Abstract
OBJECTIVES: The objective of this study was to evaluate the safety and efficacy of polyethylene glycol-electrolyte solution vs polyethylene glycol-3350 for the treatment of fecal impaction in pediatric patients.
METHODS: A retrospective, observational, institutional review board–approved study was conducted over a 1-year time period. Patients were included in the study if they were admitted to the hospital with a diagnosis of fecal impaction or constipation and were treated with either polyethylene glycol-electrolyte solution (PEG-ES) or polyethylene glycol-3350 (PEG-3350). Patients were excluded if they were discharged prior to resolution of treatment and/or did not receive PEG-ES or PEG-3350.
RESULTS: Fifty-one patients (ranging in age from 1 month to 15 years) were evaluated: 23 patients received PEG-ES and 28 patients received PEG-3350. Sex, race, age, and weight were not statistically different between the 2 groups. Resolution of fecal impaction was not significantly different between PEG-ES vs PEG-3350 (87% and 86%, respectively; p = 0.87). There was only 1 reported side effect with PEG-3350, vs 11 reported side effects with PEG-ES (p < 0.01).
CONCLUSIONS: Theses results suggest that PEG-3350 is as effective as PEG-ES for the treatment of fecal impaction in pediatric patients and is associated with fewer side effects.
INDEX TERMS: constipation, fecal impaction, pediatrics, polyethylene glycol-electrolyte solution, polyethylene glycol-3350
INTRODUCTION
Functional constipation is defined as constipation without an underlying organic abnormality. It occurs in approximately 3% of preschool-aged children and 1% to 2% of school-aged children.1 Functional constipation is responsible for 3% of pediatric outpatient visits and 25% of consultations with a pediatric gastroenterologist.2–5 In children with functional constipation, 30% to 75% experience impaction.6 Fecal impaction is diagnosed by either physical examination or abdominal radiograph or a combination of both and is defined as a large amount of stool in a dilated rectum or colon or a hard mass in the lower abdomen.2,6,7 Fecal impaction can be treated both non-pharmacologically with manual or surgical disimpaction or rectal stimulation or with drug therapy, including oral laxatives, suppositories, and enemas.2
Polyethylene glycol electrolyte solution (PEG-ES) is an osmotic agent that has been studied for efficacy in the treatment of fecal impaction.3 However, it is difficult to administer because of the need to administer a large fluid volume and often requires nasogastric (NG) tube placement.3,8 Polyethylene glycol-electrolyte solution is also associated with many side effects, such as nausea/vomiting, abdominal cramping, bloating, and electrolyte disturbances. Polyethylene glycol-3350 (PEG-3350) is an effective alternative in children with constipation and anecdotally is more tolerable and associated with fewer side effects than is PEG-ES.1,2,4,7 Clinical observation suggests its efficacy in the treatment of fecal impaction.2,7 To date, there are no trials comparing these 2 formulations for the treatment of fecal impaction.
The aims of this study were 1) to evaluate the efficacy of PEG-ES compared to PEG-3350 for the treatment of fecal impaction in pediatric patients and 2) to evaluate the side effects associated with both PEG-ES and PEG-3350.
METHODS
This was a retrospective, observational study evaluating the medical records of pediatric patients (age 0–18 years) with a diagnosis of fecal impaction or constipation who received either of the 2 PEG formulations (PEG-ES [GoLYTELY] or PEG-3350 [MiraLAX]). Patients admitted between October 1, 2010. and September 30, 2011, at Le Bonheur Children's Hospital, Memphis, Tennessee, a freestanding academic children's hospital, were included in this review. This study was approved by the Institutional Review Boards of The University of Tennessee Health Science Center and Le Bonheur Children's Hospital.
