Abstract
Background:
Benincasa hispida (BH) and Carissa congesta (CC) are regarded as ethnopharmacological imperative plants in Asian countries.
Objective:
Phytochemical screening of the extracts has shown the presence of steroids, flavonoids, saponins, glycosides, tannins, phenolic compounds, fixed oils, and fats in the BH and CC extracts. The presence of lupeol has been reported previously by us using high-performance thin-layer chromatography and high-performance liquid chromatography.
Materials and Methods:
Present research studies encompasses identification of chemical constituents in BH seeds and CC roots petroleum ether extracts by hyphenated technique such as gas chromatography-mass spectroscopy (MS) which when coupled gives a clear insight of constituents.
Results:
The components were identified by matching mass spectra with MS libraries. There were 13 and 10 different compounds analyzed from CC and BH, respectively. The components present were Pentanoic acid, 5-hydroxy, 2,4-butylphenyl; n-Hexadecanoic acid (Palmitic acid); Sulfurous acid, 2-ethylhexylhepatdecyl ester; n-Tridecane; 6-methyltridecane; (9E, 12E)-9,12-Octadecadienyl chloride, Hexadecanoic acid, 3-(trimethylsilyl)-oxy] propyl ester; 9,12-Octadecadenoic acid, 2 hydroxy-1-(hyroxymethylethyl) ester; 9,12-Octadecadienoic acid, 2,3 dihydroxypropyl ester; n-Propyl-9,12-Octadecadienoate, Lupeol; Taraxasterol; 6a, 14a-Methanopicene, perhydro-12,4a, 61a, 9,9,12a-hepatmethyl-10-hydoxy and 9-Octadecene; 2-Isoprpenyl-5-methyl-6-hepten-1-ol; n-Hexadecanoic acid, 2-hyroxy-1-(hydroxymethyl) ethyl ether; Butyl-9,12-Octadecadieonate; Friedoolean-8-en-3-one; friedours-7-en-3-one; 13,27-Cyclosuran-3-one; Stigmaste-7,25-dien-3-ol (3β, 5α); Stigmasta-7,16-dien-3-ol; chrondrillasterol in BH seeds and CC roots extracts respectively.
Conclusion:
Eluted components from the extracts could provide further researchers to work with various pharmacological activities related models and studies.
Keywords: Apocynaceae, Benincasa hispida, Carissa congesta, chrondrillasterol, Cururbitaceae, gas chromatography, mass spectroscopy, lupeol, Stigmaste-7, 25-dien-3-ol
INTRODUCTION
Discovery of natural plant-derived drugs from natural botanical herbarium has been the major breakthrough in paving the way for natural product chemistry.[1] Plants are common and important sources in contrast to marine organisms, microorganisms and fungi which are studied to the highest level in order their phytoconstituents.[2,3] But very few research has been done in the field of natural product chemistry, hence it is an indeed a need of an hour to explore these resources in search of new products which have significant pharmacotherapeutic potential as anticancer agents.[1,4,5,6,7] In recent times, the research on plants as medicines has been focusing on isolation and characterization of bioactive constituents and secondary metabolites.[8,9]
Plant kingdom exhibiting triterpenes in ample quantity shows structural multiplicity. Terpenoids have common biosynthetic origin based on isoprene units [CH2 = C (CH3)−CH = CH2 ] (1). Carbon skeletons in them are built from the union of two or more of C5 units. The classification is based on number of units as follows: C10 -Monoterpenoids (2 carbon atoms), C15 -Sesquiterpenoids (3 carbon atoms), C20 -Diterpenoids (4 carbon atoms), C30- Triterpenoids (6 carbon atoms) from squalene parent molecule and C40 -Tetraterpenoids (8 carbon atoms).
