Abstract
Caffeic acid phenethyl ester (CAPE), a naturally occurring compound isolated from propolis extract, has been reported to have a number of biological and pharmacological properties, exerting antioxidant, anti-inflammatory, anticarcinogenic, antibacterial and immunomodulatory effects. Recent in vivo and in vitro study findings have provided novel insights into the molecular mechanisms involved in the anti-inflammatory and immunomodulatory activities of this natural compound. CAPE has been reported to have anti-inflammatory properties involving the inhibition of certain enzyme activities, such as xanthine oxidase, cyclooxygenase and nuclear factor-κB (NF-κB) activation. Since inflammation and immune mechanisms play a crucial role in the onset of several inflammatory diseases, the inhibition of NF-κB represents a rationale for the development of novel and safe anti-inflammatory agents. The primary goal of the present review is to highlight the anti-inflammatory and immunomodulatory activities of CAPE, and critically evaluate its potential therapeutic effects.
Keywords: caffeic acid phenethyl ester, anti-inflammatory activity, immunomodulatory effect
1. Introduction
Caffeic acid phenethyl ester (CAPE) is an important active component of honeybee propolis extract and has been used in traditional medicine for a number of years. CAPE is a polyphenol that contains hydroxyl groups within a catechol ring, the molecular formula of CAPE is C!17H16O4 (1,2) (Fig. 1). It has been shown that this active component of propolis possesses anti-inflammatory, immunomodulatory, antineoplastic, antioxidant and wound-healing properties (1–4). Inflammation is induced by the release of chemical mediators from damaged tissue and migratory cells. Mediators identified in the inflammatory process include biogenic amines, metabolites of arachidonic acid (eicosanoids), platelet aggregation factors, cytokines [interleukins (ILs) and tumor necrosis factor-α (TNF-α)] and free oxygen radicals. These substances are produced by inflammatory cells, such as polymorphonuclear leukocytes (neutrophils, eosinophils and basophils), endothelial cells, mast cells, macrophages, monocytes and lymphocytes (5,6). CAPE inhibits cytokine and chemokine production, the proliferation of T cells and lymphokine production, and thus suppresses the inflammatory process. Specifically, CAPE is a potent and a specific inhibitor of nuclear factor-κB (NF-κB) activation, and this may provide the molecular basis for its multiple anti-inflammatory and immunomodulatory activities (2,7). The aim of this review is to highlight the anti-inflammatory and immunomodulatory activities of CAPE, focusing on the mechanisms of action (already identified) underlying this activity.
Figure 1.
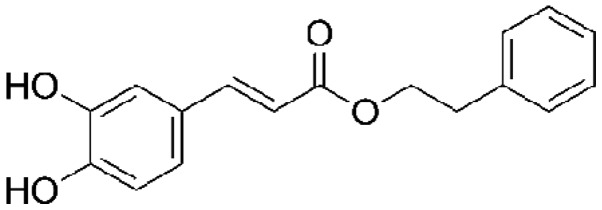
Chemical structure of caffeic acid phenethyl ester.
2. Overview to the inflammatory response
Inflammation is an immunological response to pathogens and damage that is initiated to protect the body, and contributes to physiological and pathological processes, such as wound healing and infection at the compromised site. The process is accompanied by adhesion, migration and chemotaxis of leukocytes to the inflammatory environment (6). In response to tissue injury, a multifactorial network of chemical signals initiates and maintains a host response designed to ‘heal’ the afflicted tissue. The first effectors recruited in the acute inflammatory response are neutrophils. These are followed by monocytes, which undergo differentiation in the tissue into macrophages and migrate to the site of tissue injury under the guidance of chemotactic factors (5,8). The activated leukocytes provide proinflammatory cytokines, reactive oxygen species (ROS) and matrix metalloproteinases to remove the invading pathogen (9). The pathogens and damaged tissue are then phagocytosed, and the inflammatory process is eventually terminated when lipoxins start to overrule the proinflammatory signals (10). In general, IL-1 and TNF target the endothelium and initiate the inflammatory mediator cascade following exposure to certain stimuli, including infection, trauma, ischemia, immune-activated T cells or toxins. The inflammatory cascade can be summarized as follows: i) Activation of inflammatory cytokine-secreting cells, and increases in the levels of proinflammatory cytokines, such as IL-1, TNF-α and interferon-γ (IFN-γ); ii) activation/synthesis of phospholipase A2, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS); increased endothelial adhesion molecules and synthesis of chemokines; iii) increased platelet-activating factor (PAF), leukotriene, prostanoid (prostaglandin E2) and NO levels, neutrophil endothelial adhesion, and the migration and activation of neutrophils; and iv) inflammation, tissue destruction and loss of function (10–12).
