Abstract
OBJECTIVES: To characterize off-label prescribing among US pediatric intensive care units (PICUs), determine characteristics associated with off-label use, and identify medications in highest need for additional study.
METHODS: Medications prescribed for ≥1% PICU patients (age < 18 years) in 2010 were identified from 39 children's hospitals. Use in a patient younger than the Food and Drug Administration (FDA)-approved age for any indication was considered off-label. Hierarchical multivariable modeling was used to identify characteristics associated with off-label use, accounting for center effects. Highest-impact drugs were defined by: 1) high off-label use (off-label use in at least 5% of the PICU cohort), 2) high risk medication, and 3) high priority status by the FDA or Best Pharmaceuticals for Children Act (BPCA).
RESULTS: A total of 66,896 patients received ≥1 medication of interest (n = 162) during their PICU stay. A median of 3 (interquartile range, 2–6) unique drugs per patient were used off-label. Those who received ≥1 drug off-label (85% of the cohort) had longer median PICU (2 days vs 1 day) and hospital (6 days vs 3 days) lengths of stay and higher mortality (3.6% vs 0.7%), p < 0.001. Factors independently associated with off-label drug use included: age 1 to 5 years, chronic conditions, acute organ failures, mechanical ventilation, arterial or venous catheters, dialysis, and blood products. Half of prescribed medications (n = 84) had been used off-label: 26 with significant off-label use, 30 high-risk medications, and 47 with high FDA/BPCA priority. The highest impact medications identified were: dexmedetomidine, dopamine, hydromorphone, ketamine, lorazepam, methadone, milrinone, and oxycodone.
CONCLUSIONS: Most PICU patients are exposed to off-label medication use, with uncertain evidence. Future medication research in this population should focus on medications with high impact potential.
INDEX TERMS: off label use, pediatric intensive care units, pharmacoepidemiology, research priorities, risk factors
INTRODUCTION
Prior to market release in the United States, prescribed medications must go through a rigorous approval process with the Food and Drug Administration (FDA). This approval process generally includes demonstration of safety and efficacy supported by randomized controlled trials. Such trials, however, are challenging in the pediatric population for a number of reasons including: small sample sizes, low financial incentive, and ethical concerns.1,2 As a result, a majority of available medications do not have an FDA-approved pediatric indication leading to the widespread and well-recognized practice of off-label prescribing in pediatrics.3–6
Pharmacotherapy is an essential part of caring for the critically ill child, whether targeting disease treatment or providing supportive care. In fact, a patient admitted to the pediatric intensive care unit (PICU) may be exposed to a median of 14 different medications (interquartile range [IQR], 9–19) during their PICU admission, and the total number increases with severity of illness and degree of organ failure.7 With the large number of medications administered to any single patient, the likelihood of receiving a medication off-label is significant. This is supported by prior single-center studies on off-label medication use in the PICU.8–13 However, there has been limited data on national off-label prescribing patterns in the PICU and identification of children at highest risk of exposure to medications off-label.
Therefore, we conducted this study to determine the degree of off-label prescribing among US pediatric centers and determine patient characteristics associated with high risk of off-label use. Furthermore, because of the significant number of medications utilized in the PICU, we identified the medications that might have the greatest impact with additional study based on frequency of off-label use, high risk profile, and prioritization by national agencies.
MATERIALS AND METHODS
The Colorado Multiple Institution Review Board determined this study was not human subject research.
Data Source
Data for this study were obtained from the Pediatric Health Information System (PHIS), an administrative database that contains inpatient, emergency department, ambulatory surgery, and observation data from 43 not-for-profit, tertiary care pediatric hospitals in the United States. These hospitals are affiliated with the Children's Hospital Association (Overland Park, KS) and were a mix of stand-alone children's hospitals and hospital-within-a-hospital structures. Data quality and reliability are assured through a joint effort between the Children's Hospital Association and participating hospitals. The data warehouse function for the PHIS database is managed by Truven Health Analytics (Ann Arbor, MI). For the purposes of external benchmarking, participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures. Certain hospitals also submit resource utilization data (e.g. pharmaceuticals, imaging, and laboratory) into the PHIS database. Data are deidentified at the time of data submission, and data are subjected to a number of reliability and validity checks before being included in the database. For this study, 39 hospitals contributed data.