Pentacyclic triterpenes such as α- and β-amyrins and derived acids such as oleanolic acid and ursolic acid are found in waxy coatings of leaves and fruits like apple and pear as well as in resins, latex and bark of trees. Lupeol, betulin and betulinic acid of lupane class, oleanane or ursane groups have shown enormous pharmacological potential comprising of antiviral,[10,11,12] anti-inflammatory[13] and antitumor activity. Stigmasterol and its analogues have shown potentiality of acting as chemopreventive agent in cancer by inhibiting the cells in colon and breast cancer.[14,15,16,17,18,19,20,21,22]
During last few decades, infrared spectroscopy has been introduced as a very efficient and nondestructive analytical tool for the reliable identification of triterpenes.[23] Our previous studies[24,25] confirmed the presence of lupeol by high performance thin layer chromatography (HPTLC) and high performance liquid chromatography (HPLC) in Benincasa hispida (BH) and Carissa congesta (CC) petroleum ether plant extracts. Gas-liquid chromatography is an important hyphenated technique which provides an insight to qualitative, as well as quantitative estimation for volatile components such as terpenes.[1] In the present work, we identified and confirmed the structures of the different elucidated constituents by gas chromatography-mass spectroscopy (GC-MS) from the petroleum ether extracts of BH seeds and CC roots for the first time.
MATERIALS AND METHODS
Part A: Collection, authentication, extraction and preliminary phytochemical identification
Fresh selected parts of CC and BH were collected, authenticated, extracted, preliminary phytochemical constituents were recognized followed by HPTLC and HPLC of lupeol as reported by us.[24,25]
Part B: Gas chromatography-mass spectroscopy
Mobile phase: Petroleum ether (BH and CC) electron impact (EI)-MS Spectrum was scanned at 70 eV with instrument details as follows:
Model of MS: Joel
Model: Accu time of flight analyzer gross calorific value analyzer
Specification: Mass range-10–2000 amu and resolution-6000.
Make of GC: Agilent 7890
Detector: Flame ionization detector. Run time: 40 minutes
Gas chromatography-MS analysis was performed by splitless injection of 1.0 μL of the sample in petroleum ether on a Hewlett Packard 6890 (USA) gas chromatograph fitted with a cross-linked 5% phenyl methyl siloxane HP-5 MS capillary column (30 m × 0.32 mm × 0.25 μm coating thickness), coupled with a mass detector. GC-MS operating conditions were as follows: Injector temperature 200°C, transfer line 230°C, oven temperature program 60°C-280°C with ramping 5°C min-1, carrier gas: Helium at 1.5 mL min-1, mass spectra: EI+, ion source temperature: 280°C. Individual components were identified by NIST MS 2.0 f Structural Library.
RESULTS AND DISCUSSION
We have already reported BH and CC extracts yield in our previous studies.[24,25] In the present study, constituents such as lupeol and stigmasterol were eluted in GC-MS studies. The GC-MS analysis in the recent research studies reveals that the identified compounds belong to the class of triterpenoids has a potential pharmacological role.
The identified compounds are probably lipid soluble and found in the plant cell's cytoplasm. The general procedure for their extraction is using light petroleum ether or chloroform since these solvents help to separate the compounds by suitable chromatography techniques. GC is considered as a vital technique to interpret essential oils and volatile compounds.[1] In the current research study, various compounds [Tables 1 and 2] were elucidated by GC-MS technique from BH and CC petroleum ether extracts.[10,11,12,13,14,15,16,17,18,19,20,21,22] Mass spectrum peaks of BH and CC extracts [Figures 1 and 15].
Table 1.
Various components and their fragments in BH extract