3. Proinflammatory cytokines and signaling pathways
Cytokines are polypeptide regulators of host responses to infection, immune responses, inflammation and trauma. Cytokine secretion by immune cells has a pivotal role in directing the course of an inflammatory response. Cellular cytokine production is regulated at transcriptional and translational levels and via cell signaling (11). Certain cytokines act to enhance the effects of the disease (proinflammatory), whereas others are involved in reducing inflammation and promoting healing (anti-inflammatory). Methods to block potentially harmful cytokines, particularly during an overwhelming infection, have been an area of particular interest. Administration of the proinflammatory cytokines IL-1 and TNF-α to humans can lead to fever, inflammation, tissue destruction and, in certain cases, shock and mortality (9,12). TNF-α is a ‘master regulator’ among cytokines and is responsible for mediating the inflammatory responses and innate immunity. The major pathways activated by TNF-α include caspases, NF-κB and mitogen-activated protein kinases (MAPKs). Crosstalk between these signaling pathways plays a role in determining the physiological outcome of the responses to TNF-α (13). The network response is further complicated by the phases associated with TNF-α signaling: In the early phase, TNF-α signaling induces the expression of inflammatory cytokines; this then initiates a secondary cytokine-mediated cellular response that contributes to the biological activity of TNF-α (14).
The transcription factor NF-κB plays a central role in regulating inflammatory, immune and anti-apoptotic responses. It is composed of homodimers and heterodimers of the Rel family of proteins, including p65/RelA, RelB, c-Rel, p50/p105 and p52/p100 (15,16). The activation of inactive NF-κB proteins existing in the cytoplasm is induced by numerous factors, including inflammatory cytokines (IL-1 and TNF-α), bacterial products and protein synthesis inhibitors (17); therefore, agents that can downregulate the activation of NF-κB have potential for therapeutic interventions, whereas the activation of NF-κB promotes inflammation in animals. The binding of TNF-α to cell surface receptors engages multiple signal transduction pathways, including three groups of MAPKs: Extracellular-signal-regulated kinases, c-Jun N-terminal kinases and p38 MAPKs. These MAPK signaling pathways induce a secondary response by increasing the expression of several inflammatory cytokines that contribute to the biological activity of TNF-α. MAPKs, therefore, function both upstream and downstream of signaling by TNF-α receptors (13,18). In almost all cell types, the exposure of the cells to TNF-α induces the activation of NF-κB and leads to the expression of a range of genes associated with inflammation. NF-κB is a protein complex that controls the transcription of DNA and is a central regulator of cellular stress in all cell types in humans. NF-κB plays a key role in regulating the immune response to infection and in acute and chronic inflammation. The activation of NF-κB in rats can induce the expression of IL-1β, which increases the expression of proinflammatory molecules (17,19).
4. Anti-inflammatory effects of CAPE
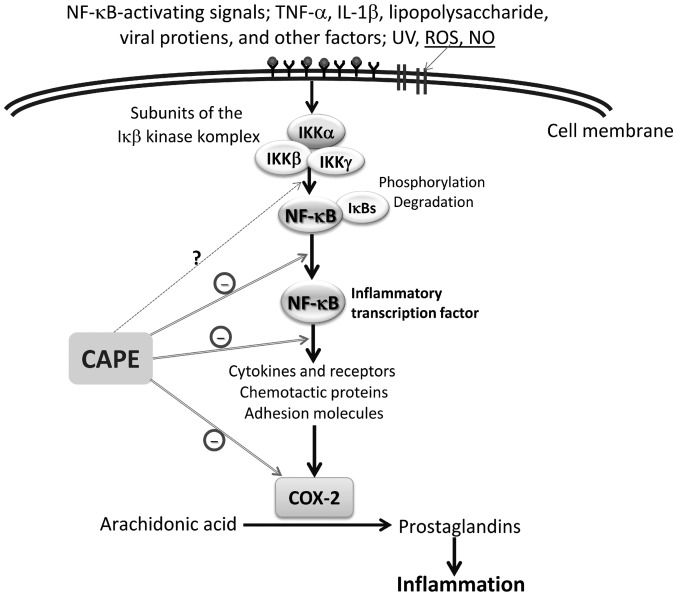
The transcription factor NF-κB has a pivotal role in a variety of physiological processes throughout the body, including immune responses, cell proliferation and inflammation. NF-κB elicits its effects by promoting the transcription of a range of cytokines, enzymes, chemokines and antiapoptotic and cell growth factors (20). Several in vitro and in vivo studies have described diverse biological activities of CAPE (at micromolar concentrations), such as a specific inhibition of NF-κB and a suppression of the lipoxygenase pathway of arachidonic acid metabolism during inflammation (2–4). It has also been shown that CAPE acts to suppress the NF-κB activation induced by ROS-generating agents in human histiocytic and coronary artery endothelial cells (2). It is believed that, rather than preventing the degradation of κB inhibitor-α (IκB-α), CAPE suppresses NF-κB activation by inhibiting the interaction between NF-κB proteins and DNA (21) (Fig. 2). Ilhan et al (22) suggested that the anti-inflammatory effect of CAPE is most likely due to the inhibition