carried out by the method of Misra and Fridovich[31] with slight modification in our laboratory.[32] Glutathione peroxidase (GPX) activity was measured by the method of Rotruck et al.[33] Glutathione S-transferase (GST) was estimated by the method of Habig et al.[34] using 1-chloro-2,4-dinitrobenzene as substrate. Myeloperoxidase (MPO) activity was determined by the method of Xia and Zweier.[35]
Histopathology
To examine the morphological changes associated with NaAsO2 administration in the mucosa of the GIT, small portions of the stomach, duodenum, ileum and colon were immediately fixed in 10% formal saline buffer, embedded in paraffin wax, and sections of 5–6 mm in thickness were made and thereafter stained with hematoxylin and eosin for histopathological examination according to Drury et al.[36] The sections were examined by light microscopy.
Statistical analysis
All values are expressed as mean ± standard deviation. The test of significance between two groups was estimated by Student's t-test using SPSS (student version 7.5; SPSS Inc., Surrey, UK). Values <0.05 were considered statistically significant.
RESULTS
Effects of Kolaviron on oxidative stress parameters and Myeloperoxidase activity in gastrointestinal tissues of sodium arsenite-treated rats
The protective effect of KV on NaAsO2 -induced lipid peroxidation in stomach and intestines is presented in Figure 2. Although NaAsO2 did not produce significant changes in MDA values when compared to control values, KV pretreatment, followed by the single dose of NaAsO2 produced significant reduction (P < 0.05) in lipid peroxidation, compared to both normal control and NaAsO2 control. This was indicated by reduced MDA level which was more obvious in the stomach and colon. The reduction in MDA was observed to be dose-dependent.
Figure 2.
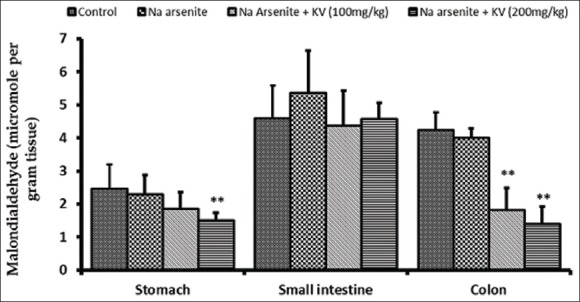
Effect of Kolaviron (KV) on malondialdehyde levels in the stomach and intestines of sodium arsenite-treated Wistar rats. Values are expressed as mean ± standard deviation of six rats *P < 0.05 by Student's t-test when the values of group II (Na arsenite) are compared with those of group I (control) **P < 0.05 by Student's t-test when the values of groups III (Na arsenite + 100 mg/kgKV) and IV (Na arsenite + 200 mg/kg KV) are compared with those of group II (Na arsenite)
As shown in Figure 3, MPO activity was found to be significantly increased (P < 0.05) in all the segments of the GIT (stomach, small intestine, and colon), compared to normal controls. However, KV pretreatment at the two doses tested produced a significant reduction (P < 0.05) in MPO activity when compared with NaAsO2 control. The higher dose of KV (200 mg/kg) produced a significant reduction in MPO activity even below that of normal control.
Figure 3.
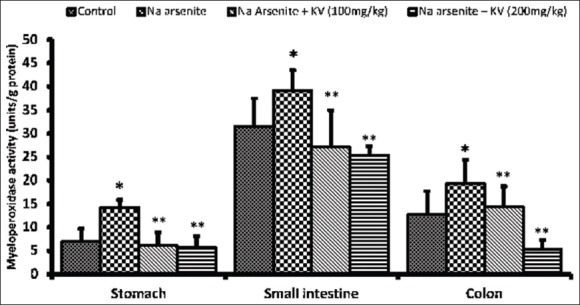
Effect of Kolaviron (KV) on myeloperoxidase activity in the stomach and intestines of Sodium arsenite-treated Wistar rats. Values are expressed as mean ± standard deviation of six rats *P < 0.05 by Student's t-test when the values of group II (Na arsenite) are compared with those of group I (control) **P < 0.05 by Student's t-test when the values of groups III (Na arsenite + 100 mg/kg KV) and IV (Na arsenite + 200 mg/kg KV) are compared with those of group II (Na arsenite)
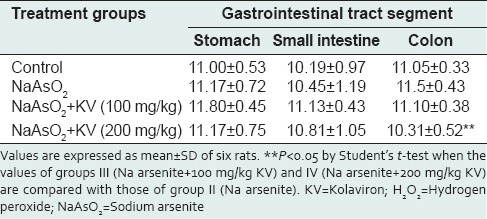
Hydrogen peroxide concentration was not significantly altered in all the different groups [Table 1]. KV at 200 mg/kg produced a significant reduction (P < 0.05) in H2 O2 in the colon. A reduction in H2 O2 levels was only observed in the colon at the higher dose of KV.