Table 2.
Various components and their fragments in CC extract

Figure 1.

Mass spectrum of Benincasa hispida extract
Figure 15.

Mass spectrum of Carissa congesta extract
Pentanoic acid, 5-hydroxy-2,4-dibutylphenyl esters [Figure 2] [33.5%] with m/z 206 and fragment ions of 41, 57, 74, 91, 107, 115, 163, 175, 191 is seen most commonly from seeds of BH (Cururbitaceae). Main component elucidated was Palmitic acid or n-Hexadecanoic acid Figure 3 [86.6%] with m/z 256 is found commonly in plants and animals with fragment ions of 41, 43, 45, 57, 60, 67, 71, 73, 83, 87, 97, 101, 111, 115, 125, 129, 143, 157, 171, 185, 194, 199, 213, 227. Sulfurous acid, 2-Ethylhexylhepatdecyl ester Figure 4 with m/z 239 and fragment ions of 29, 41, 43, 48, 55, 64, 69, 71, 83, 97, 113, 127, 139, 155 along with 6-Methyltridecane Figure 5 as m/z 183 and Tridecane Figure 6 as m/z 184 with fragment ions of 27, 29, 33, 41, 43, 53, 55, 57, 69, 71, 77, 83, 85, 98, 111, 126, 140, 154, 169 and 27, 29, 41, 43, 53, 55, 57, 71, 77, 85, 99, 112, 126, 141, 154, respectively, are also seen prominently. 9,12-Octadeca-dienylchloride Figure 7 [9.97%] with m/z 263 and fragment ions of 41, 43, 55, 57, 65, 67, 71, 77, 79, 81, 87, 95, 109, 121, 135, 149, 155, 163, 177, 185 is also characterized. Hexadecanoic acid, 3-(trimethylsilyl) oxy] propyl ester Figure 8 having m/z 371 with fragment ions of 41, 43, 55, 59, 67, 69, 73, 81, 85, 95, 109, 117, 130, 149, 190, 203, 239, 267, 315 and 9,12-Octadecadenoic acid, 2-Hydroxy-1-(hyroxymethylethyl) ester Figure 9 having m/z 354 with fragment ions of 27, 29, 31, 39, 41, 43, 45, 47, 51, 55, 57, 61, 67, 69, 71, 77, 79, 81, 95, 109, 121, 127, 135, 145, 149, 163, 166, 171, 177, 182, 185, 191, 196, 205, 210, 220, 234, 245, 262, 280, 305, 336 are also characterized. 9,12-Octadecadenoic acid, 2,3-Dihydroxypropyl ester Figure 10 having m/z 354 with fragment ions of 27, 29, 31, 39, 41, 43, 45, 47, 51, 55, 57, 61, 67, 69, 71, 75, 79, 81, 93, 95, 109, 121, 135, 149, 164, 171, 177, 182, 191, 205, 210, 225, 234, 237, 245, 251, 262, 280, 297, 305, 321, 336 is seen. Another compound called n-propyl-9,12-octadecadienoate Figure 11 having m/z 322 with fragment ions of 51, 55, 57, 61, 65, 67, 71, 77, 81, 93, 95, 101, 109, 123, 127, 133, 135, 149, 164, 178, 191, 205, 210, 234, 245, 263, 279 was obtained. Lupeol Figure 12 having m/z 426 with fragment ions of 41, 43, 53, 55, 57, 59, 65, 68, 71, 77, 79, 81, 83, 85, 91, 95, 97, 99, 109, 121, 135, 147, 161, 175, 189, 203, 207, 218, 234, 247, 257, 272, 286, 299, 315, 325, 344, 357, 370, 383, 393, 411 and Taraxasterol Figure 13 having m/z 426 with fragmentions of 107, 121, 135, 147, 161, 175, 189, 207, 218, 229, 243, 257, 272, 287, 299, 344, 357, 365, 393, 408. 6a, 14a-Methanopicene Figure 14 having m/z 426 with fragment ions of 29, 43, 45, 53, 55, 57, 60, 67, 69, 71, 77, 81, 83, 85, 93, 95, 97, 99, 109, 123, 135, 138, 161, 175, 190, 205, 208, 221, 229, 245, 257, 271, 288, 297, 303, 309, 315, 323, 329, 341, 351, 357, 366, 381, 399, 411 was also present. All the components and their fragments are depicted in Table 1.
Figure 2.
Mass spectrum showing presence of 2,4-di-tert-butylphenyl 5-hydroxypentanoate (2) in Benincasa hispida extract
Figure 3.
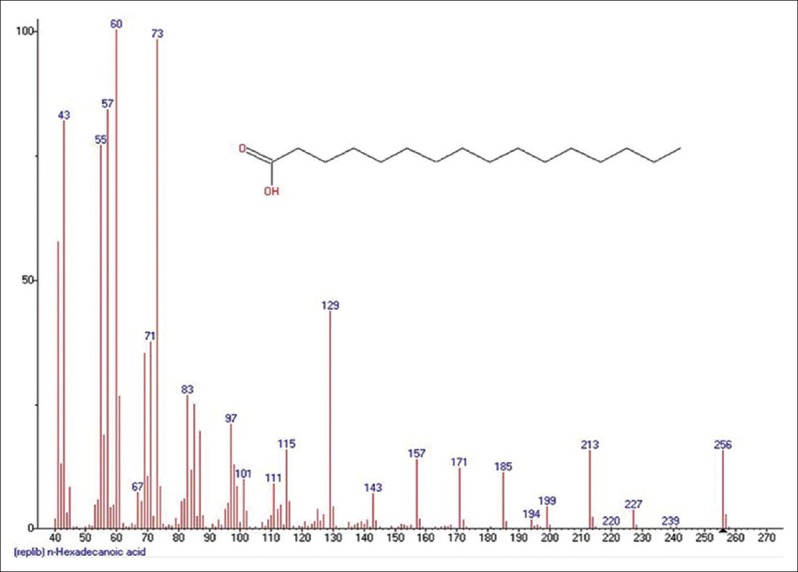
Mass spectrum showing presence of Palmitic acid (3) in Benincasa hispida extract
Figure 4.