of ROS production at the transcriptional level, through the suppression of NF-κB activation, and the direct inhibition of the catalytic activity of iNOS. Toyoda et al (23) reported that CAPE treatment inhibited Helicobacter pylori-induced NF-κB activation via the suppression of IκB-α degradation and the phosphorylation of p65 in a gastric cancer cell line. Furthermore, the results clearly demonstrated that the mRNA expression levels of inflammatory factors known to be induced by NF-κB transcriptional activation, such as TNF-α, IFN-γ, IL-2, IL-6, iNOS and KC, an IL-8 homologue chemokine, were all significantly decreased by CAPE treatment in the pyloric mucosa of H. pylori-infected Mongolian gerbils (23,24). Colonization of gastric epithelial cells with H. pylori induces NF-κB and results in the increased production of the proinflammatory cytokines TNF-α, IL-1, IL-6 and IL-8, all of which are regulated by NF-κB (25,26). It has also been demonstrated that local administration of CAPE leads to increased levels of leukocyte apoptosis and marked reductions in the concentrations of leukocytes, neutrophils and monocytes in the inflammatory site exudate. Furthermore, CAPE decreases the levels of cytosolic IκB-α and increases the nuclear translocation of p65 (27).
Figure 2.
Schematic presentation of the anti-inflammatory effects of CAPE in the inhibition of inflammation. COX-2, cyclooxygenase-2; IκB-α, κB inhibitor-α; IKK, IκB-kinase; IL-1β, interleukin-1β; NF-κB, nuclear factor κB; NO, nitric oxide; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α; UV, ultraviolet.
CAPE possesses strong antioxidant, anti-inflammatory and healing properties, and its effects on the wound healing have been attributed to the inhibition of NF-κB (28,29). Consistent with these two cited studies, Santos et al (30) reported that treatment with CAPE enhanced wound healing, particularly wound healing following burns; decreased inflammatory parameters and oxidative damage; and inhibited the activity of cyclooxygenase and lipooxygenase. Under most inflammatory conditions, such as in thermal injury, NO production is enhanced. In addition to performing histological and biochemical analyses, Santos et al (30) evaluated the anti-cluster of differentiation 68 (CD68) and NO levels, as well as myeloperoxidase (MPO) activity. CAPE exhibited an anti-inflammatory action on rat burn healing by reducing MPO activity, NO levels and the number of CD68-positive cells (30). Khan et al (31) demonstrated that CAPE reduced neurovascular inflammation and protected the rat brain following transient focal cerebral ischemia by downregulating NF-κB and certain mediators, such as cytokines and iNOS (31).
CAPE classically exerts anti-inflammatory effects by reducing prostaglandin and leukotriene synthesis. Furthermore, it has been suggested that the anti-inflammatory action exhibited by CAPE is a result of the inhibition of arachidonic acid release from the cell membrane. As a consequence of this inhibition, the activity of COX-1 and −2 and the activation of the COX-2 gene expression are suppressed (32,33). Recently, the protective effect of CAPE on a model of eccentric exercise-induced muscle injury was investigated (34). The study results showed that inflammatory skeletal muscle injury enhanced the expression of COX-2 and iNOS, as well as the production of IL-1β and monocyte chemoattractant protein-1 (MCP-1). It was proposed that these pathological changes in the rats were suppressed by CAPE, which blocked the NF-κB-dependent activation of the inflammatory response (34). In another in vitro study, CAPE significantly suppressed the levels of lipopolysaccharide (LPS)-induced IL-1β, TNF-α and MCP-1 from a macrophage cell line, RAW264.7 (35). Furthermore, in a recent study of RAW264.7 murine macrophage in vivo models, CAPE reduced the production of cytotoxic molecules, such as NO and peroxynitrite, and thus suppressed the inflammatory responses that could have resulted in cell damage and, potentially, cell death. According to the study results, RAW264.7 cells under LPS/IFNγ stimulation exhibited significantly improved viability following treatment with CAPE, which also inhibited NO production in a similar manner to an iNOS inhibitor. This indicated that CAPE exhibits therapeutic potential in a variety of inflammatory disorders (36).
