Abstract
OBJECTIVE: Conventionally, intravenous N-acetylcysteine (IV-NAC) administration is a 3-bag regimen administered over the course of 21 hours, which increases the risk of reconstitution and administration errors. To minimize errors, an alternative IV-NAC regimen consists of a loading dose (150 mg/kg) followed by a maintenance infusion (15 mg/kg/hr) until termination criteria are met. The aim was to determine the clinical outcomes of an alternative IV-NAC regimen in pediatric patients.
METHODS: A retrospective review of pharmacy dispensing records and diagnostic codes at a pediatric hospital identified patients who received alternative IV-NAC dosing from March 1, 2008, to September 10, 2012, for acetaminophen overdoses. Exclusion criteria included chronic liver disease, initiation of oral or other IV-NAC regimens, and initiation of standard IV-NAC infusion prior to facility transfer. Clinical and laboratory data were abstracted from the electronic medical record. Descriptive statistics were utilized. Clinical outcomes and adverse drug reaction incidences were compared between the alternative and Food and Drug Administration (FDA)–approved IV-NAC regimens.
RESULTS: Fifty-nine patients (mean age 13.4 ± 4.3 years; range: 2 months-18 years) with acetaminophen overdoses were identified. Upon IV-NAC discontinuation, 45 patients had normal alanine transaminase (ALT) concentrations, while 14 patients' ALT concentrations remained elevated (median 140 units/L) but were trending downward. Two patients (3.4%) developed hepatotoxicity (aspartate transaminase/ALT > 1000 units/L). No patients developed hepatic failure, were listed for a liver transplant, were intubated, underwent hemodialysis, or died. Two patients (3.4%) developed anaphylactoid reactions. No known medication or administration errors occurred. Clinical outcome incidences of the studied endpoints with the alternative IV-NAC regimen are at the lower end of published incidence ranges compared to the FDA IV-NAC regimen for acetaminophen overdoses.
CONCLUSIONS: This alternative IV-NAC regimen appears to be effective and well tolerated among pediatric patients when compared to the FDA-approved regimen. It may also result in fewer reconstitution and administration errors, leading to improved patient safety.
INDEX TERMS: acetaminophen, anaphylactoid, hepatotoxicity, N-acetylcysteine, pediatrics
INTRODUCTION
Acetaminophen, which is available in hundreds of single-ingredient and combination products, is the most common drug taken in overdoses around the world as a result of its extensive availability and low cost.1 Intravenous N-acetylcysteine (IV-NAC) is approved by the Food and Drug Administration (FDA) for the treatment of acetaminophen overdoses. The FDA-approved IV-NAC dosing regimen consists of a loading dose of 150 mg/kg infused over 60 minutes, followed by a second dose of 50 mg/kg infused over 4 hours (12.5 mg/kg/hr), and finally, a third dose of 100 mg/kg infused over 16 hours (6.25 mg/kg/hr).2 Each administered dose has different mg/kg dosing, additive volumes, and infusion rates.
Although the FDA-approved dosing regimen is effective and safe, the complex 3-step process is associated with an increased risk of medication errors.3,4 In a retrospective chart review, Hayes et al3 reported 84 medication errors in 74 patients (33%), including incorrect dose (1.4%), greater than 1 hour of interruption in therapy (18.6%), incorrect infusion rate (5%), and unnecessary administration (13.1%). Another study4 collected samples of IV-NAC–reconstituted solutions before and after infusion of acetylcysteine in 66 patients and analyzed the samples by lab assay; the chance of dosing calculations was estimated to be 5%, measurement of acetylcysteine was estimated to be 3%, and inadequate mixing was estimated to be 9%.
To minimize errors, alternative dosing regimens with simpler reconstitution and mixing processes as well as easier administration steps have been utilized. A retrospective chart review evaluated an alternative dosing regimen in adult patients that consisted of a 150 mg/kg loading dose administered over 60 minutes followed by an infusion of 14 mg/kg/hr for 20 hours.5 This study found the alternative dosing regimen safe and effective; however, this regimen has not been studied in the pediatric population.
An alternative IV-NAC dosing regimen at a pediatric hospital consists of a loading dose of 150 mg/kg over 60 minutes followed by a maintenance infusion of 15 mg/kg/hr until the serum acetaminophen concentration measures less than 10 mg/L and the serum concentration of liver enzymes remain normal or are trending downward. The main difference between the FDA-approved IV-NAC regimen and the alternative IV-NAC dosing regimen at the pediatric hospital is the maintenance infusion dose. The purpose of the study was to determine the clinical outcomes of an alternative IV-NAC regimen in the treatment of acetaminophen overdoses in pediatric patients.