Patients were identified based on International Classification of Diseases, Ninth Edition (ICD-9) codes of 560 (fecal impaction) and/or 564 (constipation). Patients were excluded from the study if they were discharged prior to resolution of symptoms, did not receive either agent as primary treatment, or were not prescribed treatment doses (≥0.8 g/kg/day). Data collection consisted of demographic information, past medical history, outpatient and inpatient bowel regimens, impatient complications (manual or surgical disimpaction, perforation), time to resolution of fecal impaction, and discharge bowel regimen. For the purpose of this study, resolution of fecal impaction was defined as a discontinuation or de-escalation in therapy (decreased dose or frequency). Time to resolution was defined as time to discontinuation or de-escalation of therapy.
A power analysis, using a 2-sided test with a significance level of 0.05 and a beta of 0.2, determined that a sample size of 30 patients (i.e., 15 patients in each arm) would be sufficient to detect a 25% difference in days until resolution of fecal impaction, assuming 25% variability in data. Statistical analysis was performed using mean ± SD, Student's t-test, and the chi-square test.
RESULTS
A total of 488 patients with ICD-9 codes of 560 (fecal impaction) and/or 564 (constipation) were screened for inclusion in this study. Of these, 437 patients were excluded because they did not receive either treatment agent, were discharged prior to resolution of symptoms, or were just on a continuation of their home regimen. A total of 51 patients were included for evaluation in the study. Twenty-eight patients were treated with PEG-3350, and 23 patients were treated with PEG-ES (Figure 1).
Figure 1.
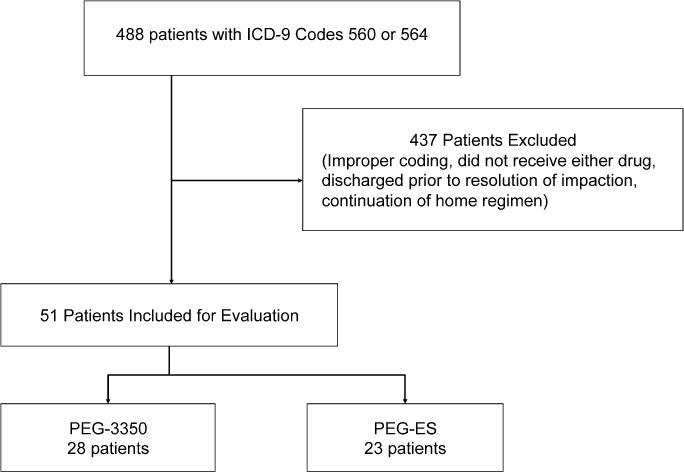
Study inclusion and exclusion. Polyethylene glycol-electrolyte solution (PEG-ES), polyethylene glycol 3350 (PEG-3350), International Classification of Diseases, Ninth Revision (ICD-9).
Patient demographics, including sex, race, age, and weight, were not statistically different between the 2 groups (Table 1). However, a documented past medical history of chronic constipation was statistically different, with more patients in the PEG-ES group having a history of chronic constipation. Additionally, 10 patients were prescribed a regimen of PEG-3350 at home before they started PEG-ES in the hospital, although at-home compliance cannot be confirmed.
Table 1.
Patient Demographics
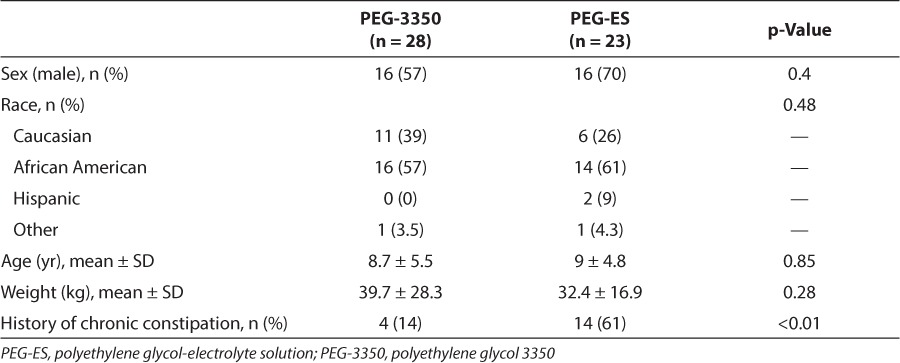
In the PEG-3350 group, 24 of the 28 patients (86%) had resolution with the primary treatment. Four patients failed PEG-3350 therapy and were effectively treated with PEG-ES. In the PEG-ES group, 20 of the 23 patients (87%) had resolution with the primary treatment. Three patients had resolution only after surgical disimpaction (Figure 2). Based on resolution of fecal impaction with primary therapy, there was not a statistically significant difference in efficacy between PEG-3350 and PEG-ES (p = 0.87).