NF-κB signaling additionally has central roles in precancerous chronic inflammation and cancer-induced inflammation (37). CAPE acts to downregulate inflammation by blocking NF-κB, and affects a variety of mediators, including adhesion molecules, cytokines and iNOS. CAPE is a well-documented inhibitor of NF-κB, which may be an action mechanism for the CAPE-mediated anti-inflammatory and anticancer effects (4,38). Although CAPE has been described to conduct its anti-inflammatory activities by modulating different inflammatory pathways, including inhibition of the transcription factors NF-κB and signal transducer and activator of transcription 3 (acute-phase response factor), the compound has already been evaluated for antitumor efficacy in numerous in vitro and in vivo studies (39,40). Coimbra et al (41), for example, investigated the antitumor efficacy of liposomal formulations of CAPE that are known to interfere with inflammatory signaling pathways and have been described to exert antitumor effects. Furthermore, CAPE has been demonstrated to be selectively cytotoxic to cancer cells (42–44). Previous studies found that CAPE could rapidly enter HL-60 cells and induce glutathione depletion (42), mitochondrial dysfunction and caspase-3 activation (43). In a study by Park et al (45) it was observed that CAPE suppressed the expression of phospholipase D1 (PLD1) at the transcriptional level by preventing the binding of NF-κB to the PLD1 promoter. This suggested that the CAPE-induced suppression of matrix metalloproteinase-2 and invasion was mediated by the downregulation of PLD1 by CAPE in glioma cells. Several proposed molecular anti-inflammatory mechanisms of CAPE have been suggested by in vivo and in vitro studies. Table I (22,23,30,34,35,46–50) summarizes the anti-inflammatory effects of CAPE.
Table I.
Potential mechanism underlying the effects or progression pathways of CAPE in its anti-inflammatory/immunomodulatory action.
| Mechanism for the effect or progression pathway(s) | In vivo/in vitro | Cells/animals used | Reported outcomes (ref.) |
|---|---|---|---|
| Inhibiting ROS production; suppressing NF-κB activation | In vivo, EAE (animal model of MS) | Rats | Inhibited ROS production (XO activity, levels of MDA); reduced infiltration of inflammatory cells (22) |
| Suppressing inflammation and ocular tissue damage | In vivo, LPS-induced inflammation | Rats | Suppressed number of inflammatory cells and MPO activity (46) |
| Inhibiting NF-κB activation and mRNA expression; preventing degradation and phosphorylation of p65; suppressed of p65 subunit | In vitro, cell culture; in vivo, H. pylori-induced chronic gastritis | AGS cells, Mongolian gerbils | Inhibited NF-κB activation by suppression of IκB-α degradation of IκB-α and phosphorylation NF-κB p50; reduced mRNA expression of TNF-α, IL-2, IL-6, iNOS and KC (23) |
| Inhibiting NF-κB transcriptional activation | In vitro | Jurkat, MT2 human T-cell lines | Inhibited NF-κB transcriptional activation induced by Tax (47) |
| Inhibiting TNF-α-dependent NF-κB activation via direct inhibition of IKK as well as activation of the Nrf2 pathway | In vitro, cell culture | HCT116 (human coloncarcinoma) cells | Inhibited NF-κB activation by TNF-α and LPS, and directly inhibited IKK in HCT116 cells. Nrf2 activation is associated with the inhibition of the NF-κB pathway (48) |
| Inhibiting the inflammatory pathway | In vivo | Mice | Reduced NF-κB activation and levels of COX-2 (49) |
| Inhibiting cytokine and chemokine production associated with the NF-κB signaling pathway | In vitro, peripheral blood sampling | MoDCs | Inhibited cytokine and chemokine production, IκB-α phosphorylation and NF-κB activation in human MoDCs (50) |
| Inhibiting gene expression of proinflammatory cytokines from LPS-stimulated macrophages | In vitro, cell culture | LPS-stimulated RAW264.7 cells | Reduced mRNA expression of MCP-1, TNF-α, IL-6 and IL-1β (35) |
| Blocking NF-κB-dependent activation of the inflammatory responses | In vivo, eccentric exercise-induced skeletal muscle injury | Rats | Suppressed high COX-2 and iNOS expression and IL-1β and MCP-1 levels (34) |
| Anti-inflammatory action on rat burn healing | In vivo, burn injury | Rats | Reduced MPO activity, NO levels and CD68 expression; improved wound healing after burn (30) |
CAPE, caffeic acid phenethyl ester; CD68, cluster of differentiation 68; COX-2, cyclooxygenase-2; EAE, experimental autoimmune encephalomyelitis; IκB-α, κB inhibitor-α; IKK, IκB-kinase; IL, interleukin; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; MODC, monocyte-derived dendritic cell; MPO, myeloperoxidase; MS, multiple sclerosis; NF-κB, nuclear factor κB; Nrf2, nuclear-factor-E2-related factor 2; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α; XO, xanthine oxidase.