Mass spectrum showing presence of 2-Ethylhexyl hepatdecyl sulfite (4) in Benincasa hispida extract
Figure 5.

Mass spectrum showing presence of 6-Methyltridecane (5) in Benincasa hispida extract
Figure 6.
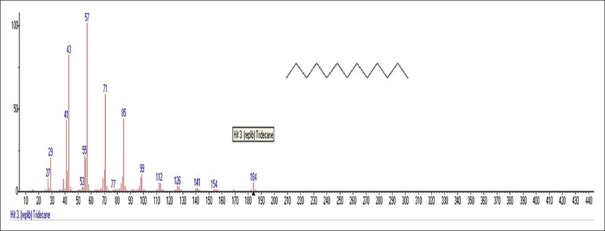
Mass spectrum showing presence of Tridecane (6) in Benincasa hispida extract
Figure 7.
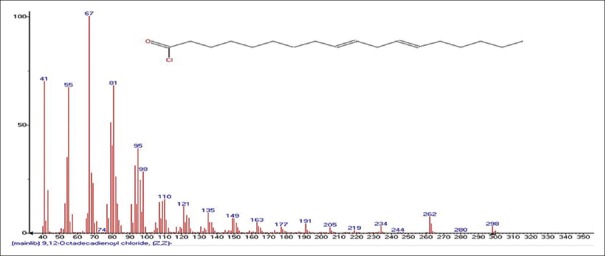
Mass spectrum showing presence of 9,12-Octadeca-9,12-dienoyl chloride (7) in Benincasa hispida extract
Figure 8.

Mass spectrum showing presence of Hexadecanoic acid, 3-(trimethylsilyl)oxy]propyl ester (8) in Benincasa hispida extract
Figure 9.

Mass spectrum showing presence of 9,12-Octadecadenoic acid (Z,Z), 2-Hydroxy-1-(hyroxymethylethyl) ester (9) in Benincasa hispida extract
Figure 10.

Mass spectrum showing presence of 9,12-Octadecadenoic acid (Z,Z), 2,3-dihydroxy propyl ester (10) in Benincasa hispida extract
Figure 11.

Mass spectrum showing presence of n-propyl-9,12-octadecadienoate (11) in Benincasa hispida extract
Figure 12.

Mass spectrum showing presence of lupeol (12) in Benincasa hispida extract
Figure 13.

Mass spectrum showing presence of Taraxasterol (13) in Benincasa hispida extract
Figure 14.

