Abstract
OBJECTIVES: The physical and chemical stability of a preservative-free oral solution of hydrocortisone succinate was studied at different pH values and storage temperatures.
METHODS: Oral solutions of hydrocortisone 1 mg/mL were prepared by dissolving hydrocortisone succinate powder in citrate buffers at pH 4.0, 5.5, and 6.5, or with sterile water (pH 7.4) stored in amber glass vials. Three identical samples of the formulations were prepared and stored under refrigeration (3–7°C), ambient temperature (20–22°C) and high temperature (29–31°C). A 200-μL sample was withdrawn from each of the 3 samples immediately after preparation and at 1, 7, 14, 21, and 35 days. Samples were assayed in duplicate using stability-indicating liquid chromatography. Stability was determined by evaluating the percentage of the initial concentration remaining at each time point; stability was defined as the retention of at least 90% of the initial concentration of hydrocortisone succinate.
RESULTS: At least 92% of the initial hydrocortisone succinate concentration in solutions pH 5.5, 6.5, and 7.4 remained throughout the 14-day study period under refrigeration. There were no detectable changes in color, odor, or pH and no visible microbial growth in these samples. In other storage conditions, hydrocortisone succinate was rapidly degraded.
CONCLUSIONS: The hydrocortisone succinate preservative-free oral solutions at pH 5.5, 6.5, or 7.4 are chemically stable when stored under refrigeration for at least 14 days. They provide flexible and convenient dosage forms without any preservatives for pediatric patients.
INDEX TERMS: drug stability, hydrocortisone, infant, pharmaceutical solutions
INTRODUCTION
Congenital adrenal hyperplasia is a group of disorders resulting from the deficiency of 1 of the 5 enzymes required for the synthesis of cortisol in the adrenal cortex. The most frequent is steroid 21-hydroxylase deficiency.1,2 Clinical manifestations are postnatal virilization and a salt-wasting syndrome in severe form with a concurrent defect in aldosterone biosynthesis.3,4 Patients require long-term glucocorticoid treatment to inhibit excessive secretion of corticotropin-releasing hormone and corticotrophin by the hypothalamus and pituitary gland, and to reduce elevated levels of adrenal sex steroids.1,5 In children, the proffered drug is hydrocortisone in maintenance doses of 10 to 20 mg/m2/day.1,6 Hydrocortisone is commercially available in tablets; however, the smallest dose is 5 mg. For very young children, tablets are usually crushed into powder to be mixed with food vehicles. This practice cannot assure good dissolution and hence therapeutic activity of hydrocortisone. An oral liquid form would be highly desirable for patients with swallowing problems such as pediatric patients. In addition a liquid form allows the dose to be easily adjusted. Few data exist on the stability of hydrocortisone oral suspension and all preparations used preservatives such as parabens.7,8 The purpose of this study was to design a hydrocortisone oral liquid formulation that allows for the optimal stability conditions of the active principle and that minimizes the number of necessary excipients. This formulation contained any excipients with notorious effect such as parabens, alcohol, and tween. Therefore, an aqueous solution of hydrocortisone was considered.
The corticosteroids are a group of compounds having poor aqueous solubility that are frequently solubilized as a labile ester prodrug for use by the parenteral route. Hydrocortisone 21-succinate ester is water soluble derivative and commercially available as a lyophilized powder for reconstitution. Consequently we chose to study the stability of hydrocortisone succinate in aqueous solution at different pH values and at 3 controlled storage temperatures.
METHODS
Sample Preparation
Various oral solutions of hydrocortisone 1 mg/mL were prepared by dissolving appropriate amount of hydrocortisone sodium succinate powder (1.34 mg/mL; hydrocortisone; Upjohn, Paris, France) in citric acid (2 g/L; Cooper, Melun, France) adjusted to pH 4.0, 5.5, or 6.5 with sodium hydroxide (1N). An oral solution exempt of citrate was obtained by dilution of hydrocortisone sodium succinate powder (1.34 mg/mL) in sterile water (Versylene, Fresenius Kabi, Sèvres, France) to yield a concentration of hydrocortisone base at 1 mg/mL and a sodium concentration at 2.8 mmol/L. The measured pH of this solution was 7.4. Another solution of hydrocortisone 1 mg/mL was obtained by addition of hydrochloric acid (1N) in sterile water to reach pH 4.0.