Study Population and Off-Label Medication Status
Subjects were included in the study if they were: 1) less than 18 years of age at hospital discharge, 2) had at least 1 day in a non-neonatal intensive care unit during the year 2010, and 3) had pharmacy charges submitted by their hospital during the study year. Demographic information (age, sex, and race), discharge diagnoses, procedures performed, and outcomes were obtained for each subject meeting inclusion criteria. Complex chronic conditions (CCCs) and acute organ dysfunction were identified by International Classification of Diseases, Ninth Revision (ICD-9) codes and were categorized based on previously developed algorithms.14–17
All unique pharmacy charges with a date occurring within the subjects' intensive care unit (ICU) stay were obtained. We excluded intravenous fluids, electrolyte replacements, topical medications, vitamins, vaccines, and inhaled anesthetics. Because the regulatory process differs for over-the-counter medications as compared to prescribed medications, over-the-counter medications were further excluded from the analysis. High use medications (defined as prescribed in at least 1% of the PICU cohort) were then identified and analyzed for off-label use, defined as use in a subject younger than the FDA-approved age for any indication.18
Schema for Prioritization
Medications of interest, as identified above, were first categorized by: 1) frequency of off-label use, 2) high-risk status, and/or 3) high priority status by the FDA or the Best Pharmaceuticals for Children Act (BPCA). High off-label use was defined as off-label use in at least 5% of the PICU cohort. If a medication was identified by the Institute for Safe Medication Practices (ISMP) as a “high alert” medication, it was categorized as high risk in our study.19 ISMP defines their “high alert” medications as those that “bear a heighted risk of causing significant patient harm when used in error,” irrespective of the frequency of actual medication error. FDA or BPCA high-priority status was determined by either: 1) a written request to the manufacturer by the FDA for pediatric studies, or 2) presence on the BCPA therapeutic priority list.20 Presence on either list was taken as a proxy for lack of pediatric data for a particular medication.
Medications under more than 1 category (frequency of off-label use, high risk, or high priority) were then identified, and those drugs present in all 3 categories were considered to be of potentially highest impact with future research efforts.
Statistical Analyses
The ICU cohort and patterns of off-label use were characterized using descriptive statistics. Subject outcomes (ICU and hospital lengths of stay, death) were compared using Wilcoxon rank sum testing (for the non-normally distributed lengths of stay) and chi-squared analysis. Logistic regression was used to measure the associations between patient characteristics and the receipt of at least 1 medication off-label. Variables with an alpha ≤ 0.05 on bivariate analysis were candidate variables for the multivariable model. Candidate variables with potential for significant collinearity were assessed using Pearson's correlation coefficient. To account for potential center effects, all regression analyses were performed using mixed modeling with PROC GLIMMIX.21 Risk estimates were presented as odds ratios with 95% confidence intervals (CIs). All analyses were performed using STATA 9.2 (Stata Corp, College Station, TX) or SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Cohort Characteristics and Outcomes
In 2010, among the 39 centers, 66,896 subjects met inclusion criteria and received at least 1 of the frequently prescribed medications (n = 163 unique drugs) during their ICU stay. Among the cohort, 56,968 (85%) had received at least 1 of these medications off-label. The average number of off-label medications a patient was exposed to was 4.5 (median, 3; IQR, 2–6). The average age of the cohort was 5.4 ± 5.7 years with a slight male predominance (55%). The most common identified races were white (64%) and black (17%). Over half had at least 1 CCC present during their hospitalization. The average PICU and hospital lengths of stay were 6 days (median, 2; IQR, 1–5) and 12 days (median, 5; IQR, 3–12). The overall hospital mortality rate for the entire ICU cohort was 3%.
Risk Factors for Receiving Off-Label Medications
In the unadjusted analysis, many characteristics were significantly associated with an increased risk of receiving a medication off-label (Table 1). Children age 5 years and younger were more likely to receive a drug off-label as were patients from the Asian/Pacific Islander race. Additionally, children who had received a medication off-label were more likely to have at least 1 CCC as compared to those who had not received any medications off-label. They were also more likely to have at least 1 organ failure and require critical care interventions. Similarly, children who had been exposed to at least 1 medication off-label had longer median PICU and hospital lengths of stay (2 days vs 1 day, and 6 days vs 3 days, respectively, p < 0.001) and more frequent deaths (3.6% vs 0.7%, p < 0.001).
Table 1.