METHODS
Study Design
This study is a retrospective chart review of pediatric patients who received IV-NAC according to the dosing regimen recommended by the Section of Clinical Toxicology at a pediatric institution. All pediatric patients (newborn to 18 years) who received the alternative IV-NAC dosing regimen for the treatment of acute and chronic acetaminophen overdoses from March 1, 2008, to September 10, 2012, were eligible for inclusion. Indications for administration of NAC were determined by the nature of the overdose. Acute acetaminophen overdoses received IV-NAC treatment if the serum acetaminophen concentration plotted above the lower treatment line of the Rumack Matthew Nomogram.6,7 Chronic overdoses, defined as ingestions occurring over more than 8 hours, were assessed for risk based on the American Association of Poison Control Centers' consensus guideline.6,7 Patients who were considered at risk underwent laboratory testing and were treated with IV-NAC if the serum aspartate transaminase (AST) or serum alanine transaminase (ALT) were abnormal or if the serum acetaminophen concentration was greater than 10 mg/L. This criterion was chosen because it has been shown to be safe and sensitive in determining the development of hepatotoxicity in chronic ingestions.8 Exclusion criteria included the presence of chronic liver disease, initiation of oral or other IV-NAC regimens, and initiation of the standard 4- or 16-hour NAC infusion prior to facility transfer. Reasons for patient exclusion were collected. Patients were identified by pharmacy dispensing records and International Classification of Diseases, Ninth Revision (ICD-9), codes. Institutional Review Board approval was obtained.
The traditional NAC maintenance dosing of 6.25 mg/kg/hr may not be adequate in all overdose patients. There is evidence that the plasma concentration of NAC continues to decline even on maintenance dosing.9,10 Thus, in patients with large ingestions the typical dosing may be inadequate to detoxify ongoing N-acetyl-p-benzoquinone imine (NAPQI) production and replenish glutathione stores. The Section of Medical Toxicology therefore chose to use only a larger dose of NAC (15 mg/kg/hr) for acetaminophen overdoses. This was done to eliminate situations in which individual physicians have to decide when the NAC dose should be increased, thereby attempting to limit the risk of inadequate NAC dosing.
Patients were classified as early or late presentations. Early presentations were defined as patients who received the loading dose of IV-NAC less than or equal to 8 hours from the acute overdose, while late presentations were defined as patients who received the loading dose of IV-NAC more than 8 hours after the acute overdose.7 Major clinical outcomes included the development of hepatotoxicity, hepatic failure, kidney failure, referral for liver transplant, and mortality rate. Hepatotoxicity was defined as serum AST or ALT concentrations greater than 1000 units/L. Monitoring parameters to evaluate the clinical outcomes of the alternative regimen included serum AST concentrations, serum ALT concentrations, encephalopathy grade, ammonia concentrations, international normalized ratios (INR) values, administration of blood products or vitamin K, serum creatinine concentrations, and initiation of hemodialysis. All documented adverse reactions and their treatment were recorded and reviewed.
Data Collection and Analysis
Clinical and laboratory data were abstracted by a single investigator from the electronic medical record using a standardized data collection tool and were imported into Excel; all patient information was deidentified. Any discrepancies regarding the data were adjudicated by 2 of the other authors until a consensus was reached. Baseline demographic characteristics, length of the IV-NAC continuous infusion, and length of stay were collected.
Descriptive statistics were used to analyze the data. Clinical outcomes were assessed based on comparison to published literature that utilized the FDA-approved IV-NAC regimen. Additionally, the clinical outcomes of the alternative IV-NAC dosing regimen in the pediatric population were compared to those in the published literature of the alternative IV-NAC dosing regimen in the adult population.
RESULTS
Patient Characteristics
Intravenous N-acetylcysteine was dispensed to 87 patients from March 1, 2008, through September 10, 2012, at the pediatric hospital. Of the patients who received this IV-NAC, 28 were excluded, while 59 patients met the inclusion criteria (Figure 1). The mean age was 13 ± 4.3 years and the median age was 14 years (range, 2 months-18 years), with 49 females and 10 males.
Figure 1.