Table 1.
Effect of KV on H2O2 generation (micromole per minute per mg protein) in the stomach and intestines of NaAsO2-treated Wistar rats
Effects of Kolaviron on antioxidant systems in gastrointestinal tissues of sodium arsenite-treated rats
Glutathione S-transferase activity was significantly reduced (P < 0.05) in NaAsO2-treated rats in the stomach and intestine, whereas, the activity of GST increased significantly (P < 0.05) in animals pretreated with KV in a dose-dependent manner [Figure 4]. CAT activity was also found to be significantly reduced in the small intestine and colon, but not in the stomach upon NaAsO2 exposure, compared to normal controls [Figure 5]. Again, KV pretreatment caused significant increases (P < 0.05) in CAT activity in all the GIT segments compared to rats treated with NaAsO2 only.
Figure 4.
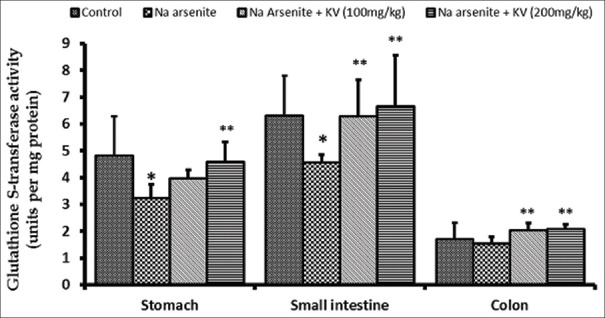
Effect of Kolaviron (KV) on Glutathione S-transferase activity in the stomach and intestines of sodium arsenite-treated Wistar rats. Values are expressed as mean ± standard deviation of six rats *P < 0.05 by Student's t-test when the values of group II (Na arsenite) are compared with those of group I (control) **P < 0.05 by Student's t-test when the values of groups III (Na arsenite + 100 mg/kgKV) and IV (Na arsenite + 200 mg/kg KV) are compared with those of group II (Na arsenite)
Figure 5.
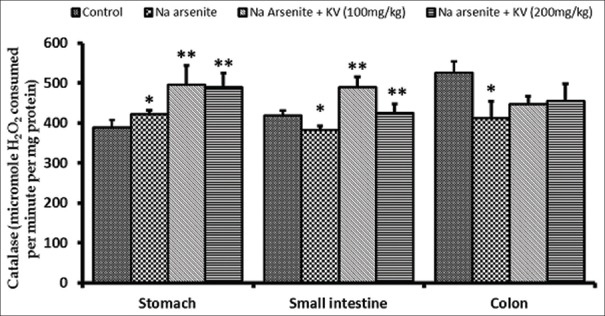
Effect of Kolaviron (KV) on Catalase activity in the stomach and intestines of sodium arsenite-treated Wistar rats. Values are expressed as mean ± standard deviation of six rats *P < 0.05 by Student's t-test when the values of group II (Na arsenite) are compared with those of group I (control) **P < 0.05 by Student's t-test when the values of groups III (Na arsenite + 100 mg/kg KV) and IV (Na arsenite + 200 mg/kg KV) are compared with those of group II (Na arsenite)
Neither NaAsO2 administered alone nor following KV pretreatment caused any significant alterations in GSH concentration [Table 2] in the different GIT segments. NaAsO2-treated animals showed a statistically significant reduction (P < 0.05) in GPX activity when compared with control animals [Table 3]. In a similar fashion to GST and CAT activities, KV produced a significant increase (P < 0.05) in GPX activity in animals treated with NaAsO2 following KV pretreatment. SOD activity in the stomach and small intestine was significantly enhanced (P < 0.05) by KV pretreatment when compared with animals treated with NaAsO2 only [Table 4].
Table 2.