Mass spectrum showing presence of 6a,14a-Methanopicene (14) in Benincasa hispida extract
Carissa congesta (Apocynaceae) showed the presence of 9-Octadecene Figure 16 [4.62%] having m/z 139 with fragment ions of 41, 43, 44, 50, 53, 55, 57, 58, 66, 67, 69, 70, 71, 74, 79, 81, 83, 84, 85, 91, 95, 97, 98, 99, 105, 111, 125 and 2-Isopropenyl-5-methyl-6-hepten-1-ol Figure 17 [8.64%] having m/z 168 with fragment ions of 41, 43, 45, 51, 53, 55, 57, 68, 71, 77, 79, 81, 86, 91, 93, 95, 97, 99, 107, 122, 135, 150, 153 were characterized. Hexadecanoic acid, 2-hydroxyl-1-(hydroxymethyl) ethyl ether Figure 18 [13.9%] having m/z 330 with fragment ions of 43, 57, 69, 74, 84, 98, 112, 134, 154, 168, 182, 194, 213, 239, 257, 270, 299, 312 along with Butyl-9,12-octadecadieonate Figure 19 (8.45%) having m/z 336 with fragment ions of 55, 60, 67, 73, 81, 95, 109, 123, 135, 150, 164, 178, 191, 205, 220, 234, 243, 263, 279 were also characterized. Friedoolean-8-en-3-one Figure 20 having m/z 424 with fragment ions of 121, 135, 149, 159, 173, 206, 218, 239, 245, 257, 300, 409 was also seen. Friedours-7-en-3-one Figure 21 having m/z 424 with fragment ions of 109, 123, 133, 147, 159, 173, 187, 205, 218, 227, 245, 257, 271, 285, 298, 313, 325, 357, 367, 408 and 13,27-Cyclosuran-3-one Figure 22 having m/z 424 with fragment ions of 27, 29, 31, 41, 45, 49, 53, 55, 57, 60, 67, 69, 71, 73, 77, 79, 81, 83, 85, 93, 95, 97, 99, 109, 123, 135, 138, 148, 161, 177, 189, 206, 218, 231, 245, 257, 271, 286, 299, 313, 327, 339, 364, 367, 381, 396, 408 were characterized. 3 β,5α-Stigma-7,25-dien-3-ol Figure 23 having m/z 412 with fragment ions of 41, 43, 55, 57, 69, 81, 95, 99, 107, 119, 133, 147, 159, 173, 201, 213, 231, 246, 255, 271 and 3 β,5α-Stigma-7,16-dien-3-ol Figure 24 having m/z 412 with fragment ions of 28, 43, 55, 57, 69, 71, 81, 85, 95, 107, 119, 133, 147, 161, 173, 187, 201, 213, 231, 246, 255, 271, 283, 300, 314, 369, 397 were characterized along with chrondrillasterol Figure 25 with fragment ions of 43, 55, 57, 69, 71, 81, 91, 93, 107, 119, 133, 147, 161, 173, 187, 201, 213, 219, 238, 246, 255, 271, 285, 300, 351, 369, 379, 397 were also obtained as some of the compounds. The elucidated important compounds are depicted in graphical abstract with Figure 26.
Figure 16.
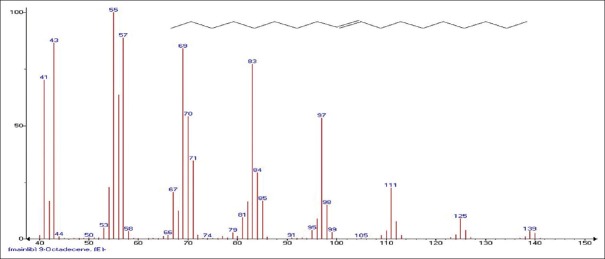
Mass spectrum showing presence of 9-Octadecene (15) in Carissa congesta extract
Figure 17.

Mass spectrum showing presence of 2-Isopropenyl-5-methyl-6-hepten-1-ol (16) in Carissa congesta extract
Figure 18.
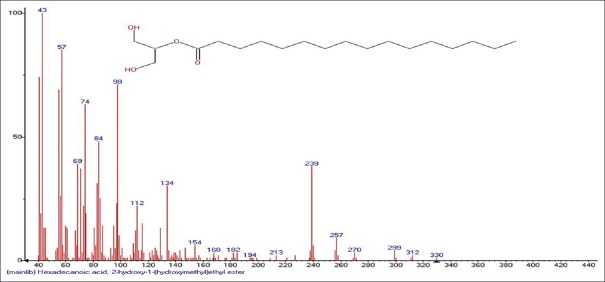
Mass spectrum showing presence of 2-Hydroxy-1-(hydroxymethyl)ethyl ether (17) in Carissa congesta extract
Figure 19.

Mass spectrum showing presence of Butyl 9,12-octadecadieonate (18) in Carissa congesta extract
Figure 20.