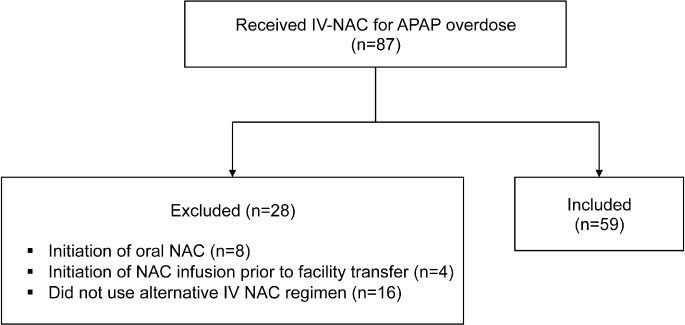
Study sample.
Three patients (5.1%) presented with chronic overdoses and 56 patients (94.9%) presented with acute overdoses. In the acute overdoses, the reported average dose ingested was 13.5 ± 11.0 g (median 10 g; range, 2–50 g), or 248.8 mg/kg. In children less than 12 years old, the reported average dose ingested was 3.8 ± 1.5 g (median 3.75 g; range, 2–6 g), or 229 mg/kg, while in children greater than 12 years old, the reported average dose ingested was 14.8 ± 11.2 g (median 10 g; range, 2.5–50 g), or 248 mg/kg. Doses were based on patient-reported information, with the highest maximum possible dose recorded. In 16 patients doses were unknown; therefore, these patients were not included in the data analysis of the dose ingested.
Of the 59 patients, 49 patients were intentional overdoses and 10 patients were unintentional overdoses. Other medications were ingested in addition to acetaminophen in 35 patients, with 4 of these being unintentional overdoses. These medications included ibuprofen, diphenhydramine, caffeine, aspirin, dextromethorphan, chlorpheniramine, pyrilamine, antidepressants (sertraline, citalopram, and paroxetine), anti-psychotics (quetiapine and risperdone), benzodiazepines, hydrocodone, and oxycodone. Diphenhydramine was a co-ingested medication in 11 patients. Diphenhydramine was separated from other co-ingested medications since it is an anticholinergic and has been associated with delayed absorption of acetaminophen.11,12
Patient Severity
Risk of hepatic injury was assessed using the Rumack Matthew Nomogram in the 56 patients with acute overdoses (Figure 2). Of the patients categorized as low risk, 4 patients had IV-NAC discontinued following toxicology consults, and 3 patients had extended treatment as a result of elevations in serum ALT or AST concentrations, although the serum acetaminophen concentration was less than 10 mg/L. Of the 6 patients with unknown risk, 4 patients had unknown ingestion times, 1 patient presented after 36 hours, and 1 patient would not state the time of the ingestion until the following day.
Figure 2.
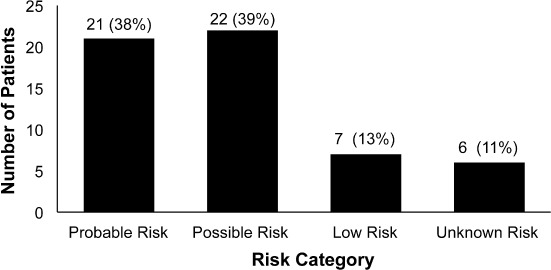
Number of patients by risk category (n = 56).
Patients with acute acetaminophen overdoses were classified as early or late presentations (Figure 3). Seven patients were not classified since 4 patients had unknown ingestion times and the time of the loading dose of IV-NAC could not be located in 3 patients' charts. Average IV-NAC infusion start time for patients with early presentations was 5.2 ± 2.7 hours (median 5 hours; range, 2–8 hours), compared to 13.6 ± 8.6 hours (median 12 hours; range, 8.25–26 hours) for patients who presented late. The mean duration of the IV-NAC continuous infusion was 30 ± 17.7 hours (median 27 hours; range, 4.25–89 hours) (Figure 4). The mean duration of IV-NAC continuous infusion for acute overdoses was 30.3 ± 18.1 hours (median 26.3 hours; range, 4.25–89 hours). Average time until serum acetaminophen concentrations reached less than 10 mg/L was 31.5 ± 9.7 hours (median 32 hours; range, 0–58.5 hours).
Figure 3.