Effect of KV on reduced GSH levels (micromole per gram tissue) in the stomach and intestines of NaAsO2-treated Wistar rats
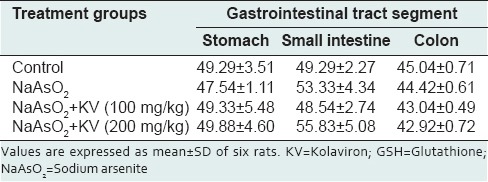
Table 3.
Effect of KV on GPx activity (units per mg protein) in the stomach and intestines of NaAsO2-treated Wistar rats
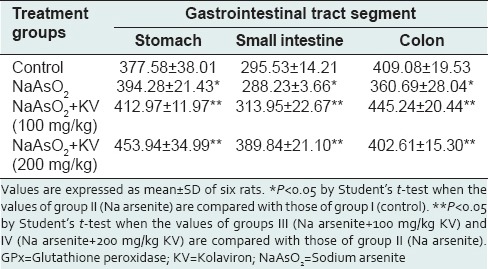
Table 4.
Effect of KV on SOD activity (units per mg protein) in the stomach and intestines of NaAsO2-treated Wistar rats
Microscopy
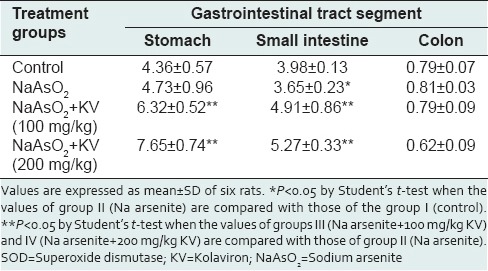
Microscopic examination of the gastrointestinal epithelium revealed considerable pathology associated with NaAsO2 exposure, much of which were reduced with KV pretreatment. Lesions in the stomach epithelium were predominantly infiltration of the mucosa and submucosa by inflammatory cells [Figure 6]. KV-treated rats, however, exhibited significantly lesser degrees of inflammatory cell infiltration. In the ileum and duodenum [Figures 7–9 respectively], NaAsO2 treatment-induced pronounced hyperplasia of the crypts and cells lining the glands at the base of the mucosa. Some of the nuclei appeared vesicular with increased nucleocytoplasmic ratio. In these GIT segments, there was also marked inflammatory cell infiltration as seen in the stomach of NaAsO2 treated rats. Some focal areas of erosion and ulceration were observed in the duodenum and ileum (not shown). However, with KV treatment, these lesions were considerable reduced.
Figure 6.
Photomicrographs showing the gastric mucosa of (a) control rats with predominantly normal parietal cells (black arrow) with only few inflammatory cells; (b) Sodium arsenite-treated rats, showing marked infiltration of the mucosa and submucosa with inflammatory cells; (c) rats pretreated with Kolaviron (KV) 100 mg/kg and (d) rats pretreated with KV 200 mg/kg, both showing only mild infiltration at the base of the mucosa
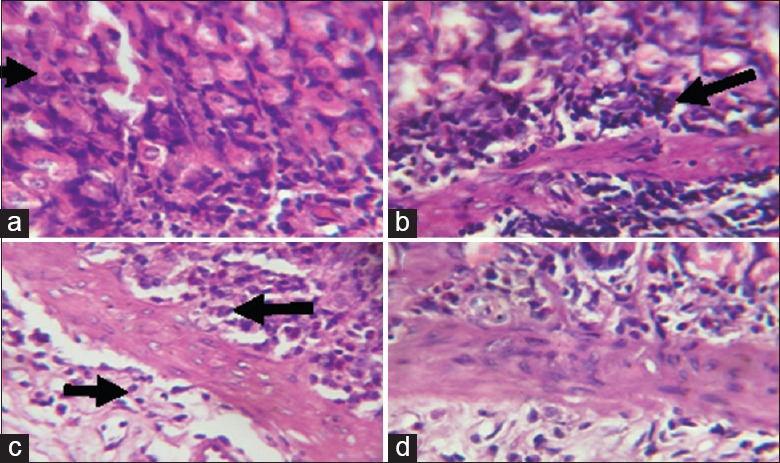
Figure 7.