Three identical samples of each formulation were prepared in amber glass vials and stored shielded from light at temperatures of 5 ± 2°C, in a controlled environment room at 21 ± 1°C, and in the incubator at 30 ± 1°C. From each solution, a 200-μL sample was taken and transferred to glass tubes. Two hundred microliters of dexamethasone acetate (1 mg/mL in methanol, Cooper) was added as the internal standard (IS). Sample was then diluted to 10 mL with sterile water to give a hydrocortisone 20 mg/L concentration. Diluted 25-μL solution was assayed in duplicate using high-performance liquid chromatography (HPLC). The physical appearance of each solution was assessed visually. The apparent pH was measured by digital pH-meter (Mettler-Toledo, Viroflay, France) at the beginning and at the end of the study. Samples were analyzed after preparation and at 1, 7, 14, 21, and 35 days.
HPLC Analysis
An HPLC method was adapted for separating and quantifying hydrocortisone succinate.9 The equipment consisted of a liquid chromatographic system (Dionex Ultimate 3000, Courtaboeuf, France) with a photodiode array detector set at a wavelength of 242 nm, an injector delivering 25 μL, a recording integrator, and a C18 column (Nova-Pak, 4.6 × 150 mm, 4 μm, Waters, Guyancourt, France) regulated at 21°C. Hydrocortisone succinate, its degradation products, and the IS (dexamethasone acetate 20 mg/L) were separated isocratically. The mobile phase consisted of a 60:40 mixture of methanol (Lichrosolv, Merck, Fontenay, France) and 0.01 M acetic acid delivered at a rate of 1.2 mL/min. Hydrocortisone succinate and IS eluted at 4.6 minutes and 8.1 minutes, respectively (Figure 1).
Figure 1.
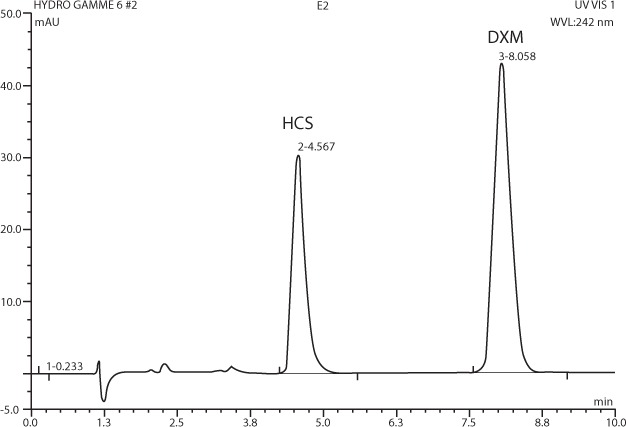
Chromatogram of hydrocortisone succinate (HCS; retention time = 4.6 min) and dexamethasone acetate (DXM) used as an internal standard (retention time = 8.1 min).
The stability-indicating capability of the assay was evaluated in the laboratory. Decomposition of hydrocortisone succinate solution was forced by exposing 3 separate samples with addition of hydrogen peroxide (Cooper), hydrochloric acid (pH 2), or sodium hydroxide (pH 12; Figure 2). Accelerated degradation was also obtained by heating 3 samples at 30 ± 1°C. The sample pH was adjusted to neutral pH and diluted with mobile phase to an expected concentration of 50 mg/L, and assayed without IS.
Figure 2.