Patient Characteristics Associated With Receiving at Least 1 Medication Off-Label
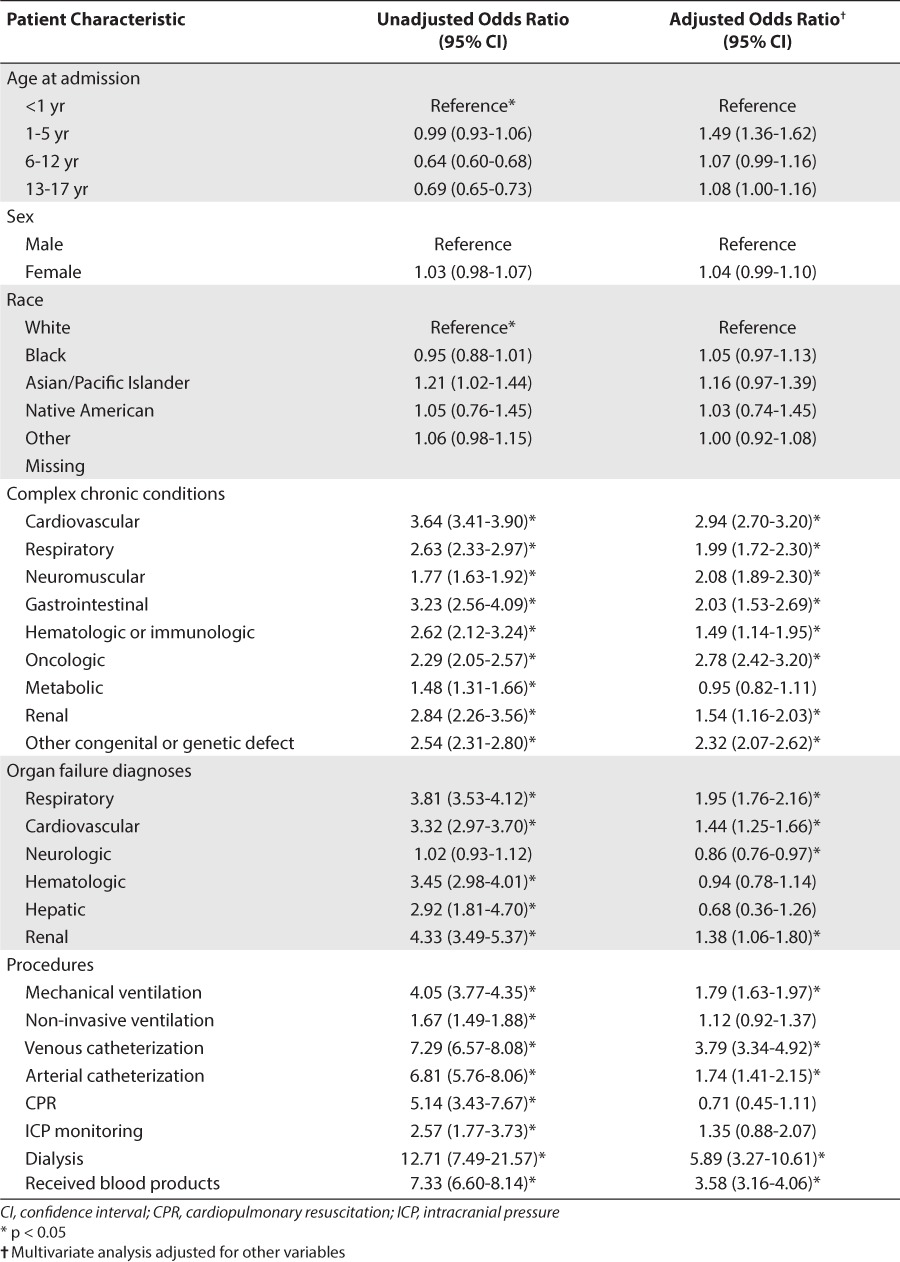
Through multivariable modeling, many of the same characteristics were identified as independently associated with the receipt of at least 1 medication off-label (Table 1). The highest risk age group was 1 to 5 years, while race and gender were not associated with a difference in risk. All of the CCC except metabolic remained significantly associated with an increased risk estimate. The CCCs with the strongest associations were in the categories of oncologic, cardiovascular, neuromuscular, and congenital or genetic syndromes. Children with respiratory, cardiovascular, or renal failure were at increased risk, while those with neurological failure had a slightly decreased risk. Requirement for mechanical ventilation, venous or arterial catheterization, and dialysis and blood products were all independently associated with an increased risk of receiving an off-label medication.
Medications Prescribed, Off-Label Status, and Prioritization
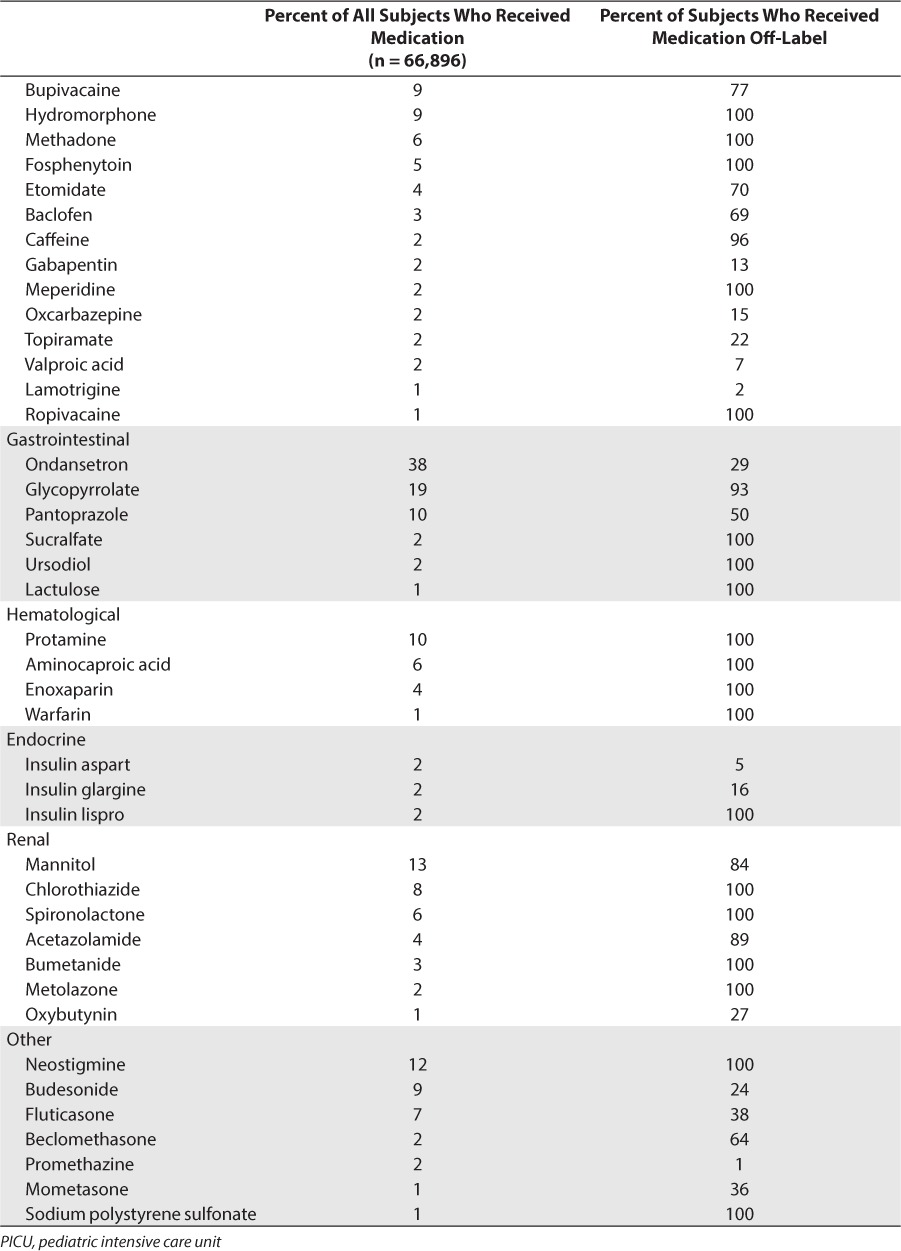
For the entire cohort, 163 individual drugs met inclusion criteria and were considered “high use” medication in the ICU population. Most of these drugs came from the therapeutic categories of neurological (24%), antimicrobial (22%), and cardiovascular (18%). Of