Photomicrographs showing the duodenum of (a) control rats with normal villi (b) sodium arsenite-treated rats, disseminated inflammatory cell infiltration as well as marked hyperplasia of the cells lining the glands (blue arrow) (c) rats pretreated with Kolaviron (KV) 100 mg/kg, also showing hyperplasia of the glands and (d) rats pretreated with KV 200 mg/kg, showing only mild inflammatory cell infiltration
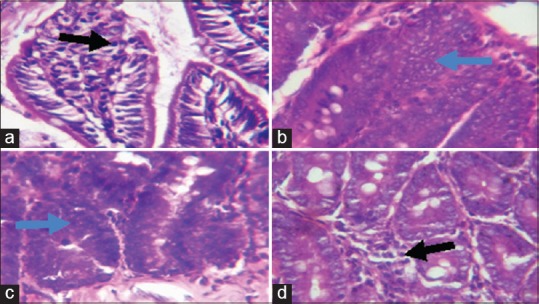
Figure 9.
Kolaviron protects against sodium arsenite-induced gastrointestinal toxicity
Figure 8.
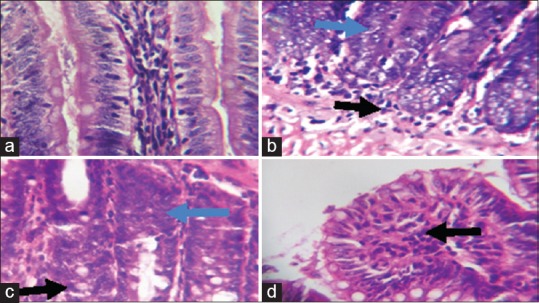
Photomicrographs showing the ileum of (a) control rats with well-arranged villi and few inflammatory cells; (b) sodium arsenite-treated rats, showing hyperplasia of cells lining the glands (blue arrow, some nuclei appearing vesicular as well as increased nucleocytoplasmic ratio and inflammatory cell infiltration; (c) rats pretreated with Kolaviron (KV) 100 mg/kg showing hyperplasia of the crypts and mild inflammatory cell infiltration, and (d) rats pretreated with KV 200 mg/kg, showing only mild infiltration at the base of the mucosa
DISCUSSION
This study was designed to investigate the nature of gastrointestinal involvement in NaAsO2 -induced toxicity, when the latter is administered intraperitoneally. We also examined the possibility of protection by KV on alterations produced. NaAsO2 exposure is known to produce clinical signs of gastrointestinal dysfunction including nausea, vomiting, and diarrhea while hemorrhagic gastrointestinal lesions have been reported in some animal studies.[2,37,38] Earlier studies in rats have shown that orally administered NaAsO2 in rats produced histological alterations in the gastric and intestinal epithelium with vacuolation and rupture and degeneration of villi and the gastric mucosa.[24] In this study, pretreatment with KV and eventual NaAsO2 administration did not cause any significant changes in the weights of the stomach, small intestine and colon in all the groups (values not presented).
Arsenic exposure has been associated with the production of reactive oxygen intermediates causing lipid peroxidation and cellular damage.[22] Our results indicated that there were no significant changes in H2 O2 generation and MDA concentration in rats treated with NaAsO2 alone compared to the control in all the segments of the GIT. MDA measured as an index of lipid peroxidation represents an important end product in the process. This finding from the present study may suggest that the single administration of NaAsO2 through the intraperitoneal route may not have caused significant alterations in these parameters to cause sufficient distribution of metabolites of NaAsO2 to the GIT.