5. Immunomodulatory effects of CAPE
Although the mechanisms underling CAPE-induced NF-κB inhibition have yet to be fully elucidated, the anti-inflammatory and immunomodulatory effects of the compound have been demonstrated in human and experimental models. Immunological studies have indicated that CAPE strongly inhibits mitogen-induced T-cell proliferation, lymphokine production and NF-κB activation (22,27,50). Furthermore, CAPE has been shown to regulate the nuclear binding of the NF-κB subunit p65/RelA, attenuate the expression of cytosolic IκB-α and suppress the dephosphorylation and T-cell transcriptional activity of nuclear factor of activated T cells (51). It has additionally been demonstrated that CAPE can inhibit eicosanoid synthesis, and NF-κB activation may be a causative factor for the increased expression of numerous inflammatory genes in asthma (52,53). NF-κB is expressed in the majority of cell types, and is known to play a central role in immune and inflammatory responses, including asthma. In a study by Jung et al (54) CAPE was found to be capable of downregulating NF-κB activity and reducing the levels of eosinophil peroxidase, indicating that CAPE could be considered as an adjuvant therapy for patients with bronchial asthma. Consistent with these observations, Choi et al (55) demonstrated the importance of NF-κB in the pathogenesis of asthma in mice. In addition, Park et al (56) showed that there was a significant decrease in the cellularity of the spleen and thymus and the thymus weight of mice treated with CAPE at a dose of 20 mg/kg. These results suggested that the treatment of CAPE directly or indirectly caused the immune cells to decrease in cell number, particularly T cells. A different study strongly indicated that the anti-allergy effect of CAPE was a result of the suppression of IgE levels occurring due to the inhibition of NF-κB activation and PAF release (57). Furthermore, it has been reported that CAPE suppresses the contraction of guinea-pig trachea induced by histamine and adenosine. CAPE may therefore be an effective therapeutic agent for allergic diseases (58).
6. Conclusion
In conclusion, CAPE, a compound recognized as the active component of propolis extract, has anti-inflammatory, antioxidant and immunomodulatory properties. Additionally, CAPE inhibits the transcriptional activity of the COX-2 gene in epithelial cells, iNOS gene expression and NO production in macrophage cell lines, and suppresses eicosanoid synthesis and the release of arachidonic acid from cell membranes. In accordance with the above effects, it has been demonstrated that CAPE is a potent and specific inhibitor of NF-κB, lipid peroxidation and lipoxygenase. NF-κB therefore represents a potential target for novel therapeutic agents developed to block the inflammatory response in cases where this process has become chronic or dysregulated. In addition, abnormalities in the NF-κB pathway are frequently observed in a variety of types of human cancer. NF-κB pathway activation is associated with the pathogenesis of chronic inflammatory diseases, including asthma, atherosclerosis, rheumatoid arthritis, inflammatory bowel disease and cancer (59). Furthermore, CAPE demonstrates potential health benefits for the prevention of obesity and associated metabolic disorders and is a potential drug candidate for ischemic stroke treatment due to its inhibition of oxidative stress and inflammation, examples that illustrate how clinically relevant it can be across a wide therapeutic window (49,59). The findings described in this review provide novel insights into the molecular mechanisms underlying the immunomodulatory and anti-inflammatory activities of CAPE. Several of the widely used anti-inflammatory agents inhibit the NF-κB pathway, at least in part, as one of their targets. The effect of CAPE in the treatment of inflammatory diseases may be mediated through the inhibition of NF-κB activation, and it is believed that CAPE is a safe, natural compound and a promising drug candidate for anti-inflammation therapy.