Mass spectrum showing presence of Friedoolean-8-en-3-one (19) in Carissa congesta extract
Figure 21.
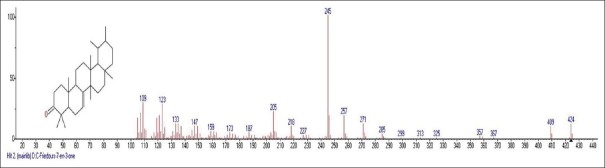
Mass spectrum showing presence of Friedours-7-en-3-one (20) in Carissa congesta extract
Figure 22.

Mass spectrum showing presence of 13,27-Cyclosuran-3-one (21) in Carissa congesta extract
Figure 23.
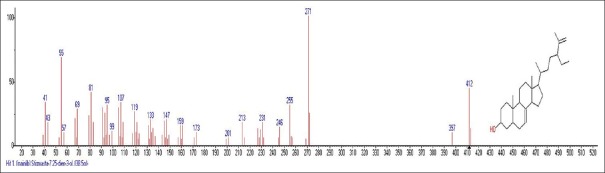
Mass spectrum showing presence of (3 β,5α)-Stigma-7,25-dien-3-ol (22) in Carissa congesta extract
Figure 24.

Mass spectrum showing presence of (3 β,5α)-Stigma-7,16-dien-3-ol (23) in Carissa congesta extract
Figure 25.
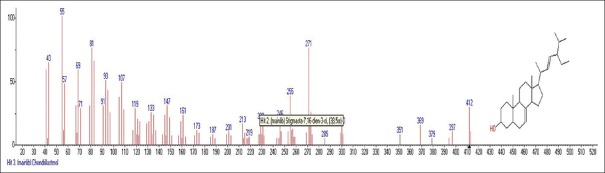
Mass spectrum showing presence of chrondrillasterol (24) in Carissa congesta extract
Figure 26.
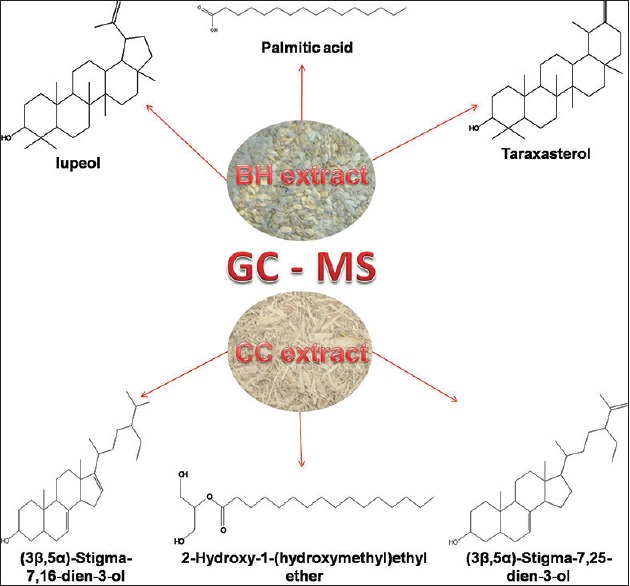
Graphical Abstract
ACKNOWLEDGMENT
We hereby acknowledge the management of VESCOP for providing all facilities to carry out this work. We would also like to acknowledge Indian Institute of Technology, Mumbai, for their help in GC-MS analysis on special discounted rates.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 3rd ed. New Delhi, India: Springer (India) Private Limited Publication House; 2005. pp. 1–36. [Google Scholar]
- 2.Iwashina T. The structure and distribution of the flavonoids in plants. J Plant Res. 2000;113:287–99. [Google Scholar]
- 3.Samuelsson G. Drugs of Natural Origin: A Textbook of Pharmacognosy. 5th ed. Sweden, Stockholm: Swedish Pharmaceutical Press; 2004. pp. 154–5. [Google Scholar]
- 4.Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17:215–34. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 5.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141–53. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 6.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 7.van Der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R. The Catharanthus alkaloids: harmacognosy and biotechnology. Curr Med Chem. 2004;11:607–28. doi: 10.2174/0929867043455846. [DOI] [PubMed] [Google Scholar]
- 8.Hanson JR. Natural Products, the Secondary Metabolites. Cambridge, United Kingdom: The Royal Society of Chemistry; 2003. The biosynthesis of secondary metabolites; pp. 112–1. [Google Scholar]
- 9.Douglas Kinghorn A. Pharmacognosy in the 21st century. J Pharm Pharmacol. 2001;53:135–48. doi: 10.1211/0022357011775334. [DOI] [PubMed] [Google Scholar]
- 10.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 11.Barroso-González J, El Jaber-Vazdekis N, García-Expósito L, Machado JD, Zárate R, Ravelo AG, et al. The lupane-type triterpene 30-oxo-calenduladiol is a CCR5 antagonist with anti-HIV-1 and anti-chemotactic activities. J Biol Chem. 2009;284:16609–20. doi: 10.1074/jbc.M109.