However, it was observed that there was a significant increase in MPO activity in all the GIT segments with NaAsO2 exposure compared to the controls. MPO is the most abundant pro-inflammatory enzyme stored in the azurophilic granules of neutrophils. It catalyzes the formation of hypochlorus acid from H2 O2. Extracellular MPO activity gives an estimate of the oxidative stress in inflammatory diseases while intracellular MPO activity correlates well with tissue neutrophil content.[39]
Kolaviron appears to be a very potent gastrointestinal protective agent from the results of our study. Pretreatment of rats before NaAsO2 exposure caused significant reductions in MDA content and MPO activity in a largely dose-dependent manner compared to the rats given NaAsO2 alone. Its anti-inflammatory action on MPO activity appeared to be much more pronounced than its effect on MDA, and this was obtained in all of the GI segments. Results from this study support previous studies demonstrating the antioxidant and anti-inflammatory potentials of KV against GI mucosal injury.[18,19]
Increased MPO activity in this study correlates with the extent of inflammatory cell infiltration observed with histological examination in the different GI segments studied. One consistent feature of NaAsO2 exposure in the GIT was inflammatory cell infiltration of the mucosa and submucosa of the stomach, ileum, and colon. This points to a general inflammatory state induced by NaAsO2 exposure. In addition, there were areas of marked hyperplasia of the crypts and cells lining the glands at the base of the mucosa. These findings represent a generalized response to the inflammatory state of the tissues.[40] Crypt hyperplasia is caused by an increase in mitotic activity of crypt epithelial cells and is generally thought to be a compensatory response to toxicity in the gastrointestinal epithelium.[41] Sloughing of the villi and inflammatory cell infiltration were observed in broiler chicks fed 150 ppm of sodium arsenate.[42] It was, however, observed that rats pretreated with KV exhibited these pathologies only to mild degrees, with reductions in inflammatory cell infiltration and hyperplasia of crypts. It is noteworthy that KV at the higher dose exhibited greater protection against histopathological alterations.
Further protection of the GI tissues by KV obtained in this study included an enhancement of the activities of the antioxidant enzymes including SOD, CAT, GST, and GPX. SOD and CAT represent two basic components of the first line of defense against oxidative stress. While SOD converts superoxide radicals to H2 O2, CAT converts H2 O2 into water molecules.[43]
The reduction in the activities of SOD and CAT obtained in this study indicates increased production of superoxide radicals.[44] The GSH-related enzymes GST and GPX cause reduction in hydroperoxides within membranes in the presence of GSH. GSH itself is an important tri-peptide component of the cellular defense mechanism against peroxidative attacks.[43] Both GST and GPX activities were reduced with NaAsO2 exposure in this study. This could ordinarily be attributed to an inhibition of GSH, which is required as a co-factor for both enzymes. However, since levels of GSH measured in this study did not show significant alterations, the inhibition of GST and GPX could be due to other mechanisms. The presence of selenium in GPx, for example, could lead to an inhibition of its activity through the formation of arsenic-selenium complexes.[45] It is recognized that the primary biochemical mechanism of arsenic toxicity is binding of the metal to the sulfhydryl groups of proteins, resulting in the inhibition of numerous cellular enzyme systems.[46] The resulting cellular destruction of damaged thiol proteins may enhance production of toxic oxygen radicals.[47]
CONCLUSION
The present study gives evidence to alterations caused by NaAsO2 manifested mainly by an inflammatory response in virtually all the segments of the GIT, even when the compound was administered intraperitoneally. KV, however, showed very promising attributes as a protective agent against the NaAsO2 -induced alterations. The anti-inflammatory properties of KV in this study particularly warrant more comprehensive studies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mandal BK, Suzuki KT. Arsenic round the world: A review. Talanta. 2002;58:201–35. [PubMed] [Google Scholar]