Glossary
Abbreviations
- CAPE
caffeic acid phenethyl ester
- CD68
cluster of differentiation 68
- COX-2
cyclooxygenase-2
- EAE
experimental autoimmune encephalomyelitis
- IFN-γ
interferon-γ
- IκB-α
κB inhibitor-α
- IKK
IκB-kinase
- IL-1
interleukin-1
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MDA
malondialdehyde
- MPO
myeloperoxidase
- MoDC
monocyte-derived dendritic cell
- MS
multiple sclerosis
- NF-κB
nuclear factor κB
- NO
nitric oxide
- Nrf2
nuclear-factor-E2-related factor 2
- PAF
platelet-activating factor
- PLD1
phospholipase D1
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor-α
- XO
xanthine oxidase
References
- 1.Sud'ina GF, Mirzoeva OK, Pushkareva MA, Korshunova GA, Sumbatyan NV, Varfolomeev SD. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993;329:21–24. doi: 10.1016/0014-5793(93)80184-V. [DOI] [PubMed] [Google Scholar]
- 2.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B; Proc Natl Acad Sci USA; 1996; pp. 9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koltuksuz U, Mutuş HM, Kutlu R, Ozyurt H, Cetin S, Karaman A, Gürbüz N, Akyol O, Aydin NE. Effects of caffeic acid phenethyl ester and epidermal growth factor on the development of caustic esophageal stricture in rats. J Pediatr Surg. 2001;36:1504–1509. doi: 10.1053/jpsu.2001.27032. [DOI] [PubMed] [Google Scholar]
- 4.Akyol S, Ozturk G, Ginis Z, Armutcu F, Yigitoglu MR, Akyol O. In vivo and in vitro antineoplastic actions of caffeic acid phenethyl ester (CAPE): Therapeutic perspectives. Nutr Cancer. 2013;65:515–526. doi: 10.1080/01635581.2013.776693. [DOI] [PubMed] [Google Scholar]
- 5.Czermak BJ, Friedl HP, Ward PA. Complement, cytokines, and adhesion molecule expression in inflammatory reactions; Proc Assoc Am Physicians; 1998; pp. 306–312. [PubMed] [Google Scholar]
- 6.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Pang J, Maffucci JA, Pade DS, Newman RA, Kerwin SM, Bowman PD, Stavchansky S. Pharmacokinetics of caffeic acid phenethyl ester and its catechol-ring fluorinated derivative following intravenous administration to rats. Biopharm Drug Dispos. 2009;30:221–228. doi: 10.1002/bdd.657. [DOI] [PubMed] [Google Scholar]
- 8.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leder C, Ziegler M, Gawaz M. Modulating immune responses and inflammation. Semin Thromb Hemost. 2010;36:219–222. doi: 10.1055/s-0030-1251507. [DOI] [PubMed] [Google Scholar]
- 11.Stow JL, Murray RZ. Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 2013;24:227–239. doi: 10.1016/j.cytogfr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 13.Sabio G, Davis RJ. TNF and MAP kinase signalling pathways. Semin Immunol. 2014;26:237–245. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK. The response of human epithelial cells to TNF involves an inducible autocrine cascade. Cell. 2006;124:1225–1239. doi: 10.1016/j.cell.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 16.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 17.Tak PP, Firestein GS. NF-kappaB: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35:193–201. doi: 10.1161/01.HYP.35.1.193. [DOI] [PubMed] [Google Scholar]
- 19.Jura J, Wegrzyn P, Korostyński M, Guzik K, Oczko-Wojciechowska M, Jarzab M, Kowalska M, Piechota M, Przewlocki R, Koj A. Identification of interleukin-1 and interleukin-6-responsive genes in human monocyte-derived macrophages using microarrays. Biochim Biophys Acta. 2008;1779:383–389. doi: 10.1016/j.bbagrm.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Song YS, Park EH, Hur GM, Ryu YS, Lee YS, Lee JY, Kim YM, Jin C. Caffeic acid phenethyl ester inhibits nitric oxide synthase gene expression and enzyme activity. Cancer Lett. 2002;175:53–61. doi: 10.1016/S0304-3835(01)00787-X. [DOI] [PubMed] [Google Scholar]
- 22.Ilhan A, Akyol O, Gurel A, Armutcu F, Iraz M, Oztas E. Protective effects of caffeic acid phenethyl ester against experimental allergic encephalomyelitis-induced oxidative stress in rats. Free Radic Biol Med. 2004;37:386–394. doi: 10.1016/j.freeradbiomed.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Toyoda T, Tsukamoto T, Takasu S, Shi L, Hirano N, Ban H, Kumagai T, Tatematsu M. Anti-inflammatory effects of caffeic acid phenethyl ester (CAPE), a nuclear factor-kappaB inhibitor, on Helicobacter pylori-induced gastritis in Mongolian gerbils. Int J Cancer. 2009;125:1786–1795. doi: 10.1002/ijc.24586. [DOI] [PubMed] [Google Scholar]