these medications, 84 (52%) had been prescribed off-label in at least 1 subject (Table 2). Approximately half (46/84) of the medications prescribed off-label did not have any FDA-approved pediatric indications, with the remainder having at least 1 FDA-approved indication for some less than 18 years of age. The largest number of unique drugs that had been prescribed off-label came from the cardiovascular (20/84, 24%) and neurological (22/84, 26%) therapeutic categories.
Table 2.
Most Frequently Prescribed Medications During PICU Stay (Received by at Least 1% of Patients) With Off-Label Use
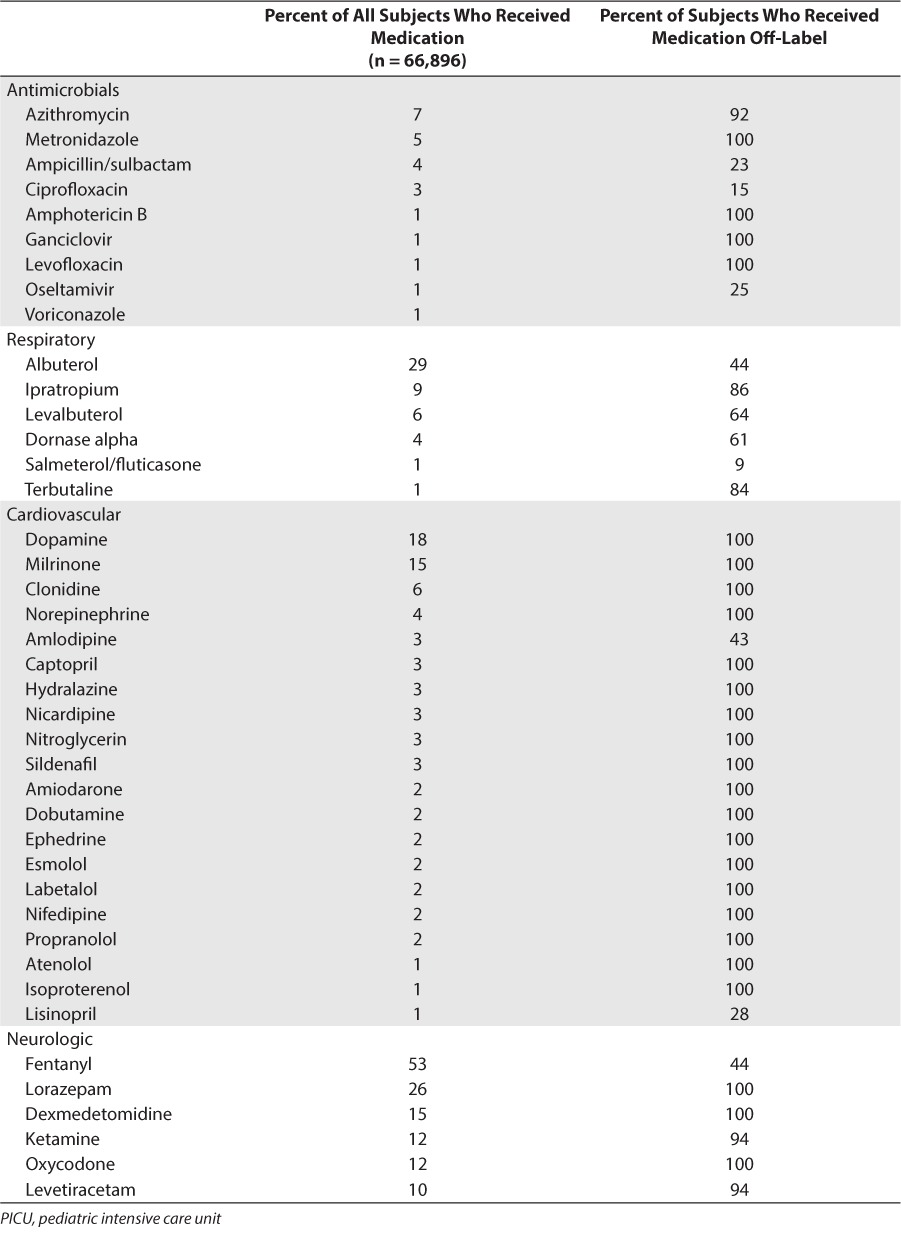
Table 2.
Most Frequently Prescribed Medications During PICU Stay (Received by at Least 1% of Patients) With Off-Label Use (cont.
Prioritization by Frequency, Risk, and the FDA/BPCA
Seventy of the 84 medications (83%) that had been used at least once in an off-label manner met at least 1 category of prioritization (significant off-label use, high risk category, or high prioritization by the FDA or the BPCA; Table 3). Twenty-six of the 162 medications of interest had been used off-label in at least 5% of the PICU cohort, with half having been used in at least 10% of the cohort. Eleven medications (42%) were of the neurological category. Thirty medications were considered as high-risk medication by ISMP, most of which are within