Figure 2.
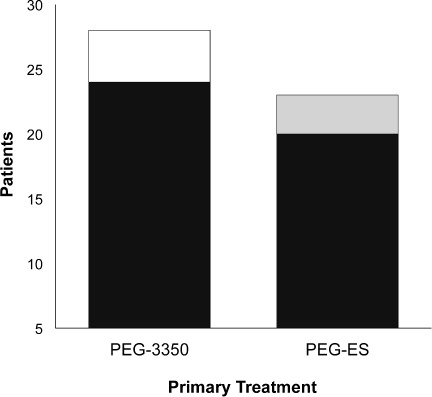
Resolution of fecal impaction. There was no significant difference in the resolution of fecal impaction with polyethylene glycol-electrolyte solution (PEG-ES) (87%) or polyethylene glycol 3350 (PEG-3350) (86%) as a primary treatment.
▪ = resolution with surgical disimpaction; □ resolution when changed to PEG-ES;  resolution with primary treatment.
resolution with primary treatment.
Patients receiving PEG-3350 had a time to resolution of 2.1 days, compared to 2.6 days with PEG-ES (Figure 3). These results were not statistically significant (p = 0.45).
Figure 3.
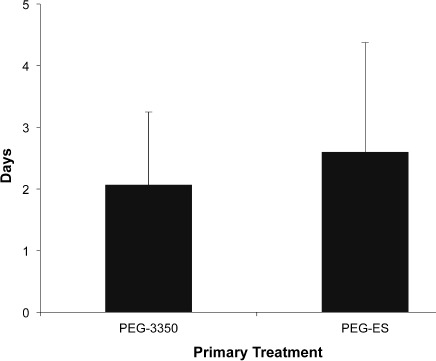
Time to resolution of fecal impaction. There was no significant difference in time to resolution of fecal impaction with polyethylene glycol-electrolyte solution (PEG-ES) or polyethylene glycol 3350 (PEG-3350) as a primary treatment.
Data are represented as mean ± SD.
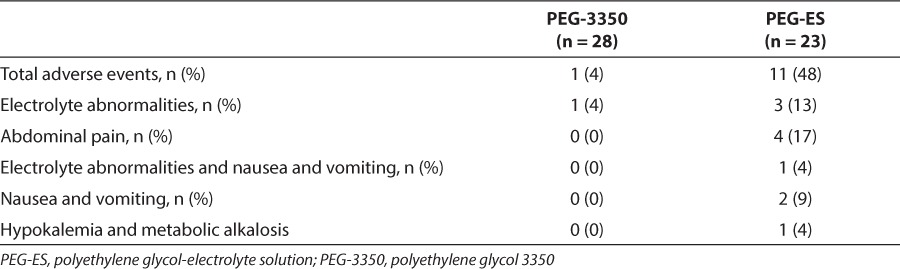
There was only 1 reported side effect in the PEG-3350 group. This patient had electrolyte abnormalities. However, this patient also had an underlying diagnosis of nephrotic syndrome. There were 11 patients (48%) with reported side effects in the PEG-ES group (Table 2). These included 4 patients (17%) with abdominal pain, 3 patients (13%) with electrolyte abnormalities, 1 patient (4%) with electrolyte abnormalities and nausea/vomiting (N/V), and 2 patients (9%) with N/V alone. One patient (4%) had severe symptomatic hypokalemia and metabolic alkalosis that required treatment and transfer to the intensive care unit (ICU) with cardiac monitoring and discontinuation of PEG-ES.