- 2.Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, et al. Arsenic: Toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 3.Chou S, Harper C, Ingerman L, Llados F, Colman J, Chappell L, et al. Toxicological Profile for Arsenic. US Department of Health and Human Service, Agency for Toxic Substances and Disease Registry. 2007 [Google Scholar]
- 4.Jones FT. A broad view of arsenic. Poult Sci. 2007;86:2–14. doi: 10.1093/ps/86.1.2. [DOI] [PubMed] [Google Scholar]
- 5.Nepusz T, Petróczi A, Naughton DP. Food alert patterns for metal contamination analyses in seafoods: Longitudinal and geographical perspectives. Environ Int. 2009;35:1030–3. doi: 10.1016/j.envint.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Gebel T. Confounding variables in the environmental toxicology of arsenic. Toxicology. 2000;144:155–62. doi: 10.1016/s0300-483x(99)00202-4. [DOI] [PubMed] [Google Scholar]
- 7.Zaw M, Emett MT. Arsenic removal from water using advanced oxidation processes. Toxicol Lett. 2002;133:113–8. doi: 10.1016/s0378-4274(02)00081-4. [DOI] [PubMed] [Google Scholar]
- 8.Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J. 2003;79:391–6. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IPCS. Health Criteria and Other Supporting Information. 2nd ed. Vol. 2. Geneva: WHO; 1996. Guidelines for Drinking Water Quality. [Google Scholar]
- 10.Marquardt H, editor. Lehrbuch der Toxikologie, Spektrum Akademischer Verlag. Berlin: Heidelberg; 1997. p. 510. [Google Scholar]
- 11.Burchiel SW, Mitchell LA, Lauer FT, Sun X, McDonald JD, Hudson LG, et al. Immunotoxicity and biodistribution analysis of arsenic trioxide in C57Bl/6 mice following a 2-week inhalation exposure. Toxicol Appl Pharmacol. 2009;241:253–9. doi: 10.1016/j.taap.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendrich S, Wang GJ, Lin HK, Xu X, Tew BY, Wang HJ, et al. Isoflavone metabolism and bioavailability. In: Papas AM, editor. Antioxidant Status, Diet, Nutrition, and Health. Boca Raton, Fla: CRC Press; 1999. pp. 211–30. [Google Scholar]
- 13.Halliwell B, Zhao K, Whiteman M. The gastrointestinal tract: A major site of antioxidant action? Free Radic Res. 2000;33:819–30. doi: 10.1080/10715760000301341. [DOI] [PubMed] [Google Scholar]
- 14.Buettner GR. The pecking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–43. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 15.Flora SJ, Bhadauria S, Kannan GM, Singh N. Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: A review. J Environ Biol. 2007;28:333–47. [PubMed] [Google Scholar]
- 16.Iwu MM. Antihepatoxic constituents of Garcinia kola seeds. Experientia. 1985;41:699–700. doi: 10.1007/BF02007729. [DOI] [PubMed] [Google Scholar]
- 17.Farombi EO. Locally derived natural antioxidant substances in Nigeria: Potential role as new chemotherapeutic agents. In: Bahorun T, Gurib-Fakim A, editors. Molecular and Therapeutic Aspects of Redox Biochemistry. Ch. 16. London: OICA International (UK) Limited; 2003. pp. 207–26. [Google Scholar]
- 18.Onasanwo SA, Singh N, Olaleye SB, Palit G. Anti-ulcerogenic and proton pump (H+, K+ATPase) inhibitory activity of Kolaviron from Garcinia kola Heckel in rodents. Indian J Exp Biol. 2011;49:461–8. [PubMed] [Google Scholar]
- 19.Farombi EO, Adedara IA, Ajayi BO, Ayepola OR, Egbeme EE. Kolaviron, a natural antioxidant and anti-inflammatory phytochemical prevents dextran sulphate sodium-induced colitis in rats. Basic Clin Pharmacol Toxicol. 2013;113:49–55. doi: 10.1111/bcpt.12050. [DOI] [PubMed] [Google Scholar]
- 20.Farombi EO, Adedara IA, Akinrinde SA, Ojo OO, Eboh AS. Protective effects of kolaviron and quercetin on cadmium-induced testicular damage and endocrine pathology in rats. Andrologia. 2012;44:273–84. doi: 10.1111/j.1439-0272.2012.01279.x. [DOI] [PubMed] [Google Scholar]
- 21.Manna P, Sinha M, Sil PC. Arsenic-induced oxidative myocardial injury: Protective role of arjunolic acid. Arch Toxicol. 2008;82:137–49. doi: 10.1007/s00204-007-0272-8. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh M, Paul G. Intestinal dysfunction and alteration of various systemic and morphometric characters in albino rats (Charles foster) under stress of inorganic arsenic (IAS) compounds: A pilot study. Int J Pharm Bio Sci. 2013;4:1008–16. [Google Scholar]