- 24.Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic Biol Med. 2002;33:323–336. doi: 10.1016/S0891-5849(02)00868-7. [DOI] [PubMed] [Google Scholar]
- 25.Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 26.Aihara M, Tsuchimoto D, Takizawa H, Azuma A, Wakebe H, Ohmoto Y, Imagawa K, Kikuchi M, Mukaida N, Matsushima K. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect Immun. 1997;65:3218–3224. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orban Z, Mitsiades N, Burke TR, Jr., Tsokos M, Chrousos GP. Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation. Neuroimmunomodulation. 2000;7:99–105. doi: 10.1159/000026427. [DOI] [PubMed] [Google Scholar]
- 28.Hoşnuter M, Gürel A, Babucçu O, Armutcu F, Kargı E, Işikdemir A. The effect of CAPE on lipid peroxidation and nitric oxide levels in the plasma of rats following thermal injury. Burns. 2004;30:121–125. doi: 10.1016/j.burns.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Armutcu F, Gürel A, Hoşnuter M, Pabuçcu O, Altnyazar C. Caffeic acid phenethyl ester improves oxidative erythrocyte damage in a rat model of thermal injury. J Burn Care Rehabil. 2004;25:171–178. doi: 10.1097/01.BCR.0000111765.08625.D4. [DOI] [PubMed] [Google Scholar]
- 30.dos Santos JS, Monte-Alto-Costa A. Caffeic acid phenethyl ester improves burn healing in rats through anti-inflammatory and antioxidant effects. J Burn Care Res. 2013;34:682–688. doi: 10.1097/BCR.0b013e3182839b1c. [DOI] [PubMed] [Google Scholar]
- 31.Khan M, Elango C, Ansari MA, Singh I, Singh AK. Caffeic acid phenethyl ester reduces neurovascular inflammation and protects rat brain following transient focal cerebral ischemia. J Neurochem. 2007;102:365–377. doi: 10.1111/j.1471-4159.2007.04526.x. [DOI] [PubMed] [Google Scholar]
- 32.Jung WK, Choi I, Lee DY, et al. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-kappaB pathways. Int J Biochem Cell Biol. 2008;40:2572–2582. doi: 10.1016/j.biocel.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Lee KW, Chun KS, Lee JS, Kang KS, Surh YJ, Lee HJ. Inhibition of cyclooxygenase-2 expression and restoration of gap junction intercellular communication in H-ras-transformed rat liver epithelial cells by caffeic acid phenethyl ester. Ann NY Acad Sci. 2004;1030:501–507. doi: 10.1196/annals.1329.062. [DOI] [PubMed] [Google Scholar]
- 34.Shen YC, Yen JC, Liou KT. Ameliorative effects of caffeic acid phenethyl ester on an eccentric exercise-induced skeletal muscle injury by down-regulating NF-κb mediated inflammation. Pharmacology. 2013;91:219–228. doi: 10.1159/000348412. [DOI] [PubMed] [Google Scholar]
- 35.Juman S, Yasui N, Ikeda K, Ueda A, Sakanaka M, Negishi H, Miki T. Caffeic acid phenethyl ester suppresses the production of pro-inflammatory cytokines in hypertrophic adipocytes through lipopolysaccharide-stimulated macrophages. Biol Pharm Bull. 2012;35:1941–1946. doi: 10.1248/bpb.b12-00317. [DOI] [PubMed] [Google Scholar]
- 36.Kassim M, Mansor M, Kamalden TA, Shariffuddin II, Hasan MS, Ong G, Sekaran SD, Suhaimi A, Al-Abd N, Yusoff KM. Caffeic acid phenethyl ester (CAPE): Scavenger of peroxynitrite in vitro and in sepsis models. Shock. 2014;42:154–160. doi: 10.1097/SHK.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 37.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 38.Carrasco-Legleu CE, Márquez-Rosado L, Fattel-Fazenda S, Arce-Popoca E, Pérez-Carreón JI, Villa-Treviño S. Chemoprotective effect of caffeic acid phenethyl ester on promotion in a medium-term rat hepatocarcinogenesis assay. Int J Cancer. 2004;108:488–492. doi: 10.1002/ijc.11595. [DOI] [PubMed] [Google Scholar]
- 39.Cao Q, Kaur C, Wu CY, Lu J, Ling EA. Nuclear factor-kappa B regulates Notch signaling in production of proinflammatory cytokines and nitric oxide in murine BV-2 microglial cells. Neuroscience. 2011;192:140–154. doi: 10.1016/j.neuroscience.2011.06.060. [DOI] [PubMed] [Google Scholar]