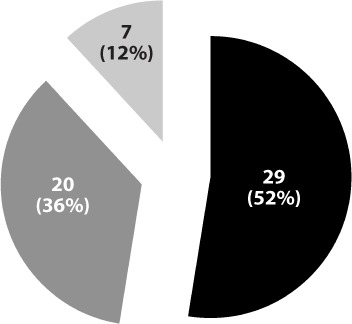
Time from ingestion to treatment (n = 56).
▪ = Late presentation (>8 hours);  = Early presentation (<8 hours);
= Early presentation (<8 hours);  = Unknown presentation.
= Unknown presentation.
Figure 4.
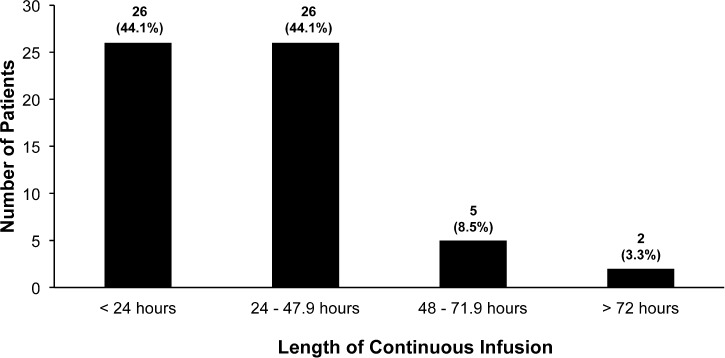
Intravenous N-acetylcysteine (IV-NAC) continuous infusion duration (n = 59).
Since co-ingesting diphenhydramine slows absorption of acetaminophen, serum acetaminophen concentrations may cross the hepatotoxicity line plotted on the Rumack Matthew Nomogram after the first 4-hour serum acetaminophen concentration; therefore, it is recommended that a second serum acetaminophen concentration be drawn 4 to 6 hours after the first 4-hour concentration. If the second serum acetaminophen concentration is still elevated, additional IV-NAC may be required.11,12 The mean duration of the IV-NAC continuous infusion in patients who co-ingested diphenhydramine was 36 ± 23.6 hours (median 31.75 hours; range, 10.75–89 hours). Of the 11 patients who co-ingested diphenhydramine, 9 patients did not experience an increase in serum acetaminophen concentrations following the initial concentration; 2 patients could not be assessed since they only had 1 serum acetaminophen concentration documented.
Development of Hepatotoxicity
Hepatotoxicity (AST/ALT > 1000 units/L) developed in 2 patients (3.4%). One patient was a 14-year-old female who ingested 25 g of acetaminophen (213 mg/kg) and diphenhydramine with a serum acetaminophen concentration of 132 mg/L at 11 hours after ingestion. Her peak serum AST concentration was 746 units/L, and her peak serum ALT concentration was 1280 units/L. The second patient was a 17-year-old female who ingested 32 g of acetaminophen (319 mg/kg) with an unknown risk because her serum acetaminophen concentration was less than 2 mg/L at 36 hours after ingestion. Her peak serum AST concentration was 1414 units/L, and her peak serum ALT concentration was 1428 units/L. Both patients were intentional acute overdoses and were classified as late presentations.
At the time of IV-NAC discontinuation, 44 patients had normal serum ALT concentrations, while in 15 patients serum ALT concentrations remained elevated but were trending downward. The median peak serum ALT concentration was 140 units/L (range, 64–1428 units/L). Hepatology was not consulted on any patient. No patients required liver transplantation.
Documented Adverse Reactions
Two patients (3.4%) developed minor anaphylactoid reactions, including flushing, facial redness, and itching. These reactions occurred at the end of the loading dose infusion of IV-NAC. Both patients were treated with intravenous diphenhydramine and completed the IV-NAC therapy without any further complications. One patient had asthma, with a serum acetaminophen concentration of less than 2 mg/L, while the other patient did not have asthma, with a serum acetaminophen concentration of 187.9 mg/L. Patients diagnosed with asthma and a history of allergies appear to be at a higher risk for development of anaphylactoid reactions.13,14 Seven patients had asthma and did not have a documented adverse reaction.
Additional Outcomes
No patients developed hepatic failure. No patients developed encephalopathy or experienced an active bleed or received fresh frozen plasma, packed red blood cells, or vitamin K. No patients were intubated or underwent hemodialysis. No patients died. No known medication or administration errors were documented in the electronic medical record. Access to reports to view medication errors was not allowed by the pediatric hospital.