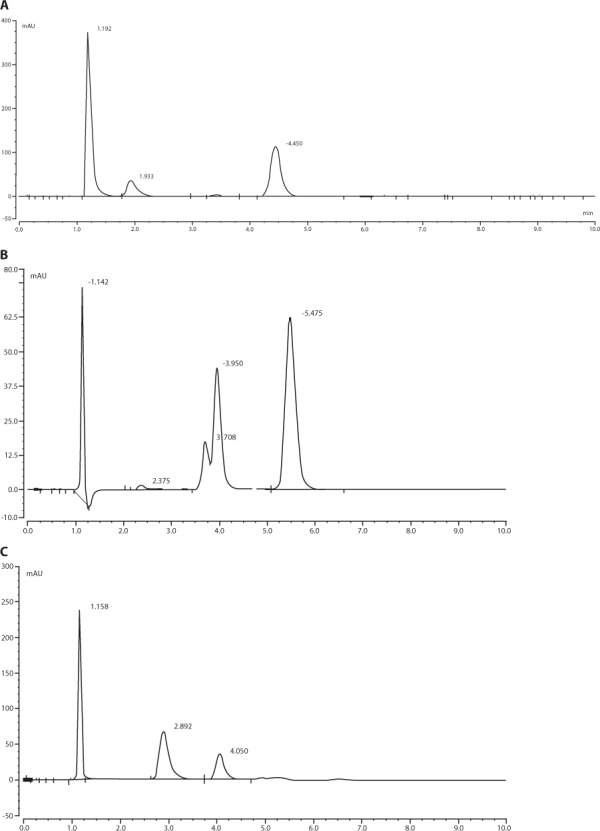
Chromatograms of hydrocortisone succinate (50 μg/mL) incubated at room temperature for 60 min with hydrogen peroxide (A), with sodium hydroxide for 90 min (B), and with hydrochloric acid for 90 min (C).
Preparations of Standard Solutions and Standard Curve
A 1 mg/mL (base) stock solution of hydrocortisone succinate was prepared with sterile water on each day sample analysis. Standard dilutions were diluted in sterile water to yield hydrocortisone concentrations of 5, 10, 20, 40, and 50 mg/L with IS. The standard curve was constructed by linear regression of the peak heights ratio of the hydrocortisone succinate/IS against hydrocortisone (base) concentration. It was used to calculate the drug concentrations in the samples (Figure 3). The least squares method showed that the curve was linear (r2 = 0.9972 ± 0.0040; n = 5) over the working range concentration and give an equation with a slope of 0.0814 ± 0.0129 (n = 5) and an intercept at −0.0270 ± 0.0536 (n = 5). The intraday and interday coefficients of variation for the hydrocortisone assay, measured with 10, 20, and 40 mg/L, were less than 1.3% (n = 3) and 2.1% (n= 5), respectively. The detection limit was 2 mg/L (signal to noise 3/1).
Figure 3.
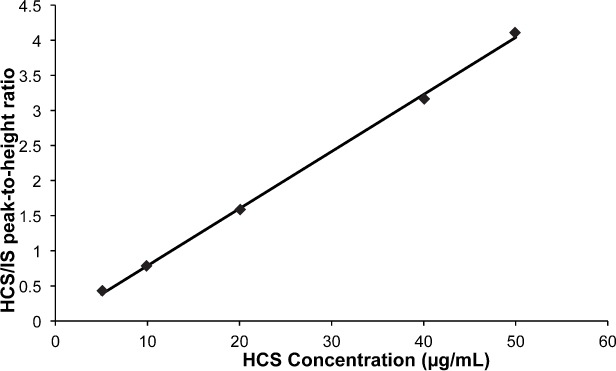
Hydrocortisone calibration curve obtained by linear regression of the peaks heights ratio versus hydrocortisone (base) concentration.
Sample Analysis
Each sample was vortexed and mixed as described above, immediately before sampling of an aliquot. Twenty-five microliters of each sample was injected into the HPLC system, and each sample was assayed in duplicate. Three quality control samples (10, 20, and 40 mg/L, assayed in duplicate) were used to validate each day run. The samples were visually inspected for color change and evaluated for pH on each day of analysis.