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian K, Yu D, Chen CH, Huang L, Morris-Natschke SL, Nitz TJ, et al. Anti-AIDS agents 78. Design, synthesis, metabolic stability assessment, and antiviral evaluation of novel betulinic acid derivatives as potent anti-human immunodeficiency virus (HIV) agents. J Med Chem. 2009;52:3248–58. doi: 10.1021/jm900136j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deepak M, Handa SS. Antiinflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother Res. 2000;14:463–5. doi: 10.1002/1099-1573(200009)14:6<463::aid-ptr611>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Nguemfo EL, Dimo T, Dongmo AB, Azebaze AG, Alaoui K, Asongalem AE, et al. Anti-oxidative and anti-inflammatory activities of some isolated constituents from the stem bark of Allanblackia monticola Staner L.C (Guttiferae) Inflammopharmacology. 2009;17:37–41. doi: 10.1007/s10787-008-8039-2. [DOI] [PubMed] [Google Scholar]
- 15.Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, et al. Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res. 1998;58:717–23. [PubMed] [Google Scholar]
- 16.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control. 1991;2:325–57. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Kohno H, Tsukio Y, Honjo S, Tanino M, Miyake M, et al. Citrus limonoids obacunone and limonin inhibit azoxymethane-induced colon carcinogenesis in rats. Biofactors. 2000;13:213–8. doi: 10.1002/biof.5520130133. [DOI] [PubMed] [Google Scholar]
- 18.Oliveria EM, Freitas SL, Martins FS, Couto RO, Pinto MV, Paula JR, et al. Isolation and quantitative HPLC-PDA analysis of lupeol in phytopharmaceutical intermediate products from Vernonanthura ferruginea (Less.) Quim Nova. 2012;35:1041–5. [Google Scholar]
- 19.Gallo MB, Sarachine MC. Biological activities of lupeol. Int J Pharm Biomed Sci. 2009;31:46–62. [Google Scholar]
- 20.Raphael TJ, Kuttan G. Effect of naturally occurring triterpenoids glycyrrhizic acid, ursolic acid, oleanolic acid and nomilin on the immune system. Phytomedicine. 2003;10:483–9. doi: 10.1078/094471103322331421. [DOI] [PubMed] [Google Scholar]
- 21.Fulda S, Kroemer G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today. 2009;14:885–90. doi: 10.1016/j.drudis.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Saleem M, Murtaza I, Tarapore RS, Suh Y, Adhami VM, Johnson JJ, et al. Lupeol inhibits proliferation of human prostate cancer cells by targeting beta-catenin signaling. Carcinogenesis. 2009;30:808–17. doi: 10.1093/carcin/bgp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dehelean CA, Cîntă PS, Peev CI, Soica C, Antal DS. Characterization of birch tree leaves buds and bark dry extracts with antitumor activity. J Optoelectron Adv Mater. 2007;9:83–787. [Google Scholar]
- 24.Doshi GM, Chaskar PK, Zine SP, Une HD. Cold extraction of Carissa congesta Wight monitored by a comparative revision of HPLC and HPTLC. Pharmacogn Commun. 2014;4:29–33. [Google Scholar]
- 25.Doshi GM, Une HD. Chromatographic studies on Benincasa hispida (thunb.) Cogn Seed extract scrutinized by HPLC and HPTLC. Pharmacogn J. 2014;6:42–8. [Google Scholar]