DISCUSSION
The traditional FDA-approved IV-NAC regimen is a 21-hour protocol; however, various studies15,16 have examined different IV-NAC dosing and therapy durations ranging in length from 21 to 48 hours. Patient-tailored NAC therapy individualizes the duration of treatment with NAC based on the patient's risk of toxicity. A retrospective case series17 evaluated a patient-tailored protocol based on serum acetaminophen and AST concentrations in 27 patients. No hepatotoxicity developed in patients treated for less than 36 hours with IV-NAC; however, these patients had NAC initiated within 10 hours of the acetaminophen overdoses. There was a lower incidence of hepatotoxicity in patients who presented late (>10 hours) and who received IV-NAC treatment for 80 to 164 hours based on the patient-tailored protocol compared to the FDA-approved IV-NAC regimen for 72 hours. Continued use of IV-NAC is important in patients with persistently elevated serum acetaminophen and AST/ALT concentrations or signs of hepatic injury.
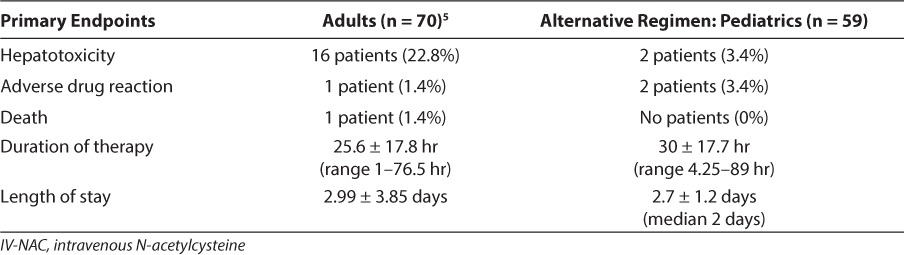
A retrospective study5 of an alternative IV-NAC regimen in an adult population found 69 (99%) of 70 patients were discharged home, while 1 patient died. Hepatotoxicity, defined as an AST value of >1000 units/L, occurred in 16 patients. One adverse reaction was attributed to the IV-NAC; the patient developed facial and truncal flushing with respiratory difficulty. The duration of therapy was 25.6 ± 17.8 hours (range, 1–76.5 hours) and the length of stay was 2.99 ± 3.85 days. The severity of patients was not reported.
The incidences of the studied endpoints with administration of the alternative IV-NAC dosing regimen are at the lower end of published incidence ranges with administration of the FDA IV-NAC regimen for acetaminophen overdoses. The incidences of outcomes between the alternative IV-NAC dosing regimen and the FDA IV-NAC regimen were compared (Table 1).16,18–25 Published literature does not separate the data by pediatric and adult populations, and the ranges and comparison groups are widely variable.
Table 1.
Comparison to FDA IV-NAC Regimen
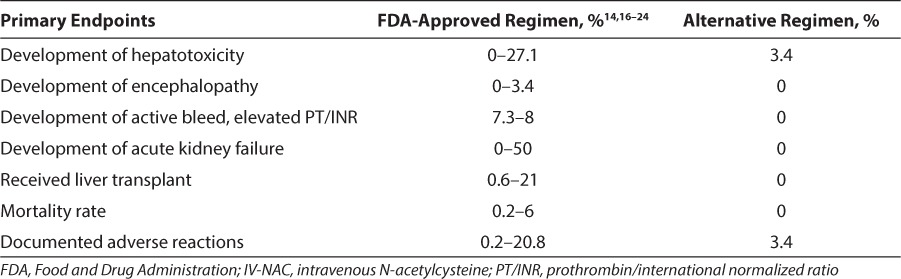
Adverse drug reactions, mean duration of therapy, and length of stay were comparable between the adult and pediatric populations (Table 2). However, only 2 (3.4%) patients developed hepatotoxicity in the pediatric population at the pediatric hospital, which was lower than the number reported in the adult population. The average duration of the continuous infusion in the pediatric population was slightly longer than the duration in the adult population. It has been reported26 that hepatotoxicity occurs less frequently in young children; this finding is presumed to be secondary to increased sulfation in children. Long-term ethanol use, larger acetaminophen doses, or underlying hepatic disease may explain the differences in the rates of hepatotoxicity seen between children and adults.26
Table 2.