Table 2.
Adverse Events
Placement of an NG tube for the sole purpose of administration of PEG-3350 was not necessary in any of the patients. However, 18 of the 23 patients receiving PEG-ES required NG tube placement for administration (Figure 4). These results were statistically significant (p = 0.01).
Figure 4.
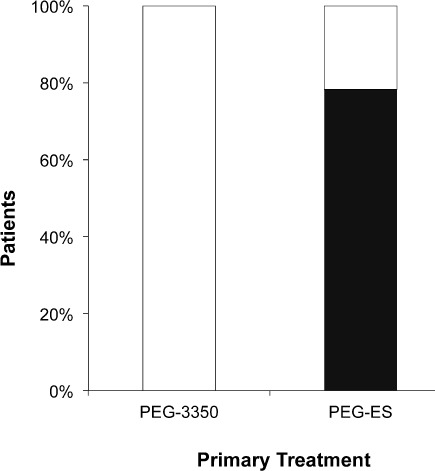
Nasogastric (NG) tube placement. No patients in the polyethylene glycol 3350 (PEG-3350) group required placement of a NG tube, as compared to 78% in the polyethylene glycol-electrolyte solution (PEG-ES) group (p < 0.01).
□ No NG-tube placed; ▪ = NG-tube placed.
DISCUSSION
This is the first reported comparison of the efficacy of PEG-ES and PEG-3350 for the treatment of fecal impaction and constipation in pediatric patients. No significant difference was found in efficacy between the 2 treatment options; however, PEG-ES was associated with more side effects. Nasogastric tube placement was not required for the delivery of PEG-3350, which is not yet labeled for use in the treatment of constipation and fecal impaction in pediatric patients, but as a result of its lower side effect profile, it has become a favorite treatment option by pediatric gastroenterologists and is often prescribed at our institution. Several studies9,10 have already evaluated the efficacy of PEG-3350 in comparison to that of other common laxatives, such as lactulose and magnesium hydroxide, and found that its efficacy was greater than or equal to that of these other laxatives. In a randomized, double-blinded study9 examining the efficacy of PEG-3350 and lactulose, researchers found no significant difference between the groups but higher rates of vomiting and flatulence in the lactulose treatment arm. In a similar study10 comparing PEG-3350 to Milk of Magnesia, no differences in efficacy were found between the 2 groups, though PEG-3350 was better accepted by the pediatric patients. Based upon the minimal existing published research, it is reasonable to question the efficacy and tolerability of PEG-3350 compared to PEG-ES as an alternate treatment for fecal impaction and constipation, thus the reason for conducting this review.
In our study, patients who received PEG-ES as their primary treatment did have a statistically significant history of chronic constipation. Patients with chronic constipation typically require a bowel regimen at home. This regimen oftentimes includes PEG-3350 at prophylactic doses.2 Additionally, the success rates of PEG-ES and PEG-3350 (87% and 86%, respectively) reported in this study were similar to those of other studies of PEG reviewed by Candy et al,11 who reported success rates that range from 56% to 84%.
For the purpose of our retrospective evaluation, time to resolution was defined in the study as discontinuation or de-escalation of therapy. This definition was used for the end point of time to resolution, because oftentimes in practice, time to resolution for the 2 agents differs. Time to resolution for PEG-ES is defined as clear stools, whereas time to resolution for PEG-3350 is defined as first bowel movement. Even though the time to resolution was not statistically different between the 2 agents, it could be considered clinically significant, as this could lead to fewer days in the hospital. There was a half-day difference in time to resolution between PEG-3350 and PEG-ES. This difference could result in earlier discharges, decreased hospitalization costs, and quicker relief of discomfort.