the cardiovascular (12/30, 40%) or neurological categories (11/30, 37%). Forty-seven medications had already been identified by the FDA and/or BPCA as a high priority drug for pediatric research with cardiovascular and neurologic medications as the most commonly identified.
Table 3.
Top Medications Used Off-Label in the Pediatric Intensive Care Unit (PICU) and Suggested Prioritization Based on High Off-Label Use (Off Label Use in at Least 5% of the Cohort), High Risk Status (As Identified by the Institute of Safe Medication Practices) and High Regulatory Priority (As Identified by Either the US Food and Drug Administration or Through the Efforts Under the Best Pharmaceuticals for Children Act [BPCA])
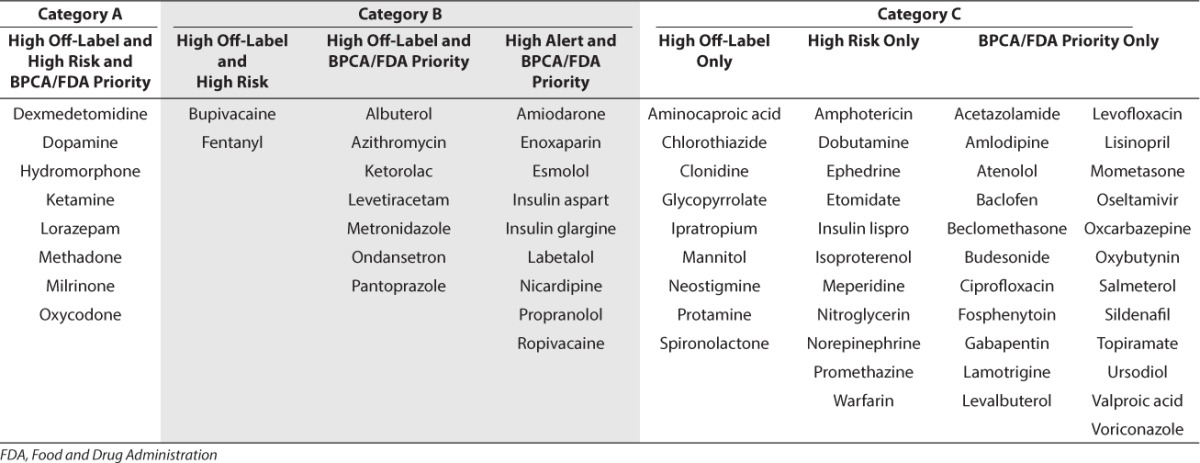
Twenty-six off-label medications had overlap in at least 2 categories, with only 8 meeting the criteria of significant frequency, high risk, and FDA/BPCA priority status. These medications were dexmedetomidine, dopamine, hydromorphone, ketamine, lorazepam, methadone, milrinone, and oxycodone. All but 1 (methadone) had been used in at least ~10% of the ICU cohort. All of the highest priority drugs were cardiovascular or neurological medications, and none other than ketamine (approved for ages 16 years and above) had FDA approval for use in patients younger than 18 years for any indication at the time of our analysis.