- 40.Omene CO, Wu J, Frenkel K. Caffeic Acid Phenethyl Ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Invest New Drugs. 2012;30:1279–1288. doi: 10.1007/s10637-011-9667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coimbra M, Crielaard BJ, Storm G, Schiffelers RM. Critical factors in the development of tumor-targeted anti-inflammatory nanomedicines. J Control Release. 2012;160:232–238. doi: 10.1016/j.jconrel.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Chen YJ, Shiao MS, Wang SY. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs. 2001;12:143–149. doi: 10.1097/00001813-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFkappaB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279:6017–6026. doi: 10.1074/jbc.M306040200. [DOI] [PubMed] [Google Scholar]
- 44.Lee YT, Don MJ, Hung PS, Shen YC, Lo YS, Chang KW, Chen CF, Ho LK. Cytotoxicity of phenolic acid phenethyl esters on oral cancer cells. Cancer Lett. 2005;223:19–25. doi: 10.1016/j.canlet.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 45.Park MH, Kang DW, Jung Y, Choi KY, Min S. Caffeic acid phenethyl ester downregulates phospholipase D1 via direct binding and inhibition of NFκB transactivation. Biochem Biophys Res Commun. 2013;442:1–7. doi: 10.1016/j.bbrc.2013.09.105. [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz A, Yildirim O, Tamer L, et al. Effects of caffeic acid phenethyl ester on endotoxin-induced uveitis in rats. Curr Eye Res. 2005;30:755–762. doi: 10.1080/02713680590967962. [DOI] [PubMed] [Google Scholar]
- 47.Shvarzbeyn J, Huleihel M. Effect of propolis and caffeic acid phenethyl ester (CAPE) on NFκB activation by HTLV-1 Tax. Antiviral Res. 2011;90:108–115. doi: 10.1016/j.antiviral.2011.03.177. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y, Shin DH, Kim JH, et al. Caffeic acid phenethyl ester-mediated Nrf2 activation and IkappaB kinase inhibition are involved in NFkappaB inhibitory effect: Structural analysis for NFkappa B inhibition. Eur J Pharmacol. 2010;643:21–28. doi: 10.1016/j.ejphar.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 49.Bezerra RM, Veiga LF, Caetano AC, Rosalen PL, Amaral ME, Palanch AC, de Alencar SM. Caffeic acid phenethyl ester reduces the activation of the nuclear factor κB pathway by high-fat diet-induced obesity in mice. Metabolism. 2012;61:1606–1614. doi: 10.1016/j.metabol.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Wang LC, Lin YL, Liang YC, et al. The effect of caffeic acid phenethyl ester on the functions of human monocyte-derived dendritic cells. BMC Immunol. 2009;10:39. doi: 10.1186/1471-2172-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Márquez N, Sancho R, Macho A, Calzado MA, Fiebich BL, Muñoz E. Caffeic acid phenethyl ester inhibits T-cell activation by targeting both nuclear factor of activated T-cells and NF-kappaB transcription factors. J Pharmacol Exp Ther. 2004;308:993–1001. doi: 10.1124/jpet.103.060673. [DOI] [PubMed] [Google Scholar]
- 52.Mirzoeva OK, Calder PC. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot Essent Fatty Acids. 1996;55:441–449. doi: 10.1016/S0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- 53.Hart LA, Krishnan VL, Adcock IM, Barnes PJ, Chung KF. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am J Respir Crit Care Med. 1998;158:1585–1592. doi: 10.1164/ajrccm.158.5.9706116. [DOI] [PubMed] [Google Scholar]
- 54.Jung WK, Lee DY, Choi YH, Yea SS, Choi I, Park SG, Seo SK, Lee SW, Lee CM, Kim SK, et al. Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced asthma. Life Sci. 2008;82:797–805. doi: 10.1016/j.lfs.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Choi IW, Kim DK, Ko HM, Lee HK. Administration of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-kappaB inhibits established asthmatic reaction in mice. Int Immunopharmacol. 2004;4:1817–1828. doi: 10.1016/j.intimp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 56.Park JH, Lee JK, Kim HS, Chung ST, Eom JH, Kim KA, Chung SJ, Paik SY, Oh HY. Immunomodulatory effect of caffeic acid phenethyl ester in Balb/c mice. Int Immunopharmacol. 2004;4:429–436. doi: 10.1016/j.intimp.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Park SG, Lee DY, Seo SK, Lee SW, Kim SK, Jung WK, Kang MS, Choi YH, Yea SS, Choi I, Choi IW. Evaluation of anti-allergic properties of caffeic acid phenethyl ester in a murine model of systemic anaphylaxis. Toxicol Appl Pharmacol. 2008;226:22–29. doi: 10.1016/j.taap.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Nader MA. Caffeic acid phenethyl ester attenuates IgE-induced immediate allergic reaction. Inflammopharmacology. 2013;21:169–176. doi: 10.1007/s10787-012-0138-4. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]