Data Analysis
All values are means ± standard deviation. The initial concentration of hydrocortisone in each solution was set at 100% and subsequent concentrations are expressed as percentages of the initial concentration of the corresponding solution. A preparation was considered stable if the concentration of hydrocortisone succinate remaining was above 90% of the initial concentration.
RESULTS AND DISCUSSION
The stability indicating capability of the method for hydrocortisone succinate assay was demonstrated using diverse forced degradation experiments (Figure 2) and summarized in Table 1. These forced degradation data proved that the method is specific for the analytes and free from the interference of degradation products.
Table 1.
Summary of Forced Degradation Results
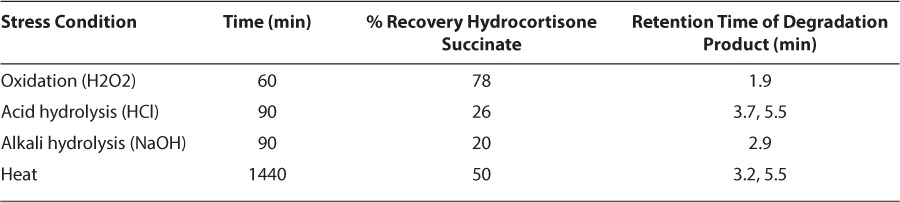
At least 50% loss of the initial concentration of hydrocortisone and a precipitate formation were shown after 1-hour at pH 4.0 regardless of the presence of citrate. Hydrocortisone succinate solutions stored at pH 5.5, 6.5, or 7.4 was degraded after the 4-day study period at 21°C (Table 2; Figure 4A), and 48 hours at 30°C. At least 92% of the initial concentration of hydrocortisone succinate stored under refrigeration at pH 5.5 and 6.5 remained throughout the 21-day study period (Table 3; Figure 4B). The solution of hydrocortisone succinate at pH 7.4 was stable under refrigeration throughout the 14-day study period (Table 2; Figure 4A).
Table 2.
Stability of Hydrocortisone Succinate Solutions (1 mg/mL, Base) at pH 5.5, 6.5, and 7.4, Stored at 20–22°C
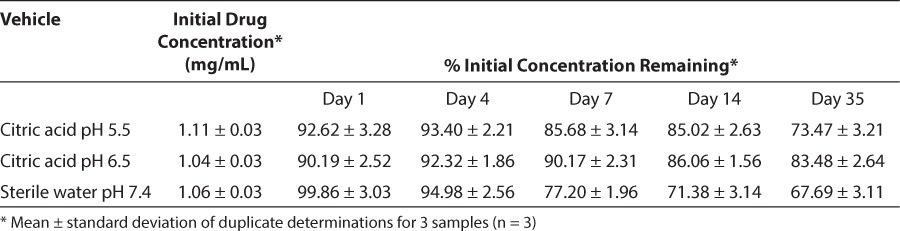
Figure 4.
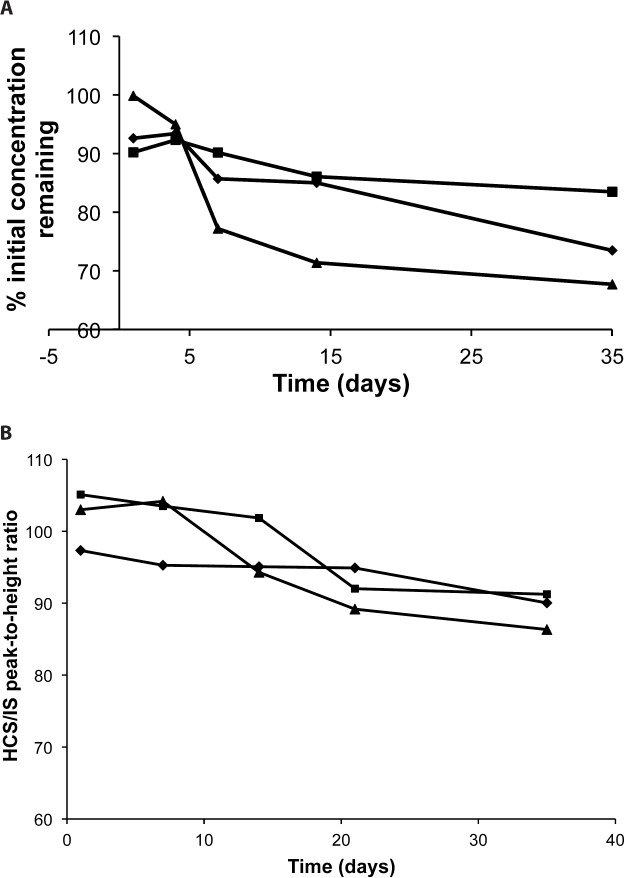
Relationship between hydrocortisone succinate solutions at pH 5.5 (♦), 6.5 (▪), 7.4 (▴), remaining concentration (%), and time of storage at 19–22°C (A) and 3–5°C (B).