DISCUSSION
In this multicenter evaluation of off-label medication use, we found that a majority of patients received at least 1 medication in an off-label manner during their PICU admission. These patients were more likely to be young, have existing chronic complex conditions, more frequent organ failures, and require more intensive support. They were also more likely to have longer lengths of stay and higher mortality. Of the most frequently prescribed medications, over half had been used in an age group younger than that approved by the FDA for any indication. Based on frequent off-label use, high-risk status, and national prioritization, most of these off-label medications met at least 1 criterion for high prioritization with almost half meeting more than 1. Eight drugs met all 3 criteria, all of which were in cardiovascular or neurological therapeutic categories.
Our findings of high off-label medication exposure in the PICU population are consistent with prior single-center studies.8,9,11,12 There are certainly benefits to off-label use of medications.22 Off-label use has allowed access to potentially therapeutic benefits not otherwise available to pediatric patients. Additionally, using medications for other non-approved indications has led to innovative new therapies for certain pediatric diseases.23 Yet, using a medication off-label may mean prescribing with limited information about drug dosing, effectiveness, and side effects, although this is not always the case. In the field of pediatrics, medication dosing is often extrapolated from adult studies. This strategy, however, may not be appropriate for the developing child with varying ability for drug metabolism and elimination.1,24 This can be further complicated by the disturbed physiology of the critically ill child, often with multiorgan failure, who was at greatest risk of receiving an off-label medication in our study. These differences can then result in under or overdosing of a medication and the associated risk of therapeutic failures or adverse events.
Not surprisingly, the medications used off-label with greatest frequency were from the cardiovascular or neurological therapeutic categories. Aside from antimicrobials, these are some of the most frequently used medications in the ICU in both a therapeutic as well as supportive manner. The implications of off-label use, however, may be more significant given the potential for immediate adverse events as well as longer-term ones.25–27 Cardiovascular medications, if under- or overdosed, may result in hemodynamic instability and/or impaired oxygen delivery. Neurological medications, similarly, could result in acute instability in cardiopulmonary status as well as neurologic. Furthermore, as evidence has been emerging on the potential for longer-term adverse effects of sedatives, unclear dosing or unknown therapeutic benefits raises additional questions about the risk-vs-benefit profile of certain neurological medications.28,29 These concerns, in addition to the high frequency of use, make these drugs particularly good targets for additional pediatric studies.
Pediatric patients have frequently been referred to as “therapeutic orphans” because of the limited pediatric data on a majority of medications. Recognizing this, there has been national and legislative efforts to improve our knowledge of medications used in pediatrics. Significant legislative efforts to advance pediatric drug research have included the Pediatric Research Equity Act (previously Pediatric Final Rule), the Pediatric Exclusivity Provision, and the BPCA.30 These legislative changes support increased financial incentives to industry, increase power by the FDA to require pediatric studies on drugs with high likelihood of pediatric use, and provide a mechanism to study pediatric medications outside of the manufacturer's purview. Such efforts have led to increased pediatric clinical studies but the progress can be slow.24,31,32
One highly promising effort is the Pediatric Trials Network (PTN; pediatrictrials.org), sponsored by the National Institute of Child Health and Human Development.33 The PTN is a network of clinical research sites within the United States that is “studying the formulation, dosing, efficacy, and safety, of drugs…used in pediatric patients.” One example of an on-going study that may directly involve critically ill children is the Pharmacokinetics of Understudied Drugs Administered to Children Per Standard of Care (PTN_POPS). This study is specifically interested in obtaining pharmacokinetic information on medications that are understudied in pediatric patients and special populations such as obese patients or those requiring extra-corporeal support. Currently, 7 of the actively studied medications in the PTN_POPS study met at least 1 criterion for high priority in our study, 1 (methadone) of which was identified as particularly high impact in our study (personal communication: Peter Mourani, Children's Hospital Colorado, June 4, 2013). The findings from such studies and others will hopefully lead to more safe and effective use in our patients.