We report a statistically significant difference in side effects. The majority of side effects seen were related to treatment with PEG-ES. Common side effects reported in the literature with PEG-ES include nausea, bloating, abdominal cramps, vomiting, and anal irritation.3,7 Less common but serious side effects associated with PEG-ES are aspiration, pneumonia, pulmonary edema, and Mallory-Weiss tear.7 Previous studies2,7,12 with PEG-3350 show minor adverse effects that did not interfere with compliance with therapy. An earlier study13 examining the safety of poly-ethylene glycol in chronic constipation found adverse effects of PEG-3350 to include diarrhea (10%), bloating/flatulence (6%), and abdominal pain (2%); these patients were on polyethylene glycol for an average duration of 8.7 months. Adverse effects were reported if they happened at any time during this period, and resolution was achieved with dose reduction. No patients in this study13 discontinued PEG-3350 as a result of adverse effects. Several studies1,7,13 evaluating PEG-3350 dosing have found that doses of at least 1 to 1.5 g/kg/day were sufficient in the treatment of fecal impaction and chronic constipation. However, dose titration to reach an effective daily dose while minimizing uncomfortable side effects is appropriate.13
Bekkali et al6 evaluated enemas vs high doses of oral PEG (1.5 g/kg/day) for the treatment of fecal impaction. During this study, the investigators used a questionnaire to evaluate side effects associated with these 2 treatment options. Patients/caregivers reported difficulty in administration, anxiety, and abdominal pain as side effects associated with treatment. Of these, abdominal pain was significantly higher in the enema group compared to the PEG group. These adverse effects include diarrhea, flatulence, and abdominal pain. Additionally, adverse effects are more likely to occur with larger doses of PEG.
Nasogastric tube placement was required in a significant number of patients treated with PEG-ES. On the other hand, PEG-3350 did not require NG tube placement for administration. Placement of an NG tube can lead to discomfort and carries risks such as tissue tearing, bleeding, and tube misplacement into the lungs.3 Because of the smaller volume required for PEG-3350 administration, NG tube placement is not necessary in patients managed with this treatment option. While the efficacy of different formulations of PEG has not been compared in clinical trials, Lam et al14 conducted a randomized, crossover, double-blind study to evaluate the taste of 3 different PEG-3350 formulations available in Europe. The investigators determined that the formulation palatability may affect compliance in patients with chronic constipation.
Our study does have some limitations due to the retrospective observational nature of the study. Although the sample size was adequate to detect a 25% difference in days until resolution of fecal impaction, a larger sample size is needed to detect statistical significance of our secondary outcomes. As previously mentioned, patients who received PEG-ES had a significantly greater history of chronic constipation, which may suggest that they require more aggressive treatment or that it would take longer for fecal impaction to resolve, indicating why PEG-3350 seemed to result in more rapid resolution of symptoms. Additionally, there were some patients who were on a regimen of PEG-3350 at home before they started PEG-ES in the hospital.
Based on this retrospective study, the following conclusions have been made: PEG-3350 is as effective as PEG-ES for the treatment of non-chronic fecal impaction in pediatric patients; PEG-3350 is associated with fewer and less serious side effects compared to PEG-ES; and PEG-3350 administration is less invasive to pediatric patients. Prospective randomized studies are needed to confirm these results.
ACKNOWLEDGMENTS
Poster was presented at ASHP Mid-Year Clinical Meeting in December 2011 in New Orleans, Louisiana. Preliminary data were presented at the PPAG Annual Conference in April 2012 in Houston, Texas. Completed research was presented at the Mid-South Pharmacy Resident's Annual Conference in May 2012 in Memphis, Tennessee.