Comparison to Alternative IV-NAC Regimen in the Adult Population
In pediatric patients, adverse reactions have occurred in 3% to 14% of patients with the FDA-approved IV-NAC regimen; adverse reactions include bronchospasms, injection site erythema, urticaria, fever, and rarely anaphylaxis and death. These adverse reactions are caused by the histamine-related response and more commonly occur when the largest dose of NAC is given, especially during the loading dose, and diminish with discontinued infusions or administration of antihistamines.16 The incidence of adverse reactions with administration of the alternative IV-NAC regimen is at the lower end of the published incidence compared with administration of the FDA-approved IV-NAC regimen.
Limitations
Limitations of the study include a retrospective study design, 13 patients classified as low or unknown risk on the Rumack Matthew Nomogram, and unknown time of the NAC loading dose administration in 3 patients. These patients were inadvertently included initially and were only determined to be at low risk later in the course of treatment when further history or laboratory data became available. One implication of the inclusion of these patients is the increased risk of potential anaphylactoid reactions, since low serum acetaminophen concentrations have been shown27 to be a risk factor for anaphylactoid reactions; high serum acetaminophen concentrations appear to stabilize mast cells. Additionally, these patients may have made the alternative IV-NAC regimen appear more effective when in reality these patients may have done well without treatment.
Since there were only 3 patients with chronic overdoses, the ability to generalize the assessments to this population is limited. Another limitation is that the alternative IV-NAC regimen was not prospectively compared to the traditional FDA-approved regimen. Although no known medication or administration errors were noted, access to reports to review errors was not allowed at the pediatric hospital.
CONCLUSIONS
This alternative IV-NAC dosing regimen appears to be effective and well tolerated among pediatric patients when compared to the FDA-approved regimen. In addition, it may result in fewer compounding and administration errors, leading to improved patient care and patient safety.
ACKNOWLEDGMENT
Kathryn Pauley had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This research has been presented during a platform session at the Annual Meeting of the North American Congress of Clinical Toxicology in Atlanta, Georgia, on October 1, 2013. The authors would like to acknowledge Gary S. Wasserman, DO, who was instrumental in developing the alternative IV-NAC dosing regimen and provided pediatric toxicology mentorship until his death in 2014.
ABBREVIATIONS
- ALT
serum alanine transaminase
- AST
serum aspartate transaminase
- FDA
Food and Drug Administration
- ICD-9
International Classification of Diseases, Ninth Revision
- INR
international normalized ratio
- IV-NAC
intravenous N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15(6):398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- 2.Product information Acetadote (N-Acetylcysteine) Nashville, TN: Cumberland Pharmaceuticals; 2008. [Google Scholar]
- 3.Hayes BD, Klien-Schwarz W, Doyon S. Frequency of medication errors with intravenous acetylcysteine for acetaminophen overdose. Ann Pharmacother. 2008;42(6):766–770. doi: 10.1345/aph.1K685. [DOI] [PubMed] [Google Scholar]
- 4.Ferner RE, Langford NJ, Anton C et al. Random and systematic medication errors in routine clinical practice: a multicentre study of infusions, using acetylcysteine as an example. Br J Clin Pharmacol. 2001;52(5):573–577. doi: 10.1046/j.0306-5251.2001.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson MT, McCammon CA, Mullins ME, Halcomb SE. Evaluation of a simplified n-acetylcysteine dosing regimen for the treatment of acetaminophen toxicity. Ann Pharmacother. 2011;45(6):713–720. doi: 10.1345/aph.1P613. [DOI] [PubMed] [Google Scholar]
- 6.Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975;55(6):871–876. [PubMed] [Google Scholar]
- 7.Dart RC, Erdman AR, Olson KR et al. Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol. 2006;44(1):1–18. doi: 10.1080/15563650500394571. [DOI] [PubMed] [Google Scholar]