Several limitations to our study deserve acknowledgement. First, we used an administrative database in which a medication charge was used as proxy for actual receipt of medication. While this allowed for a large national sample, this could increase the risk of misclassification. However, we believe the likelihood of this occurring would be sufficiently low to not impact our findings. We also were unable to determine whether certain medications (e.g. non-intravenous medications) were initiated with the PICU or were continued from outpatient (reflecting outpatient rather than PICU off-label prescribing). Additionally, because we did not have the indications for prescribed medications, we were limited to using age only as an indicator for off-label use. Therefore, the estimated rate of off-label use in our study could have been an underestimate if medications were used for a different indication than that approved for a particular pediatric age range, resulting in a different prioritization list. Furthermore, the data only came from pediatric centers, limiting the generalizability of our findings to children cared for in non-pediatric centers. Finally, our prioritization scheme was developed empirically and did not include costs as a consideration. Other approaches may result in a different set of identified medications for high-impact future research.
CONCLUSIONS
Off-label prescribing remains a challenging reality for pediatric providers, and the decision-making becomes even more difficult with critically ill children. Although they comprise only a small percentage of the pediatric population, children who require ICU support are a uniquely vulnerable population deserving of special attention. They often have underlying vulnerability related to chronic conditions, which may be further exacerbated when critically ill.34 Additionally, as seen in our study, they are exposed to multiple different medications, often in an off-label manner. It is unreasonable to expect that off-label prescribing cease, for children deserve access to potentially beneficial therapies. However, this practice mandates careful consideration of risk vs benefit in medical decision-making by providers based on the existing evidence including expert guidelines. Parents and children, alike, trust their health care professionals to make the best decision in this respect.35,36 Furthermore, as stated by the American Academy of Pediatrics Committee on Drugs, “Physicians who choose to prescribe a medication with limited pediatric data have a public and professional responsibility to assist in the systematic development of the information about that drug for the benefit of other patients.”3 Hopefully, our findings serve as a starting point for focusing our attention on which lines of drug research might have the greatest impact on safe and effective use in critically ill children.
ACKNOWLEDGMENT
A portion of these results were presented at the annual meeting of the Pediatric Academic Societies, Washington, DC, May 4–7, 2013.
ABBREVIATIONS
- BPCA
Best Pharmaceuticals for Children Act
- CCC
complex chronic condition
- CI
confidence interval
- FDA
Food and Drug Administration
- ICU
intensive care unit
- ISMP
Institute for Safe Medication Practices
- IQR
interquartile range
- PHIS
Pediatric Health Information System
- PICU
pediatric intensive care unit
- PTN
Pediatric Trials Network
- PTN_POPS
Pharmacokinetics of Understudied Drugs Administered to Children Per Standard of Care
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Meadows M. Drug research and children. FDA Consum. 2003;37(1):12–17. [PubMed] [Google Scholar]
- 2.Bourgeois FT, Murthy S, Pinto C et al. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012;130(2):285–292. doi: 10.1542/peds.2012-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee on Drugs, American Academy of Pediatrics Uses of drugs not described in the package insert (off-label uses) Pediatrics. 2002;110(1 pt 1):181–183. [PubMed] [Google Scholar]
- 4.Sachs AN, Avant D, Lee CS et al. Pediatric information in drug product labeling. JAMA. 2012;307(18):1914–1915. doi: 10.1001/jama.2012.3435. [DOI] [PubMed] [Google Scholar]
- 5.Cuzzolin L, Zaccaron A, Fanos V. Unlicensed and off-label uses of drugs in paediatrics: a review of the literature. Fundam Clin Pharmacol. 2003;17(1):125–131. doi: 10.1046/j.1472-8206.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr. 2005;164(9):552–558. doi: 10.1007/s00431-005-1698-8. [DOI] [PubMed] [Google Scholar]
- 7.McDonnell C, Hum S, Frndova H, Parshuram CS. Pharmacotherapy in pediatric critical illness: a prospective observational study. Paediatr Drugs. 2009;11(5):323–331. doi: 10.2165/11310670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Zuppa AF, Adamson PC, Mondick JT et al. Drug utilization in the pediatric intensive care unit: monitoring prescribing trends and establishing prioritization of pharmacotherapeutic evaluation of critically ill children. J Clin Pharmacol. 2005;45(11):1305–1312. doi: 10.1177/0091270005280966. [DOI] [PubMed] [Google Scholar]
- 9.Doherty DR, Pascuet E, Ni A, Stewart P et al. Off-label drug use in pediatric anesthesia and intensive care according to official and pediatric reference formularies. Can J Anaesth. 2010;57(12):1078–1088. doi: 10.1007/s12630-010-9395-0. [DOI] [PubMed] [Google Scholar]
- 10.Yang CP, Veltri MA, Anton B et al. Food and Drug Administration approval for medications used in the pediatric intensive care unit: a continuing conundrum. Pediatr Crit Care Med. 2011;12(5):e195–e199. doi: 10.1097/PCC.0b013e3181fe25b9. [DOI] [PubMed] [Google Scholar]
- 11.Hsu B, Brazelton T. Off-label medication use in an academic hospital pediatric critical care unit. WMJ. 2009;108(7):343–348. [PubMed] [Google Scholar]
- 12.Turner S, Gill A, Nunn T et al. Use of “off-label” and unlicensed drugs in paediatric intensive care unit. Lancet. 1996;347(9000):549–550. [PubMed] [Google Scholar]
- 13.Eiland LS, Knight P. Evaluating the off-label use of medications in children. Am J Health Syst Pharm. 2006;63(11):1062–1065. doi: 10.2146/ajhp050476. [DOI] [PubMed] [Google Scholar]
- 14.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–209. [PubMed] [Google Scholar]
- 15.Simon TD, Berry J, Feudtner C et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655. doi: 10.1542/peds.2009-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston JA, Yi MS, Britto MT, Mrus JM. Importance of organ dysfunction in determining hospital outcomes in children. J Pediatr. 2004;144(5):595–601. doi: 10.1016/j.jpeds.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123(3):849–857. doi: 10.1542/peds.2008-0856. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration Drug Information Website. Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed May 2013.