ABBREVIATIONS
- ICD-9
International Classification of Diseases, Ninth Revision
- ICU
intensive care unit
- NG
nasogastric
- N/V
nausea/vomiting
- PEG-ES
polyethylene glycol-electrolyte solution
- PEG-3350
polyethylene glycol 3350
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Bell EA, Wall GC. Pediatric constipation therapy using guidelines and polyethylene glycol 3350. Ann Pharmacother. 2004;38(4):686–693. doi: 10.1345/aph.1D297. [DOI] [PubMed] [Google Scholar]
- 2.Constipation Guideline Committee of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Evaluation and treatment of constipation in infants and children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2006;43(3):e1–e13. doi: 10.1097/01.mpg.0000233159.97667.c3. [DOI] [PubMed] [Google Scholar]
- 3.Candy DCA, Edwards D, Geraint M. Treatment of faecal impaction with polyethylene glycol plus electrolytes (PEG+E) followed by a double-blind comparison of PEG-E versus lactulose as maintenance therapy. J Pediatr Gastroenterol Nutr. 2006;43(1):65–70. doi: 10.1097/01.mpg.0000228097.58960.e6. [DOI] [PubMed] [Google Scholar]
- 4.Philichi L. When the going gets tough: pediatric constipation and encopresis. Gastroenterol Nurs. 2008;31(2):121–130. doi: 10.1097/01.SGA.0000316531.31366.27. [DOI] [PubMed] [Google Scholar]
- 5.Walia R, Mahajan L, Steffen R. Recent advances in chronic constipation. Curr Opin Pediatr. 2009;21(5):661–666. doi: 10.1097/MOP.0b013e32832ff241. [DOI] [PubMed] [Google Scholar]
- 6.Bekkali NLH, van den Berg MM, Dijkgraaf MGW et al. Rectal fecal impaction treatment in childhood constipation: enemas versus high doses oral PEG. Pediatrics. 2009;124(6):e1108–e1115. doi: 10.1542/peds.2009-0022. [DOI] [PubMed] [Google Scholar]
- 7.Youssef NN, Peters JM, Henderson W et al. Dose response of PEG 3350 for the treatment of childhood fecal impaction. J Pediatr. 2002;141(3):410–414. doi: 10.1067/mpd.2002.126603. [DOI] [PubMed] [Google Scholar]
- 8.Biggs WS, Dery WH. Evaluation and treatment of constipation in infants and children. Am Fam Physician. 2006;73(3):469–477. [PubMed] [Google Scholar]
- 9.Dupont C, Leluyer B, Maamri N et al. Double-blind randomized evaluation of clinical and biological tolerance of polyethylene glycol 4000 versus lactulose in constipated children. J Pediatr Gastroenterol Nutr. 2005;41(5):625. doi: 10.1097/01.mpg.0000181188.01887.78. [DOI] [PubMed] [Google Scholar]
- 10.Loening-Baucke V, Pashankar DS. A randomized, prospective, comparison study of polyethylene glycol 3350 without electrolytes and Milk of Magnesia for children with constipation and fecal incontinence. Pediatrics. 2006;118(2):528. doi: 10.1542/peds.2006-0220. [DOI] [PubMed] [Google Scholar]
- 11.Candy D, Belsey J. Macrogol (polyethylene glycol) laxatives in children with functional constipation and faecal impaction: a systematic review. Arch Dis Child. 2009;94(2):156–160. doi: 10.1136/adc.2007.128769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller MK, Dowd MD, Friesen CA, Walsh-Kelly CM. A randomized trial of enema versus polyethylene glycol 3350 for fecal disimpaction in children presenting to an emergency department. Pediatr Emerg Care. 2012;28(2):115–119. doi: 10.1097/PEC.0b013e3182442c0a. [DOI] [PubMed] [Google Scholar]
- 13.Pashankar DS, Loening-Baucke V, Bishop WP. Safety of polyethylene glycol 3350 in the treatment of chronic constipation in children. Arch Pediatr Adolesc Med. 2003;157(7):661. doi: 10.1001/archpedi.157.7.661. [DOI] [PubMed] [Google Scholar]
- 14.Lam TJ, Mulder CJ, Felt-Bersma JF. Differences in taste between three polyethylene glycol preparations: a randomized double-blind study. Patient Preference Adherence. 2011;5:423–426. doi: 10.2147/PPA.S22780. [DOI] [PMC free article] [PubMed] [Google Scholar]