Table 3.
Stability of Hydrocortisone Succinate Solutions (1 mg/mL, Base) at pH 5.5, 6.5, and 7.4 Stored at 3–7°C
There were no changes from the initial color, odor, and taste, and no physical indications of microbial growth were observed of any sample except for the solution pH 4.0, which a precipitate occurred almost immediately. No significant changes from the initial pH occurred in any samples (solutions at pH 4.0 were not assessed).
CONCLUSIONS
The hydrocortisone preservative-free oral solutions at different pH are chemically stable when stored under refrigeration for at least 14 days. They provide flexible and convenient pediatric dosage forms without any preservatives.
Abbreviations
- HPLC
high-performance liquid chromatography
- IS
internal standard
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Deslauriers JR, Lenz AM, Root AW et al. Gender related differences in glucocorticoid therapy and growth outcomes among pubertal children with 21-hydroxylase deficiency congenital adrenal hyperplasia (CAH) J Pediatr Endocrinol Metab. 2012;25(9–10):977–981. doi: 10.1515/jpem-2012-0125. [DOI] [PubMed] [Google Scholar]
- 2.Wedell A. Congenital adrenal hyperplasia. Clin Biochem. 2011;44(7):505–506. doi: 10.1016/j.clinbiochem.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Reisch N, Arlt W, Krone N. Health problems in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. 2011;76(2):73–85. doi: 10.1159/000327794. [DOI] [PubMed] [Google Scholar]
- 4.Bonfig W, Schwarz HP. Growth pattern of untreated boys with simple virilising congenital adrenal hyperplasia indicates relative androgen insensitivity during the first six months of life. Horm Res Paediatr. 2011;75(4):264–268. doi: 10.1159/000322580. [DOI] [PubMed] [Google Scholar]
- 5.Claahsen-van der Grinten HL, Stikkelbroeck NM, Otten BJ et al. Congenital adrenal hyperplasia–pharmacologic interventions from the prenatal phase to adulthood. Pharmacol Ther. 2011;132(1):1–14. doi: 10.1016/j.pharmthera.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Bonfig W, Schmidt H, Schwarz HP. Growth patterns in the first three years of life in children with classical congenital adrenal hyperplasia diagnosed by newborn screening and treated with low doses of hydrocortisone. Horm Res Paediatr. 2011;75(1):32–37. doi: 10.1159/000316973. [DOI] [PubMed] [Google Scholar]
- 7.Fawcett JP, Boulton DW, Jiang R et al. Stability of hydrocortisone oral suspensions prepared from tablets and powder. Ann Pharmacother. 1995;29:987–990. doi: 10.1177/106002809502901005. [DOI] [PubMed] [Google Scholar]
- 8.Santovena A, Llabres M, Farina JB. Quality control and physical and chemical stability of hydrocortisone oral suspension: an interlaboratory study. Int J Pharm Compound. 2010;4(5):430–435. [PubMed] [Google Scholar]
- 9.Gupta V. Chemical stability of hydrocortisone in Humco simple syrup and Ora-Sweet vehicle. Int J Pharm Compound. 2010;14(1):76–77. [PubMed] [Google Scholar]