- 23.Patlolla AK, Todorov TI, Tchounwou PB, van der Voet G, Centeno JA. Arsenic-induced biochemical and genotoxic effects and distribution in tissues of Sprague-Dawley rats. Microchem J. 2012;105:101–7. doi: 10.1016/j.microc.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemalatha P, Reddy AG, Rani MU, Anandkumar A, Shivakuma P. Arsenic-induced histological alterations in various organs in rats. Int J Life Sci Biotechnol Pharm Res. 2013;2:119–27. [Google Scholar]
- 25.PHS (PUBLIC HEALTH SERVICE) Public Health Service Policyon Humane Care and the Use of Laboratory Animals. Washington, DC: US Department of Health and Humane Services; 1996. pp. 99–158. [Google Scholar]
- 26.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–66. [PubMed] [Google Scholar]
- 27.Wolff SP. Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Method Enzymol. 1994;233:182–9. [Google Scholar]
- 28.Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron - A Garcinia kola seed extract. Food Chem Toxicol. 2000;38:535–41. doi: 10.1016/s0278-6915(00)00039-9. [DOI] [PubMed] [Google Scholar]
- 29.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–69. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 30.Claiborne A. Catalase activity. In: Greewald AR, editor. Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press; 1995. pp. 237–242. [Google Scholar]
- 31.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;25(247):3170–5. [PubMed] [Google Scholar]
- 32.Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O. Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol. 2014 Apr 5; doi: 10.1002/tox.21994. doi: 10.1002/tox.21994. [DOI] [PubMed] [Google Scholar]
- 33.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 34.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;25(249):7130–9. [PubMed] [Google Scholar]
- 35.Xia Y, Zweier JL. Measurement of myeloperoxidase in leukocyte-containing tissues. Anal Biochem. 1997;245:93–6. doi: 10.1006/abio.1996.9940. [DOI] [PubMed] [Google Scholar]
- 36.Drury R A, Wallington E A, Cancerson R. Carlton's Histopathological Techniques, fourth ed. Oxford/London/New York: Oxford UniversityPress; 1976. [Google Scholar]
- 37.Uede K, Furukawa F. Skin manifestations in acute arsenic poisoning from the Wakayama curry-poisoning incident. Br J Dermatol. 2003;149:757–62. doi: 10.1046/j.1365-2133.2003.05511.x. [DOI] [PubMed] [Google Scholar]
- 38.Vantroyen B, Heilier JF, Meulemans A, Michels A, Buchet JP, Vanderschueren S, et al. Survival after a lethal dose of arsenic trioxide. J Toxicol Clin Toxicol. 2004;42:889–95. doi: 10.1081/clt-200035344. [DOI] [PubMed] [Google Scholar]
- 39.Pulli B, Ali M, Forghani R, Schob S, Hsieh KL, Wojtkiewicz G, et al. Measuring myeloperoxidase activity in biological samples. PLoS One. 2013;8:e67976. doi: 10.1371/journal.pone.0067976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boudreau MD, Mellick PW, Olson GR, Felton RP, Thorn BT, Beland FA. Clear evidence of carcinogenic activity by a whole-leaf extract of Aloe barbadensis miller (aloe vera) in F344/N rats. Toxicol Sci. 2013;131:26–39. doi: 10.1093/toxsci/kfs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilgore SP, Sigel JE, Goldblum JR. Hyperplastic-like mucosal change in Crohn's disease: An unusual form of dysplasia? Mod Pathol. 2000;13:797–801. doi: 10.1038/modpathol.3880138. [DOI] [PubMed] [Google Scholar]
- 42.Khan A, Hussain HI, Sattar A, Khan MZ, Abbas RZ. Toxico-pathological aspects of arsenic in birds and mammals: A review. Int. J. Agric. Biol. 2014;16:1213–1224. [Google Scholar]
- 43.Muthumani M. Tetrahydrocurcumin Potentially Attenuates Arsenic Induced Oxidative Hepatic Dysfunction in Rats. J Clin. Toxicol. 2013;3:168. doi: 10.4172/2161-0495.1000. [Google Scholar]
- 44.Yamanaka K, Hasegawa A, Sawamura R, Okada S. Cellular response to oxidative damage in lung induced by the administration of dimethylarsinic acid, a major metabolite of inorganic arsenics, in mice. Toxicol Appl Pharmacol. 1991;108:205–13. doi: 10.1016/0041-008x(91)90111-q. [DOI] [PubMed] [Google Scholar]
- 45.Zeng H, Uthus EO, Combs GF., Jr Mechanistic aspects of the interaction between selenium and arsenic. J Inorg Biochem. 2005;99:1269–74. doi: 10.1016/j.jinorgbio.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Sqibb K S, Fowlar B A. The Toxicity of Arsenic and its Compounds. In: Fowlar B A, editor. Biological and Environmental Effects of Arsenic. New York: Elsevier; 1983. pp. 233–269. [Google Scholar]
- 47.Lee TC, Ho IC. Differential cytotoxic effects of arsenic on human and animal cells. Environ Health Perspect. 1994;102(Suppl 3):101–5. doi: 10.1289/ehp.94102s3101. [DOI] [PMC free article] [PubMed] [Google Scholar]