- 8.Daly F, O'Malley G, Heard K, Dart RC. Prospective Eealuation of repeated supra-therapeutic acetaminophen ingestion. Ann Emerg Med. 2004;44(4):393–398. doi: 10.1016/j.annemergmed.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Waring WS. Novel acetylcysteine regimens for treatment of paracetamol overdose. Ther Adv Drug Saf. 2012;3(6):305–315. doi: 10.1177/2042098612464265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rumack B, Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present, and future. Clin Toxicol. 2012;50(2):91–98. doi: 10.3109/15563650.2012.659252. [DOI] [PubMed] [Google Scholar]
- 11.Burda T, Sigg T. Double peak and prolonged absorption after large acetaminophen extended-release and diphenhydramine ingestion. Am J Ther. 2012;19(2):e101–e104. doi: 10.1097/MJT.0b013e3181e7a536. [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Monte A, Bagdure D, Heard K. Hepatic failure despite early acetylcysteine following large acetaminophen-diphenhydramine overdose. Pediatrics. 2011;127(4):e1077–e1080. doi: 10.1542/peds.2010-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt LE, Dalhoff K. Risk factors in the development of adverse reactions to n-acetylcysteine in patients with paracetamol poisoning. Br J Clin Pharmacol. 2005;51(1):87–91. doi: 10.1046/j.1365-2125.2001.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appelboam AV, Dargan PI, Knighton J. Fatal anaphylactoid reaction to n-acetylcysteine: caution in patients with asthma. Emerg Med J. 2002;19(6):594–595. doi: 10.1136/emj.19.6.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whyte AJ, Kehrl T, Brooks DE, Sokolowski D. Safety and effectiveness of acetadote for acetaminophen toxicity. J Emerg Med. 2010;39(5):607–611. doi: 10.1016/j.jemermed.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Perry HE, Shannon MW. Efficacy of oral versus intravenous n-acetylcysteine in acetaminophen overdose: results of an open-label, clinical trial. J Pediatr. 1998;132(1):149–152. doi: 10.1016/s0022-3476(98)70501-3. [DOI] [PubMed] [Google Scholar]
- 17.Yarema MC, Johnson DW, Berlin RJ et al. Comparison of the 20-hour intravenous and 72-hour oral acetylcysteine protocols for the treatment of acute acetaminophen poisoning. Ann Emerg Med. 2009;54(4):606–614. doi: 10.1016/j.annemergmed.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 18.James LP, Wells E, Beard RH, Farrar HC. Predictors of outcomes after acetaminophen poisoning in children and adolescents. J Pediatr. 2002;140(5):522–526. doi: 10.1067/mpd.2002.122936. [DOI] [PubMed] [Google Scholar]
- 19.Tsai CL, Chang WT, Weng TI et al. A patient-tailored n-acetylcysteine protocol for acute acetaminophen intoxication. Clin Ther. 2005;27(3):336–341. doi: 10.1016/j.clinthera.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Prescott LF, Illingworth RN, Critchley JA et al. Intravenous n-acetylcysteine: the treatment of choice for paracetamol poisoning. Br Med J. 1979;2(6198):1097–1100. doi: 10.1136/bmj.2.6198.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison PM, Keays R, Bray GP et al. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 1990;335(8705):1572–1573. doi: 10.1016/0140-6736(90)91388-q. [DOI] [PubMed] [Google Scholar]
- 22.Buckley NA, Whyte IM, O'Connoll DL, Dawson AH. Oral or intravenous n-acetylcysteine: which is the treatment of choice for acetaminophen (paracetamol) poisoning? J Toxicol Clin Toxicol. 1999;37(6):759–767. doi: 10.1081/clt-100102453. [DOI] [PubMed] [Google Scholar]
- 23.Bebarta VS, Kao L, Froberg B et al. A multi-center comparison of the safety of oral versus intravenous acetylcysteine for treatment of acetaminophen overdose. Clin Toxicol (Phila) 2010;48(5):424–430. doi: 10.3109/15563650.2010.486381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11(3):525–548. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Whyte IM, Francis B, Dawson AH. Safety and efficacy of intravenous n-acetylcysteine for acetaminophen overdose: analysis of the Hunter Area Toxicology Service (HATS) database. Curr Med Res Opin. 2007;23(10):2359–2368. doi: 10.1185/030079907X219715. [DOI] [PubMed] [Google Scholar]
- 26.Alander SW, Dowd MD, Bratton SL, Kearns GL. Pediatric acetaminophen overdose: risk factors associated with hepatocellular injury. Arch Pediatr Adolesc Med. 2000;154(4):346–350. doi: 10.1001/archpedi.154.4.346. [DOI] [PubMed] [Google Scholar]
- 27.Coulson J, Thompson JP. Paracetamol (acetaminophen) attenuates in vitro mast cell and peripheral blood mononucleocyte cell histamine release induced by n-acetylcysteine. Clin Toxicol (Phila) 2010;48(2):111–114. doi: 10.3109/15563650903520959. [DOI] [PubMed] [Google Scholar]