- 19.Institue for Safe Medication Practices List of High-Alert Medication. http://www.ismp.org/Tools/institutionalhighAlert.asp. Accessed June 6, 2013.
- 20.BPCA Priority List of Pediatric Therapeutic Needs. http://bpca.nichd.nih.gov/prioritization/status.cfm. Accessed June 6, 2013.
- 21.Greenland S. Principles of multilevel modelling. Int J Epidemiol. 2000;29(1):158–167. doi: 10.1093/ije/29.1.158. [DOI] [PubMed] [Google Scholar]
- 22.Bright JL. Positive outcomes through the appropriate use of off-label prescribing. Arch Intern Med. 2006;166(22):2554–2555. doi: 10.1001/archinte.166.22.2554-b. author reply 2555. [DOI] [PubMed] [Google Scholar]
- 23.Leibovitch L, Matok I, Paret G. Therapeutic applications of sildenafil citrate in the management of paediatric pulmonary hypertension. Drugs. 2007;67(1):57–73. doi: 10.2165/00003495-200767010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez W, Selen A, Avant D et al. Improving pediatric dosing through pediatric initiatives: what we have learned. Pediatrics. 2008;121(3):530–539. doi: 10.1542/peds.2007-1529. [DOI] [PubMed] [Google Scholar]
- 25.Conroy S. Association between licence status and medication errors. Arch Dis Child. 2011;96(3):305–306. doi: 10.1136/adc.2010.191940. [DOI] [PubMed] [Google Scholar]
- 26.Neubert A, Dormann H, Weiss J et al. The impact of unlicensed and off-label drug use on adverse drug reactions in paediatric patients. Drug Saf. 2004;27(13):1059–1067. doi: 10.2165/00002018-200427130-00006. [DOI] [PubMed] [Google Scholar]
- 27.Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr. 1999;88(9):965–968. doi: 10.1080/08035259950168469. [DOI] [PubMed] [Google Scholar]
- 28.Grant MJ, Scoppettuolo LA, Wypij D, Curley MA. Prospective evaluation of sedation-related adverse events in pediatric patients ventilated for acute respiratory failure. Crit Care Med. 2012;40(4):1317–1323. doi: 10.1097/CCM.0b013e31823c8ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith HA, Fuchs DC, Pandharipande PP et al. Delirium: an emerging frontier in the management of critically ill children. Crit Care Clin. 2009;25(3):593–614. x. doi: 10.1016/j.ccc.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zajicek A. The National Institutes of Health and the Best Pharmaceuticals for Children Act. Paediatr Drugs. 2009;11(1):45–47. doi: 10.2165/0148581-200911010-00015. [DOI] [PubMed] [Google Scholar]
- 31.Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;290(7):905–911. doi: 10.1001/jama.290.7.905. [DOI] [PubMed] [Google Scholar]
- 32.Dimasi JA. Innovating by developing new uses of already-approved drugs: trends in the marketing approval of supplemental indications. Clin Ther. 2013;35(6):808–818. doi: 10.1016/j.clinthera.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Pediatric Trials Network. https://pediatric-trials.org/. Accessed June 6, 2013.
- 34.Kane-Gill SL, Jacobi J, Rothschild JM. Adverse drug events in intensive care units: risk factors, impact, and the role of team care. Crit Care Med. 2010;38(suppl 6):S83–S89. doi: 10.1097/CCM.0b013e3181dd8364. [DOI] [PubMed] [Google Scholar]
- 35.Yoon EY, Clark SJ, Butchart A et al. Parental preferences for FDA-approved medications prescribed for their children. Clin Pediatr (Phila) 2011;50(3):208–214. doi: 10.1177/0009922810385105. [DOI] [PubMed] [Google Scholar]
- 36.Mukattash T, Trew K, Hawwa AF, McElnay JC. Children's views on unlicensed/off-label paediatric prescribing and paediatric clinical trials. Eur J Clin Pharmacol. 2012;68(2):141–148. doi: 10.1007/s00228-011-1110-8. [DOI] [PubMed] [Google Scholar]


